Abstract
Background
Hospitalization for heart failure (HHF) is among the most important problems confronting medicine. Late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) robustly identifies intrinsic myocardial damage. LGE may indicate inherent vulnerability to HHF, regardless of etiology, across the spectrum of heart failure stage or left ventricular ejection fraction (LVEF).
Methods and Results
We enrolled 1068 consecutive patients referred for CMR where 448 (42%) exhibited LGE. After a median of 1.4 years (Q1 to Q3: 0.9 to 2.0 years), 57 HHF events occurred, 15 deaths followed HHF, and 43 deaths occurred without antecedent HHF (58 total deaths). Using multivariable Cox regression adjusting for LVEF, heart failure stage, and other covariates, LGE was associated with first HHF after CMR (HR: 2.70, 95% CI: 1.32 to 5.50), death (HR: 2.13, 95% CI: 1.08 to 4.21), or either death or HHF (HR: 2.52, 95% CI: 1.49 to 4.25). Quantifying LGE extent yielded similar results; more LGE equated higher risks. LGE improved model discrimination (IDI: 0.016, 95% CI: 0.005 to 0.028, P=0.002) and reclassification of individuals at risk (continuous NRI: 0.40, 95% CI: 0.05 to 0.70, P=0.024). Adjustment for competing risks of death that shares common risk factors with HHF strengthened the LGE and HHF association (HR: 4.85, 95% CI: 1.40 to 16.9).
Conclusions
The presence and extent of LGE is associated with vulnerability for HHF, including higher risks of HHF across the spectrum of heart failure stage and LVEF. Even when LVEF is severely decreased, those without LGE appear to fare reasonably well. LGE may enhance risk stratification for HHF and may enhance both clinical and research efforts to reduce HHF through targeted treatment.
Keywords: late gadolinium enhancement, magnetic resonance imaging, myocardial delayed enhancement, myocardial fibrosis, myocardial infarction
Introduction
Myocardial “scar” visualized by late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) indicates irreversible damage to the myocardium, and this insult to myocardial structure may increase the risk of subsequent hospitalization for heart failure (HHF).1 HHF remains one of the most important problems confronting medicine.2 HHF is a sentinel event that indicates disease progression from outpatient heart failure3–4 and a clear decrement in patients' health status. Mortality and rehospitalization after HHF remain unacceptably high after discharge.2–3 Furthermore, HHF costs nearly $40 billion annually and incurs the largest costs for Medicare.5 Yet, HHF remains poorly understood.6 Clinical risk scores derived from clinical parameters do not predict HHF well.7–8 More than 20 phase 3 drug trials have not improved intermediate to long‐term clinical outcomes.5,9 Similarly, increased adherence to performance measures has not improved outcomes.10 A common theme underlying these sobering findings might be the failure to address a potential root cause of HHF, myocardial damage. There remains an urgent need to identify and understand HHF substrates in order to enhance risk stratification and ultimately develop targeted prevention strategies.
LGE mostly indicates fundamental and irreversible derangements in myocardial structure, including myocardial infarction;11–13 focal replacement myocardial fibrosis from nonischemic causes;14 or infiltrative disease such as amyloidosis.15 LGE is associated with poor response to medical therapy,16 revascularization,17–18 or device‐based therapy19 and is associated with poor prognosis.1,20–23 Whether the presence and extent of myocardial “scar” or “damage” detected by LGE culminates in actual HHF events across the spectrum of heart failure stage and left ventricular ejection fraction (LVEF) is unknown. This issue is important because prognostically relevant LGE is prevalent in heart failure1,24 and other cohorts.20–23 LGE may stratify risk of HHF, identify vulnerable patients, and indicate higher risks of nonresponse to conventional treatment.
To investigate the associations between the presence and extent of myocardial scar detectable by LGE and first HHF after CMR, we followed a large cohort of consecutive patients after referral for LGE CMR. We hypothesized that after adjusting for LVEF by CMR and other important covariates, the presence and extent of LGE would be associated with subsequent HHF, mortality, or both. These data may be relevant for both clinical and research efforts to reduce HHF through targeted treatment.
Methods
Patient Population
After Institutional Review Board approval, we prospectively recruited 1439 adult patients referred for clinical CMR with contrast at the University of Pittsburgh Medical Center (UPMC) CMR Center at the time of their CMR scan from December 4, 2009 to September 20, 2012 and followed them through December 20, 2012. Patients were approached at the time of CMR scanning to acquire their informed consent to use their data for research purposes. This cohort derived entirely from clinical referrals was formed a priori to examine whether CMR data predict patient outcomes. We excluded those with adult congenital heart disease (n=127), which is a unique pathophysiologic subgroup. Otherwise, we minimized exclusions because we were interested in studying the entire spectrum of the heart failure continuum, including those with subclinical disease who still may be vulnerable to HHF. Inclusion criteria were written informed consent and completion of a contrast‐enhanced CMR scan. We excluded 244 patients who did not follow‐up within the UPMC health system, which may have compromised our ability to retrieve medical records related to HHF. The UPMC health system is the largest healthcare provider in Western Pennsylvania and includes a large, integrated network of hospitals and clinics sharing a common electronic medical record. While results were very similar with or without this exclusion, the exclusion avoids introducing bias related to differential ability to identify death or HHF outcomes, which is important for the competing risk analysis.
Data Elements
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Pittsburgh.25 Hospitalization status and baseline comorbidity data were determined according to the medical record at the time of CMR scanning. Such data represent the actual data used for medical decision‐making, which is relevant for generalizability. Therefore, prior heart failure diagnosis and adjudication for first HHF admission after CMR required documentation during the admission from the physicians responsible for the patient's care. Vital status was ascertained by both Social Security Death Index queries and medical record review.
“First HHF after CMR” included any HHF following CMR scanning (regardless of any prior HHF), and was identified by medical record review using a definition from prior epidemiologic studies.26 HHF required physician documentation and: (1) documented symptoms (eg, shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea) and physical signs (eg, edema, pulmonary rales) consistent with heart failure; (2) supporting clinical findings (eg, pulmonary edema on chest x‐ray); or (3) therapy for heart failure, including diuretics, digitalis, angiotensin‐converting enzyme inhibitors, or beta‐blockers.26 HHF was confirmed by 2 investigators blinded to LGE. There were no disagreements.
