Abstract
Glioblastoma remains the most clinically challenging tumor of the CNS, as evidenced by the dismal change in overall survival over the past 50 years. However, recent advances in high-throughput screening techniques have given rise to a wealth of new information regarding the aberrant signaling pathways that drive the tumor phenotype. Two of these so-called ‘oncopathways’ are NF-κB and JAK/STAT. This review will describe the basic mechanisms of these pathways, explore the relevance of NF-κB and JAK/STAT signaling in glioblastoma, and look ahead to experimental compounds that will integrate our knowledge of these pathways into existing therapies.
Keywords: cytokine, glioblastoma, intracellular signaling, JAK/STAT, NF-κB
Glioblastoma
Glioblastoma (formerly known as glioblastoma multiforme; GBM) is the most frequent and the most aggressive primary brain tumor in adults. GBM is also one of the most lethal of all human neoplasms, with the majority of patients succumbing within 1 year of diagnosis, and only a few surviving 24 months [1]. Incidence peaks in the sixth to eighth decades of life [2], with men being afficted 1.5-times more often than women [3]. Approximately 14,000 new cases of malignant glioma are diagnosed each year in the USA, with 60–70% of those tumors being GBM [4].
Neurons, the information-transmitting cells of the CNS, are postmitotic and, thus, rarely develop the mutational profile required for malignant transformation. Instead, the majority of CNS tumors arise from cells with glial-like characteristics (gliomas), most commonly expressing markers of the astrocyte lineage. The WHO divides astrocytomas into four grades. Of these, WHO Grade III (anaplastic astrocytoma) and WHO Grade IV (GBM) are the malignant subtypes [5]. Histopathologically, both Grades III and IV feature increased cellularity, nuclear atypia and mitotic activity, with GBM also displaying endothelial cell proliferation and/or a characteristic pseudopalisading pattern of tumor cell proliferation and necrosis [6]. Another hallmark of GBM is its propensity for invasion of the surrounding normal brain tissue. This feature is one of the most difficult obstacles in treatment, because it makes full resection nearly impossible. Only months after the primary lesion is excised, the remaining invasive tumor cells give rise to one or more recurrent masses to which the patient eventually succumbs.
Glioblastoma can arise de novo (primary GBM) or as the result of a transformation event by a lower grade tumor (secondary GBM). Each are characterized by distinct genetic events [7]. Primary GBM frequently has EGF receptor (EGFR) amplification and phosphatase and tensin homolog (PTEN) deletion, while TP53 mutations are more common in secondary GBM [8]. Furthermore, primary and secondary GBM arise at distinct times in life, with primary GBMs occurring in the sixth to seventh decade and secondary GBMs usually affecting somewhat younger individuals in the ffth to sixth decade [7]. Primary GBM is also more frequent than secondary [9]. Interestingly, although genetically distinct, the histopathology of primary and secondary GBM is identical [4].
The standard protocol for treating this disease begins with an immediate surgical debulking of operable tumors followed by a combination of radiation and temozolomide (TMZ) [10]. Unfortunately, this regimen provides only a modest survival advantage. Radiotherapy and concomitant TMZ therapy, followed by six rounds of adjuvant TMZ has been demonstrated to enhance 2-year survival from 10 to 27% [11]. Many experimental therapies have been tested in clinical trials, but none have yet been able to significantly extend patients' lives. The need for effective new therapies is evident even in the few patients who survive long-term, as they tend to suffer serious cognitive impairment from the effects of radiotherapy [12]. In this regard, basic scientists and clinicians are attacking GBM from a number of different vantage points, from new forms of radiation [13], to oncolytic herpes simplex virus vector-delivery of antitumor therapies [14], to molecular therapies [15]. As researchers learn more about the intracellular signaling pathways that drive the tumor's phenotype, therapies designed to interrupt such aberrant signaling may help improve outcomes for patients with GBM and other types of malignant gliomas.
Aberrant signaling pathways in glioma
Intracellular signaling pathways are the communication lines between the extracellular environment and the genome of a cell. Through sequential post-translational modifications, the information from the soluble factors of other cells can be integrated into the cell's signaling network and affect its phenotype. Signaling pathways also allow for amplification of signals, meaning that picomolar amounts of an extracellular factor may cause drastic changes in gene expression and cell phenotype. Malignant gliomas and other tumors hijack these pathways for their own goal of relentless proliferation. In a sense, one way to understand cancer is as a series of genetic aberrations that lead to enhancements in protumor signaling pathways. Indeed, while the origins of cancer are in the DNA, the functional players of the disease are the protein products that disrupt normal signaling and encourage uncontrolled proliferation.
Studies on human GBM samples have revealed a number of genetic alterations that suggest the importance of particular onco-pathways in these tumors [16–19]. For instance, EGFR amplification is found in 40–50% of primary GBM, with approximately half of these amplification events involving a variant of the receptor termed EGFRvIII [4]. EGFRvIII lacks exons 2–7, which normally code for the extracellular domain of the receptor, causing the receptor to be constitutively active [20]. The downstream effects of EGFR overactivation are many, but importantly, include a direct increase in signal transducer and activator of transcription (STAT) 3 activity in GBM cells [21]. Another common genetic event in GBM is a loss-of-function mutation in the tumor suppressor, PTEN [22]. PTEN is a phosphatase that negatively regulates the PI3K–AKT–mTOR signaling cascade and, thus, mutations in PTEN lead to constitutive activation of this pathway in GBM [20]. VEGF signaling also plays an important role in GBM biology and its expression is upregulated in GBM. Activation of its receptor, VEGFR-2, which is found on brain endothelial cells, promotes tumor growth by increasing blood supply to the highly metabolic tumor [23].
There are still other pathways that, while largely overlooked in current GBM drug trials, show great promise for drug development. Two such pathways are the NF-κB and JAK/STAT pathways, both of which are aberrantly upregulated in a variety of human cancers [24,25]. Both pathways drive the expression of a lrge number of genes, which, when overexpressed, are beneficial to cancer cells. For example, VEGF expression is upregulated in tumor cells by NF-κB and STAT3 signaling [26,27]. The investigation of these pathways holds great promise in bringing more effective, molecularly targeted drugs to GBM therapy regimens. In this review, we discuss NF-κB and JAK/STAT signaling, outline preclinical studies that aim to inhibit these pathways and discuss the implications of targeting these pathways in GBM.
