Abstract
Background
This study evaluated the prevalence of ideal cardiovascular (CV) health in the Atherosclerosis Risk in Communities Study and determined its relationship with prevalent retinopathy, wider retinal venular diameters, and narrower arteriolar diameters, which are risk markers for subclinical cerebrovascular disease and are associated with increased stroke and coronary heart disease (CHD) morbidity and mortality.
Methods and Results
We used gradings of fundus photography measurements from the Atherosclerosis Risk in Communities Study to examine the association of retinopathy and retinal arteriolar and venular calibers to the number of ideal CV health metrics. Prevalent retinopathy showed a graded relationship with the CV health categories and number of ideal CV health metrics present: retinopathy prevalence was 2.1% among those with ≥5 ideal CV health metrics compared with 13.1% among those with zero ideal CV health metrics (odds ratio [CI]), 4.8 [2.5 to 8.9]). Central retinal venule equivalent and central retinal arteriolar equivalent diameters also showed graded relationships with CV health categories and number of ideal CV health metrics: after adjustment for age, race, sex, and education, mean central retinal venular equivalent was 187.8 μm (95% CI, 186.9 to 188.6 μm) among those with ≥5 ideal CV health metrics compared with 201.1 μm (95% CI, 199.1 to 203.1 μm) among those with zero ideal CV health metrics. Mean central retinal arteriolar equivalent was 163.8 μm (95% CI, 163.0 to 164.5 μm) among those with ≥5 ideal CV health metrics compared with 157.9 μm (95% CI, 156.1 to 159.7 μm) among those with zero ideal CV health metrics.
Conclusions
Few adults had ideal cardiovascular health. Those with the best level of health were less likely to have retinopathy signs, wide retinal venules, and narrow retinal arterioles, which are associated with increased stroke and coronary heart disease risk.
Keywords: cardiovascular diseases, cardiovascular health metrics, cerebrovascular circulation, epidemiology, risk factors
Introduction
To further its goal to improve the cardiovascular (CV) health of the population, the American Heart Association (AHA) recently developed criteria to measure the CV health of the US population, based on 7 risk factors and health behaviors.1 These metrics are combined to define “ideal,” “intermediate,” and “poor” CV health. Ideal CV health comprises the absence of cardiovascular disease (CVD) and the following: no smoking in the past year, body mass index (BMI) <25 kg/m2, untreated blood pressure of <120/<80 mm Hg, untreated total cholesterol <200 mg/dL, adequate physical activity, a healthy diet, and untreated fasting serum glucose <100 mg/dL. Intermediate CV health consists of having ≥1 intermediate metric but no poor metric, and poor CV health consists of ≥1 poor metric, defined by certain cut points. The AHA has labeled the 7 ideal CV health metrics as “Life's Simple 7” in its campaign to improve CV health. A positive association between poorer AHA CV metrics and increased incidence of CVD has been demonstrated.2 However, no study has yet examined the relation between ideal CV health and retinal microvascular findings. Potentially relevant retinal microvascular findings include retinopathy signs—retinal microaneurysms, hemorrhages (flame‐shaped and blot), and soft exudates—and retinal vessel caliber measurements, namely, narrower arteriolar and wider venular diameters.
Previous studies have established that retinal microvascular findings are associated with individual CV risk factors. In the Atherosclerosis Risk in Communities Study (ARIC), the incidence of retinopathy was associated positively with blood pressure, fasting serum glucose, total cholesterol,3–4 and physical inactivity.5 In the Beaver Dam Eye Study, incident retinopathy and narrower central retinal arteriolar equivalent (CRAE) were independently associated with higher concurrent and past levels of blood pressure, smoking, BMI, and drinking.6–9 Greater dietary fiber intake was associated with more favorable wider retinal arteriolar and narrower venular diameters in ARIC.9
Prior studies have also linked retinal findings with incidence of CVD. For example, retinopathy, a marker for diabetic, hypertensive, or other retinal microvascular disease, is a risk factor for stroke,10–14 heart failure,15 and coronary heart disease (CHD) in population‐based samples and among individuals with diabetes and/or hypertension.6,16–17 Smaller retinal arteriolar diameters and larger venular calibers are associated with increased CHD incidence in ARIC women.6 Wider retinal venular calibers are also associated with increased stroke risk with10,18 or without narrow retinal arterioles.19–22
Despite previous evidence associating individual risk factors with retinal microvascular findings, it also might be helpful to understand how the CV health metrics promoted by the AHA relate to retinal findings. Our cross‐sectional study therefore examined whether AHA CV health metrics are associated with prevalent retinopathy and retinal vessel measurements in the ARIC study.
