Abstract
Background
Sleep disturbance is prevalent among women with metastatic breast cancer (MBC). Our study examined the relationship of depression and marital status to sleep assessed over 3 nights of polysomnography (PSG).
Methods
Women with MBC (N=103) were recruited; they were predominately white (88.2%) and 57.8±7.7 years of age. Linear regression analyses assessed relationships among depression, marital status, and sleep parameters.
Results
Women with MBC who reported more depressive symptoms had lighter sleep (e.g., stage 1 sleep; P<.05), less slow-wave sleep (SWS) (P<.05), and less rapid eye movement (REM) sleep (P<.05). Single women had less total sleep time (TST) (P<.01), more wake after sleep onset (WASO) (P<.05), worse sleep efficiency (SE) (P<.05), lighter sleep (e.g., stage 1; P<.05), and less REM sleep (P<.05) than married women. Significant interactions indicated that depressed and single women had worse sleep quality than partnered women or those who were not depressed.
Conclusion
Women with MBC and greater symptoms of depression had increased light sleep and reduced SWS and REM, and single women had worse sleep quality and greater light sleep than married counterparts. Marriage was related to improved sleep for women with more depressive symptoms.
Keywords: Metastatic Breast Cancer, Polysomnography, Sleep Quality, Sleep Architecture, Depression, Women
1. Introduction
Women diagnosed with breast cancer have high rates of sleep disruption [1–5]. Sleep disturbance often begins before or during treatment and may continue long after treatment completion, often worsening for women with metastatic breast cancer (MBC) [6–8]. One recent study demonstrated that those with breast cancer had the highest number of sleep quality complaints among cancer patients [9]. The majority of studies examining sleep in women with breast cancer have relied on self-report or indirect measurement [10]. Relatively few studies have used polysomnography (PSG), the gold standard for objectively assessing sleep [10], to examine sleep patterns in individuals with breast cancer. Of the studies that utilized PSG, dysregulation of sleep architecture in cancer patients was evidenced by lower sleep efficiency (SE) [11], more time in lighter nonrapid eye movement (NREM) sleep (stages 1 and 2) and less time in deep NREM or slow-wave sleep (SWS) (stages 3 and 4), as well as less rapid eye movement (REM) sleep than experienced by the general population [12]. However, another study found little change in PSG-assessed sleep before and after completion of chemotherapy [13]. Evidence regarding objective changes in sleep architecture of women with MBC is inconclusive and further study is warranted.
There is a well-described correlation between depression and sleep disturbance in the general population [14,15]. Among women with breast cancer, nearly 20% to 30% experience depression [2,16,17], a higher prevalence than that seen in the general population. Depression further increases as breast cancer advances [18,19]. Palesh et al [8] found that higher baseline and worsening depression among women with MBC predicted progressive sleep problems and that sleep disruption was associated with autonomic dysregulation during the day, particularly loss of vagal tone [20]. Although many precipitating factors may engender sleep disturbance and depression in MBC (e.g., stress, pain) the physiologic or psychological changes underlying these phenomena are unknown.
In addition, the relationship of marriage and sleep quality in women with breast cancer has been understudied, with investigations primarily focusing on healthy adults. Studies have demonstrated that divorced women have higher rates of sleep disturbance and insomnia [21]. Further there is evidence that marital happiness is related to improved sleep quality in women, relative to those experiencing marital discord [22]. Spousal sleep problems also negatively affect sleep and mood as well as the health of their partners [23]. Thus marriage appears to be an important variable affecting sleep quality in cancer patients. We aimed to examine the relationship of marital status to sleep among metastatic breast cancer patients. Our study examined the relationship of depression and marital status with sleep parameters assessed by 2 nights of consecutive at-home PSG and 1 night of laboratory PSG. Our a priori hypothesis was that higher depression scores among women with MBC would be related to significantly greater sleep disturbance. Exploratory aims were to examine the role of a potential moderator, marital status, in relation to sleep quality and architecture. We explored potential interactions between depression and marital status as they related to sleep in women with MBC.
