Abstract
As survival of mouse oocytes subjected to vitrification depends far more on the warming rate than on the cooling rate, we wished to determine whether the lack of correlation between survival and cooling rate was mirrored by a lack of correlation between cooling rate and vitrification of the medium (EAFS), and between survival and the vitrification of the medium. The morphological and functional survival of the oocytes showed little or no relation to whether or not the EAFS medium vitrified or froze. We studied if the droplet size and the elapsed time (between placing the droplet on the Cryotop and the start of cooling) affects the result through modification of the cooling rate and solute concentration. Dehydration was rapid; consequently, the time between the placing the droplets into a Cryotop and cooling must be held to a minimum. The size of the EAFS droplet that is being cooled does not seem to affect vitrification. Finally, the degree to which samples of EAFS vitrify is firmly dependent on both its solute concentration and the cooling rate.
Keywords: Vitrification, mouse oocytes, EAFS concentration, evaporation rate, droplet volume, cooling rate, survival
Our laboratory at the University of Tennessee has recently demonstrated that the survival of mouse oocytes subjected to vitrification procedures depends far more on the warming rate than on the cooling rate. Furthermore, if the warming rate is high, survival is high not only in full-strength vitrification solutions but also in a 2-fold dilution of that solution [11]. The experiments cited were carried out by transferring ~6 oocytes in 0.1 to 0.2 µl drops of the vitrification solution (EAFS 10/10 ) that had been placed on the blade of a Cryotop. EAFS 10/10 is composed principally of etheylene glycol (EG), acetamide, Ficoll, and sucrose. The total solute concentration is 7.4 molal. It was developed by Pedro [5] and Pedro et al.[6] as a vitrification medium for mouse oocytes. Cryotops (Kitazato Corp, Japan) consist of a minute blade of plastic measuring 0.1 × 0.7 × 20mm attached to a plastic handle. The Cryotop was then immersed into liquid nitrogen (LN2), either naked or surrounded by some form of insulation to lower the cooling rate. These rates ranged from 100 to 70,000°C/min. They were warmed at rates ranging from 600 to 118,000°C/min. When warmed at the latter rate in full-strength EAFS, survivals ranged from 70 to 85% for the four cooling rates studied. When warmed at 610°C/min, survivals ranged from 0 to 25% for the four cooling rates. Although we referred to these as vitrification procedures, we in fact had no direct evidence as to whether the oocytes and the medium vitrified or froze. Determining the former would be difficult, but determining the latter was not. In more general terms, we wished to determine whether our previously published lack of correlation between survival and cooling rate was mirrored by a lack of correlation between cooling rate and vitrification of the medium, and between survival and the vitrification of the medium. Two secondary questions were whether the droplet size and the elapsed time between placing the droplet on the Cryotop and the start of cooling affects the results through alteration of the cooling rate and the solute concentration.
Evaporation rate of droplets of EAFS solutions
