Abstract
Celiac disease (CD) is associated with both lymphoproliferative malignancy (LPM) and increased death from LPM. Research suggests that co-existing autoimmune disease may influence survival in LPM. Through Cox regression we examined overall and cause-specific mortality in 316 individuals with CD+LPM vs. 689 individuals with LPM only. CD was defined as having villous atrophy according to biopsy reports at any of Sweden’s 28 pathology departments, and LPM as having a relevant disease code in the Swedish Cancer Register. During follow-up, there were 551 deaths (CD: n=200; non-CD: n=351). Individuals with CD+LPM were at an increased risk of death compared with LPM-only individuals (adjusted hazard ratio (aHR)=1.23; 95% confidence interval (CI)=1.02–1.48). However, this excess risk was only seen in the first year after LPM diagnosis (aHR=1.76), with HRs decreasing to 1.09 in years 2–5 after LPM diagnosis and to 0.90 thereafter. Individuals with CD and non-Hodgkin lymphoma (NHL) were at a higher risk of any death as compared with NHL-only individuals (aHR=1.23; 95%CI=0.97–1.56). This excess risk was due to a higher proportion of T-cell lymphoma in CD patients. Stratifying for T- and B-cell status, the HR for death in individuals with CD+NHL was 0.77 (95%CI=0.46–1.31
In conclusion, we found no evidence that co-existing CD influences survival in individuals with LPM. The increased mortality in the first year after LPM diagnosis is related to the predominance of T-NHL in CD individuals. Individuals with CD+LPM should be informed that their prognosis is similar to that of individuals with LPM only. However, this study had low statistical power to rule our excess mortality in patients with CD and certain LPM subtypes.
Keywords: cancer, celiac, coeliac, death, lymphoproliferative, malignancy mortality
Introduction
Celiac disease (CD) occurs in about 1% of the Western population.[1] It is an immune-mediated disorder triggered by gluten exposure in genetically sensitive individuals.[2] Although CD was first regarded as restricted to the small intestine, it has now become evident that it also affects extra-intestinal organs. CD has been associated with both autoimmune disorders (such as type 1 diabetes),[3] and non-autoimmune complications, including adverse pregnancy outcome,[4] tuberculosis,[5] and excess mortality.[6]
One of the main complications in CD is cancer, both any cancer[6 7] and more specifically lymphoproliferative malignancy (LPM).[8 9] Relative risks have typically varied between 1.2 and 1.4 for any malignancy[6 7 10 11] and 2 to 6 for LPM.[6–8 12–18] The largest study to date reported an overall hazard ratio (HR) for LPM of 2.82 (95% confidence interval (CI) 2.36–3.37)[8], decreasing to around 2 beyond the first year of follow-up. The highest relative risks for LPMs in CD are usually seen for non-Hodgkin lymphoma (NHL). NHL is also a common form of cancer in non-celiac inflammatory conditions, including rheumatoid arthritis, systemic lupus erythematosus, and Hashimoto’s disease.[19]
LPM is the most common form of hematological malignancy, accounting for about 5% of all cancers in the USA. While demographic factors, age at LPM diagnosis, and disease characteristics (e.g., malignancy stage and tumor location) influence the prognosis of LPM, it is unclear whether co-existing CD can affect survival in LPM.
There is evidence that patients with an earlier diagnosis of an autoimmune disease (rheumatoid arthritis) have better survival in NHL than individuals without rheumatoid arthritis (but higher death rates from causes other than NHL).[20] In contrast, another study of individuals with a diagnosis of rheumatoid arthritis before cancer diagnosis found poorer survival in patients with rheumatoid arthritis.[21] Some data suggest that autoimmune disease may influence survival in individuals with other subtypes of LPM than NHL (e.g., Hodgkin’s lymphoma).[22] Interestingly, patients with a small intestinal adenocarcinoma in the setting of CD have improved survival compared with those without CD.[23] Low Hb[24] and low albumin[25] are common characteristics of CD, and have been associated both with lower survival rates in CD[26 27], and in LPM[28 29]. We therefore hypothesized that celiac patients with LPM had a lower survival rate than non-celiac patients with LPM.
We linked nationwide data on biopsy-verified CD to data from the Swedish Cancer Register, the Total Population Register, and the Cause of Death Register. We then estimated the survival of LPM individuals in relation to CD status.
Methods
Study participants
Data on small intestinal biopsy reports were collected in 2006–2008. The biopsies had been performed in 1969–2008 and later examined at one of Sweden’s 28 pathology departments. CD was defined as having villous atrophy (VA, equivalent to Marsh stage III) [30] on biopsy. We did not require a positive CD serology for the CD diagnosis; however, 88% of a random subset of individuals with available data on CD serology were serologically positive at time of biopsy. [24]
IT personnel at each department of pathology searched computerized biopsy report databases and then delivered data on biopsy date, personal identity number of the patient,[31] morphology according to the Swedish SnoMed classification (see Appendix), and topography (duodenum and jejunum). Because we limited our search to computerized data, most individuals in this study had been biopsied since 1990 (85.1%). Details on the data collection procedure have been published elsewhere.[24 32]
In the current study we used the same cohort (n=29,096) as in our “parent study” on mortality in CD.[32] Each individual with CD was matched with up to five reference individuals from the Total Population Register. Matching criteria included sex, age, county, and year of biopsy (reference individuals: n=144,522).