2. Methods
2.1. Participants
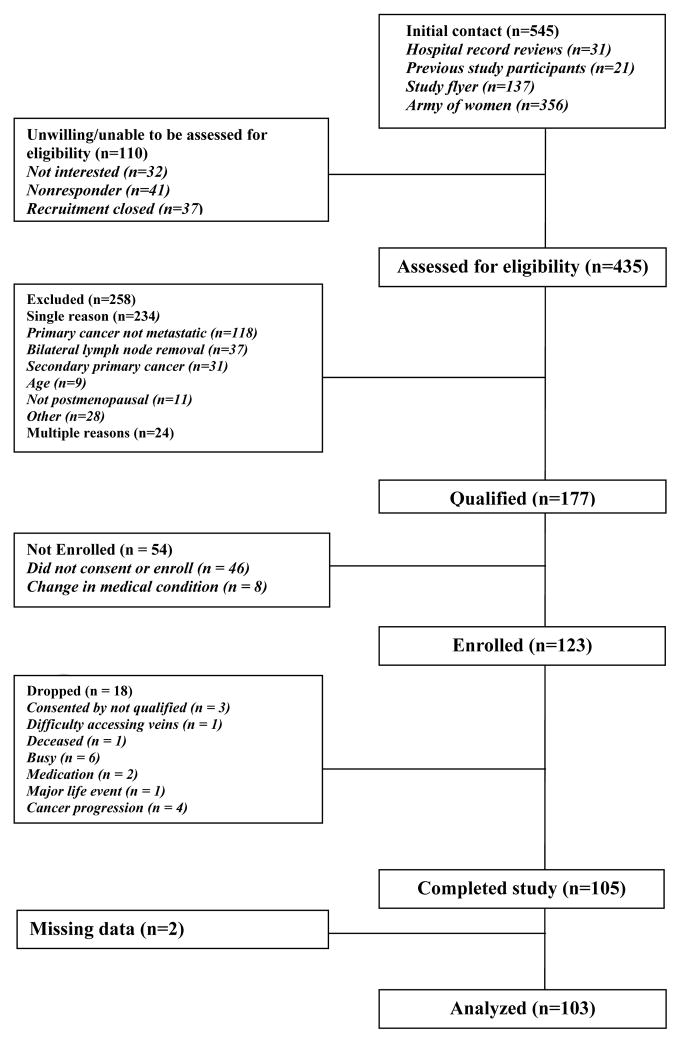
Predominately white women (88.2%) with MBC (N=103) were recruited and consented to participate (Fig. 1). The women were postmenopausal and were between the ages of 45 to 75 years (mean age, 57.8±7.7 y) (Table 1). They also had documented metastatic or recurrent breast cancer, with Karnofsky Performance Status Scale ratings of at least 70% (physical ability measure for medically ill patients) [24]. Participants were at least 2 months postchemotherapy or hormonal treatment. Women were excluded if they had (1) previous bilateral lymph nodes removal; (2) had active cancers (other than breast cancer) within the past 10 years; (3) had taken corticosteroids, glucocorticoids, or benzodiazepines within the week preceding and during their in-laboratory sleep study; (4) had a history of major psychiatric illness that required hospitalization in the preceding year; (5) had substance abuse or dependence; or (6) had engaged in regular travel involving 2 or more time zones or shift work (e.g., working 4:00 PM–midnight or 10:00 PM–6:00 AM) during the 3 months before the beginning of the study. Participants were recruited by letter of request through breast cancer clinics at Stanford University, by breast surgeons and oncologists at Stanford and University of California San Francisco (UCSF), through Army of Women mailing list, and through advertisements in the local newspapers and the Internet.
Fig. 1.
Patient flow diagram.
Table 1.
Baseline means and standard deviations of demographic variables by condition.
| Demographic characteristics (N=103) | Medical characteristics (N=103) | ||
|---|---|---|---|
| Mean/%/N±SD | Mean/%/N±SD | ||
| Age, y | 57.8±7.7 | Age at diagnosis, y | 47.3±8.3 |
| BMI | 27.3±5.5 | Stage at initial diagnosis | |
| 0/I | 32.1% | ||
| Education | II | 35.9% | |
| HS/GED/trade school | 8.7% | III | 15.5% |
| Some college/Graduate school | 91.3% | IV | 16.5% |
| Employment | Site of metastatic spread | ||
| Unemployed/retired | 52.4% | Soft tissue or chest wall | 33 |
| Part time | 20.4% | Bone | 28 |
| Fulltime | 27.2% | Organs | 16 |
| Multiple sites | 26 | ||
| Total gross income | |||
| <$20,000–$59,900 | 42.2% | Disease-free interval (mo) | 70.5±64.1 |
| $60,000–$99,900 | 36.2% | Treatment type | |
| ≥$100,000 | 33.3% | Radiation | 1 |
| D/K or refused | 2.0% | Chemotherapy | 16 |
| Hormonal therapy | 66 | ||
| Ethnicity | Monoclonal antibody | 18 | |
| Non-Hispanic | 97.1% | ||
| Hispanic/Latino | 2.9% | Hormone receptor status | |
| ER+ | 82.5% | ||
| Race | ER − | 16.5% | |
| Asian | 3.9% | PR+ | 67.0% |
| Black | 6.9% | PR − | 28.2% |
| White | 88.2% | HER2+ | 23.2% |
| Black/white | 1.0% | HER2 − | 67.0% |
| Marital status | Medications | ||
| Married/partnered | 57.3% | Pain | 53.4% |
| Single/NM | 8.7% | Sleep | 39.8% |
| Separated | 3.9% | Antidepressants | 29.1% |
| Divorced | 24.3% | Steroids | 16.5% |
| Widowed | 4.9% | Benzodiazepines | 12.6% |
| Other | 1.0% | ||
Abbreviations: N, number of patients; SD, standard deviation; y, years; BMI, body mass index; HS, high school; GED, general equivalency diploma; mo, months; D/K, do not know; NM, not married; ER, estrogen receptor; PR, progesterone receptor.