NF-κB
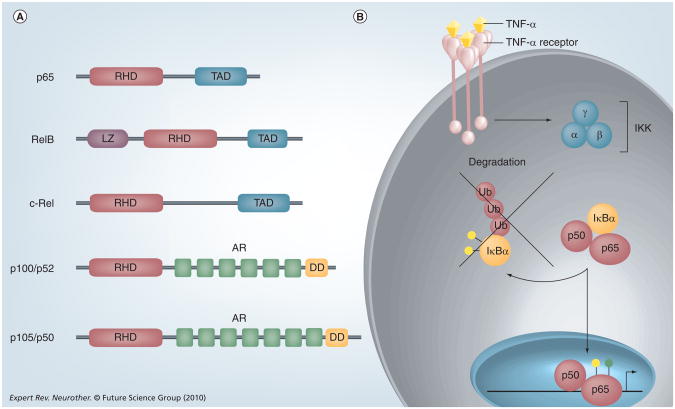
The mammalian NF-κB family includes five members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-kB1) and p52/p100 (NF-κB2) (Figure 1A) [28]. In an unstimulated state, these proteins exist in the cytoplasm as homo- or heterodimers, bound to an inhibitory family of proteins called IκBα [29]. Classical or ‘canonical’ signaling of this pathway (Figure 1B) consists of one of many extracellular ligands (e.g., TNF-α, IL-1) binding to its receptor, which, in turn, activates the IκB Kinase (IKK) complex. IKK is comprised of three proteins: IKKα, IKKβ and the regulatory protein NEMO (or IKKγ) [28]. Signaling through the classical pathway is driven primarily through IKKβ [30]. This complex phosphorylates IκBα, which marks it for degradation via the ubiquitin-proteasome pathway. Free NF-κB dimers then translocate to the nucleus where they activate a number of pro inflammatory target genes, such as IL-8, MCP-1 and IL-6 (Table 1) [31].
Figure 1. The NF-κB signaling pathway.
(A) The structure of NFkB proteins. NF-κB proteins are defined by a C-terminal RHD, which can be activated by phosphorylation. p65, RelB and c-Rel contain TAD, which can activate NF-κB target genes. p100 and p105 lack TADs but contain AR domains and N-terminal DDs. RelB also contains a LZ. (B) TNF-α is one of the most potent activators of NF-κB,and is produced in the CNS by microglia, astrocytes, endothelial cells and some neurons. TNF-α binds to the TNF-α receptor and activates the IKK kinase, which consists of three subunits: α, β and γ. IKK phosphorylates (yellow circles) the inhibitory protein IκBα and targets this protein for Ub mediated degradation. The liberated NF-κB dimer translocates into the nucleus, undergoes phosphorylation (yellow circle) and/or acetylation (green circle), and binds to κB elements in the promoters of target genes to regulate gene expression.
AR: Ankyrin repeat; DD: Death domain; IKK: IκB kinase; LZ: Leucine zipper domain; RHD: Rel homology domain; TAD: Transactivation domains; Ub: Ubiquitin.
Table 1.
NF-κB and STAT3 target genes.
| NF-κB | STAT3 |
|---|---|
| Cell growth & proliferation | |
|
| |
| c-Myc | c-Myc |
| MDM2 | Pim1 |
| Cyclin D1 | Cyclin D1 |
| Pim1 | AKT |
| AKT | JunB |
| p53↓ | p53↓ |
|
| |
| Apoptosis & drug resistance | |
|
| |
| Bcl-2 | Bcl-2 |
| Bcl-XL | Bcl-X L |
| Survivin | Survivin |
| cIAP2 | cIAP2 |
| MGMT | MDR1 |
| Mcl-1 | |
| STAT3 | |
|
| |
| Cell migration & invasion | |
|
| |
| MMP-9 | MMP-2 |
| ICAM-1 | MMP-9 |
| VCAM-1 | |
| E-selectin | |
|
| |
| Angiogenesis | |
|
| |
| I L- 8 | VEGF |
| VEGF | HIF-1α |
| iNOS | |
| COX-2 | |
|
| |
| Cytokines/signaling pathways | |
|
| |
| IL-6 | IL-10 |
| IL-10 | IL-17 |
| TNF-α | IL-27 |
| Sonic Hedgehog | Notch1 |
| Jagged-1 | |
| δ-like ligand | |
cIAP: Cellular inhibitor of apoptosis protein; iNOS: Inducible nitric oxide synthase; MDR: Multi-drug resistance; MGMT: O6-methylguanine–DNA methyltransferase; MMP: Matrix metalloproteinase; STAT: Signal transducer and activator of transcription.
An alternative or so-called ‘noncanonical’ pathway was recognized in 2001 [32]. This pathway utilizes IKKα and, rather than IκB proteins acting as inhibitors, this function is ascribed instead to the C-terminal domain of p100 [30]. In the canonical pathway, the primary transcriptionally active dimer is p65–p50, while p52–RelB dimers serve this function in the alternative pathway [28]. Although its physiological context is not yet fully understood, the alternative pathway plays an important role in secondary lymphoid development and B-cell maturation through the induction of a subset of NF-κB target genes [30].
All NF-κB proteins contain an N-terminal Rel homology domain, which contains a DNA-binding domain, a dimerization domain and a nuclear-localization signal. p65, RelB and c-Rel also contain a C-terminal transactivation domain, which gives them the ability to activate gene transcription (Figure 1A). The lack of a transactivation domain but presence of a DNA-binding domain in p50 and p52 allows some NF-κB dimers, such as p50–p50 homodimers, to function as dominant-negative transcription factors by blocking κB elements [33]. Although almost all hetero- and homodimer combinations are possible, it is generally accepted that the most common active NF-κB dimer in cancer is p65–p50.
The NF-κB proteins undergo a number of post-translational modifications, which result in important changes in their physical interactions with other NF-κB proteins, cofactors and DNA. These modifications include ubiquitination, acetylation and, most importantly, phosphorylation [34]. In general, most phosphorylation events on NF-κB are activating modifications. For instance, phosphorylation of p65 at serine residue 276 is critical for protein–protein interactions with CBP/p300 [35], one of the necessary cofactors for NF-κB-regulated gene transcription [36]. Another important site of phosphorylation on p65 is serine residue 536, which can be phosphorylated by IKK, also increasing the activity of the protein [37].