In prior work, the volume of the droplet of medium placed on the Cryotops has been estimated to be 0.1 to 0.2 µl. We wanted to determine that more quantitatively and at the same time determine the rates at which droplets evaporated. The former might influence the likelihood of vitrification or freezing by an effect on cooling rate; the latter might affect the likelihood of vitrification or freezing of the medium by concentrating the solutes.
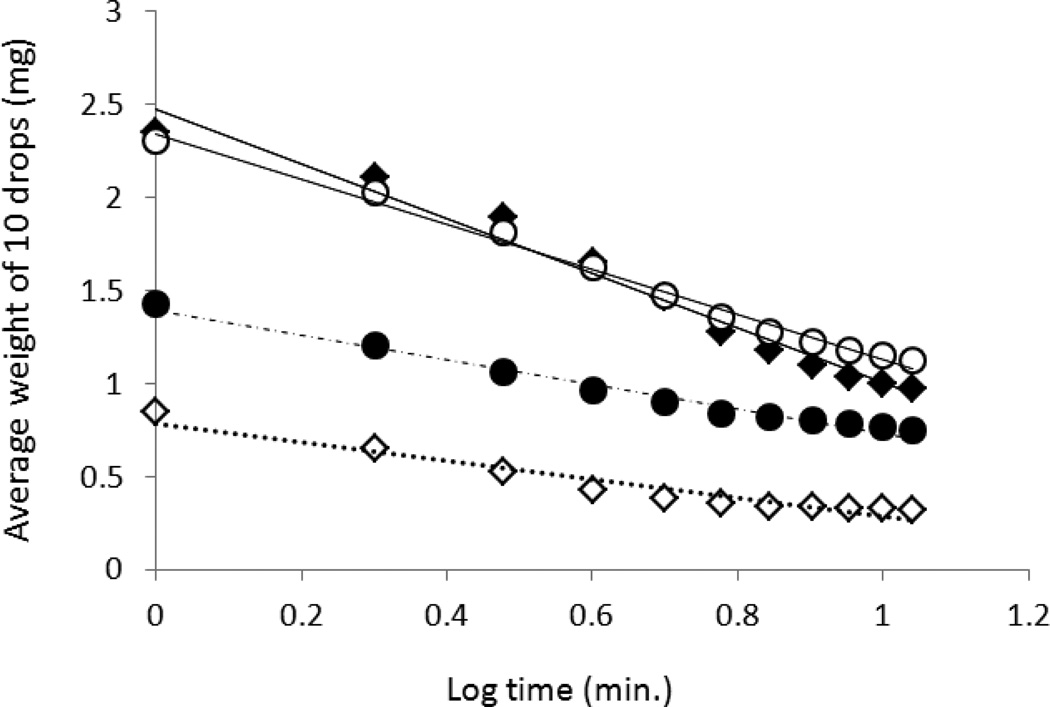
Using a micro-mouth pipette, 10 separate small drops of two EAFS dilutions- 0.5× or 0.33× -were placed into a 35 mm plastic Petri dish (process took 10–20 seconds) which in turn was placed on an analytical balance with 0.05 mg sensitivity. The weight was recorded immediately and monitored each minute for 10 minutes. The temperature in the laboratory was 23°C and the relative humidity runs about 45%. The evaporation measurements were repeated two additional times for each EAFS concentration and for each mode of delivery. The observed mass at zero time of the 10 droplets was converted to volume by dividing by the densities of the two solutions; namely, 1.076 and 1.051g/ml, respectively [9]. The whole experiment was then replicated using a 10µl Hamilton syringe (Hamilton Company, Reno, Nevada, USA) to deliver the droplets, Figure 1 shows the observed weights of 10 droplets of the two different EAFS dilutions over~10 min (Log 10 = 1). The mean mass of an individual droplet is 1/10th of the values on the ordinate. The mass of the droplets is seen to be a linear function of the logarithm of time. The mean volume of a droplet at zero time was 0.22µl for the mouth-delivered droplets and 0.08, and 0.13 µl for the droplets delivered by Hamilton syringe in the four runs, values that confirm the previous qualitative estimates. As would be expected, the evaporation rate was higher in the 0.33× EAFS solution than in the 0.5× solution (60% and 50% respectively after 10 min). The differences in the initial droplet size, however, had no apparent effect on the evaporation rate.
Fig. 1.
| EAFS | Drops add by | Symbol in fig.* |
Initial mass (mg) |
Mass @ 10 min (mg) |
% loss mass in 10 minutes |
Position of curve |
Equation of linear fit** |
|---|---|---|---|---|---|---|---|
| 0.33× | Pipette | 2.35 | 0.97 | 59% | 1 (top) | Y = −1.472x +2.475 | |
| Hamilton | 0.84 | 0.32 | 62% | 4 | Y = −0.506x + 0.788 | ||
| 0.5× | Pipette | ○ | 2.31 | 1.12 | 52% | 2 | Y= −1.215x + 2.344 |
| Hamilton | 1.43 | 0.75 | 48% | 3 | Y = −0.657x + 1.391 |
Two factors that influence the evaporation rate are the concentration of solutes in the drop and its surface to volume ratio. The higher the concentration of solutes, the lower the vapor pressure or chemical potential of the water in the drop and the lower is the difference between it and the ambient vapor pressure of water. This reduces the driving force for dehydration and thus slows it. With respect to the second factor, the higher the surface to volume ratio (S/V), the faster will be the dehydration. If the drops remain spherical, larger drops will have a lower S/V than smaller ones and should dehydrate more slowly. But the droplets are not spherical because of surface tension and probably do not maintain a constant S/V as drying progresses.
Dehydration was rapid. In three of the four cases, they lost 20 to 26% of their mass in 2 min. In the fourth they lost 38%. In our prior experiments involving oocytes, the loss was less than that because less that 1 min elapsed between the time the droplet of EAFS was placed on a Cryotop, the oocytes inserted into the drop, and vitrification initiated. Furthermore, most of our previous experiments have used 1× EAFS which would have evaporated more slowly.