Methods
The ARIC study is a prospective cohort study of 15 792 men and women in 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and the northwest suburbs of Minneapolis, Minnesota. At baseline, in 1987–1989, the participants were between 45 and 64 years of age and were selected either by list or area probability sampling.23 African Americans were the only participants included in Jackson. The differences between participants and nonparticipants have been described.24 The present study is based on data from the third examination (1993–1995), which included 12 887 participants (86% of baseline) and was the first ARIC visit to obtain retinal photographs. We excluded participants whose race was neither black nor white (n=38); those who had prevalent CHD (n=895), prevalent stroke (n=252), ungradable photographs or missing data for retinal vessel caliber and other retinal signs (n=1854); and those with missing data for any of the CV health metrics (n=590). Our final analytic sample included 9258 participants (72% of those present for the third examination), consisting of 3856 men (42%) and 5402 women (58%); see Figure1. The study population was made up of 80% (7368) whites and 20% (1890) blacks.
Figure 1.
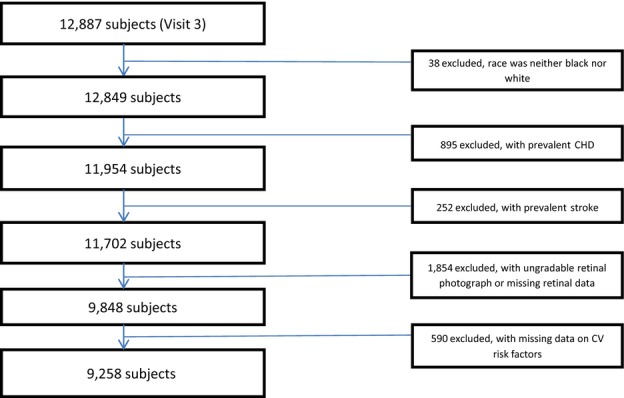
Participant inclusion. CHD indicates coronary heart disease; CV, cardiovascular.
Institutional review boards at each study site approved the study, and written informed consent was obtained for examination.
The ARIC study protocols have been described previously.2,23–25 We obtained smoking status from personal interviews. Trained technicians obtained height and weight measurements in a scrub suit to calculate BMI. Certified phlebotomists collected fasting blood, which was used to measure plasma cholesterol and serum glucose concentrations. We measured sitting blood pressure 3 times using a random‐zero sphygmomanometer after a 5‐minute rest and used the mean of the last 2 measurements for analysis. We identified via interviews the medications used for lowering blood pressure, cholesterol, and glucose, verified from participants' prescription bottles. Physical activity was computed from the response of participants to the Baecke questionnaire.25 A slightly modified, 66‐item Harvard food frequency questionnaire 26 was used in calculating a healthy diet score. We then used the AHA definitions of CV health 1 to classify each CV health metric into ideal, intermediate, or poor categories, as shown in Table 1.
Table 1.