NF-κB activation in glioma
Although NF-κB has not received the same attention as some other oncopathways in glioma, those studies that have examined it have yielded interesting results. Levels of NF-κB activity, as assessed by serine phosphorylation, are much higher in GBM tissue compared with non-GBM tissue [38,39], and correspond with increasing grade in astrocytic tumors [40,41]. While the precise mechanism of NF-κB activation in GBMs is largely undefined, there are numerous proteins and pathways dysregulated in GBMs that may cause NF-κB activation. TNF-α is one of the most potent activators of NF-κB (Figure 1B), and is produced in the CNS by microglia, astrocytes, endothelial cells and some neurons [42]. TNF-α and other proinflammatory soluble factors, such as IL-6 and IL-1β, are important regulators of paracrine signaling in brain tumor cells. These cytokines cause changes in gene expression in neighboring cells through direct ligand–receptor interactions, in addition to causing inflammation through the recruitment of immune cells to the tumor environment. TNF-α predominantly signals through TNF receptor (TNFR) 1, and upon binding to TNFR1 induces the formation of an intracellular complex that is either cytoprotective or cytotoxic. In gliomas, TNF-α signaling through TNFR1 promotes NF-κB activation and subsequent anti-apoptotic responses [43,44]. Moreover, the levels of TNFR1 expression are elevated in GBM as compared with low-grade gliomas [45]. As such, TNF-α and TNFR1, via NF-κB activation, promote tumor growth and development. NF-κB can also be aberrantly activated by numerous growth factors or signaling pathways that are dysregulated in gliomas [46]. NF-kB is activated by EGF, which is frequently overexpressed, and/or its receptor, EGFR, which is often mutationally activated [4]. PDGF has also been shown to activate NF-κB in GBM cells [47]. Through binding to their respective receptors, both EGF and PDGF activate NF-κB via a PI3K–AKT–IKK-dependent mechanism [48,49]. Additionally, PDGF-induced AKT has been shown to directly phosphorylate p65 [50]. As mentioned previously, GBMs often fail to express PTEN, a tumor suppressor and negative regulator of the AKT pathway. In the absence of PTEN, AKT is constitutively active and can, in turn, activate NF-κB [51]. The ubiquitin-editing protein A20 is a negative regulator of NF-κB whose expression is diminished in GBMs [52]. The downregulation of A20 in glioma cells is also associated with acquired resistance to O6-alkalyating agents, such as TMZ [52]. We have recently shown that inhibitor of growth protein 4, a negative regulator of NF-κB, is expressed at very low levels in GBMs, and that the lack of ING4 enables NF-κB to remain constitutively active [39]. Conversely, Pin1, a positive activator of NF-κB, is overexpressed in GBMs, and also contributes to constitutive NF-κB activation [53]. Finally, inhibition of NF-κB activity or of NF-κB-regulated genes has been demonstrated to reduce brain tumor growth, invasion and angiogenesis [54]. Thus, there is a strong correlation between constitutive NF-κB activation and gliomagenesis.
NF-κB signaling in glioma: preclinical data
During the past 5 years, one of the more promising preclinical drugs for glioma was the anti-inflammatory drug sulfasalazine. One of the first studies examining the effects of this drug on glioma used a model wherein U87-MG glioma cells were injected into the brains of immunocompromised (nude) mice [55]. Mice receiving daily intraperitoneal injections of sulfasalazine developed tumors that were 80% smaller than control animals [55]. Concurrent in vitro data from the same study suggested that the mechanism of action was via inhibition of NF-κB activity. However, a competing theory on the mechanism of action of sulfasalazine has been proposed [56,57], which claims that rather than inhibiting NF-κB, sulfasalazine inhibits a cystine-glutamate transporter [57]. The prospect of an already US FDA-approved drug showing strong antitumor effects in glioma was very exciting. Unfortunately, a recent Phase I/II trial using this drug on advanced-stage recurrent anaplastic astrocytoma or GBM patients was terminated owing to a lack of response [58]. The reasons for the failure in human GBM patients is unclear, but the authors of this study speculate that the lack of response may be due to differences between mice and humans, the O6-methylguanine–DNA methyltransferase (MGMT) promoter methylation status of patient's tumors or the small n-value of the study. Regardless, further studies will be required to fnd other compounds that effectively target NF-κB in GBM.
JAK/STAT signaling
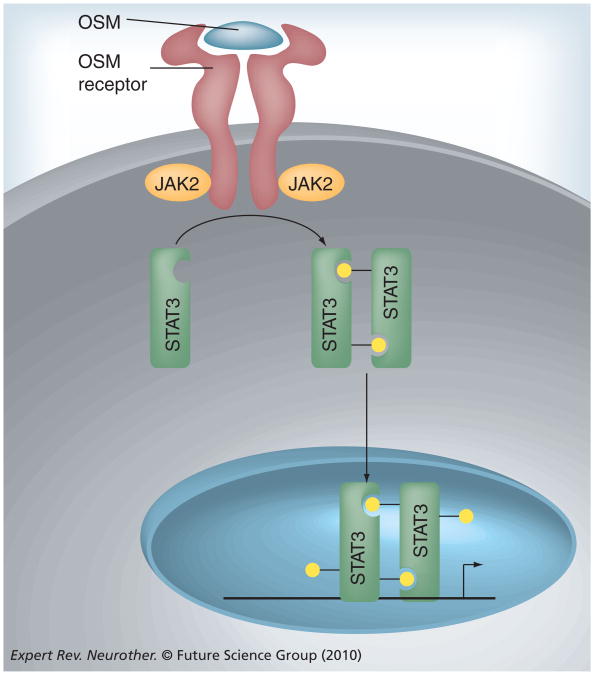
There are four members of the JAK family, including JAK1, JAK2, JAK3 and TYK2, and seven STAT family proteins: STATs 1, 2, 3, 4, 5A, 5B and 6 [59]. The JAK/STAT signaling pathway can be activated by a number of different stimuli including growth factors, interferons and the IL-6 family of cytokines [60]. Oncostatin-M (OSM), a member of the IL-6 family, provides a prototypical example of JAK/STAT pathway activation (Figure 2). The pathway begins through the binding of OSM to its receptor, which is composed of two protein subunits: gp130 and OSM receptor β [61]. Upon cytokine binding, associated JAK proteins, primarily JAK2, trans- and autophosphorylate themselves and the cytoplasmic tail of the receptor. When the receptor becomes phosphorylated, it serves as a docking site for another protein, STAT3. JAK2 proteins then phosphorylate STAT3 on tyrosine 727, a modification that allows STAT3 molecules to form homodimers through reciprocal SH2 domain interactions [62]. STAT3 dimers then translocate to the nucleus and function as transcription factors by binding to γ activated sequences elements in the promoters of STAT-responsive genes, such as VEGF, IL-10 and Bcl-2 (Table 1). JAK/STAT signaling also induces an important family of negative regulators, the suppressors of cytokine signaling proteins, which terminate JAK/STAT activity [63]. JAK2/STAT3 signaling is critical for the growth and survival of a variety of human malignancies, making it a focal point of cancer research [64–66].
Figure 2. IL-6 family signaling through the JAK/STAT-3 pathway.
OSM binding to the OSM receptor activates intracellular JAK kinases, predominantly JAK2, which phosphorylate (yellow circles) STAT-3. Once phosphorylated, STAT-3 molecules dimerize, translocate to the nucleus and bind to STAT response elements in the promoters of target genes. OSM: Oncostatin-M.