We also addressed the question of whether LGE adds incremental prognostic value beyond ischemic cardiomyopathy, which is believed to have worse outcomes than nonischemic cardiomyopathy.27 We employed the definition of ischemic cardiomyopathy by Felker et al,27 which required LVEF ≤40% as well as one of the following: (1) patients with history of myocardial infarction (MI) or revascularization (CABG or PCI); (2) patients with ≥75% stenosis of left main or proximal LAD; or (3) patients with ≥75% stenosis of ≥2 epicardial vessels. We added LGE to a model with risk adjustment similar to the approach adopted by Felker and colleagues, which adjusted for LVEF, age, mitral regurgitation, heart failure stage (a surrogate for NYHA class8 because we did not assess/record NYHA class), and finally ischemic cardiomyopathy.
Cine CMR
Patients received clinical CMR scans by dedicated CMR technologists with a 1.5 Tesla Siemens Magnetom Espree (Siemens Medical Solutions) and a 32‐channel phased array cardiovascular coil. The exam included standard breath held segmented cine imaging with steady‐state free precession (SSFP). Left ventricular dimensions, myocardial mass (indexed to body surface area), volume indices, and LVEF were measured without geometric assumptions from short‐axis stacks of end diastolic and end systolic cine frames. LVEF <55% was considered abnormal and we used age‐ and gender‐appropriate thresholds to identify ventricular remodeling manifest by increases in left ventricular mass and volume,28 important for the algorithm specifying heart failure stage assignment (0, A, B, C, D). Because there were very few patients with stage D heart failure, which is a nuanced distinction from stage C, these were included with stage C for analysis purposes. Mitral regurgitation was classified as mild, moderate, or severe.
Late Gadolinium Enhancement
Late gadolinium enhancement (LGE) imaging was performed 10 minutes after a 0.2 mmol/kg intravenous gadoteridol bolus (Prohance, Bracco Diagnostics) with optimization of inversion times. To optimize LGE, we used a phase‐sensitive inversion‐recovery (PSIR) pulse sequence to increase signal‐to‐noise ratios, correct for surface coil intensity variation, and render signal intensity proportional to T1 recovery.29 When patients could not hold their breath or had arrhythmia, single‐shot steady‐state free precession (SSFP) LGE and motion corrected, averaged PSIR images were acquired.30
Myocardial infarction was identified when LGE involved the subendocardium in a coronary distribution; other “atypical” patterns of LGE were designated “nonischemic.” This strategy yields sensitivities and specificities >90% for MI detection.14 Atypical LGE and MI were not necessarily mutually exclusive. LGE required confirmation in orthogonal planes and was interpreted with the intent of prioritizing specificity over sensitivity to avoid confusing LGE with artifact and noise. The extent of LGE was assessed visually in terms of the segmental extent of LGE (none, <25%, 26% to 50%, 51% to 75%, >75%) from which the percent of left ventricular myocardium exhibiting LGE was computed.21
Statistical Analysis
Categorical variables were summarized as frequencies and percentages, and continuous variables were summarized as median and interquartile range, because some continuous variables exhibited skewed distributions on visual inspection, and the Shapiro‐Wilk test indicated non‐normal distributions. Chi square (χ2) tests or Fisher's exact tests compared categorical variables. Wilcoxon rank sum tests compared continuous variables. Survival analysis for first HHF after CMR right censored for mortality and examined only the first HHF event. Statistical tests were 2 sided, and P<0.05 was considered significant.
The Kaplan‐Meier method with log‐rank test and Cox regression examined associations between covariates and outcomes, and the number of covariates was constrained to yield roughly 10 events per predictor variable.31 Multivariable Cox regression models were stratified for hospitalization stage and heart failure stage.8 These disease severity variables do not provide insight into etiology and are known to indicate a different clinical trajectory than outpatients without heart failure. Because we were not interested in quantifying their association with first HHF after CMR, stratification still permitted adjustment for hospitalization and baseline heart failure stage with further risk adjustment from additional covariates. We confirmed the proportional hazards assumption with nonsignificant time interaction terms for LGE. We tested for interactions between variables in the HHF model by adding terms that were the product of the variables. We used the integrated discrimination improvement (IDI) and net reclassification improvement (NRI) indices to evaluate the added predictive ability of survival models with the introduction of the LGE variable.32
HHF survival analysis right censored for mortality, but deaths were not random events (informative dropouts) and may share some of the same risk factors as HHF. To further evaluate the association between LGE and HHF while accounting for competing risks of death, we used a joint modeling approach with the “proc nlmixed” procedure in SAS.33 The 2 submodels included a Weibull regression model for time to HHF (censored for death) and a Weibull regression model for time to death (censored for HHF), which were then linked via a shared random term φ. Both submodels included the covariates previously selected for each stratified Cox model (based on univariable models for each outcome) and stratified by hospitalization and baseline heart failure. Statistical analyses were performed using SAS 9.2.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1 according to LGE. Those with LGE generally had higher comorbidity. Myocardial infarction was less prevalent than nonischemic scar (203 versus 273, P<0.001). Similar to previous community‐based epidemiologic studies, our cohort revealed a similar bimodal distribution of LVEF in those with baseline heart failure (Figure 1).34 Examples of LGE are shown in Figure 2.
Table 1.