Cancer data
Data on cancer were obtained through the Swedish Cancer Register.[33] This register was established in 1958. About 99% of all malignancies are morphologically verified and almost 100% of malignancies are reported to the Swedish Cancer Register. We defined LPM as having an ICD7 code of 200–204. Subtypes were defined as follows: NHL (200 and 202) and “non-NHL” (includes Hodgkin’s lymphoma 201; myeloma/plasmacytoma 203, ALL 204.0; and chronic leukemia 204.1).
Through linkage to the Swedish Cancer Register, we identified 1005 individuals with LPM before the end of the follow-up (CD: n=316; individuals: n=689).
Statistics and analysis plan
We estimated the risk of overall death and death from LPM using Cox’ regression models. The time-scale of analysis was time since LPM diagnosis. Because the parent study [32] included CD individuals enrolled at biopsy date and matched controls that were alive at the corresponding date, the model allowed for staggered entry, i.e. individuals with a prevalent LPM diagnosis at entry of the parental study started their follow-up at the corresponding time since LPM diagnosis. Follow-up ended at date of death, emigration, or end of study, whichever occurred first. The proportional hazard assumption was investigated by assessing the HRs according to time since LPM diagnosis, and given the presence of non-proportionality we calculated both overall HRs and time-specific HRs (≤1 year; 1 > to ≤5 years, and > 5years). HRs were also adjusted for sex, age at LPM diagnosis, subtype of LPM (NHL vs. non-NHL), calendar period, socioeconomic position and education. The adjusted survival curve was standardized to the covariate distribution for the CD individuals and was estimated from the cumulative hazard function in a Cox’ regression model with separate base-line hazard functions for CD and controls. Different base-line hazard functions were applied only when estimating the survival curves and the reason for that was to allow the data to display the suggested non-proportionality of the CD-effect, with a more pronounced risk difference in the first years since diagnosis.
We also examined mortality in CD patients and controls diagnosed specifically with T- or B-cell NHL. We restricted these sub-analyses to individuals diagnosed in the 2000 or later in that earlier histopathology SnoMed codes for malignancy may lack in specificity. To consider the overall influence of T- and B-cell lymphoma on mortality in CD patients with NHL, we also carried out a stratified analysis allowing for different baseline hazards for T- and B-cell lymphoma.
Given that CD is sometimes undiagnosed for many years, [34] there is a risk that some patients diagnosed with CD after LPM actually had CD at the time of LPM diagnosis. In a sub-analysis we therefore examined survival in LPM among patients with CD diagnosed before LPM diagnosis.
Socioeconomic status and education were only available in a subset of individuals (Table 1), and missing data were fitted as a separate category in the statistical analyses. The proportion of individuals with missing data on these two parameters was greater than in our earlier study on socioeconomic position,[35] which is due to the high average age of study participants in the current study. Data on socioeconomic position originated from occupational data from the Swedish Occupational Register.
Table 1.
Characteristics of individuals with lymphoproliferative malignancy
| Characteristics | Controls, n (%) | Celiac disease, n (%) |
|---|---|---|
| Total | 689 (100) | 316 (100) |
| Age at LPM diagnosis (years) | ||
| 0–9 | 37 (5.4) | 8 (2.5) |
| 10–19 | 23 (3.3) | 2 (0.6) |
| 20–29 | 22 (3.2) | 4 (1.3) |
| 30–39 | 21 (3.0) | 9 (2.8) |
| 40–49 | 53 (7.7) | 12 (3.8) |
| 50–59 | 111 (16.1) | 80 (25.3) |
| 60–69 | 168 (24.4) | 91 (28.8) |
| 70–79 | 181 (26.3) | 83 (26.3) |
| 80+ | 73 (10.6) | 27 (8.5) |
| Sex | ||
| Female | 321 (46.6) | 157 (49.7) |
| Male | 368 (53.4) | 159 (50.3) |
| Year of LPM diagnosis | ||
| -1979 | 40 (5.8) | 11 (3.5) |
| 1980–89 | 67 (9.7) | 36 (11.4) |
| 1990–99 | 224 (32.5) | 104 (32.9) |
| 2000- | 358 (52.0) | 165 (52.2) |
| Country of birth | ||
| Nordic | 661 (95.9) | 296 (93.7) |
| Not Nordic | 28 (4.1) | 20 (6.3) |
| Socioeconomic status* | ||
| Highest | 85 (12.3) | 38 (12.0) |
| Intermediate | 63 (9.1) | 18 (5.7) |
| Lowest | 173 (25.1) | 59 (18.7) |
| Missing | 368 (53.4) | 201 (63.6) |
| Education | ||
| <9 years | 296 (43.0) | 136 (43.0) |
| High school, 2years (Manual programs) | 150 (21.8) | 67 (21.2) |
| High school, 3years (theoretical programs) | 65 (9.4) | 21 (6.6) |
| University/College | 129 (18.7) | 58 (18.3) |
| Missing | 49 (7.1) | 34 (10.8) |
| LPM, subtype | ||
| NHL | 348 (50.5) | 243 (76.9) |
| “ Non-NHL” | 341 (49.5) | 73 (23.1) |
| (HL) | 65 (9.4) | 13 (4.1) |
| (Myeloma) | 128 (18.6) | 32 (10.1) |
| (CLL) | 98 (14.2) | 18 (5.7) |
| (ALL) | 50 (7.3) | 10 (3.2) |
LPM, Lymphoproliferative malignancy
HR, Hazard ratio; NHL, Non-Hodgkin lymphoma; HL, Hodgkin lymphoma, CLL, Chronic lymphatic leukemia; ALL, Acute lymphatic leukemia;
“Non-NHL” = HL, Myeloma, CLL and ALL.