JAK2/STAT3 activation in GBM
Since STAT3 affects transcription of genes involved in apoptosis and the cell cycle [67], tight control of STAT3 activity is imperative to prevent malignant transformation of cells. However, aberrant activation of STAT3 is observed in the majority of human neoplasms, indicating that endogenous regulatory mechanisms have failed [64,65,68]. In GBMs, STAT3 was shown to be constitutively active, as assessed by tyrosine phosphorylation status [69–73]. Phosphorylated STAT3 correlated with poor survival in patients with anaplastic astrogliomas [72]. In another study, tyrosine-phosphorylated STAT3 was shown to be a frequent occurance in high-grade gliomas, and was positively associated with tumor grade [70]. Our group has determined that total levels of STAT3 and activation of STAT3, as assessed by both tyrosine and serine phosphorylation, are elevated in GBM tissues compared with control tissue [73]. We also detected elevated levels of phosphorylated JAK2 in GBM tissue [73]. An elegant study by Carro et al. demonstrated that STAT3 is one of the master regulators of mesenchymal transformation, leading to the mesenchymal phenotype that is a hallmark of tumor aggressiveness in GBM [74]. Futhermore, in malignant glioma, expression of STAT3 correlated with mesenchymal differentiation and predicted poor clinical outcome. Similar to NF-κB activation, there are many proteins and pathways dysregulated in GBMs that can lead to JAK2 and/or STAT3 activation. Owing to their ability to activate STAT3, IL-6 cytokines have been strongly implicated in GBMs [69,71,75]. IL-6 is one of the most ubiquitously dysregulated cytokines in cancer, with elevated levels observed in all tumors studied to date [76]. Human GBMs express elevated levels of IL-6 and the IL-6 family member OSM [61,77,78], which correlates with increasing tumor grade. In addition, IL-6 gene amplification events occur in 40–50% of GBM patients, and this is associated with decreased patient survival [79]. A survey of the available Cancer Genome Atlas data confirms these findings. IL-6 and OSM stimulation of GBM cells regulates VEGF expression, contributing to the angiogenic process [77]. We have recently shown that protein inhibitor of activated STAT3 (PIAS3), a negative regulator of activated STAT3, is expressed at very low levels in GBMs, which contributes to constitutive STAT3 signaling and cell proliferation [73]. Our preliminary results also demonstrate that Pin1, which is overexpressed in GBMs, can serve as a positive activator of S TAT3 [Atkinson GP et al., Unpublished Observation]. These fndings collectively indicate that STAT3 activation is associated with gliomagenesis.
STAT3 has also been studied in the context of the tumor-associated immune response [66]. The relationship of the immune system to tumor initiation and progression is complex and controversial, as inflammation can conspire with or against a tumor in a context- and cell-type-dependent fashion. What has become clear is that with the proper costimulatory signals, cytotoxic CD8+ T cells are capable of controlling and even eliminating tumor cells. It has been demonstrated that microglia/macrophages associated with human gliomas show dramatically lower costimulatory molecule expression than the same cells from normal controls [80], and this expression can be enhanced through the use of a small-molecule STAT3 inhibitor [81]. These data suggest that STAT3 inhibition in antigen-presenting cells may enhance the T-cell-mediated antitumor immune response in glioma. Data from other types of tumors have also shown the protumor STAT3-mediated immune-suppressive functions of myeloid-derived cells [82] and the STAT3-dependent protumor expansion of both Th17 cells [83,84] and Tregs [85]. On the whole, there is an abundance of data that suggest STAT3 activation in tumor-associated immune cells suppresses both the innate and adaptive immune responses [86].
The role of STAT3 in gliomagenesis is complex, however, and may depend on the mutational profle of the tumor. The laboratory of Azad Bonni has shown that in the context of mutated EGFR, STAT3 functions as an oncogene, but that in tumors with PTEN mutations, STAT3 can function as a tumor suppressor [87,88]. These fndings illustrate the need for an integration of the molecular profle of a patient's tumor, with information about signaling aberrancies, to develop tailored therapeutic interventions.
Inhibition of JAK2/STAT3 in glioma: preclinical data
According to the National Clinical Trials Database, there have not been any human clinical trials using experimental compounds intended to directly target oncogenic JAK/STAT signaling in glioma [201]. However, a number of studies using animal models of glioma have shown great promise that effective inhibition of JAK2/STAT3 signaling can reduce the malignancy of these tumors [75,89,90]. In 2004, a study using a transgenic mouse model of glioma demonstrated that IL-6 is required for gliomagenesis [75]. In this system, glioma formation was caused by v-src expression under the control of the GFAP promoter. At 65 weeks, the incidence of glioma in v-src+/-/IL-6+/+ mice was approximately 20%, while the incidence in v-src+/-/IL-6-/- animals was only 3% [75]. Importantly, the v-src+/-/IL-6-/- mice showed no detectable STAT3 activation in astrocytes, suggesting that IL-6-mediated STAT3 activation is a crucial component of glioma initiation [75]. This study suggests that an anti-IL-6 therapy may be useful in controlling glioma, although it did not explore the effect of IL-6 signaling on established tumors. IL-6-targeted therapies are already being used to treat other human diseases. In the past few years, tocilizumab, a humanized monoclonal antibody against the IL-6 receptor, has been found to be safe and have impressive effects on rheumatoid arthritis. A recent study found that after 5 years of monotherapy with this drug, over half of the rheumatoid arthritis patients enrolled in the study achieved clinical remission [91]. Based on our knowledge of the importance of IL-6 signaling in GBM, these already approved anti-IL-6 therapies could be useful in treating this tumor.
A more recent study examined the effect of a small-molecule inhibitor of STAT3, LLL-3, on glioma growth and animal survival [89]. The primary model used in this study involved injection of an established human glioma cell line, U87-MG, into the brains of nude mice. Mice that received a single stereotactic injection of LLL-3 into their nascent U87-MG brain tumor had a median survival of 28.5 days compared with 16 days in the vehicle-treated controls [89]. Although posthumous immunohistochemistry was not performed, MRI of both groups showed significantly smaller tumors in the brains of LLL-3 treated mice, confirming the role of STAT3 in tumor growth [89]. Another group used a similar model to investigate the effects of a dominant-negative STAT3 in glioma tumor formation [90]. Results showed that orthotopically implanted U87-MG cells expressing a dominant-negative STAT3 formed small tumors, but failed to show significant growth over a period of 7 weeks [90]. Control tumors quadrupled in size over the same period [90]. Immunohistochemical ana lysis showed not only decreased proliferation, but also enhanced levels of apoptosis in tumors expressing the dominant-negative form of STAT3 [90]. In a clever study of tumor immunity, the STAT3 inhibitor WP1066 was demonstrated to effectively decrease levels of activated STAT3 in GBM patient macrophages and T cells [81]. This study also found that WP1066 treatment enhanced the levels of CD86 and CD80 in GBM patient microglia, suggesting that with this type of treatment, the patients' antitumor T cells might be more potently activated [81]. These preclinical studies have confirmed the importance of STAT3 in glioma initiation, growth and immune evasion, and support further exploration of these inhibitors in future glioma therapies.