Patient Characteristics (n=1068)
| Variable | LGE (N=448) | No LGE (N=620) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, median (Q1 to Q3), y | 59 (51 to 69) | 53 (39 to 63) | <0.001 |
| Female, n (%) | 123 (27) | 313 (50) | <0.001 |
| White race, n (%) | 397 (89) | 545 (88) | 0.72 |
| Black race, n (%) | 44 (10) | 52 (8) | 0.42 |
| General indication for CMR exam | |||
| Known or suspected cardiomyopathy, n (%) | 211 (47) | 274 (44) | 0.35 |
| Possible coronary disease/viability/vasodilator stress testing, n (%) | 233 (52) | 215 (35) | <0.001 |
| Evaluation for arrhythmia substrate*, n (%) | 85 (19) | 207 (33) | <0.001 |
| Possible mass or thrombus, n (%) | 25 (6) | 23 (4) | 0.15 |
| Comorbidity | |||
| Diabetes, n (%) | 117 (26) | 93 (15) | <0.001 |
| Hypertension, n (%) | 268 (60) | 260 (42) | <0.001 |
| Dyslipidemia, n (%) | 206 (46) | 196 (32) | <0.001 |
| Current cigarette smoking, n (%) | 79 (18) | 81 (13) | 0.04 |
| Atrial fibrillation or flutter, n (%) | 38 (8) | 57 (9) | 0.69 |
| Hospitalized/Inpatient status, n (%) | 188 (42) | 165 (27) | <0.001 |
| Prior coronary revascularization, n (%) | 149 (33) | 35 (6) | <0.001 |
| Body mass index, median (Q1 to Q3), kg/m2 | 28 (25 to 33) | 28 (24 to 34) | 0.44 |
| Baseline heart failure, n (%) | 123 (27) | 91 (15) | <0.001 |
| Heart failure stage, n (%) | |||
| 0 | 25 (6) | 169 (27) | <0.001 |
| A | 60 (13) | 223 (36) | |
| B | 248 (55) | 164 (26) | |
| C or D | 115 (26) | 64 (10) | |
| Medications | |||
| ACE inhibitor, angiotensin receptor blocker, or mineralocorticoid antagonist | 233 (52) | 200 (32) | <0.001 |
| Beta‐blockers | 299 (67) | 240 (39) | <0.001 |
| Aspirin or other antiplatelet | 277 (62) | 248 (40) | <0.001 |
| Statin | 227 (51) | 183 (30) | <0.001 |
| Loop diuretic | 127 (28) | 97 (16) | <0.001 |
| Laboratory and CMR characteristics | |||
| Creatinine, median (Q1 to Q3), mg/dL | 0.9 (0.9 to 1.2) | 0.9 (0.8 to 1.0) | <0.001 |
| Glomerular filtration rate, median (Q1 to Q3), mL/min per 1.73 m2 | 81 (64 to 93) | 90 (73 to 92) | 0.001 |
| Ejection fraction, median (Q1 to Q3), % | 49 (34 to 61) | 60 (54 to 66) | <0.001 |
| Left ventricular mass index, median (Q1 to Q3), g/m2 | 69 (57 to 88) | 53 (44 to 64) | <0.001 |
| End diastolic volume index, median (Q1 to Q3), mL/m2 | 91 (72 to 112) | 78 (66 to 91) | <0.001 |
| End systolic volume index, median (Q1 to Q3), mL/m2 | 44 (28 to 72) | 30 (23 to 40) | <0.001 |
| Moderate or severe mitral regurgitation by cine CMR, n (%) | 22 (5) | 17 (3) | 0.06 |
| Myocardial infarction, n (%) | 203 (45) | — | — |
| Acute myocardial infarction, n (%) | 63 (14) | — | — |
| Nonischemic or atypical scar evident on LGE images, n (%) | 273 (61) | — | — |
| Amyloid suspected by LGE, n (%) | 15 (3) | — | — |
ACE indicates angiotensin‐converting enzyme; CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement.
Arrhythmia substrate refers to evaluating patients with known or suspected ventricular arrhythmia for structural heart disease.
Figure 1.
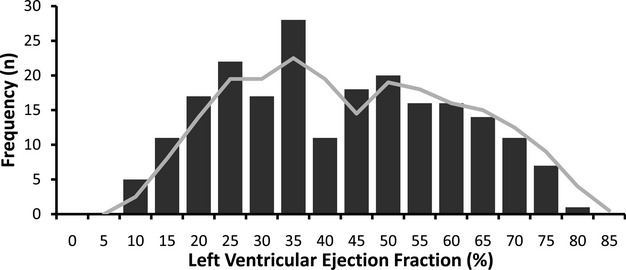
The distribution of left ventricular ejection fraction (LVEF) measures by cardiovascular magnetic resonance (CMR) (n=214) in those with heart failure approximates a bimodal distribution (shown by the moving average trend line) observed in previous reports from community‐based epidemiologic studies.34
Figure 2.
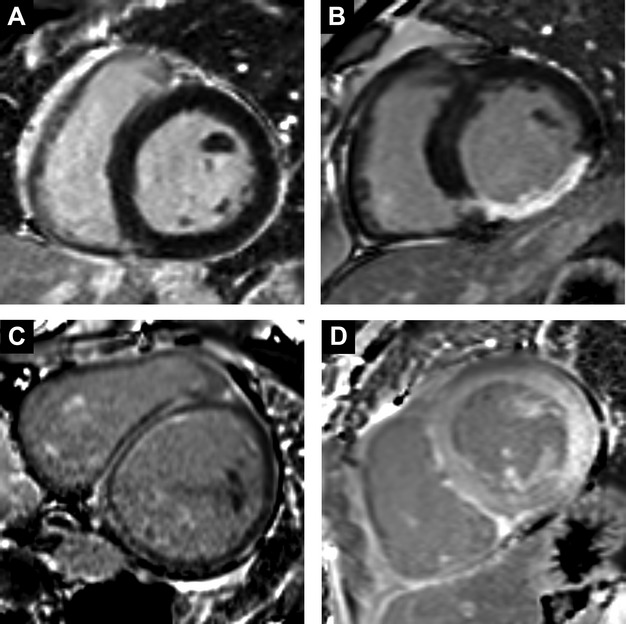
Clinical examples of significant late gadolinium enhancement (LGE) are shown, including: normal myocardium without significant LGE (A); inferior wall transmural myocardial infarction (B); midwall fibrosis following anthracycline chemotherapy (C); cardiac amyloidosis pattern from light chain (AL) systemic amyloidosis with brighter signal in the myocardium compared to blood pool from diffuse myocardial LGE and rapid gadolinium contrast clearance from the blood (D).