Distribution of Baseline Cardiovascular Health Metrics in the ARIC Study Population Without Prevalent Cardiovascular Disease, 1993–1995
| Health Metric | Definition | Total Sample, % (n=9258) | Women, % (n=5402) | Men, % (n=3856) |
|---|---|---|---|---|
| Smoking | ||||
| Ideal | Never or quit >12 months | 81.2 | 82.3 | 79.6 |
| Intermediate | Former ≤12 months | 1.6 | 1.3 | 2.0 |
| Poor | Current | 17.3 | 16.4 | 18.5 |
| Body mass index | ||||
| Ideal | <25 kg/m² | 27.9 | 31.3 | 23.1 |
| Intermediate | 25 to 29.99 kg/m² | 39.5 | 33.7 | 47.7 |
| Poor | ≥30 kg/m² | 32.6 | 35.0 | 29.2 |
| Physical activity | ||||
| Ideal | ≥150 min/week moderate or ≥75 min/week vigorous or ≥150 min/week moderate+vigorous | 41.8 | 37.5 | 47.9 |
| Intermediate | 1 to 149 min/week moderate or 1 to 74 min/week vigorous or 1 to 149 min/week moderate+vigorous | 23.2 | 26.0 | 19.4 |
| Poor | None | 34.9 | 36.5 | 32.8 |
| Healthy diet score | ||||
| Ideal | 4 to 5 components | 2.3 | 2.9 | 1.3 |
| Intermediate | 2 to 3 components | 54.7 | 60.4 | 46.8 |
| Poor | 0 to 1 components | 43.0 | 36.6 | 51.9 |
| Total cholesterol | ||||
| Ideal | <200 mg/dL, without medication | 40.7 | 35.6 | 47.8 |
| Intermediate | 200 to 239 mg/dL or treated to <200 mg/dL | 41.1 | 42.5 | 39.1 |
| Poor | ≥240 mg/dL | 18.2 | 21.9 | 13.1 |
| Blood pressure | ||||
| Ideal | <120/<80 mm Hg, without medication | 34.2 | 34.8 | 33.5 |
| Intermediate | SBP 120 to 139 mm Hg or DBP 80 to 89 mm Hg or treated to <120/<80 mm Hg | 46.9 | 46.7 | 47.0 |
| Poor | SBP ≥140 mm Hg or DBP ≥90 mm Hg | 18.9 | 18.5 | 19.5 |
| Fasting serum glucose | ||||
| Ideal | <100 mg/dL, without medication | 50.4 | 56.8 | 41.4 |
| Intermediate | 100 to 125 mg/dL or treated to <100 mg/dL | 37.9 | 32.4 | 45.8 |
| Poor | ≥126 mg/dL | 11.7 | 10.9 | 12.8 |
ARIC indicates Atherosclerosis Risk in Communities Study; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Trained technicians performed retinal photography according to standardized procedures.27 After 5 minutes of dark adaptation, a 45 degree‐retinal photograph was obtained of 1 randomly selected eye. These photographs were digitized, and trained graders estimated the caliber of individual retinal arterioles and venules by a computer‐assisted technique. Trained graders also evaluated the retinal photographs for retinopathy signs: microanerysms, flame‐shaped hemorrhages, blot hemorrhages, or soft exudates. The graders' retinal vascular measurements had high reproducibility.27–28
Statistical Analysis
We computed the prevalence of the combinations of ideal CV health metrics and used logistic regression to calculate the odds ratio and 95% confidence interval of prevalent retinopathy associated with the number of ideal CV health metrics. We adjusted for age, sex, race, and education, with P values <0.10 as covariates. We also computed the prevalence according to AHA CV health categories stratified by sex and used logistic regression to calculate the odds ratios and 95% confidence intervals of prevalent retinopathy according to the CV health categories, adjusted for age, race, and education. Interactions of retinopathy with sex and poor CV health were tested by including cross‐product terms in the logistic regression models.
General linear modeling was used to calculate the mean and 95% confidence interval of central arteriolar and venular diameters (equivalents) according to the number of ideal CV health metrics. For linear modeling of arteriolar equivalents, we used SAS Proc GLM and adjusted for corresponding venular diameters, age, sex, race, and education as covariates. For the venular diameters, covariates were the corresponding arteriolar diameters, age, sex, race, and education. SAS (version 9.3) was used for all analyses.
Results
Prevalence of Ideal Cardiovascular Health
The distribution of ideal CV health metrics in the ARIC study population is shown in Table 1. Of the participants, 81.2% did not smoke in the past year, 27.9% had a BMI <25 kg/m2, 41.8% had adequate physical activity, 2.3% regularly ate a healthy diet, 40.7% had untreated total cholesterol <200 mg/dL; 34.2% had untreated blood pressure <120/<80 mm Hg, and 50.4% had untreated fasting serum glucose <100 mg/dL. A higher proportion of women than men met ideal levels for each metric, except for total cholesterol and physical activity.
As shown in Table 2, only 0.1% of ARIC study participants had all 7 ideal CV health metrics; 2.3% had 6 and 8.5% had 5 ideal health metrics; ≈45% had either 3 or 4 ideal health metrics; 42.5% had either 1 or 2 ideal health metrics; and 1.9% of participants had no ideal health metric.