2.2. Design and procedures
All study procedures were approved by the Stanford University Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki. Participants underwent 2 nights of at-home PSG and one night of laboratory PSG, which consisted of a reduced montage (LOC/ROC, C3/C4, Fz/Cz, electrocardiogram 1/2, and electromyogram 1/2). The laboratory study was conducted for 28 hours at Stanford Hospital’s Clinical Translational Research Unit or at the Stanford Sleep Medicine Center during which participants were scheduled to sleep at their habitual sleep time for 8 hours. For participants who did not live near the clinics, the at-home protocol was conducted at a hotel prior to or after the 28-hour laboratory study. Computerized psychosocial measures were administered during the second night of at-home PSG.
2.3. Measures
2.3.1. Sleep measures
Our study utilized 3 nights of PSG-measured sleep (2 at-home and one laboratory PSG), with home sleep measured approximately 2 weeks before laboratory sleep. Surface electrodes (Grass Instruments Co, Braintree, MA) were taped to standard (International 10–20 System) locations on the face (bilateral electrooculogram, above and below the outer canthi; electromyogram, chin) and to the scalp (electroencephalogram, C3, C4, Fz, Oz) using Quik-Cap (Neuroscan, Charlotte, NC). Signals were collected by a Siesta system (Compumedics, El Paso, TX) onto compact flash memory cards. Data were analyzed using Pro Fusion PSG software (Compumedics, El Paso, TX) and scored in 30-second epochs after the method of Rechtschaffen and Kales [25]. Sleep scoring yielded both total amount and percentage of stages 1, 2, 3, and 4 of NREM sleep, REM sleep, and wake. Other calculated metrics include time available for sleep, total sleep time (TST), SE, sleep- or REM-onset latency, and wake after sleep onset (WASO).
2.3.2. Depression
To examine depressive symptoms among women with MBC, we administered the Center for Epidemiologic Studies Depression scale (CES-D) [26,27] and the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) [28], and also assessed the participants’ use of antidepressant medication. The CES-D was selected because the items of the scale minimize medical illness symptoms; it also has high internal consistency (α=0.89) and adequate test-retest reliability in individuals with cancer (r=0.57; P<.001) [26,29]. For between-group analyses involving the presence or absence of depression, we first used a median split (8.0) on the CES-D to separate those with less or more depressive symptoms for clinical relevance. Studies among nondepressed nonpatient and community samples have demonstrated that CES-D scores below 8 indicate no depression, thus supporting our use of the median split to differentiate depression in our sample [30–32]. Second, we utilized the SCID to determine lifetime or current depression. Lastly, we included a third variable that combined women who had a positive screening for clinical depression on the CES-D (e.g., a score ≥16 was considered the clinical cutoff point for high depressive symptoms) [27] or on the SCID, or those who were taking antidepressant medication. Fifteen women met criteria for depression on the CES-D and 6 women on the SCID; additionally, 21 women were taking selective serotonin reuptake inhibitors or other antidepressants for mood disturbance. A total of 33 women met criteria for depression on either the CES-D (score ≥16) or the SCID, or were taking antidepressants. Therefore, we captured a clinical range of women who reported subthreshold depressive symptoms (e.g., score ≥8), who met full criteria for depression, or who were being treated for depression.
2.3.3. Marital status
The role of marriage as a moderator was assessed through the marital status item collected in the demographic data. This variable was recoded and women were dichotomized into 2 groups of either married or single (e.g., single, divorced, widowed).
2.4. Data analysis
At-home PSG data were averaged over 2 nights and the means were used in the analyses. Laboratory PSG data were separately analyzed. To assess if sleep differed between home and laboratory environments, we examined these associations. Only approximately 3% of at-home PSG and laboratory PSG variables were moderately or less correlated (0.40<Spearman rho<0.60), thus indicating differences between at-home vs. in-laboratory sleep. Although we were not surprised that women had greater sleep disturbance in the laboratory, significant findings that emerged in the laboratory environment suggest the relationships between psychosocial and sleep variables are robust outside of their habitual sleep environments. We corrected for multiple comparisons equivalent to other studies using PSG in cancer populations (e.g., Bonferroni correction) [33]. Our between-group rather than continuous analysis of depression was designed to be clinically relevant and address the floor effect of our sample, as the majority of women with MBC did not meet full criteria for depression. To examine the effects of depression and marriage and their interaction on sleep, linear regression analysis was employed. Scheffé tests (α=0.05) were conducted to follow-up on significant interactions. Medical variables (e.g., treatment type, disease status, hormone receptor status, medications)(Table 1) did not show any significant or sizable (r>0.30) association with the predictor or outcome variables in our hypotheses, and therefore were not included in our main regression analyses. Analyses were conducted using SPSS 19.0. Data are presented as mean ± standard deviation.
3. Results
3.1. Depression and Sleep
3.1.1. Home PSG
Consistent with our hypothesis regarding depression and sleep disruption, significant effects of depression emerged for REM sleep. Women who reported more symptoms of depression on the CES-D (score of ≥8) spent less time in REM sleep (i.e., minutes of REM sleep divided by minutes of TST) and had fewer minutes of REM sleep than those reporting fewer symptoms of depression. Table 2 presents the CES-D means and standard deviations, and Table 3 shows the standardized regression coefficients and effect sizes for at-home and laboratory PSG sleep by depression severity and marital status.
Table 2.