Droplet volume and vitrification of EAFS solutions
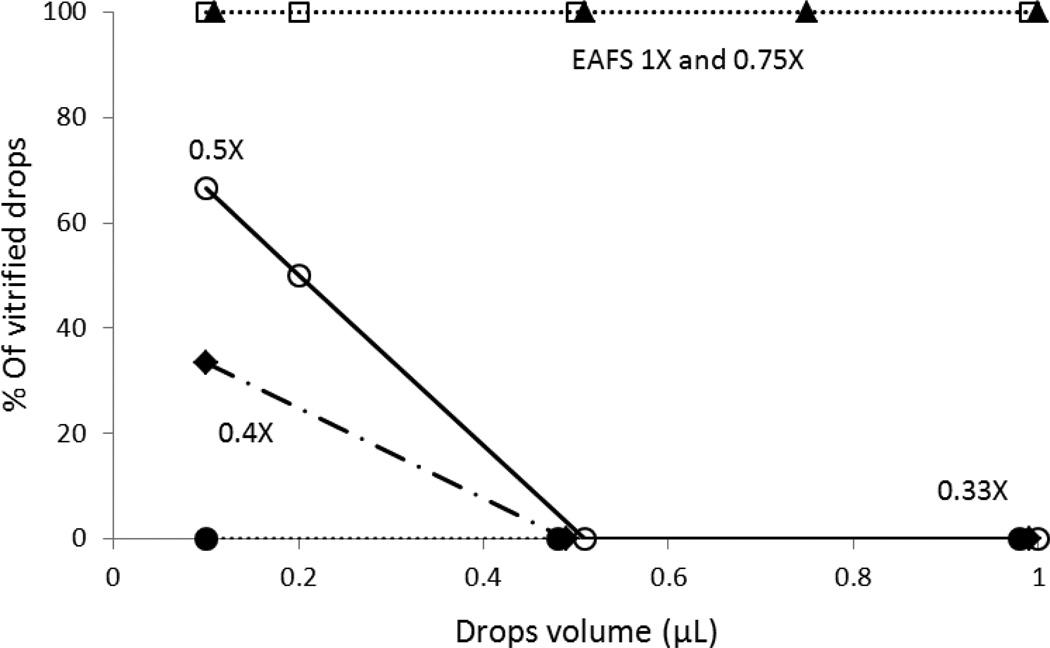
Although the evaporation rate of EAFS droplets was not significantly affected by a 2 to 3- fold difference in volume, whether or not they vitrified might be. Consequently, we checked the percentages of triplicate samples of full strength EAFS (1× EAFS) and of 0.75×, 0.5×, 0.4× and 0.33× dilutions that underwent vitrification as a function of droplet volume. Using a 1µl Hamilton syringe that we had purchased, we deposited droplets of 0.1, 0.2, 0.5, 0.75 or 1 µl on a Cryotop and plunged it directly into liquid nitrogen. The LN2 was held in an unsilvered Dewar. This enabled us to observe whether a droplet was clear (vitrified) or opaque (frozen) after cooling. Thermocouple measurements/óscillographic recordings by Kleinhans et al [3] on 0.1 µl droplets of 1× EAFS yielded a cooling rate of 69,000°C/min.
The Cryotops holding the larger and more dilute droplets would be expected to cool somewhat more slowly. In spite of that, the results (Figure 2) show that all of the droplets of 1× and 0.75× EAFS vitrified even when the droplet volume was as large as 1 µl, 10-times the normal. At the other extreme, none of the droplets of 0.33× EAFS vitrified, even using the smallest volume (0.1 µl). Droplets of 0.5× and 0.4× EAFS behaved in an intermediate fashion. One-third to two thirds of the 0.1 µl drops froze, as did all of the 0.5 µl drops.
Fig. 2.
Percentages of triplicate droplets of various concentrations of EAFS undergoing vitrification as a function of the mean volumes (µl) of individual droplets. The EAFS concentrations were 1× (□), 0.75× (▲), 0.5× (○). 0.4× ( ), and 0.33× ( ). The droplets on a Cryotop were abruptly plunged into LN2. This produces a cooling rate of ~69,000°C/min.