Table 2.
Prevalence* and Odd Ratios of Retinopathy According to Number of Ideal CV Health Metrics, Adjusted for Age, Race, Education, and Sex
| Number of Ideal CV Health Metrics | Number With Retinopathy | Total Number (%) in Category | % Retinopathy in Category* | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| 5 to 7 | 21 | 1002 (10.8) | 2.1 | 1.0 | Reference |
| 4 | 52 | 1627 (17.6) | 3.2 | 1.4 | 0.8 to 2.3 |
| 3 | 118 | 2518 (27.2) | 4.7 | 1.9 | 1.2 to 3.0 |
| 2 | 156 | 2539 (27.4) | 6.1 | 2.3 | 1.4 to 3.7 |
| 1 | 125 | 1396 (15.1) | 9.0 | 3.3 | 2.0 to 5.3 |
| 0 | 23 | 176 (1.9) | 13.1 | 4.8 | 2.5 to 8.9 |
5 to 7, Ideal CV health metrics analyzed as 1 stratum. CI indicates confidence interval; CV, cardiovascular.
Unadjusted.
The prevalence of “intermediate” CV health (having ≥1 intermediate metric and no poor metrics) was only 15.4%. A majority (84.5%) in ARIC had “poor” CV health, with 26.6% having ≥3 poor metrics (Tables 1 and 3).
Table 3.
Prevalence* and Odd Ratios of Retinopathy Associated with CV Health Categories, Adjusted for Age, Race, Education, and Sex
| CV Health Categories | Number With Retinopathy | Total Number (%) in Category | % Retinopathy in Category* | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Ideal | 0 | 8 (0.1) | 0 | ||
| Intermediate | 34 | 1421 (15.4) | 2.4 | 1.0 | Reference |
| Poor | 461 | 7829 (84.5) | 5.9 | 2.1 | 1.5 to 3.1 |
| Poor (1) | 99 | 2801 (30.3) | 3.5 | 1.5 | 1.0 to 2.1 |
| Poor (2) | 140 | 2566 (27.7) | 5.5 | 2.0 | 1.4 to 3.0 |
| Poor (3) | 119 | 1638 (17.7) | 7.3 | 2.6 | 1.7 to 3.8 |
| Poor (4 to 7) | 103 | 824 (8.9) | 12.5 | 4.4 | 2.9 to 6.6 |
Ideal and intermediate CV Health Categories analyzed as 1 stratum. Number with only 1 poor metric among those in the poor CV health category. Number with 2 poor metrics among those in the poor CV health category. Number with 3 poor metrics among those in the poor CV health category. Number with ≥4 metrics among those in the poor health category. CI indicates confidence interval; CV, cardiovascular.
Unadjusted.
Retinal Microvascular Findings
The prevalence of retinopathy was 5.4% (n=495). Prevalent retinopathy showed a graded relation to the number of ideal CV health metrics, after adjustment for age, race, sex, and education. As shown in Table 2, no retinopathy was observed among the few participants who met all 7 ideal health metrics. Retinopathy prevalence was very low among participants with 5 or 6 ideal health metrics, 2.2% and 1.9%, respectively. The prevalence of retinopathy increased as the number of ideal health metrics decreased, reaching 13.1% among participants with no ideal health metrics. The odds ratio of retinopathy, after adjustment for age, race, sex, and education, correspondingly increased with decreasing numbers of ideal CV health metrics. Participants with 4, 3, 2, 1, and 0 ideal CV health metrics had 1.4 (95% CI, 0.8 to 2.3), 1.9 (95% CI, 1.2 to 3.0), 2.3 (95% CI, 1.4 to 3.7), 3.3 (95% CI, 2.0 to 5.3), and 4.8 (95% CI: 2.5 to 8.9) higher odds of retinopathy compared with participants with ≥5 ideal CV health metrics.
As shown in Table 3, using the 3 AHA categories of health, the prevalence of retinopathy was 2.4% among participants with intermediate CV health and 5.9% among participants with poor CV health. Participants with poor CV health thus had 2.1‐fold (95% CI, 1.5 to 3.1) higher odds of retinopathy compared with those with no poor CV health metrics (ideal and intermediate categories). This odds ratio was larger in women, 3.1 (95% CI, 1.8 to 5.4), than men, 1.6 (95% CI, 1.0 to 2.6), with P=0.07 for the interaction between sex and poor CV health.