Means, standard deviations, and significant differences of home- and laboratory PSG–measured sleep by Center for Epidemiologic Studies Depression scale depressive symptom severity.
| Low depression | High depression | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Home PSG | (n=54) | (n=48) |
| Sleep quality | ||
| Time available for sleep (min) | 522.5 ± 67.5 | 518.0 ± 82.6 |
| TST (min) | 394.9 ± 58.0 | 382.5 ± 77.2 |
| Sleep efficiency (%) | 75.8 ± 8.4 | 73.7 ± 11.5 |
| Sleep-onset latency (min) | 44.0 ± 28.6 | 47.5 ± 37.4 |
| REM sleep latency (min) | 110.0 ± 51.2 | 131.5 ± 62.8 |
| WASO (min) | 70.0 ± 37.0 | 70.5 ± 37.2 |
| Sleep stages | ||
| Stage 1 (%) | 39.6 ± 8.8 | 41.5 ± 9.9 |
| Stage 2(%) | 39.8 ± 8.6 | 39.0 ± 9.0 |
| SWS (%) | 6.9 ± 5.4 | 8.0 ± 6.1 |
| REM (%) | 13.3 ± 4.5 | 11.5** ± 5.4 |
| Stage 1 (min) | 155.9 ± 39.0 | 158.8 ± 47.7 |
| Stage 2 (min) | 156.7 ± 39.9 | 148.0 ± 44.6 |
| SWS (min) | 27.3 ± 21.7 | 29.7 ± 21.7 |
| REM (min) | 53.5 ± 20.5 | 45.8* ± 24.8 |
| Laboratory PSG | ||
| Sleep quality | ||
| Time available for sleep (min) | 515.7 ± 52.2 | 506.3 ± 49.2 |
| TST (min) | 339.8 ± 73.2 | 323.9 ± 71.4 |
| Sleep efficiency (%) | 66.1 ± 14.1 | 64.3 ± 14.1 |
| Sleep-onset latency (min) | 51.4 ± 42.1 | 47.1 ± 37.3 |
| REM sleep latency (min) | 141.3 ± 77.5 | 149.5 ± 82.3 |
| WASO (min) | 105.8 ± 47.1 | 116.6 ± 53.8 |
| Sleep stages | ||
| Stage 1 (%) | 43.0 ± 12.4 | 51.6** ± 17.9 |
| Stage 2(%) | 40.2 ± 9.7 | 34.3* ± 12. 9 |
| SWS (%) | 5.2 ± 5.8 | 4.3 ± 5.0 |
| REM (%) | 11.6 ± 4.8 | 9.9 ± 7.3 |
| Stage 1 (min) | 143.4 ± 45.0 | 164.3 ± 61.5 |
| Stage 2 (min) | 137.5 ± 45.1 | 110.5** ± 48.1 |
| SWS (min) | 18.7 ± 21.0 | 14.6 ± 18.4 |
| REM (min) | 40.2 ± 20.4 | 34.4 ± 27.5 |
Abbreviations: SD, standard deviation; min, minutes; TST, total sleep time; REM, rapid eye movement sleep; WASO, wake after sleep onset; SWS, slow-wave sleep; PSG, polysomnography.
Significant at P<.05.
Significant at P<.01.
Reference group is low depression.
Median split=high depression: Center for Epidemiologic Studies Depression scale (CES-D) score of ≥8.1 (mean, 14.92±5.94).
Low depression: CES-D score of ≤8 (mean, 2.97±2.57).
Table 3.
Standardized regression coefficients and effect sizes for home- and laboratory-polysomnography sleep by depression severity and family structure.
| β | R2adj | P value | Cohen d | |
|---|---|---|---|---|
| DEPRESSION SEVERITY | ||||
| Home PSG | ||||
| High depression (CES-D) vs low depression | ||||
| REM (%) | −0.26 | 0.057 | P<.01 | −0.37 |
| REM (min) | −0.23 | 0.044 | P<.05 | −0.34 |
| High depression (SCID) vs low depression | ||||
| REM (%) | −0.20 | 0.031 | P<.05 | −0.88 |
| REM (min) | −0.20 | 0.029 | P<.05 | −0.85 |
| Stage 2 (min) | −0.26 | 0.055 | P<.01 | −1.12 |
| High depression (combined) vs low depression | ||||
| REM latency (min) | 0.32 | 0.090 | P<.001 | −0.71 |
| Laboratory PSG | ||||
| High depression (CES-D) vs low depression | ||||
| Stage 1 (%) | 0.28 | 0.065 | P<.01 | 0.41 |
| Stage 2 (%) | −0.25 | 0.053 | P<.05 | −0.35 |
| Stage 2 (min) | −0.28 | 0.068 | P .01 | −0.43 |
| High depression (SCID) vs low depression | ||||
| REM latency (min) | 0.23 | 0.042 | P<.05 | −1.13 |
| High depression (combined) vs low depression | ||||
| SWS (min) | −0.22 | 0.039 | P<.05 | −0.49 |
| MARITAL STATUS | ||||
| Home PSG | ||||
| Single/no partner vs married | ||||
| TST (min) | −0.30 | 0.081 | P<.01 | −0.64 |
| WASO (min) | 0.20 | 0.029 | P<.05 | 0.40 |
| Sleep efficiency (min) | −0.28 | 0.068 | P<.01 | −0.58 |
| Stage 2 (min) | −0.27 | 0.063 | P<.01 | −0.56 |
| REM (%) | −0.23 | 0.043 | P<.05 | −0.48 |
| Laboratory PSG | ||||
| Single vs married | ||||
| Stage 1 (%) | 0.27 | 0.060 | P<.05 | 0.55 |
| Stage 2 (min) | −0.28 | 0.067 | P<.01 | −0.59 |
Abbreviations: PSG, polysomnography; CES-D, Center for Epidemiologic Studies Depression scale; REM, rapid eye movement sleep; min, minutes; SCID, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders; SWS, slow-wave sleep; TST, total sleep time; WASO, wake after sleep onset.