Vitrification of EAFS solutions and cooling rate
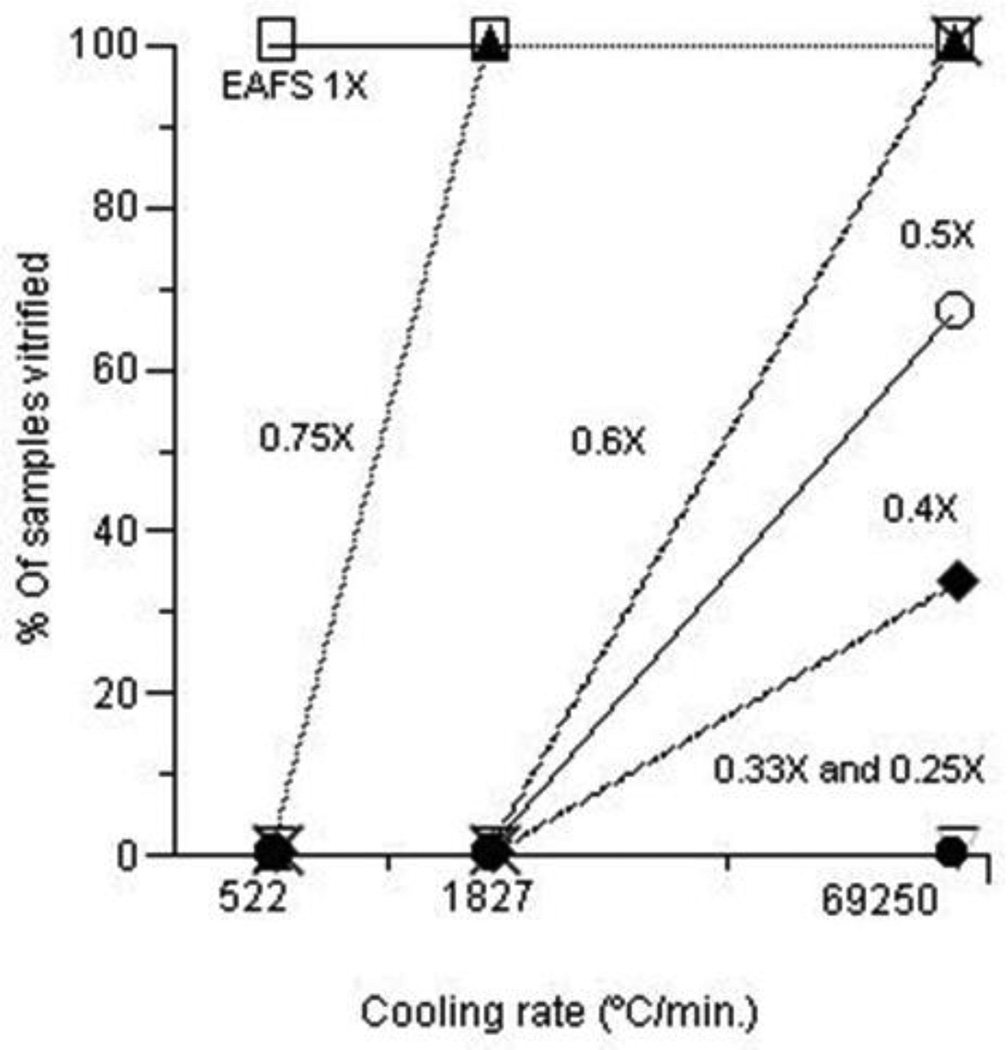
The next matter was to determine the effects and interactions of cooling rate and EAFS concentration on the percentages of triplicate samples undergoing vitrification. A 0.1 µl droplet of EAFS solution - 1×, 0.75×, 0.6×, 0.5×, 0.4×, 0.33× and 0.25×- was placed on a Cryotop and cooled to −196°C at 69,000, 1830, and 522°C/min. The highest cooling rate was achieved by plunging the naked Cryotop blade directly into liquid nitrogen. The two lower cooling rates were achieved by suitably insulating the blade of the Cryotop as indicated in Table 3 of Seki and Mazur [11]. As mentioned, the liquid nitrogen was held in an unsilvered Dewar flask, thus permitting us to observe visually whether the EAFS droplet vitrified or froze. Those droplets that remained clear were deemed to have vitrified. Those that turned sugary white were considered to have frozen. The drops in a small percentage of cases had the appearance of skim milk and they also were scored as frozen.
Fig. 3 shows the percentages of the triplicate samples of seven concentrations of EAFS that underwent vitrification after cooling at 522, 1927, and 69,250°C/min. At one extreme were the samples of 1× EAFS, all of which vitrified at all three cooling rates. At the other extreme were the samples of 0.33× and 0.25× EAFS. All of them froze at all three cooling rates. The results for 0.75×, 0.6×, 0.5×, and 0.4× EAFS were intermediate. With 0.75× EAFS, 100% vitrified when cooled at 1827°C/min or faster, but 100% froze when cooled at 522°C/min. With 0.6× EAFS the transition from 100% frozen to 100% vitrified occurred between cooling rates of 1827 and 69,250°C/min. The results for 0.5× and 0.4× were similar but only 66% and 33% vitrified even at the highest cooling rate.
Fig. 3.
Percentages of triplicate droplets of various concentrations of EAFS undergoing vitrification as a function of the cooling rate. The EAFS concentrations were 1× (□), 0.75× (▲), 0.6× (×), 0.5× (○). 0.4× (♦), 0.33× ( ), and 0.25× (Δ). The three different cooling rates were achieved as indicated in Table 2.