Survival Analysis
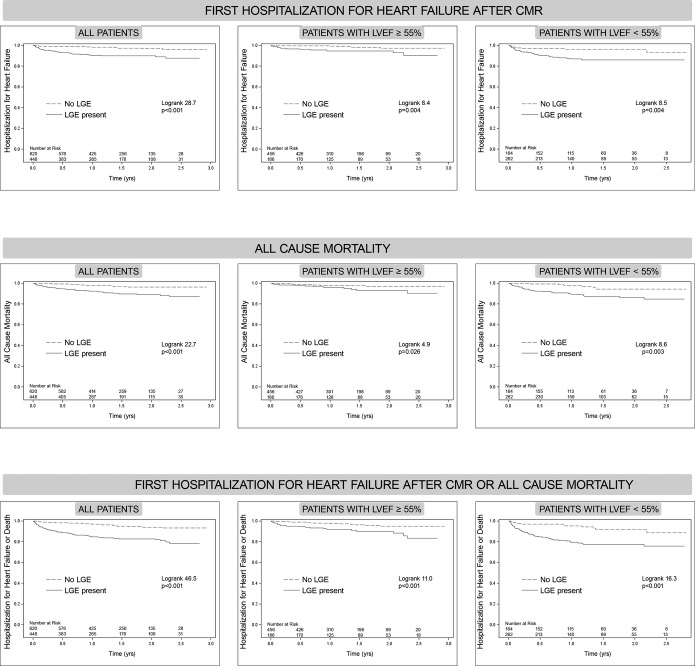
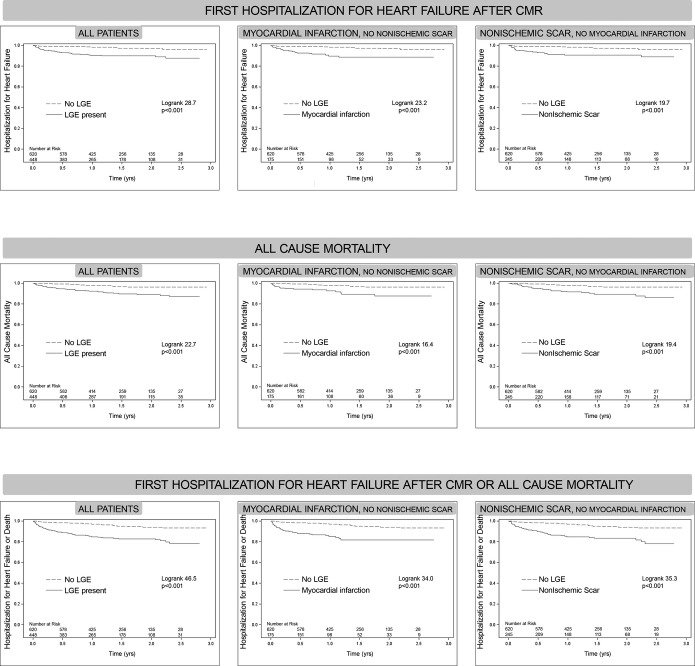
LGE was associated with HHF and/or mortality regardless of LVEF status. After a median of 1.4 years (Q1 to Q3: 0.9 to 2.0 years), 57 HHF events occurred after CMR, 15 deaths followed HHF, 43 deaths occurred without antecedent HHF (58 total deaths and 100 total death/HHF events). In those exhibiting LGE, there were 43 HHF events, 42 deaths, and 74 total death/HHF events. In those without LGE, there were 14 HHF events, 16 deaths, and 26 total death/HHF events. Cardiac amyloid evident by LGE (n=15) constituted <10% of events (5 deaths, 5 HHF, 8 death or HHF events). Only 2 of the 57 individuals (<4%) with HHF had a concurrent acute myocardial infarction (without ST elevation) with peak troponins of 6 and 13. Kaplan‐Meier curves show a relation between LGE and outcomes, regardless of whether LVEF is preserved (Figure 3), and regardless of LGE etiology (Figure 4). LGE remained associated with outcomes in those with preserved LVEF after excluding those with suspected amyloid (not shown). More extensive LGE is associated with higher risk, indicating a dose‐response relationship (Figure 5). Conversely the absence of LGE was prognostically favorable, even in the setting of severe systolic dysfunction with LVEF ≤30%, which represented the lower 10th percentile of LVEF (Figure 6).
Figure 3.
Kaplan‐Meier curves for those with or without late gadolinium enhancement (LGE) showing time to event for: hospitalization for heart failure (top row), all cause mortality (middle row), or either event (bottom row). The cohort is also stratified according to whether preserved left ventricular ejection fraction (LVEF) is preserved (≥55%, middle column), or not (<55%, right column). LGE was significantly associated with adverse events for all outcomes, even when LVEF was preserved (≥55%) or reduced (<55%). CMR indicates cardiovascular magnetic resonance.
Figure 4.
Kaplan‐Meier curves for those with or without late gadolinium enhancement (LGE) where the cohort is further stratified according to the presence of myocardial infarction (excluding those with nonischemic myocardial scar, middle column) or according to the presence of nonischemic myocardial scar (excluding those with myocardial infarction, right column). The curves show time to event for: hospitalization for heart failure (top row), all cause mortality (middle row), or either event (bottom row). Risks of adverse events were similar for the presence myocardial infarction and nonischemic scar based on their log rank statistics. CMR indicates cardiovascular magnetic resonance.
Figure 5.