High depression: CES-D score of ≥8.1 (n=48).
High depression: SCID (n = 6).
High depression: combined (n=33), CES-D score of ≥ 16 + SCID + antidepressant users.
3.1.2. Laboratory PSG
In the high depression group, women spent significantly more time in light sleep, including more time in stage 1 sleep and less time in minutes and percentage in stage 2 sleep.
3.1.3. Clinical depression
Similar to our findings above, women with clinical depression had significant sleep disturbance including reduced REM sleep and spent significantly more time in the lighter stages of sleep. Table 3 highlights the standardized regression coefficients that demonstrated significant relationships between depression severity and sleep, both at home and in the laboratory.
3.2. Post hoc analyses: marital status
3.2.1. Home PSG
Participants who were single with MBC had less TST, more WASO, and worse SE than married women. Single women also had fewer minutes of stage 2 sleep and a lower percentage of REM sleep (Table 3).
3.2.2. Laboratory PSG
Similarly sleep architecture measured in the laboratory revealed that single women spent a greater percentage of sleep in stage 1 and fewer minutes in stage 2 sleep than married women with MBC. Overall, depressed and single women with MBC who slept in the laboratory had worse sleep quality and spent more time in lighter NREM sleep than depressed and married or not depressed and single or married counterparts. Significant interactions emerged between depression (CES-D) and marital status for laboratory-measured TST (β=0 .47, P<.01; R2adj=0.08, P<.05), WASO (β= −0.45, P<.01; R2adj=0.07, P<.05), stage 1 sleep percentage (β= −0.38, P<.05; R2adj=0.14, P≤.001), and stage 2 sleep in minutes (β=0.46, P<.01; R2adj=0.17, P<.001). Scheffé tests using analysis of variance were conducted to follow-up on the significant interactions. Significant comparisons are represented in Fig. 2 and depict differences in sleep among groups in relation to those who were depressed and single.
Fig. 2.
Significant Scheffé group comparisons for polysomnographic in-laboratory sleep quality and architecture among women with metastatic breast cancer.
Abbreviations: TST, total sleep time; WASO, wake after sleep onset.
*Significant at P<.05.
**Significant at P<.01.
aAll significant group comparisons are between the high depression Center for Epidemiologic Studies Depression scale score ≥8.1 (mean, 4.92±5.94) and single group.
4. Discussion
Women with MBC who had more depressive symptoms had more disturbed sleep at home and in the laboratory. Single women with MBC had worse sleep quality at home and worse SE than married women. We also found that marriage was protective of sleep quality in women with high depressive symptoms. At home women with MBC averaged 6.5 hours of sleep and had low SE of 74.0% (i.e., normal efficiency ≥85%) [34,35]. Our sample had considerably longer sleep-onset latency [34,36], extended REM latency, and reduced percentage of REM sleep relative to other advanced-cancer patients and healthy adults [33,35]. The number of nocturnal awakenings and WASO were considerably higher than that among healthy adults [34]. The poor sleep quality of the overall sample made sleep differences among the depressed and single patients more notable. Sleep architecture differences included increased stage 1 sleep, reduced stage 2 sleep, and lower than average SWS relative to other advanced-cancer patients. Although sleep quality and architecture were significantly more disturbed in the laboratory than at home for TST, WASO, SE, NREM sleep stages 1 and 2, SWS, and REM as might have been expected (P values ranged from P<.001 to P<.05), the findings linking depression and unmarried women were consistent across measurement settings.
4.1. Depression and sleep architecture
We found that women with high depressive symptoms obtained less REM sleep at home, both in percent and minutes of REM. In studies with depressed adults, REM percentage of sleep generally is found to increase relative to the other sleep stages [36]; however, this increase generally is related to a deficiency in SWS [37–40]. In our sample, REM sleep was reduced compared to other studies with healthy adults who averaged 17 % to 25% of REM sleep [35]. Our findings reflect those of a study that found that advanced-cancer patients spent 15% of their sleep in REM sleep [33], and another study of breast cancer patients who spent 18% of sleep in REM sleep [11]. Our sample had reductions in REM sleep beyond that previously observed; thus, it was striking that depressed women had reductions in REM sleep beyond their nondepressed counterparts. Women who met all 3 criteria for depression (SCID, CES-D, or use of antidepressant agents) had increased REM sleep latency, which may be a feature of antidepressant use. Studies have found that antidepressant agents typically suppress REM sleep [41] and the use of selective serotonin reuptake inhibitors by advanced-cancer patients increases REM sleep latency [33], which is congruent with our observations.