A reviewer of this paper brought to our attention that Forsyth and MacFarland [1] published data indicating that solutions of EG and other solutes could exhibit situations during warming where differential scanning calorimetry (DSC) shows them to be frozen, but visual observation shows them to be transparent. This seeming paradox could arise if the size of the ice crystals were below the wave length of visible light. This raises the question as to whether that could be the case here during cooling. We think not for the following reasons: One is that in their experiments, all the samples were cooled at very high rates to −196°C and all their measurements were made on the state of the solutions during warming above the glass transformation temperature, Tg,. In our experiments, the samples were cooled at rates of 95°, 880°, and 69,000°C/min, and no warming was involved. In their experiments, the concentrations of EG and propylene glycol ranged from 35 to 50 wt %. In ours, the concentrations of EG, formamide, and other solutes (excluding Ficoll) ranged from 11.4 wt% for 0.33× EFAS to 31 wt% for 1× EAFS. A final point is that they were able to use DSC to determine thermal peaks during relatively slow warming at 10 or 80°C/min. DSC machines can not cool at the two higher cooling rates we used, and thermograms obtained at our lowest cooling rate would be unreliable because of supercooling.
Vitrification of external media and oocyte survival
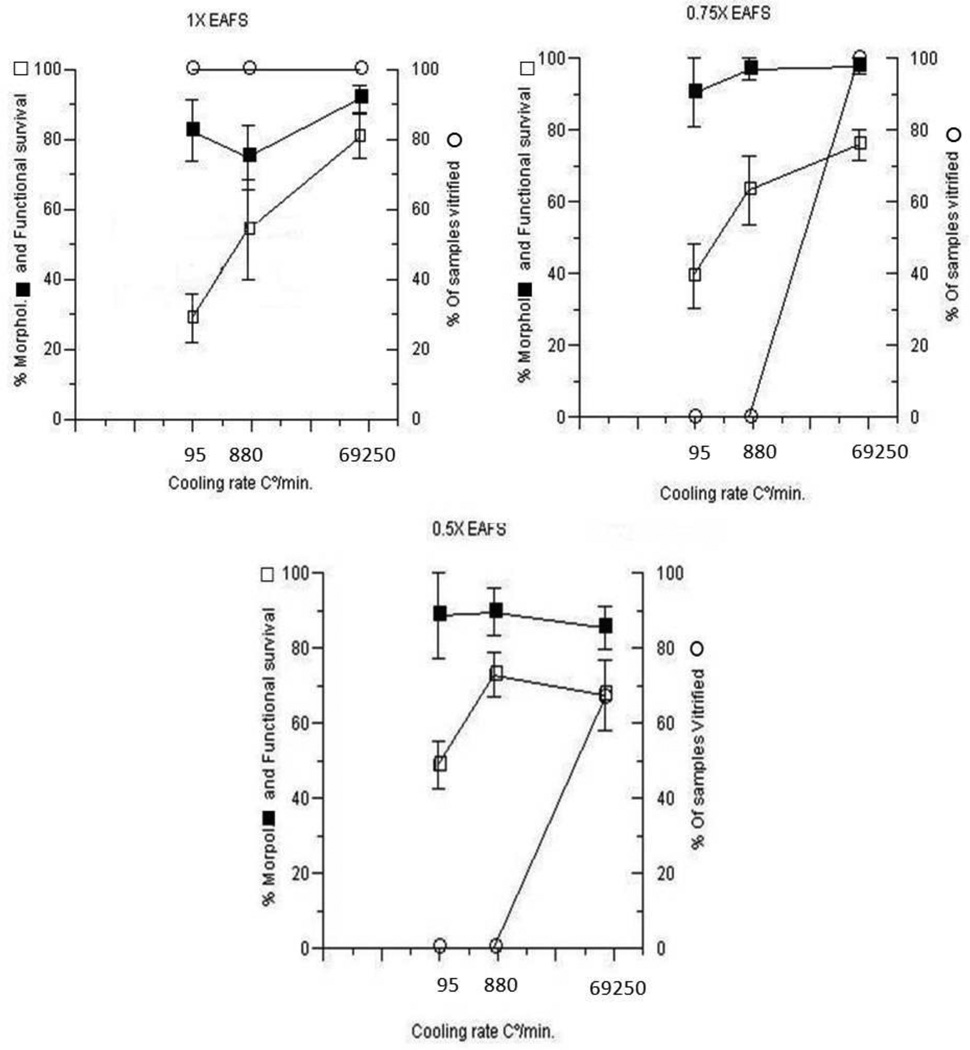
In previous experiments, Mazur and Seki and Seki and Mazur [4,11] determined the morphological survival and partial functional survival of oocytes suspended in 1×, 0.75×, and 0.5× EAFS, cooled at 95°, 880°, and 69,250°C/min, and warmed at 117,500°C/min. In their experiments, approximately six freshly collected oocytes were pipetted into a 0.1 µl droplet of each the three EAFS concentrations placed on a Cryotop a few seconds previously. The Cyotops were then appropriately insulated (Table 3 of [11]), and abruptly immersed into LN2. For ultrarapid warming, the insulation was removed from the Cryotops at −140°C or below, and the Cryotop immersed in a small volume of 0.5 M sucrose in PB1 solution. When basing survival on morphology, the criteria also included membrane integrity and osmotic responsiveness. Functional survival meant the ability of an oocyte to undergo in vitro fertilization and develop to 2-cell embryos. In the present study, we wanted to determine if there is a correlation between survival and whether or not the EAFS medium vitrified. As can be seen in Fig. 4, the correlation is poor or non-existent. In the top left graph (1× EAFS), the EAFS samples vitrified at all three cooling rates whereas in 0.75× and 0.5× EAFS, the only ones that vitrified were those cooled at the highest rate. In contrast, survivals were quite similar with all three EAFS concentrations. To make the vitrification observations, a 0.1 µl droplet of the EAFS solution was placed on the Cryotop using a 1µL Hamilton syringe. The Cryotop, appropriately insulated to achieve the three desired cooling rates (95°C/min, 880°C/min, and 69,250°C/min [See footnote in Table 1]), was then abruptly immersed in LN2 in an unsilvered Dewar and observed to see whether a given droplet froze or vitrified.