Mean CRAE showed a graded positive relation with the number of ideal CV health metrics (Table 4). The mean CRAE in participants with ≥5 ideal CV health metrics was 163.8 μm (95% CI, 163.0 to 164.5 μm), after adjustment for age, sex, race, education, and central retinal venular equivalent (CRVE). Mean CRAE gradually decreased with decreasing number of ideal CV health metrics to 157.9 μm (95% CI, 156.1 to 159.7 μm) in participants with 0 ideal CV health metrics. The mean CRAE among participants with AHA‐defined ideal or intermediate CV health (no poor metrics) was 161.2 μm (95% CI, 160.5 to 161.8 μm), whereas the mean CRAE among participants in the AHA poor CV health category was 159.2 μm (95% CI, 158.9 to 159.5 μm).
Table 4.
Retinal Vessel Calibers According to Number of Ideal CV Health Metrics, Adjusted for Age, Race, Education, and Sex
| Number of Ideal CV Health Metrics | Mean Retinal Arteriolar Equivalent (95% CI)* | Mean Retinal Venular Equivalent (95% CI)* |
|---|---|---|
| 7 | ||
| 6 | ||
| 5 | 163.8 (163.0 to 164.5) | |
| 4 | 161.8 (161.2 to 162.4) | 190.8 (190.1 to 191.4) |
| 3 | 159.2 (158.8 to 159.7) | 193.1 (192.6 to 193.6) |
| 2 | 158.2 (157.8 to 158.7) | 193.5 (193.0 to 194.1) |
| 1 | 156.7 (156.0 to 157.3) | 196.9 (196.2 to 197.6) |
| 0 | 157.9 (156.1 to 159.7) | 201.1 (199.1 to 203.1) |
5 to 7, Ideal CV health metrics analyzed as 1 stratum. CI indicates confidence interval; CV, cardiovascular.
In micrometers, also adjusted for central retinal arteriolar equivalent (CRAE).
In micrometers, also adjusted for central retinal venular equivalent (CRVE).
Mean CRVE was inversely associated with the number of ideal CV health metrics (Table 4). In participants with ≥5 ideal CV health metrics, mean CRVE was 187.8 μm (95% CI, 186.9 to 188.6 μm), after adjustment for age, sex, race, education, and CRAE. Mean CRVE increased with decreasing number of ideal CV health metrics to 201.1 μm (95% CI, 199.1 to 203.1 μm) in participants with 0 CV health metrics (Table 4). Correspondingly, mean CRVE was larger among participants in the AHA poor CV health category, 193.5 μm (95% CI, 193.2 to 193.8 μm), compared with participants who had no poor CV health metric (AHA ideal or intermediate categories), 190.1 μm (95% CI, 189.4 to 190.8 μm).
Discussion
This cross‐sectional community‐based study found that few adults at ARIC visit 3 met AHA's ideal level of CV health. We previously reported this for ARIC visit 1, and similar low prevalence of ideal CV health has been reported by other studies.2,29–31 Clearly, the US population has a long way to go in achieving ideal CV health.
The novel finding of this study was that people with better AHA CV health had a lower prevalence of retinopathy and more favorable retinal vessel diameters. This association was moderately strong and showed a dose–response relation across the number of ideal CV health metrics.
Our findings corroborate prior studies that have examined the association between some of the individual AHA health metrics and retinal microvascular abnormalities. Incident retinopathy has shown consistent positive associations with higher levels of blood pressure and fasting glucose but has been inconsistently associated with higher total cholesterol, BMI, and smoking in 3 community‐based prospective cohort studies.3–4,3–9,3–33 However, by shifting the focus to a combination of risk factors rather than emphasis on each and using metrics to assess these risk factors rather than single cutoff values as used in clinical practice for primary and secondary prevention, the AHA strategy is to promote a population‐level distribution of ideal risk factors rather than tailoring prevention only to those identified as high risk.