The signaling of cancer stem cells
In recent years, a small subpopulation of tumor cells with the ability of self-renewal has been described in a number of different tumors, including GBM [92]. Termed cancer stem cells (CSCs), these cells are best identified by the presence of the stem cell markers Nestin, Sox2 and, most notably, CD133 [93], although the validity of this final marker has recently come under question [94]. Similar to pluripotent neural stem cells, CSCs of GBM are thought to originate from the subventricular zone and are capable of differentiating into multiple CNS cell lineages [95]. Unfortunately, this crucial population of brain tumor cells has proven highly resistant to chemo- and radiotherapies, making its characterization even more important [93]. A number of in vitro and in vivo studies have demonstrated that CSCs are required for the growth and survival of glioma tumors [96]. Indeed, a mouse xenograft study showed that only human tumor cells with stem cell properties were able to form tumors in the brains of immunocompromised mice [97]. Furthermore, the CD133+ content of primary brain tumor samples has been found to correlate directly with tumor grade [98]. Another study recently found that the presence of CSCs was a highly significant independent prognostic factor for overall and progression-free survival in GBM [99].
Do the NF-κB and/or JAK2/STAT3 pathways play a role in the CSCs of gliomas? At this point the answer is not clear, but several studies have examined this possibility. It has been shown recently that STAT3 is required for the proliferation and survival of glioma CSCs [100,101]. STAT3 activation correlated with stem cell markers, and two different inhibitors and an siRNA knockdown of STAT3 also demonstrated that the regenerative properties of stem cells require STAT3 [100]. Similarly, it has also been shown that IL-6 signaling plays an important role in activating STAT3 in CSCs, and targeting IL-6 itself or the IL-6 receptor can induce apoptosis in these cells [101]. By contrast, another study found that ciliary neurotrophic factor-induced STAT3 activation promotes CSC differentiation, thus eliminating stem cell properties [102]. Part of the inconsistency of these studies may come from the lack of agreement on the defning marker(s) of CSCs.
The role of NF-κB signaling in glioma CSCs is even less defned. However, one study showed that COX-2, a gene that is regulated by NF-κB signaling, shows higher levels of expression in U87-derived CD133+ cells than in CD133- cells from the same cell line [103]. It does appear that many of the signaling aberrations that have been characterized in malignant glioma may be particularly relevant to the stem-cell population of the tumors [93]. For example, introduction of EGFRvIII-expressing neural stem cells into the mouse brain causes induction of malignant lesions that resemble high-grade glioma [104]. The PI3K–AKT signaling pathway has been shown to be constitutively active in CD133+ cells, and small-molecule inhibition of this pathway causes apoptosis [105]. Because of the relative newness of the feld of CSCs, further studies are needed to defne the function of JAK/STAT and NF-κB signaling in controlling CSC properties in the context of GBM.
Crosstalk between NF-κB & JAK-STAT in cancer
Data from our laboratory and others, as well as our unpublished data, indicate that activation of the NF-κB and JAK2/STAT3 pathways is a common feature of GBMs, and positively correlates with tumor grade [39,70,73,106]. The complex inter-relationship between the NF-κB and JAK/STAT pathways is beginning to be elucidated, illustrating that the JAK/STAT/NF-κB axis is central for tumor progression [107,108]. Crosstalk between the JAK/STAT3 and NF-κB pathways has been demonstrated at multiple levels, including activation of STAT3 by NF-κB-induced factors, such as IL-6 and COX-2, STAT3 activation of NF-κB processing, leading to proapoptotic responses [109], and STAT3 promotion of NF-κB nuclear translocation [110]. In the context of colitis-associated cancer, it has been shown that IL-6, an NF-κB regulated cytokine, is a critical tumor promoter during early colitis-associated cancer tumorigenesis, and that the proliferative and survival effects of IL-6 are mediated by STAT3 [108]. An elegant study by Lee et al. demonstrated that constitutively activated STAT3 maintains constitutive NF-κB activity in cancers by inhibiting its export from the nucleus [107]. Activated STAT3 prolongs NF-κB nuclear retention through p300-mediated NF-κB acetylation, which interferes with NF-κB nuclear export. These fndings define a cooperativity between STAT3 and NF-κB in cancer, and help explain why both transcription factors appear to stimulate a highly overlapping repertoire of prosurvival, proliferative and angiogenic genes (Table 1).
The interplay and clinical regulation of these two pathways was elegantly demonstrated in diffuse large B-cell lymphoma (DLBCL) [111]. DLBCL is characterized by constitutive NF-κB and STAT3 activity. A molecular profile was established demonstrating that STAT3 high DLBCLs had a higher expression of gene signatures that reflected NF-κB activity. DLBCL cell lines that secreted IL-6 were selectively killed by an inhibitor of STAT3 signaling, an effect that synergized with an inhibitor of the NF-κB pathway. This synergistic toxicity suggests a functional crosstalk between these two important signaling pathways that could be meaningful in a therapeutic setting. In unpublished results from our laboratory, we detect the concomitant presence of activated JAK2, activated STAT3, and acetylated and phosphorylated p65 in the majority of glioma xenografts, demonstrating the co-existence of the JAK/STAT and NF-κB pathways in glioma [Unpublished Data]. In this regard, activated STAT3 was one of the transcription factors significantly associated with The Cancer Genome Atlas worst-prognosis signature, which also correlates with marked mesenchymal features [74]. There is emerging evidence that crosstalk occurs between NF-κB and JAK/STAT3 pathways [107], leaving open the possibility that a single pharmacological agent could be used to inhibit both pathways.
Expert commentary
Although certain mutations are common in malignant glioma, there appears to be no single pathway that the tumor requires for survival. If this were the case, the pathway would become the focus of drug development and, with proper delivery, would likely lead to meaningful increases in survival. After all, this is essentially the story of chronic myelocytic leukemia (CML), a tumor with a hallmark constitutively active kinase caused by a reciprocal translocation between chromosomes 9 and 22 (the Philadelphia chromosome, Ph). Since almost all CML cases are Ph+, a specific kinase inhibitor (Gleevec®, Novartis Pharmaceuticals, NJ, USA) was developed and has been highly successful in treating CML [112]. Glioma presents a much more complex picture, with major challenges in both delivery and pathway targeting.
As such, it is not surprising that monotherapies targeting non-essential single pathways have not been effective in treating malignant gliomas [113,114]. Even if a well-delivered treatment kills 90% of tumor cells in a patient, the remaining 10% are likely to survive because they possess genetic advantages that confer resistance to the drug. Without susceptibility to the therapy, these few cells proliferate and lead to the recurrent tumors that eventually kill the patient. In this way, the escape of a tumor due to selective pressure is not unlike the development of antibiotic resistance in bacteria. For this reason, the focus of many current and upcoming clinical trials is on combining pathway-specific inhibitors. The rationale is that combining drugs with different mechanisms of action will give the tumor less of an opportunity to escape.