Fig. 4.
Percentage survival of mouse oocytes (left ordinate) and percentage of triplicate samples of EAFS that vitrified (right ordinate) as functions of the relative concentrations of EAFS (1×, 0.75×, and 0.5×) and the cooling rate (95, 880, or 69,250°C/min. The percentages vitrified (○) were determined in the present study. The three cooling rates were achieved by the methods described in Table 3 of Seki and Mazur [11]. The survivals (■, □) are from columns 3 and 5 in Table 6 of Seki and Mazur [11]. The symbol ■ refers to survival based on morphological normality and membrane integrity. The symbol □ refers to oocytes that underwent IVF and developed to 2-cell embryos.
Table 1.
Derivative solute concentrations in various dilutions of EAFS10/10 vitrification solution
| (A) Relative concn, EAFS |
(B) Total mass (g) |
(C) Total volume (ml) |
(D) Molality EG |
(E) Molality acet. |
(F) Molality sucros. |
(G) Molality NaCl |
(H) Molar EG |
(I) Molar acet. |
(J) Molar sucros. |
(K) Molar NaCl |
(L) Osmol non-perm. |
(M) Rel. equil vol cell water |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1× | 11.52 | 10 | 3.23 | 3.27 | 0.72 | 0.15 | 2.283 | 2.283 | 0.503 | 0.105 | 0.996 | 0.277 |
| 0.75× | 11.14 | 10 | 2.01 | 2.04 | 0.45 | 0.15 | 1.589 | 1.613 | 0.356 | 0.119 | 0.726 | 0.380 |
| 0.5× | 10.76 | 10 | 1.15 | 1.16 | 0.26 | 0.15 | 0.999 | 1.008 | 0.226 | 0.130 | 0.533 | 0.518 |
| 0.33× | 10.51 | 10 | 0.7 | 0.71 | 0.16 | 0.15 | 0.640 | 0.649 | 0.146 | 0.137 | 0.436 | 0.633 |
| 0.25× | 10.38 | 10 | 0.50 | 0.51 | 0.11 | 0.15 | 0.469 | 0.478 | 0.103 | 0.141 | 0.390 | 0.710 |
Columns H-K: The molarities have been calculated from the molalities by the equations in an article titled “Molality” in Wikipedia, the free encyclopedia (http://en.wikipedia.org/wiki/Molality) for solutions with more than one solute. The molarity of the ith solute in moles/liter solution is Mi = Ww mi Mw, where Ww is the molecular weight of water in kg/mole (0.0018), mi is the molality of that solute in moles/kg of water, and Mw is the molarity of the water. Mw is equal to ρmw/(1 + Σ(mj Wi)), where ρ is the density of the solution, and mi Wi are the molalities of the ith solute times the molecular weight of that solute in kg/mole. Here, the contributions of Ficoll and BSA are ignored. If those of Ficoll are included, the molarities of EG, acetamide, sucrose, and NaCl are each 21% and 11% lower for the 1× and 0.5× EAFS solutions, respectively, and are proportionally lower for the other dilutions.