We found a stronger association between AHA's poor CV health metrics and retinopathy in women than in men. This finding parallels previous studies that reported narrow retinal arterioles and wider venules are more consistently associated with higher CHD risk in women than in men.34–35
The retinal vessels share similar anatomical and physiological properties with coronary and cerebral microcirculation and have been suggested as biomarkers to estimate vascular health. Also, retinal vascular changes may parallel physiological and pathological changes both in coronary micro‐ and macrocirculation.36–39 Changes in the retinal vessel diameters (narrow arterioles and wide venules) occur early in the pathogenesis of retinal vascular disease and may reflect past and current levels of CV health, as the diameters are sensitive to endothelial dysfunction from inflammation, changes in blood flow associated with tissue hypoxia, and other physiologic changes.36–39 Abnormal retinal vessel diameters may also contribute further to the development of retinopathy.40 Retinopathy, however, is an indicator of target organ damage and is currently used clinically in risk stratification of patients with hypertension and diabetes.
Retinal abnormalities have also been shown to predict stroke and CHD independent of traditional risk factors, especially in middle‐aged people (14, 18, 34, and 35). Signs of developing retinopathy are usually asymptomatic. Studies have shown that regression of retinal abnormalities may occur if there are early detection and strict management of risk factors such as diabetes and hypertension, which are the commonest causes of retinopathy in adult populations.41–42
The AHA's goal of defining criteria for CV health is an extension of its policies to significantly reduce the burden of CVD and stroke morbidity and mortality. The AHA has intensified efforts to promote ideal CV health metrics through community interventions in addition to clinical approaches. Prevention should be sustained, as both community and clinical interventions have been estimated to account for ≈50% reduction (each) in CHD mortality over 2 decades43 and should further reduce CVD and stroke mortality if used consistently.43–45 Retinal abnormalities are risk markers for subclinical CVD, stroke, and vision impairment in diabetic and hypertensive individuals. So, improvement in ideal CV health metrics should lead to reduction in stroke and CVD burden and reduce some signs of retinopathy, especially among people with diabetes and hypertension.
This study has some important limitations. First, as a cross‐sectional study, it is difficult to establish an antecedent–consequence relationship between ideal CV health metrics and retinal microvascular abnormalities. Second, measurement of CV health behaviors—physical activity and diet—were from questionnaires and may not be as valid and reproducible as the others. Third, the retinal data from ARIC participants were obtained from only 1 eye, which would have led to underestimation of retinopathy prevalence. However, measurements of retinopathy and retinal vessel caliber were standardized, as were measurements of the CV health metrics. Data were missing on ≈6.7% of subjects analyzed, mostly because of missing retinal data. Those with versus without retinal data had similar distributions of CV health metrics (data not shown). Thus, it seems unlikely that missing data led to significant bias in our findings.
In conclusion, we found few adults with AHA‐defined ideal CV health in ARIC. Those with the best level of ideal CV health had few retinopathy signs and were less likely to have narrow arterioles and wide venules, which are associated with increased stroke and CHD risk.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
none.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Horn LV, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WDAmerican Heart Association Strategic Planning Task Force Statistics Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010; 121:586-613 [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WDAtherosclerosis Risk in Communities (ARIC) Study Investigators Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57:1690-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TY, Klein R, Amirul Islam FM, Cotch MF, Couper DJ, Klein BEK, Hubbard LD, Sharrett AR. Three‐year incidence and cumulative prevalence of retinopathy: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2007; 143:970-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Sharrett AR, Klein BEK, Chambless LE, Cooper LS, Hubbard LD, Evans G. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000; 20:1644-1650 [DOI] [PubMed] [Google Scholar]
- 5.Tikellis G, Anuradha S, Klein R, Wong TY. Association between physical activity and retinal microvascular signs: the atherosclerosis risk in communities (ARIC) study. Microcirculation. 2010; 17:381-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Myers CE, Knudtson MD, Lee KE, Gangnon R, Wong TY, Klein BEK. Relationship of blood pressure and other factors to serial retinal arteriolar diameter measurements over time: the Beaver Dam Eye Study. Arch Ophthalmol. 2012; 130:1019-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R, Klein BEK, Moss SE, Wang Q. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol. 1994; 112:92-98 [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull. 2005; 73–74:57-70 [DOI] [PubMed] [Google Scholar]