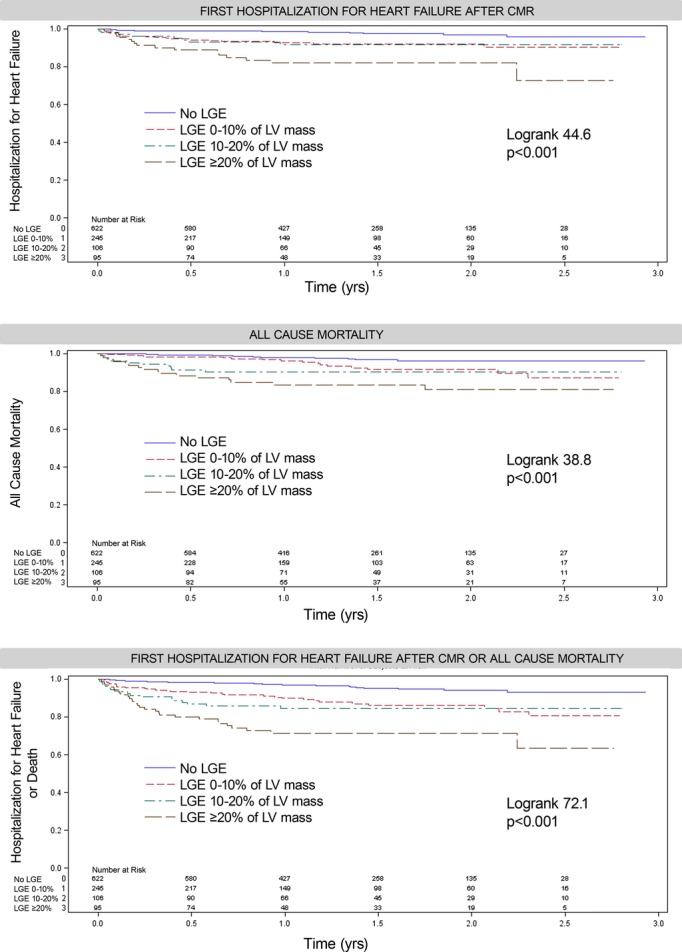
More extensive LGE is associated with higher risks of adverse outcomes demonstrating a dose–response relationship. CMR indicates cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle.
Figure 6.
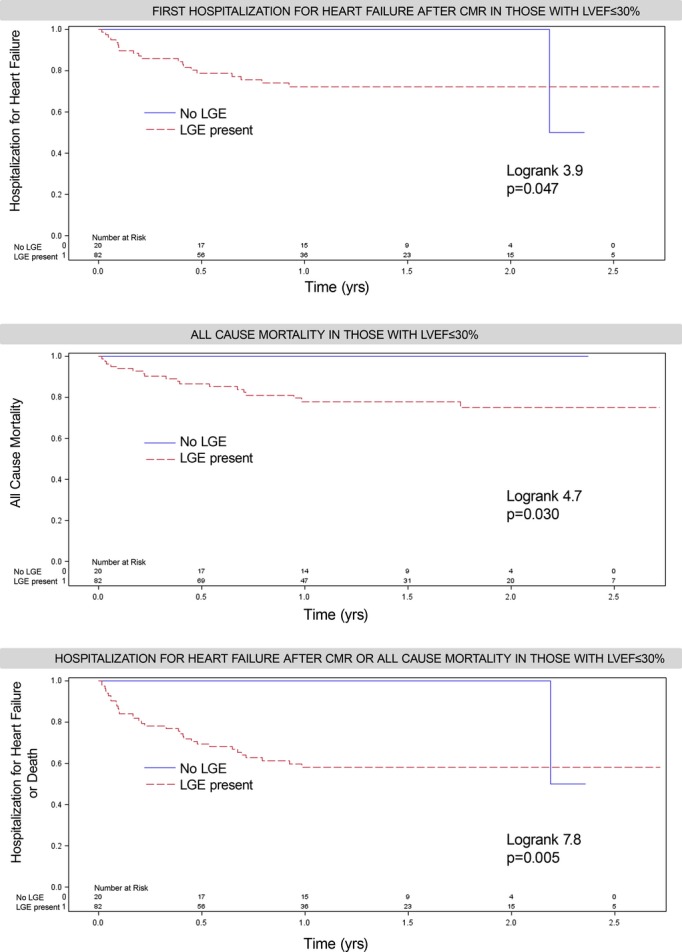
Among 102 (of 1068) patients with severely reduced systolic function (LVEF ≤30%), of whom 73 were hospitalized, absence of LGE was prognostically favorable where no events occurred during follow‐up, except in 1 of the 2 remaining patients still being followed after 2 years who experienced HHF. In contrast, those with LGE were a very high‐risk group with 1‐year event rates of 28% for HHF, 22% for mortality, and 42% for either HHF or mortality. LGE was prevalent when LVEF was ≤30%; 46 had nonischemic scar and 44 had MI (8 had both). CMR indicates cardiovascular magnetic resonance; HHF, hospitalization for heart failure; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
The relationship between LGE and outcomes remained significant in multivariable Cox regression models (Table 2). For Cox regression models with HHF as the outcome, there was no interaction between LVEF and LGE (interaction term: HR 1.01, 95% CI: 0.97 to 1.04, P=0.79) or between nonischemic LGE and myocardial infarction (interaction term: HR 0.33, 95% CI: 0.09 to 1.20, P=0.09). Adding LGE to the fully adjusted stratified Cox regression model for HHF (Table 2) also increased its discrimination (IDI: 0.016, 95% CI: 0.005 to 0.028, P=0.002) and improved the classification of individuals as shown by the continuous NRI (0.40, 95% CI: 0.05 to 0.70, P=0.024), and the categorical NRI (0.15, 95% CI: 0.04 to 0.27, P=0.006) using arbitrarily selected 0.05 and 0.25 risk categories.
Table 2.