Women with MBC who were more depressed also spent more of their TST in stage 1 sleep (in the laboratory) and spent less time sleeping in stage 2 sleep (at home and in the laboratory) or in SWS (in the laboratory). These findings are different from other studies with advanced-cancer patients (e.g., stage 1, 10.9%; stage 2, 73.8%; SWS, 0.3%) [33], breast cancer patients (e.g., stage 1, 15%; stage 2, 61%; SWS, 4%) [11], and healthy adults (e.g., stage 1, 3%–12%; stage 2, 51%–72%; SWS, 0–17%) [34]. However, Parker et al [33] and Silberfarb et al [11] observed small samples of breast cancer patients (N=32 and N=15, respectively) and patients varied on level of cancer progression. Consistent with our findings, Fiorentino et al [12] found that breast cancer patients spent more time in the light stages of sleep, with reductions in SWS and REM sleep. Studies on depression have shown reductions in SWS [42] and also that increases in SWS may improve mood [43]. SWS also is known as restorative sleep and is linked to decreased cortisol secretion [44], reduced insulin sensitivity and sympathetic nervous system arousal [42,43], and lower nocturnal blood pressure [45,46]. The reductions in SWS found among depressed women in our sample may be associated with worsened immune and cardiovascular functioning. The significant disturbances in NREM and REM sleep found among more depressed women with MBC in our study suggest a negative and synergistic effect of advanced disease and worsened mood on sleep architecture.
4.2. Marital status, sleep quality, and sleep architecture
Single women with MBC had significantly worse sleep quality than married women. Further our findings demonstrate that single women have less TST, more WASO, and worse SE than married counterparts, which is consistent with studies on sleep quality in marriage [21–23]. Sleep architecture also significantly differed, with single women having reduced REM sleep and stage 2 sleep at home. These findings demonstrate a link between marriage and improved sleep and suggest that having a stable partner also may stabilize sleep, which also is consistent with studies that have examined the role of social rhythms in entraining biologic rhythms and sleep [20,47–49].
Breast cancer and its treatment often create lasting problems with sleep including insomnia [9]. Although biologic changes that can affect sleep have been reported in women with breast cancer (e.g., diurnal cortisol disruption, menopause) [50–53], it might be that marriage provides both psychological and social benefits that support regular sleep habits and improve sleep quality [48]. In contrast, a negative relationship emerged in a study on the role of spousal sleep problems and partner health, indicating that worse health and well-being of the partner are associated with greater spousal sleep problems (e.g., depressed mood, unhappiness) [54]. Although we were not able to measure our participants’ spousal sleep problems, it is promising to see a positive association with marriage and improved sleep quality. However, more research is needed to examine the role of marriage as it relates to objective and subjective sleep quality in women with MBC. To our knowledge, our study was the first study to examine the relationship of depression and marital status on objective sleep quality and architecture.
4.3. Depressed and single: adverse effects on sleep
The collective effect of being depressed and single with MBC revealed the most significant sleep quality and architecture disturbances. Although there is little research examining the role of marriage, sleep, and cancer, studies have demonstrated considerable psychological benefits of marriage on sleep [22], especially for individuals who reported premarital depression [55]. Another study that examined the effect of cancer and marital stability demonstrated that, while having cancer does not negatively impact marriage rates in women, individuals with breast cancer were notably less likely to be married [56]. It is unclear why women with breast cancer differ in marital status, but it may be that cancer related stressors, age at onset, or the constellation of treatment side effects experienced by women with breast cancer (e.g., early menopause, sexual dysfunction) play a role [57,58]. Our findings are consistent with studies that have found that women who were separated or divorced had greater sleep disturbance [21]. Overall, married women in our sample had improved sleep even in the laboratory and away from their husbands, which suggests greater psychological effects of having a husband rather than physical effects on regulating sleep, especially among depressed women. Greater disturbances in sleep for depressed and single women with MBC suggest that these women also may be at greater risk for immune compromise [59]. Thus the benefit of marriage, especially for depressed women with metastatic breast cancer, appears to be central for improved sleep quality and architecture among women with MBC.
4.4. Comparisons of home PSG to laboratory PSG
Although the findings using at-home and laboratory PSG general were consistent, they revealed greater disturbance in REM sleep associated with depression at home. Stage 2 sleep and REM sleep latency were equally disturbed in both environments, though differences in stage 1 sleep and SWS only emerged in the laboratory setting. For women who were single relative to married women, there were greater disturbances in their sleep in TST, WASO, SE, stage 2 sleep and REM sleep at home; however, laboratory findings revealed that the only differences in sleep disturbance emerged from stage 1 and 2 sleep.