Column L: The nonpermeating solutes are sucrose and PBS (we ignore the very small contribution of the Ficoll and BSA.) Their combined osmolality is Column F + 0.276. We assume the osmolality of PBS to be equal to that of the same molality of NaCl. The osmolality of NaCl = 2φm where 2 is the number of species into which the molecule dissociates and φ is the osmotic coefficient.
Column M: The volume of water in the oocytes after equilibration with the external medium relative to the volume of water in an isotonic cell. It is 0.276/Column L.
In 2009, Seki and Mazur [10] reported that when mouse oocytes suspended in 1× EAFS 10-10 vitrification medium were cooled to −150°C at 187°C/min in straws, the medium froze. Nevertheless, 82% survived if warming was rapid. That was also the cooling rate that Pedro [5] found to yield the highest survivals.
Conclusions
The most significant finding in this study is that the morphological and functional survival of the oocytes shows little or no relation to whether or not the EAFS medium froze or vitrified. For example, for 0.75× EAFS in Fig. 4, we see two examples at the lower cooling rates where survivals are reasonably high in spite of the fact that all the samples of the EAFS froze. The same is true for 0.5× EAFS. The simplest explanation is that the interior of the oocyte vitrifies under conditions that result in the freezing of the EAFS medium. Rall [7] has argued rather persuasively that this will occur because the oocyte or embryo cytoplasm contains significant concentrations of macromolecules (proteins and nucleic acids) which will augment the glass-forming ability of the permeating solutes in EAFS. Furthermore, the concentration of these endogenous macromolecules will rise well above normal because the cells shrink extensively prior to cooling because of the strong hyperosmoticity of the solutes in EAFS.
In Fig. 4, morphological/osmotic survival is nearly independent of cooling rate. But that is not true of functional survival (development to 2-cell). It drops as the cooling rates is lowered to 880°C/min, and particularly as it is lowered to 95°C/min. Furthermore, it does so even when the oocytes are warmed at the highest rate, The simplest hypothesis is that at the lower cooling rates, some of the oocytes undergo sufficient intracellular freezing to incur injury that prevents them from undergoing IVF and further development. Such an interpretation is reasonably consistent with the data for 0.75× and 0.5× EAFS in Fig. 4. in that the drop in survival occurs at cooling rates that cause the EAFS to freeze. Mazur [3] has made the argument that external ice is the agent that nucleates intracellular supercooled water. It is not consistent with the data for 1× EAFS, for in that case, the medium vitrified at all three cooling rates.
Another possibility is that the loss in survival is a consequence of something other than intracellular ice formation (IIF). We believe that is unlikely, but it is difficult to reject or prove because of the difficulty in determining whether a given treatment results in IIF. Electron microscopy on freeze-substituted or freeze fractured cells is one method but it is labor intensive. Another potential method is to use differential scanning calorimetry to distinguish freezing exotherms inside cells from those in the external medium. Seki et al. [8] successfully applied this method to detecting IIF in yeast cells, but the method requires very high cell cytocrits, the attainment of which would require the unacceptable use of too many animals.
An important matter technically is our finding that the submicroliter drops of EAFS lose water rapidly. In 10 min., droplets of 0.33× and 0.5× EAFS in air lose 60% and 40% of their mass. In two minutes they lose 20–30%. In most of our previous experiments, only a few seconds elapsed between placing a droplet on a Cryotop, introducing the oocytes into the drop, and initiating cooling; consequently, the degree of evaporation ought to have been small. Nevertheless, the data show that unless the time between the addition of the droplets to a Cryotop and the initiation of cooling is held to a minimum, the concentration of solutes through evaporation can be a source of confounding.
Another potential source of variation might be expected to be the size of the EAFS droplet that is being cooled, but that has proved pretty much not to be the case. In Fig. 2 we see that 100% of droplets of 1× and 0.75× EAFS vitrify when the droplet varies from 0.1 to 1 µl. In contrast 100% droplets of 0.33× EAFS freeze over the same range of volumes.
Not surprisingly, the degree to which samples of EAFS vitrify is strongly dependent on both its solute concentration and the cooling rate. Thus, as shown in Fig. 3, in 1× EAFS, 100% of the samples vitrify at all three cooling rates tested (533, 1827, and 69,250°C/min.), whereas in 0.33× or 0.25× EAFS, 100% of the samples freeze at all three cooling rates. The transition between 100% vitrification and 0% vitrification occurs abruptly as the EAFS relative concentration is reduced from 0.6× to 0.4×.