Association Between Variables and Outcomes in Univariable and Multivariable Models
| Outcome | Variable | Univariable Models | Multivariable Model (Stratified by Hospitalization and Heart Failure Stage) | ||||
|---|---|---|---|---|---|---|---|
| χ2 | HR (95% CI) | P Value | χ2 | HR (95% CI) | P Value | ||
| Hospitalization for heart failure (n=57 events) | LVEF (per 5% decrement) | 40.8 | 1.27 (1.18 to 1.35) | <0.001 | 0.5 | 1.04 (0.93 to 1.16) | 0.51 |
| LGE | 24.1 | 4.54 (2.48 to 8.29) | <0.001 | 7.4 | 2.70 (1.32 to 5.50) | 0.006 | |
| Left ventricular mass index (per 10 g/m2 increment) | 21.9 | 1.18 (1.10 to 1.27) | <0.001 | 1.9 | 1.08 (0.97 to 1.20) | 0.17 | |
| eGFR (per 10 mL/min per 1.73 m2 decrement) | 19.1 | 1.32 (1.16 to 1.49) | <0.001 | 2.7 | 1.11 (0.98 to 1.24) | 0.10 | |
| Diabetes (type 2) | 17.2 | 3.07 (1.81 to 5.21) | <0.001 | 3.0 | 1.65 (0.94 to 2.90) | 0.08 | |
| Age (per 10 year increment) | 12.8 | 1.40 (1.16 to 1.68) | <0.001 | 1.1 | 1.12 (0.91 to 1.38) | 0.30 | |
| Moderate or severe mitral regurgitation by cine CMR | 12.4 | 4.15 (1.88 to 9.15) | <0.001 | ||||
| History of atrial fibrillation | 5.2 | 2.11 (1.11 to 4.00) | 0.022 | ||||
| Number of known diseased coronary vessels | 3.6 | 1.31 (0.99 to 1.72) | 0.056 | ||||
| Smoker | 2.9 | 1.71 (0.92 to 3.18) | 0.09 | ||||
| Female | 0.1 | 0.93 (0.55 to 1.58) | 0.80 | ||||
| Mortality (n=58 events) | LVEF (per 5% decrement) | 23.0 | 1.20 (1.11 to 1.29) | <0.001 | 2.7 | 1.10 (0.98 to 1.23) | 0.10 |
| Left ventricular mass index (per 10 g/m2 increment) | 21.9 | 1.18 (1.10 to 1.27) | <0.001 | 0.0 | 0.99 (0.89 to 1.11) | 0.87 | |
| Number of known diseased coronary vessels | 21.7 | 1.70 (1.36 to 2.12) | <0.001 | 0.8 | 1.12 (0.87 to 1.45) | 0.38 | |
| LGE | 20.0 | 3.72 (2.09 to 6.62) | <0.001 | 4.7 | 2.13 (1.08 to 4.21) | 0.030 | |
| Diabetes (type 2) | 16.1 | 2.94 (1.73 to 4.97) | <0.001 | 4.0 | 1.78 (1.01 to 3.15) | 0.047 | |
| Age (per 10 year increment) | 17.9 | 1.50 (1.24 to 1.81) | <0.001 | 5.7 | 1.28 (1.05 to 1.57) | 0.017 | |
| Moderate or severe mitral regurgitation by cine CMR | 11.0 | 3.80 (1.73 to 8.38) | <0.001 | ||||
| History of atrial fibrillation | 6.4 | 2.42 (1.22 to 4.81) | 0.012 | ||||
| Smoker | 5.4 | 2.01 (1.12 to 3.62) | 0.020 | ||||
| eGFR (per 10 mL/min per 1.73 m2 decrement) | 3.4 | 1.11 (0.99 to 1.24) | 0.067 | ||||
| Female | 2.0 | 1.49 (0.86 to 2.61) | 0.16 | ||||
| Hypertension | 0.5 | 1.20 (0.71 to 2.01) | 0.50 | ||||
| Hospitalization for heart failure or mortality (n=100 events) | LVEF (per 5% decrement) | 57.7 | 1.24 (1.17 to 1.31) | <0.001 | 1.6 | 1.06 (0.97 to 1.15) | 0.20 |
| LGE | 39.8 | 4.24 (2.70 to 6.59) | <0.001 | 11.8 | 2.52 (1.49 to 4.25) | <0.001 | |
| Age (per 10 year increment) | 27.6 | 1.46 (1.27 to 1.68) | <0.001 | 4.7 | 1.20 (1.02 to 1.41) | 0.031 | |
| Diabetes (type 2) | 27.3 | 2.92 (1.96 to 4.37) | <0.001 | 6.4 | 1.79 (1.14 to 2.81) | 0.011 | |
| Number of known diseased coronary vessels | 21.8 | 1.5 (1.28 to 1.84) | <0.001 | 0.0 | 1.00 (0.82 to 1.22) | 0.99 | |
| Left ventricular mass index (per 10 g/m2) | 21.2 | 1.15 (1.08 to 1.22) | <0.001 | 0.6 | 1.00 (0.99 to 1.01) | 0.45 | |
| eGFR (per 10 mL/min per 1.73 m2 decrement) | 18.8 | 1.22 (1.12 to 1.34) | <0.001 | 2.3 | 1.07 (0.98 to 1.17) | 0.13 | |
| Moderate or severe mitral regurgitation by cine CMR | 16.7 | 3.69 (1.97 to 6.91) | <0.001 | 4.0 | 1.97 (1.02 to 3.82) | 0.045 | |
| History of atrial fibrillation | 9.6 | 2.18 (1.33 to 3.57) | 0.002 | 5.2 | 1.80 (1.09 to 2.98) | 0.023 | |
| Hypertension | 8.7 | 1.89 (1.23 to 2.80) | 0.003 | 1.0 | 0.79 (0.49 to 1.25) | 0.31 | |
| Smoker | 4.1 | 1.63 (1.02 to 2.62) | 0.042 | ||||
| Female | 0.3 | 1.10 (0.74 to 1.65) | 0.63 | ||||
Covariates in univariable models are ranked in terms of strength of association with outcomes as measured by χ2 values. Multivariable models employing the strongest univariable predictors indicate a greater than 2‐fold risk of adverse outcomes for LGE. Values in bold indicate key data. CI indicates confidence interval; CMR, cardiovascular magnetic resonance; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
LGE expressed as a continuous variable (% of LV mass) remained similarly associated in multivariable stratified models with HHF (HR: 1.17 per 10% increase in myocardial mass affected, 95% CI: 1.03 to 1.33, χ2=5.8, P=0.016), death (HR: 1.28 per 10% increase, 95% CI: 1.12 to 1.45, χ2=13.5, P<0.001), and HHF or death (HR: 1.10 per 10% increase, 95% CI: 1.03 to 1.18, χ2=8.6, P=0.003) where more LGE equated higher risk.