Several studies have investigated the reliability and validity of objectively PSG measured sleep in both at-home and in-laboratory environments [60–62]. PSG-measured sleep at home is considered more reliable than laboratory-measured sleep when patients are fitted with the PSG device by a sleep technician, as in our study [60,63]. Thus it may be possible that substantive differences in sleep emerged in women based on assessment in their familiar home environment. For example, women with MBC may respond to the laboratory environment as a stressor, as many women with breast cancer experience anticipatory anxiety on returning to a hospital setting during treatment [64,65], and likely would experience this anxiety after treatment. In a study that investigated home-based and in-hospital PSG to measure obstructive sleep apnea, findings revealed improved sleep at home (e.g., improved SE, longer REM) compared to laboratory sleep [60]. Although greater sleep disturbances occurred in the laboratory for women overall, the differences that emerged among depressed or single women were robust and accounted for even greater discrepancies in sleep quality and architecture compared to nondepressed or married counterparts.
4.5. Study limitations
Our analyses were cross-sectional; however, the 3 nights of PSG-measured sleep strengthened our objective measurement of sleep in women with MBC. Analyses also were limited due to our sample size, and although we investigated a number of relationships with our sleep variables at-home and in-laboratory PSG significantly differed, indicating that these 2 measures should be separately examined. Utilizing categorical rather than continuous analysis of measures of depression might have reduced our power to detect an effect of depression on sleep. Due to the low number of women in our sample with full clinical depression on the CES-D and SCID, we chose to compare groups with clinically meaningful elevation of scores on the CES-D in comparison to those who had few depressive symptoms. Further, sleep and pain medication use may have affected sleep architecture in our sample; however, the relationships between these medications and our sleep outcomes were not sizeable (r<0.30). The generalizability of the study also was limited due to our sample of predominately non-Hispanic white women, with a relatively high education and socioeconomic status. Further, data were only collected on women with MBC, and thus we cannot generalize our results to women in earlier stages of breast cancer or to other individuals, including men with metastatic disease.
5. Conclusion
Our findings demonstrate that depressed or single women with MBC have disturbed sleep, including increased light NREM sleep, reduced SWS and REM sleep, and poor sleep quality. Marriage seemed to protect sleep quality and normalize sleep architecture in the presence of depression, even when women slept alone in the laboratory. Depressed and single women with MBC appear to be at the highest risk for objective sleep disturbances and may derive greater benefit from clinical interventions. Future studies would be useful and should examine how mood, marriage, and sleep disturbance are related to endocrine and immune function. These studies are central to identifying potential treatment methods that may improve depression, sleep, and other aspects of quality of life among women with breast cancer.
Highlights.
We examined the relationship of depression and marital status with sleep in cancer.
Sleep quality and architecture in metastatic breast cancer is characterized.
Women with greater depression had increased light sleep and reduced slow-wave sleep and rapid eye movement sleep.
Single women had worse sleep quality and greater light sleep than married women.
Marriage was related to improved sleep for women with more depressive symptoms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864–6. doi: 10.1200/JCO.2009.24.5993. [DOI] [PubMed] [Google Scholar]
- 2.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419–29. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors [published online ahead of print January 18, 2010] J Pain Symptom Manage. 2010;39:535–47. doi: 10.1016/j.jpainsymman.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26:2431–2. doi: 10.1200/JCO.2008.16.2008. [DOI] [PubMed] [Google Scholar]
- 6.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24:471–80. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 7.Koopman C, Nouriani B, Erickson V, Anupindi R, Butler LD, Bachmann MH, et al. Sleep disturbances in women with metastatic breast cancer. Breast J. 2002;8:362–70. doi: 10.1046/j.1524-4741.2002.08606.x. [DOI] [PubMed] [Google Scholar]
- 8.Palesh OG, Collie K, Batiuchok D, Tilston J, Koopman C, Perlis ML, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer [published online ahead of print December 12, 2006] Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program [published online ahead of print November 23,2009] J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Ancoli-Israel S. Sleep disturbances in cancer. Psychiatric Annals. 2008;38:627–34. doi: 10.3928/00485713-20080901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberfarb PM, Hauri PJ, Oxman TE. Assessment of sleep in patients with lung and breast cancer. J Clin Oncol. 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino L, Mason W, Parker B, Johnson S, Amador X, Ancoli-Israel S, et al. Sleep disruption in breast cancer patients post-chemotherapy. Sleep. 2005;28 (suppl):A294. [Google Scholar]
- 13.Roscoe JA, Perlis ML, Pigeon WR, O’Neill KH, Heckler CE, Matteson-Rusby SE, et al. Few changes observed in polysomnographic-assessed sleep before and after completion of chemotherapy [published online ahead of print September 22, 2011] J Psychosom Res. 2011;71:423–8. doi: 10.1016/j.jpsychores.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med. 2007;8(suppl 4):S15–20. doi: 10.1016/S1389-9457(08)70004-2. [DOI] [PubMed] [Google Scholar]