The physical measurements in this paper deal only with events occurring before the cooling of the EAFS solution (evaporation) and whether or not the EAFS samples have vitrified as they are cooled to −196°C. Survival, of course also depends on the events occurring inside cells and events occurring during warming. We have shown in previous papers [4,9–11] that the major event is whether or not the warming rate is high enough to prevent the growth of any intracellular ice by recrystallization. That continues to be our position.
Table 2.
The Percentage of EAFS samples vitrified as a function of the EAFS dilution and the cooling rate.
| EAFS | Cooling rate* | % vitrified |
|---|---|---|
| dilution | (°C/min) | |
| 1× | 69250 ± 4300 | 100 ± 0 |
| 1827 ± 210 | 100 ± 0 | |
| 522 ± 54 | 100 ± 0 | |
| 0.75× | 69250 | 100 ± 0 |
| 1827 | 100 ± 0 | |
| 522 | 0 ± 0 | |
| 0.6× | 69250 | 100 ± 0 |
| 1827 | 0 ± 0 | |
| 522 | 0 ± 0 | |
| 0.5× | 69250 | 66.7 ± 33.3 |
| 1827 | 0 ± 0 | |
| 522 | 0 ± 0 | |
| 0.4× | 69250 | 33.3 ± 33.3 |
| 1827 | 0 ± 0 | |
| 522 | 0 ± 0 | |
| 0.33× | 69250 | 0 ± 0 |
| 1827 | 0 ± 0 | |
| 522 | 0 ± 0 | |
| 0.25× | 69250 | 0 ± 0 |
| 1827 | 0 ± 0 | |
| 522 | 0 ± 0 | |
The cooling rates of (1) 69,000°C/min., (2) 1827°C/min., and (3) 522°C/min. were achieved by (1) immersing the blade of a cryotop with 0.1 µl of EAFS directly into LN2, (2) immersing a 0.25 ml insemination straw (IMV Technologies, l’Aigle, France) containing EAFS into LN2, and (3) Inserting the 0.25 ml straw into a 0.5 ml straw before immersion into LN2. The rates were measured with thermocouples by Seki and Mazur [10] and Kleinhans et al. [2].
[1] for the 1× EAFS solutions, but have not been measured for the more dilute ones. We doubt that they would be affected noticeably because the overall specific heats of the sample + container ought not to vary significantly with the EAFS solute concentration. We have calculated the heat capacities of 1 ml of 1×, 0.5×, 0.33×, and 0× EAFS to be 0.34, 0.41, 0.45, and 0.5 cal/°C, respectively. The heat capacity of the Cryotop and straws itself would of course be constant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
E. Paredes was sponsored by a fellowship from the Spanish government (FPU Research Stays). The research was supported by NIH grant 8R01 OD 011201, P. Mazur, P.I.
References
- 1.Forsyth M, MacDonald DR. Recrystallization revisited. Cryo-Letters. 1986;7:367–378. [Google Scholar]
- 2.Kleinhans FW, Seki S, Mazur P. Simple, inexpensive attainment and measurement of very high cooling and warming rates. Cryobiology. 2010;61:231–233. doi: 10.1016/j.cryobiol.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazur P. Principles of Cryobiology. In: Fuller BJ, Lane N, Benson E, editors. Life in the Frozen State. Boca Raton: CRC Press; pp. 3–65. [Google Scholar]
- 4.Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to −196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: A new paradigm for cryopservation by vitrification. Cryobiology. 2011;62:1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedro PB. Ph.D. thesis. Japan: United Graduate School of Agricultural Sciences, Ehime University University; Studies on the Cryopreservation of mammalian oocytes and embryos with reference to some cryobiologcal characteristics. [Google Scholar]
- 6.Pedro PB, Zhu SE, Makino N, Sakurai T, Edashige K, Kasai M. Effects of hypotonic stress on the survival of mouse oocytes and embryos at various stages. Cryobiology. 1997;35:150–158. doi: 10.1006/cryo.1997.2034. [DOI] [PubMed] [Google Scholar]
- 7.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196 degrees C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 8.Seki S, Kleinhans FW, Mazur P. Intracellular ice formation in yeast cells vs. cooling rate: Predictions from modeling vs. experimental observation by differential scanning calorimetry. Cryobiology. 2009;58:157–165. doi: 10.1016/j.cryobiol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seki S, Mazur P. Effect of warming rate on the survival of vitrified mouse oocytes and on the recrystallization of intracellular ice. Biology of Reproduction. 2008;79:727–737. doi: 10.1095/biolreprod.108.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59:75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to −196°C in dilutions of a standard vitrification solution. PLos One. 2012;7(4) doi: 10.1371/journal.pone.0036058. [DOI] [PMC free article] [PubMed] [Google Scholar]