For example, an ongoing clinical trial taking place at Duke University (NC, USA) is testing the safety and efficacy of combined erlotinib, an EGFR inhibitor, and bevacizumab, a specific inhibitor of VEGF signaling, in recurrent glioma (CTID: NCT00671970). Another study is combining erlotinib with an inhibitor of the mTOR pathway called temsirolimus (CTID: NCT00112736). Pathway-specific inhibitors appear to cause fewer side effects and toxicities than traditional chemotherapies, so the hope is that combinations of these drugs will not only be more effective, but also better tolerated than older drugs. Although major breakthroughs in progression-free survival and median survival have not yet been made, the use of pathway-specific inhibitors in combination will likely yield favorable progress in glioma therapy.
Five-year view
Due in large part to advances in genomics, bioinformatics, transgenic mice and antibody-based technologies, our molecular understanding of malignant gliomas has expanded significantly over the past 20 years. As with other types of tumors, inhibitors that target specific signaling pathways, rather than all proliferative cells, are being developed with the hope of higher efficacy and fewer side effects. Owing to the widespread hyperactivation of these pathways in a broad sampling of human tumors, the NF-κB and JAK2/STAT3 pathways have become prime targets for pharmacological intervention. Currently, a large number of clinical trials are underway or recruiting for the use of NF-κB or JAK2/STAT3 inhibitors for the treatment of various types of cancer (see Table 2 for an abbreviated list). In the next 5 years, we are hopeful that some of the more promising drug compounds from these trials will be tested for efficacy in malignant glioma.
Table 2.
Selected current or upcoming JAK2/STAT3 and NF-κB pathway inhibitor clinical trials.
| Clinical Trial ID | Drug | Pathway | Molecular target | Tumor | Phase | Timeline |
|---|---|---|---|---|---|---|
| NCT00910728 | AZD1480 | JAK2/STAT3 | JAK2 | Myeloproliferative disorders | I/II | Recruiting |
| NCT00639002 | INCB018424 | JAK2/STAT3 | JAK2 | Multiple myeloma | II | Ongoing |
| NCT00079482 | CEP-701 | JAK2/STAT3 | JAK2 | AML | II | Ongoing |
| NCT00696176 | STAT 3 DECOY | JAK2/STAT3 | STAT3 | Head and neck cancer | 0 | Recruiting |
| NCT00955812 | OPB-31121 | JAK2/STAT3 | STAT3 | Solid tumors | I | Recruiting |
| NCT00522574 | XL019 | JAK2/STAT3 | JAK2 | Myeloproliferative disorders | I | Ongoing |
| NCT00077467 | Bortezomib | NF-κB | Proteosome | AML, ALL | I | Completed |
| NCT00572637 | CEP-18770 | NF-κB | Proteosome | Solid tumors, NHL | I | Recruiting |
| NCT00667082 | NPI-0052 | NF-κB | Proteosome | Lung, pancreatic, melanoma, lymphoma | I | Recruiting |
| NCT00199212 | PS-341 | NF-κB | Proteosome | Metastatic breast cancer | I | Recruiting |
| NCT00396864 | NPI-0052 | NF-κB | Proteosome | Solid tumors, lymphomas | I | Recruiting |
| NCT00376948 | Genistein | NF-κB | IκBα??? | Pancreatic cancer | II | Ongoing |
ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia; NHL: Non-Hodgkin lymphoma.
Another exciting event on the horizon is the completion of the Cancer Genome Atlas pilot project in GBM. This multicenter ana lysis of approximately 500 GBM tumor samples will be the most comprehensive study of a malignant glioma subtype to date, with each sample being extensively characterized using multiple histopathological and genomic techniques. An interim analysis published in 2008 was an exciting first step toward a more quantitative understanding of the genetic and signaling aberrations in glioma [16,18]. The publicly accessible data set from this enormous effort will only increase the likelihood that more common mechanisms and pathways underlying these tumors will be discovered. Indeed, The Cancer Genome Atlas has already led to a furry of discoveries regarding GBM genetics [115–118], and the importance of the STAT3 transcription factor in glioma [74].
As a result of these studies, gliomas will be further stratified into groups that describe these tumors not only histopathologically, but molecularly as well. Accordingly, patients will be parsed into treatment cohorts that are most likely to give successful outcomes. This is already occurring to some extent, as evidenced by the prognosis data of patients with MGMT promoter methylation [119,120]. MGMT is a critical enzyme in DNA repair. Like many genes, the expression of MGMT can be epigenetically silenced by the methylation of its promoter. Recent studies have found that malignant glioma patients with silenced MGMT respond significantly better to TMZ therapy than those with normal MGMT expression [119]. Further stratification based on tumor genetics and epigenetics will allow physicians to treat patients with the therapeutic regimen that is most likely to be effective.
All of this will slowly move GBM treatment closer to the holy grail of therapies: personalized medicine. Although the economics of individually tailored medicines will be difficult, personalized medicine is the most logical way to fight most forms of cancer. After all, while we attempt to categorize cancers to find and exploit commonalities, each case is in fact unique. This is particularly true with GBM, a tumor that is well known for its genetic and histologic heterogeneity [4]. This century, we will likely see rapid profiling of biopsy samples, custom therapy development based on tumor profiles and subsequent multipathway attacks on glioma through targeted delivery. In many cases, drugs directed toward the NF-κB and JAK2/STAT3 pathways will be a major part of these therapies.
Key issues.
Glioblastomas (GBMs) are invasive brain tumors with ineffective treatment options and a generally poor prognosis.
Numerous studies have shown that patient glioma samples frequently display constitutive activation of NF-κB and JAK2/STAT3 signaling pathways.
Preclinical studies in GBM have shown that inhibition of NF-κB and STAT3 is beneficial to outcome.
A number of JAK2/STAT3 and NF-κB-targeting drugs have been and are currently being used in clinical trials in other tumor types.
Clear attempts to directly target these pathways in human glioma patients have not yet been attempted, although inhibiting NF-κB and/or JAK2/STAT3 signaling shows immense promise in vitro and in vivo.
JAK2/STAT3 signaling, and possibly NF-κB signaling, is critical for the maintenance of cancer stem cells, the regenerative cell population of GBM.
Recent data suggest crosstalk exists between the JAK/STAT and NF-κB pathways, which may have important implications for designing effective drug therapies in GBM.
Acknowledgments
The authors would like to thank to Brandi Baker (UAB) for reading the manuscript.
This work was supported in part by National Institutes of Health grants CA-97247 (ENB), NS-50665 (ENB), IRG-60-001- 47 from the American Cancer Society and CA-13148-31 from the NCI (SEN), NIH CA-13148-35 (ENB), P30 CA-13149-38 from the UAB Comprehensive Cancer Center (ENB), and the Southeastern Brain Tumor Foundation (SEN). George P Atkinson was supported by the UAB Medical Scientist Training Program and by NIH T32-AI0705.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
George P Atkinson, Department of Cell Biology, University of Alabama at Birmingham, Birmingham, AL 35294-0005, USA.