Adjusting for the competing risk of death and the covariates specified in the multivariable models from Table 2, the LGE hazard ratio for HHF events was HR 3.83 (95% CI: 1.13 to 13.0) in stratified models. The estimated coefficient of the random term φ trended positive (0.84, 95% CI: −0.06 to 1.73, P=0.07), suggesting that HHF and death shared similar risk factors (Table 2) and therefore were positively correlated to each other. Thus, patients with LGE who died before HHF still would have had a high probability for HHF if they had survived.
Those with ischemic cardiomyopathy defined by Felker et al27 were vulnerable and had worse outcomes than those without ischemic cardiomyopathy in univariable Cox regression models (HHF: HR 3.30, 95% CI: 1.67 to 6.54; death or HHF: HR 3.67, 95% CI: 2.22 to 6.05; or death: HR 4.36, 95% CI: 2.35 to 8.08). For predicting HHF, LGE added incremental prognostic value beyond the Felker et al27 definition of ischemic cardiomyopathy. Adjusting for LVEF, age, mitral regurgitation, heart failure stage, and ischemic cardiomyopathy, LGE was associated with increased risks of HHF (HR: 2.35, 95% CI: 1.23 to 4.50), IDI 0.0086 (95% CI: 0.001 to 0.0188, P=0.048), continuous NRI 0.50 (95% CI: 0.16 to 0.82, P=0.006), and categorical NRI 0.11 (95% CI: 0.03 to 0.20, P=0.002 using arbitrary 0.05 and 0.25 risk categories).
Discussion
The principal finding of this study is the association between the presence and extent of myocardial damage detected by LGE and first hospitalization for heart failure (HHF) after CMR across the spectrum of both heart failure stages and LVEF in a large cohort of consecutive patients referred for CMR. LGE was prevalent, and the association with outcomes appeared similar in our cohort regardless of ischemic or nonischemic etiology. LGE was associated with outcomes in those with depressed LVEF or preserved LVEF. These associations persisted even after adjustment for LVEF and several other important variables using a variety of statistical measures, and more extensive LGE was associated with higher risks. Those without LGE had a more favorable prognosis, even when systolic function was severely reduced. Adjustment for the competing risk of death, which shares common risk factors with HHF, further strengthened the association between LGE and HHF.
These data are important because clinical risk scores summarizing clinical information without LGE data do not estimate risk of hospital readmission well.7 LGE introduces vulnerability. LGE represents irreversible myocardial damage and is associated with poor response to: (1) medical therapy,16 (2) revascularization,17–18 or (3) device‐based therapy.19 In this study, we link LGE to HHF after CMR. While defining optimal treatment of those with scar remains requires further investigation, LGE may provide opportunities to improve cardiac care by enhancing risk stratification, which is essential to optimize medical decision‐making. Conceptually, the intensity of follow‐up as well as the selection of medical therapy,16 revascularization,17–18 or device‐based therapy19 can be calibrated to patient vulnerability to HHF and other outcomes1,20–23 that may be informed by LGE.
In a landmark study, Gulati et al1 recently reported an association between midwall fibrosis detected by LGE and HHF in the dilated cardiomyopathy subset. We extend these results by studying HHF across the full spectrum of LGE (regardless of cause), heart failure stage, and LVEF in a large cohort with minimal exclusions, and we further analyzed the competing risk of mortality. Given the apparent myocyte loss indicated by LGE, the implication of additional afterload on remaining myocytes, and the stimulus for further ventricular remodeling, LGE may lead to supply‐demand mismatch and contribute to energy starvation, a proposed mechanism of myocardial dysfunction that may culminate in HHF.
HHF remains a recalcitrant problem that imposes enormous burden on patients and society,2 reflecting disease progression3–4 and a significant decrement in health status. For the evaluation of new therapies under development, it may be important to understand how treatment responses vary by LGE status. Populations with a high prevalence of LGE may be less responsive to new therapies designed to prevent HHF compared to populations with a low prevalence of LGE. Conversely, opposite effects may be expected for anti‐arrhythmic strategies where treatment benefits may be apparent only if baseline risk, which has been linked with LGE1,35–36is sufficiently high. Notably, LGE is prevalent in heart failure1,24 and unexpected, prognostically relevant LGE is common in both referred and population‐based samples.1,22 Given the diverse and frequently international populations recruited for contemporary clinical trials where baseline characteristics can be linked with outcomes,37 LGE may be an important confounder in the evaluation of new treatments. LGE may be an important descriptor in trials and influence power calculations. These issues may be especially important given the lack of progress, cost, and challenge of developing new therapies to prevent HHF.
The similar prevalence of ischemic LGE (ie, myocardial infarction) and nonischemic scarring by LGE and their similar associations with HHF highlight nonischemic LGE as a significant contributor to HHF. If confirmed in community‐based cohorts, this finding linking nonischemic disease to HHF has important implications for heart disease prevention strategies, which currently revolve around coronary disease prevention. Prevention efforts targeted at nonischemic myocardial fibrosis and not solely coronary disease might be a useful strategy to lower the incidence of HHF but requires further investigation.
Limitations
Our study has limitations. First, the data are from a single‐center population with referral bias; thus, results may not generalize. We enrolled consecutive patients to minimize this limitation. We note that the cohort reproduced the bimodal LVEF distribution in heart failure patients observed in community‐based epidemiology studies.34 Second, the data were observational; thus, associations may reflect unmeasured confounders. The data, however, fit a general pattern of prior data linking LGE to adverse outcomes. Third, follow‐up was limited, but short‐term prognosis remains inherently important to patients and clinicians. Fourth, limited numbers of events constrained risk adjustment, statistical power, and prevented detailed examination of important subgroups. Further work is needed. Finally, we did not analyze emerging measures of myocardial extracellular matrix expansion (ie, the extracellular volume fraction) in noninfarcted myocardium which may refine further risk stratification, but these “T1 mapping” measures are not yet as available as LGE.38–40 Further work is needed.