- 15.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10:329–36. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review [published online ahead of print October 13, 2005] Eur J Cancer. 2005;41:2613–9. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008;110:9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 18.Fulton CL. The physical and psychological symptoms experienced by patients with metastatic breast cancer before death. Eur J Cancer Care. 1997;6:262–6. doi: 10.1046/j.1365-2354.1997.00057.x. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel D, Bloom JR. Pain in metastatic breast cancer. Cancer. 1983;52:341–5. doi: 10.1002/1097-0142(19830715)52:2<341::aid-cncr2820520227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Hale L. Who has time to sleep? J Public Health. 2005;27:205–11. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- 22.Troxel WM, Buysse DJ, Hall M, Matthews KA. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behav Sleep Med. 2009;7:2–19. doi: 10.1080/15402000802577736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strawbridge WJ, Shema SJ, Roberts RE. Impact of spouses’ sleep problems on partners. Sleep. 2004;27:527–31. doi: 10.1093/sleep/27.3.527. [DOI] [PubMed] [Google Scholar]
- 24.Karnofsky DA, Burchenal JH. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. [Google Scholar]
- 25.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 26.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 27.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 29.Winzelberg AJ, Classen C, Alpers GW, Roberts H, Koopman C, Adams RE, et al. Evaluation of an internet support group for women with primary breast cancer. Cancer. 2003;97:1164–73. doi: 10.1002/cncr.11174. [DOI] [PubMed] [Google Scholar]
- 30.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies-Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–87. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Eaton WW, Kessler LG. Rates of symptoms of depression in a National sample. Am J Epidemiol. 1981;114:528–38. doi: 10.1093/oxfordjournals.aje.a113218. [DOI] [PubMed] [Google Scholar]
- 32.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 33.Parker KP, Bliwise DL, Ribeiro M, Jain SR, Vena CI, Kohles-Baker MK, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–72. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 34.Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Beck SL, et al. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005;32:E98–126. doi: 10.1188/05.ONF.E98-E126. [DOI] [PubMed] [Google Scholar]
- 35.Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of Human Sleep: Clinical Applications. New York, NY: Wiley; 1974. [Google Scholar]
- 36.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 37.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaffery J, Hoffmann R, Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9:82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- 39.Sharpley AL, Cowen PJ. Effect of pharmacologic treatments on the sleep of depressed patients. Biol Psychiatry. 1995;37:85–98. doi: 10.1016/0006-3223(94)00135-P. [DOI] [PubMed] [Google Scholar]
- 40.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 41.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans [published online ahead of print January 2, 2008] Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riemann D, Berger M, Voderholzer U. Sleep and depression—results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 45.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27:1097–1103. doi: 10.1093/sleep/27.6.1097. [DOI] [PubMed] [Google Scholar]
- 46.Matthews KA, Kamarck TW, Hall MH, Strollo PJ, Owens JF, Buysse DJ, et al. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women [published online ahead of print May 15, 2008] Am J Hypertens. 2008;21:826–31. doi: 10.1038/ajh.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–94. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. a unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45:948–52. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 49.Spiegel D, Sephton S. Re: Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2002;94:530. doi: 10.1093/jnci/94.7.530. [DOI] [PubMed] [Google Scholar]
- 50.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawach I, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 51.Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stress of daily activities and risk of breast cancer: a prospective cohort study in Finland. Int J Cancer. 2001;91:888–93. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1138>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 52.Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst. 2001;93:1513–5. doi: 10.1093/jnci/93.20.1513. [DOI] [PubMed] [Google Scholar]
- 53.Abercrombie HC, Giese-Davis J, Sephton SE, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–92. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Strawbridge WJ, Shema SJ, Roberts RE. Impact of spouses’ sleep problems on partners. Sleep. 2004;27:527–31. doi: 10.1093/sleep/27.3.527. [DOI] [PubMed] [Google Scholar]
- 55.Frech A, Williams K. Depression and the psychological benefits of entering marriage. J Health Soc Behav. 2007;48:149–63. doi: 10.1177/002214650704800204. [DOI] [PubMed] [Google Scholar]
- 56.Syse A. Does cancer affect marriage rates? J Cancer Surviv. 2008;2:205–14. doi: 10.1007/s11764-008-0062-1. [DOI] [PubMed] [Google Scholar]
- 57.Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13:1–14. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 58.Fobair P, Stewart SL, Chang S, D’Onofrio C, Banks PJ, Bloom JR. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15:579–94. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy [published online ahead of print March 2, 2012] Brain Behav Immun. 2012;26:706–13. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruyneel M, Sanida C, Art G, Libert W, Cuvelier L, Paesmans M, et al. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2011;20(1, pt 2):201–6. doi: 10.1111/j.1365-2869.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 61.Fry JM, Diphillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998;21:635–42. doi: 10.1093/sleep/21.6.635. [DOI] [PubMed] [Google Scholar]
- 62.Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, et al. Polysomnography performed in the unattended home versus the attended laboratory setting–Sleep Heart Health Study methodology. Sleep. 2004;27:536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 63.Kapur VK, Rapoport DM, Sanders MH, Enright P, Hill J, Iber C, et al. Rates of sensor loss in unattended home polysomnography: the influence of age, gender, obesity, and sleep-disordered breathing. Sleep. 2000;23:682–8. [PubMed] [Google Scholar]
- 64.Jacobsen PB, Bovbjerg DH, Redd WH. Anticipatory anxiety in women receiving chemotherapy for breast cancer. Health Psychol. 1993;12:469–75. doi: 10.1037//0278-6133.12.6.469. [DOI] [PubMed] [Google Scholar]
- 65.Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, Von DM. Anticipatory nausea: the role of individual differences related to sensory perception and autonomic reactivity. Ann Behav Med. 2007;33:69–79. doi: 10.1207/s15324796abm3301_8. [DOI] [PubMed] [Google Scholar]