Susan E Nozell, Department of Cell Biology, University of Alabama at Birmingham, Birmingham, AL 35294-0005, USA.
Etty (Tika) N Benveniste, Email: tika@uab.edu, Department of, Cell Biology, THT – Room 926A, 1900 University Boulevard, Birmingham, AL 35294-0006, USA, Tel.: +1 205 934 7667, Fax: +1 205 975 6748.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85(2):171–180. doi: 10.1007/s11060-007-9405-4. [DOI] [PubMed] [Google Scholar]
- 3.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4••.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. Excellent, comprehensive review of malignant glioma. [DOI] [PubMed] [Google Scholar]
- 5.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225. doi: 10.1093/jnen/61.3.215. discussion 226–219. [DOI] [PubMed] [Google Scholar]
- 6.Frosch MP, Anthony DC, De Girolami U. The Central Nervous System. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. Elsevier Saunders; PA, USA: 2005. pp. 1347–1419. [Google Scholar]
- 7.Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131(3):397–406. doi: 10.5858/2007-131-397-G. [DOI] [PubMed] [Google Scholar]
- 8.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 9.Kanu OO, Hughes B, Di C, et al. Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol. 2009;3:39–52. doi: 10.4137/cmo.s1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry J, Laperriere N, Zuraw L, Chambers A, Spithoff K, Cairncross JG. Adjuvant chemotherapy for adults with malignant glioma: a systematic review. Can J Neurol Sci. 2007;34(4):402–410. doi: 10.1017/s0317167100007265. [DOI] [PubMed] [Google Scholar]
- 11••.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. Breakthrough study describing the benefits of temozolomide in glioma treatment. [DOI] [PubMed] [Google Scholar]
- 12.Scott JN, Rewcastle NB, Brasher PM, et al. Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci. 1998;25(3):197–201. doi: 10.1017/s0317167100034016. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Nakai K, Kageji T, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol. 2009;91(1):80–84. doi: 10.1016/j.radonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9(4):505–517. doi: 10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colman H, Aldape K. Molecular predictors in glioblastoma: toward personalized therapy. Arch Neurol. 2008;65(7):877–883. doi: 10.1001/archneur.65.7.877. [DOI] [PubMed] [Google Scholar]
- 16.Network TTR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purow B, Schiff D. Advances in the genetics of glioblastoma: are we reaching critical mass? Nat Rev Neurol. 2009;5(8):419–426. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 21.Rao RD, Mladek AC, Lamont JD, et al. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7(10):921–929. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, James CD, Frederick L, Alderete BE, Jenkins RB. PTEN/MMAC1 mutations and EGFR amplification in glioblastomas. Cancer Res. 1997;57(23):5254–5257. [PubMed] [Google Scholar]
- 23.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5(11):610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 24.Prasad S, Ravindran J, Aggarwal BB. NF-kB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;1336(1–2):25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JL, Abe T, Inoue R, Fujiki M, Kobayashi H. IκBαM suppresses angiogenesis and tumorigenesis promoted by a constitutively active mutant EGFR in human glioma cells. Neurol Res. 2004;26(7):785–791. doi: 10.1179/016164104225014139. [DOI] [PubMed] [Google Scholar]
- 27.Loeffer S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115(2):202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- 28.Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-κB signaling module. Oncogene. 2006;25(51):6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 31.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 32.Senftleben U, Cao Y, Xiao G, et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science. 2001;293(5534):1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 33.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene. 2006;25(51):6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1(5):661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 36.Janknecht R, Hunter T. Transcription A growing coactivator network. Nature. 1996;383(6595):22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai H, Suzuki S, Kawasaki N, et al. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278(38):36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NF-κB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84(8):941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 39.Nozell S, Laver T, Moseley D, et al. The ING4 tumor suppressor attenuates NF-κB activity at the promoter of target genes. Mol Cell Biol. 2008;28(21):6632–6645. doi: 10.1128/MCB.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korkolopoulou P, Levidou G, Saetta AA, et al. Expression of nuclear factor-κB in human astrocytomas: relation to pI κ Ba, vascular endothelial growth factor, Cox-2, microvascular characteristics, and survival. Hum Pathol. 2008;39(8):1143–1152. doi: 10.1016/j.humpath.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Angileri FF, Aguennouz M, Conti A, et al. Nuclear factor-κB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112(10):2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 42.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otsuka G, Nagaya T, Saito K, Mizuno M, Yoshida J, Seo H. Inhibition of nuclear factor-κB activation confers sensitivity to tumor necrosis factor-α by impairment of cell cycle progression in human glioma cells. Cancer Res. 1999;59(17):4446–4452. [PubMed] [Google Scholar]
- 44.Koul D, Takada Y, Shen R, Aggarwal BB, Yung WK. PTEN enhances TNF-induced apoptosis through modulation of nuclear factor-κB signaling pathway in human glioma cells. Biochem Biophys Res Commun. 2006;350(2):463–471. doi: 10.1016/j.bbrc.2006.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi S, Yamamoto M, Ueno Y, et al. Expression of nuclear factor-κB, tumor necrosis factor receptor type 1, and c-Myc in human astrocytomas. Neurol Med Chir (Tokyo) 2001;41(4):187–195. doi: 10.2176/nmc.41.187. [DOI] [PubMed] [Google Scholar]
- 46.Brown RE, Law A. Morphoproteomic demonstration of constitutive nuclear factor-κB activation in glioblastoma multiforme with genomic correlates and therapeutic implications. Ann Clin Lab Sci. 2006;36(4):421–426. [PubMed] [Google Scholar]
- 47.Smith D, Shimamura T, Barbera S, Bejcek BE. NF-κB controls growth of glioblastomas/astrocytomas. Mol Cell Biochem. 2007;307(1–2):141. doi: 10.1007/s11010-007-9593-4. [DOI] [PubMed] [Google Scholar]
- 48.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10(2):262. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 49.Romashkova JA, Makarov SS. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 50.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19(7):4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 52.Bredel M, Bredel C, Juric D, et al. Tumor necrosis factor-α-induced protein 3 as a putative regulator of nuclear factor-κB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24(2):274–287. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 53.Atkinson GP, Nozell SE, Harrison DK, Stonecypher MS, Chen D, Benveniste EN. The prolyl isomerase Pin1 regulates the NF-κB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene. 2009;28(42):3735–3745. doi: 10.1038/onc.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie TX, Aldape KD, Gong W, et al. Aberrant NF-κB activity is critical in focal necrosis formation of human glioblastoma by regulation of the expression of tissue factor. Int J Oncol. 2008;33(1):5–15. [PubMed] [Google Scholar]
- 55.Robe PA, Bentires-Alj M, Bonif M, et al. In vitro and in vivo activity of the nuclear factor-κB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10(16):5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- 56.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67(19):9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung WJ, Sontheimer H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-κB. J Neurochem. 2009;110(1):182–193. doi: 10.1111/j.1471-4159.2009.06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robe PA, Martin DH, Nguyen-Khac MT, et al. Early termination of ISRCTN45828668, a Phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer. 2009;9:372. doi: 10.1186/1471-2407-9-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aaronson DS, Horvath CM. A road map for those who don't know JAK–STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 60.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 61.Chen SH, Benveniste EN. Oncostatin M: a pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev. 2004;15(5):379–391. doi: 10.1016/j.cytogfr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Mertens C, Darnell JE., Jr SnapShot: JAK–STAT signaling. Cell. 2007;131(3):612. doi: 10.1016/j.cell.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 63.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30(8):392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18(2):254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE. 2006;2006(343):pe30. doi: 10.1126/stke.3432006pe30. [DOI] [PubMed] [Google Scholar]