- 9.Kan H, Stevens J, Heiss G, Klein R, Rose KM, London SJ. Dietary fiber intake and retinal vascular caliber in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007; 86:1626-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001; 358:1134-1140 [DOI] [PubMed] [Google Scholar]
- 11.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004; 3:179-183 [DOI] [PubMed] [Google Scholar]
- 12.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI‐defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006; 37:82-86 [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BEK, Liao DP, Hubbard LD, Mosley THARIC Investigators Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002; 288:67-74 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005; 65:1005-1009 [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Rosamond WD, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, Folsom AR, Klein R. Retinopathy and risk of congestive heart failure. JAMA. 2005; 293:63-69 [DOI] [PubMed] [Google Scholar]
- 16.Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol. 2002; 86:1002-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Sharrett AR, Klein BEK, Wang JJ, Chambless LE, Wong TY. Risk prediction of coronary heart disease based on retinal vascular caliber (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol. 2008; 102:58-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BEK, Wong TY, Burlutsky G, Mitchell P. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007; 28:1984-1992 [DOI] [PubMed] [Google Scholar]
- 19.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BEK, Wang JJ, Mitchell P, Vingerling JR, de Jong PTVM, Witteman JCM, Breteler MMB, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual‐participant meta‐analysis. Am J Epidemiol. 2009; 170:1323-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006; 166:2388-2394 [DOI] [PubMed] [Google Scholar]
- 21.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, de Jong PTVM, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006; 66:1339-1343 [DOI] [PubMed] [Google Scholar]
- 22.Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry. 2009; 80:158-165 [DOI] [PubMed] [Google Scholar]
- 23. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989; 129:687-702 [PubMed] [Google Scholar]
- 24.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom AR, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community‐based study vary by gender ethnicity. The atherosclerosis risk in communities (ARIC) study Investigators. J Clin Epidemiol. 1996; 49:1441-1446 [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36:936-942 [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985; 122:51-65 [DOI] [PubMed] [Google Scholar]
- 27.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999; 106:2269-2280 [DOI] [PubMed] [Google Scholar]
- 28.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, Brothers RJ, Nieto FJ. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002; 133:78-88 [DOI] [PubMed] [Google Scholar]
- 29.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011; 123:850-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012; 307:1273-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the national health and nutrition examination surveys (NHANES) 2003–2008. Circulation. 2012; 125:45-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Moss SE. The relation of systemic hypertension to changes in the retinal vasculature: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 1997; 95:329-348 [PMC free article] [PubMed] [Google Scholar]
- 33.van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003; 121:245-251 [DOI] [PubMed] [Google Scholar]
- 34.Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR, Mitchell P. Retinal vascular calibre and the risk of coronary heart disease‐related death. Heart. 2006; 92:1583-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10‐year cardiovascular mortality: a population‐based case‐control study. Ophthalmology. 2003; 110:933-940 [DOI] [PubMed] [Google Scholar]
- 36.de Jong FJ, Ikram MK, Witteman JC, Hofman A, de Jong PT, Breteler MM. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol. 2007; 61:491-495 [DOI] [PubMed] [Google Scholar]
- 37.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi‐ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006; 47:2341-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004; 45:2129-2134 [DOI] [PubMed] [Google Scholar]
- 39.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006; 124:87-94 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE, Cotch MF, Wong TY. Relationship of retinal vascular caliber with diabetes and retinopathy: the multi‐ethnic study of atherosclerosis (MESA). Diabetes Care. 2008; 31:544-549 [DOI] [PubMed] [Google Scholar]
- 41.Strachan MW, McKnight JA. Images in clinical medicine. Improvement in hypertensive retinopathy after treatment of hypertension. N Engl J Med. 2005; 352:e17. [DOI] [PubMed] [Google Scholar]
- 42.Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Aldington S, Chaturvedi N. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post‐hoc results from the DIRECT Programme. Diabet Med. 2011; 28:345-351 [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007; 356:2388-2398 [DOI] [PubMed] [Google Scholar]
- 44.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects—Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007; 167:573-579 [DOI] [PubMed] [Google Scholar]
- 45.Folsom AR, Yamagishi K, Hozawa A, Chambless LEAtherosclerosis Risk in Communities Study Investigators Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009; 2:11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]