Conclusions
Regardless of etiology, the presence and extent of scar or myocardial damage detected by LGE are associated with first HHF after CMR in a large cohort of consecutive patients referred for CMR, across heart failures stages and LVEF. In contrast, those without LGE fare better, even when LVEF is severely reduced. Collectively, these observations and other data20–23 support the general principle that myocardial structural abnormalities detectable by LGE are common and clinically important phenomena. LGE appears important regardless of LVEF, a central parameter in cardiology that currently governs medical, surgical, and device‐based therapy. Because LGE typically indicates irreversible injury, these data may be relevant to efforts to improve outcomes in HHF and develop new therapies.
Sources of Funding
Dr Schelbert is supported by a grant from The Pittsburgh Foundation, Grant M2009‐0068, and an American Heart Association Scientist Development grant (09SDG2180083) including a T. Franklin Williams Scholarship Award; funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Heart Association. Dr Wong is supported by grant K12 HS19461‐01 from the Agency for Healthcare Research and Quality. Dr Moon is supported by the UK National Institute for Health Research University College London Hospitals Biomedical Research Centre. This work was also supported by grant UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), NIH Roadmap for Medical Research.
Disclosures
None.
Acknowledgments
We gratefully acknowledge and appreciate the support of Drs Christopher R. Deible, Joan M. Lacomis, and Ferenc Czeyda‐Pommersheim from the Department of Radiology, and also Kathy Puntil, Deborah Yasko, Amritha Chandy, and Elizabeth Ruhl. We thank the patients at the University of Pittsburgh Medical Center who volunteered to participate in this research.
References
- 1.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013; 309:896-908 [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Fonarow GC, Gheorghiade M. Strategies and opportunities for drug development in heart failure. JAMA. 2013; 309:1593-1594 [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006; 296:2217-2226 [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013; 61:391-403 [DOI] [PubMed] [Google Scholar]
- 5.Pang PS, Komajda M, Gheorghiade M. The current and future management of acute heart failure syndromes. Eur Heart J. 2010; 31:784-793 [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011; 305:1702-1703 [DOI] [PubMed] [Google Scholar]
- 7.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011; 17:54-75 [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 128:e240-e319 [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Braunwald E. Hospitalizations for heart failure in the United States—a sign of hope. JAMA. 2011; 306:1705-1706 [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Peterson ED. Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009; 302:792-794 [DOI] [PubMed] [Google Scholar]
- 11.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999; 100:1992-2002 [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM. Performance of delayed‐enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double‐blinded, randomized trial. Circulation. 2008; 117:629-637 [DOI] [PubMed] [Google Scholar]
- 13.Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE. Late gadolinium‐enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010; 3:743-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non‐ischaemic cardiomyopathies. Eur Heart J. 2005; 26:1461-1474 [DOI] [PubMed] [Google Scholar]
- 15.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole‐Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005; 111:186-193 [DOI] [PubMed] [Google Scholar]
- 16.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta‐blocker therapy. Circulation. 2003; 108:1945-1953 [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000; 343:1445-1453 [DOI] [PubMed] [Google Scholar]
- 18.Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S. Value of delayed‐enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004; 110:1535-1541 [DOI] [PubMed] [Google Scholar]
- 19.Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, Auricchio A. Cardiac resynchronization therapy guided by late gadolinium‐enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011; 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed‐enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009; 120:2069-2076 [DOI] [PubMed] [Google Scholar]
- 21.Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P, Parker M, Judd RM, Kim RJ. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011; 4:610-619 [DOI] [PubMed] [Google Scholar]
- 22.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012; 308:890-896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JL, Kellman P, Cook SC, Schwartzman DS, Simon MA, Mulukutla SR, Schelbert EB. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. J Cardiovasc Magn Reson. 2013; 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourantas CV, Nikitin NP, Loh HP, Lukaschuk EI, Sherwi N, de Silva R, Tweddel AC, Alamgir MF, Wong K, Gupta S, Clark AL, Cleland JG. Prevalence of scarred and dysfunctional myocardium in patients with heart failure of ischaemic origin: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011; 13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PW, Newman AB, Harris TB, Butler J. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010; 3:495-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002; 39:210-218 [DOI] [PubMed] [Google Scholar]
- 28.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006; 8:417-426 [DOI] [PubMed] [Google Scholar]
- 29.Kellman P, Arai AE. Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging. 2012; 36:529-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piehler KM, Wong TC, Puntil KS, Zareba KM, Lin K, Harris DM, Deible CR, Lacomis JM, Czeyda‐Pommersheim F, Cook SC, Kellman P, Schelbert EB. Free‐breathing, motion‐corrected late gadolinium enhancement is robust and extends risk stratification to vulnerable patients. Circ Cardiovasc Imaging. 2013; 6:423-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995; 48:1503-1510 [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011; 30:11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu B, Chang C‐CH. Estimating Covariate Effects by Treating Competing Risks as Informative Dropouts. 2013Orlando, FL: East North American Region (ENAR), International Biometric Society [Google Scholar]
- 34.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012; 5:720-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012; 60:408-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong‐Sit P, Klein GJ, Stirrat J, Fine N, Pallaveshi L, Wisenberg G, Thompson TR, Prato F, Drangova M, White JA. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012; 5:448-456 [DOI] [PubMed] [Google Scholar]
- 37.Butler J, Subacius H, Vaduganathan M, Fonarow GC, Ambrosy AP, Konstam MA, Maggioni A, Mentz RJ, Swedberg K, Zannad F, Gheorghiade M. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST (efficacy of vasopressin antagonism in heart failure: outcome study with tolvaptan) trial. J Am Coll Cardiol. 2013; 61:571-579 [DOI] [PubMed] [Google Scholar]
- 38.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short‐term mortality. Circulation. 2012; 126:1206-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong TC, Piehler K, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2013. 10.1093/eurheartj/eht193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz‐Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013; 15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]