- 66.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DJ, Chan KS, Sano S, Digiovanni J. Signal transducer and activator of transcription 3 (STAT3) in epithelial carcinogenesis. Mol Carcinog. 2007;46(8):725–731. doi: 10.1002/mc.20342. [DOI] [PubMed] [Google Scholar]
- 68.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109(9):1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active STAT3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 70.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14(19):6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaefer LK, Ren Z, Fuller GN, Schaefer TS. Constitutive activation of STAT3α in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2) Oncogene. 2002;21(13):2058–2065. doi: 10.1038/sj.onc.1205263. [DOI] [PubMed] [Google Scholar]
- 72.Abou-Ghazal M, Yang DS, Qiao W, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14(24):8228–8235. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brantley EC, Nabors LB, Gillespie GY, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14(15):4694–4704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carro MS, Lim WK, Alvarez MJ, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weissenberger J, Loeffer S, Kappeler A, et al. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23(19):3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 76.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110(9):1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 77.Repovic P, Fears CY, Gladson CL, Benveniste EN. Oncostatin-M induction of vascular endothelial growth factor expression in astroglioma cells. Oncogene. 2003;22(50):8117–8124. doi: 10.1038/sj.onc.1206922. [DOI] [PubMed] [Google Scholar]
- 78.Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci. 1999;19(13):5236–5244. doi: 10.1523/JNEUROSCI.19-13-05236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tchirkov A, Khalil T, Chautard E, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007;96(3):474–476. doi: 10.1038/sj.bjc.6603586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/ macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. In vivo study of the glioblastoma-associated immune response and the role of STAT3. [DOI] [PubMed] [Google Scholar]
- 82.Matsukawa A, Kudo S, Maeda T, et al. STAT3 in resident macrophages as a repressor protein of inflammatory response. J Immunol. 2005;175(5):3354–3359. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 83.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 84.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 85.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 86••.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting STAT3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. Elegant study showing the importance of STAT3 in the immune response to tumors. [DOI] [PubMed] [Google Scholar]
- 87••.de la Iglesia N, Konopka G, Puram SV, et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 2008;22(4):449–462. doi: 10.1101/gad.1606508. Demonstrates a STAT3 contribution to the EGFRvIII oncopathway in gliomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de la Iglesia N, Konopka G, Lim KL, et al. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci. 2008;28(23):5870–5878. doi: 10.1523/JNEUROSCI.5385-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuh B, Sobo M, Cen L, et al. LLL-3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. Br J Cancer. 2009;100(1):106–112. doi: 10.1038/sj.bjc.6604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dasgupta A, Raychaudhuri B, Haqqi T, et al. STAT3 activation is required for the growth of U87 cell-derived tumours in mice. Eur J Cancer. 2009;45(4):677–684. doi: 10.1016/j.ejca.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68(10):1580–1584. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 93.Li Z, Wang H, Eyler CE, Hjelmeland AB, Rich JN. Turning cancer stem cells inside out: an exploration of glioma stem cell signaling pathways. J Biol Chem. 2009;284(25):16705–16709. doi: 10.1074/jbc.R900013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y, Wu PY. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009;18(8):1127–1134. doi: 10.1089/scd.2008.0338. [DOI] [PubMed] [Google Scholar]
- 95.Kappadakunnel M, Eskin A, Dong J, et al. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol. 2010;96(3):359–367. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55(5):369–374. [PubMed] [Google Scholar]
- 97.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 98.Thon N, Damianoff K, Hegermann J, et al. Presence of pluripotent CD133+ cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2010;43(1):51–59. doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 99.Pallini R, Ricci-Vitiani L, Banna G, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(24):8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- 100.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27(10):2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Lathia JD, Wu Q, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27(10):2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee J, Son MJ, Woolard K, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13(1):69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Annabi B, Laflamme C, Sina A, Lachambre MP, Beliveau R. A MT1–MMP/NF-κB signaling axis as a checkpoint controller of COX-2 expression in CD133+ U87 glioblastoma cells. J Neuro inflammation. 2009;6:8. doi: 10.1186/1742-2094-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/ Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 105.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26(12):3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6(5):675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107••.Lee H, Herrmann A, Deng JH, et al. Persistently activated STAT3 maintains constitutive NF-κB activity in tumors. Cancer Cell. 2009;15(4):283–293. doi: 10.1016/j.ccr.2009.02.015. Great paper describing a role for STAT3 in maintaining active NF-κB in the nucleus of transformed cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grivennikov S, Karin E, Terzic J, et al. IL-6 and STAT3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC. STAT3 activation of NF-κB p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA. 2006;103(19):7264–7269. doi: 10.1073/pnas.0509808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFκB. Genes Dev. 2007;21(11):1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-κB pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111(7):3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 113.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma – animal models and therapeutic challenges. Brain Pathol. 2009;19(1):112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res. 2008;14(4):957–960. doi: 10.1158/1078-0432.CCR-07-1810. [DOI] [PubMed] [Google Scholar]
- 115.Freire P, Vilela M, Deus H, et al. Exploratory analysis of the copy number alterations in glioblastoma multiforme. PLoS One. 2008;3(12):E4076. doi: 10.1371/journal.pone.0004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118••.Bredel M, Scholtens DM, Harsh GR, et al. A network model of a cooperative genetic landscape in brain tumors. JAMA. 2009;302(3):261–275. doi: 10.1001/jama.2009.997. Uses a systems biology approach to determine the network of mutations that drive the malignant glioma phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119••.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. First study to show the importance of MGMT silencing in glioma patient outcome. [DOI] [PubMed] [Google Scholar]
- 120.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
Website
- 201.National Clinical Trials Database www.clinicaltrials.gov