HRs were considered statistically significant at p<0.05 when 95% CIs did not include 1. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study was approved by the Research Ethics Committee of Karolinska Institutet, Stockholm, Sweden.
Results
Most study participants were diagnosed with LPM at age 50 years or later (Table 1, mean age at LPM diagnosis was 62.7±4.2 years in CD patients vs. 59.5±4.8 in controls). Some 50% of LPM cases were seen in males (Table 1) and half of the cases occurred in 2000 or later. Education and socioeconomic status were similar in CD patients and controls. NHL was the most common LPM subtype in both CD patients (76.9%) and controls (50.5%).
All-cause mortality
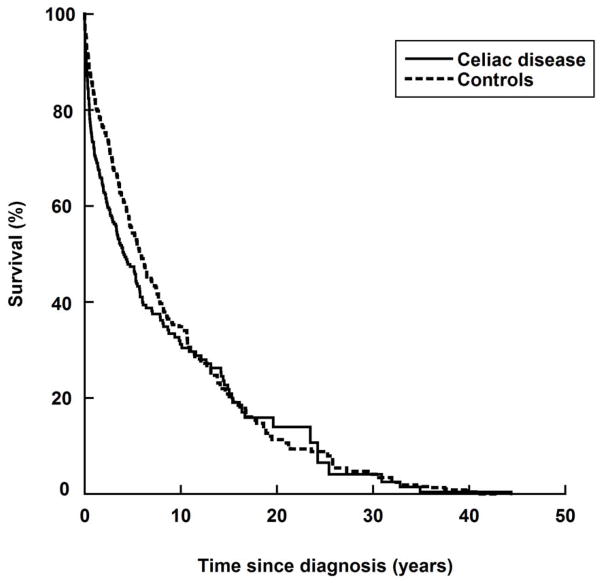
During follow-up, there were 551 deaths in 1005 study participants (CD: n=200 in 316 participants; not CD: n=351 in 689). Most of these deaths took place in the first year after LPM diagnosis (79.5% of deaths in CD individuals vs. 67.2% in individuals without CD). Individuals with CD+LPM were at an increased risk of death compared with those with LPM only (adjusted HR=1.23; 95% CI=1.02–1.48) (Figure 1). Crude HRs were similar (Table 2). The excess mortality was only seen in the first year after LPM diagnosis (adjusted HR=1.76; 95% CI=1.31–2.36) (Figure 1), with HRs decreasing to 1.09 in years 2–5 after LPM diagnosis and to 0.90 thereafter (Table 2).
Figure 1.
Overall survival in individuals with lymphoproliferative malignancy in relation to celiac disease status.
Graphs were standardized to the distribution of covariates (see text).
Table 2.
All-cause mortality according to time since diagnosis of lymphoproliferative malignancy in individuals with and without celiac disease
| Deaths in CD vs. deaths in controls | Crude | Adjusted | |||
|---|---|---|---|---|---|
| Follow-up | HR | 95% CI | HR | 95% CI | |
| Any overall | 200 vs. 351 | 1.33 | (1.12–1.59) | 1.23 | (1.02–1.48) |
| Year 1 | 159 vs. 236 | 1.89 | (1.42–2.52) | 1.76 | (1.31–2.36) |
| Years 2–5 | 26 vs. 51 | 1.11 | (0.81–1.51) | 1.09 | (0.79–1.49) |
| Years >5 | 15 vs. 64 | 1.06 | (0.77–1.47) | 0.90 | (0.64–1.26) |
| NHL* overall | 152 vs. 170 | 1.33 | (1.07–1.67) | 1.23 | (0.97–1.56) |
| Year 1 | 126 vs. 119 | 1.72 | (1.23–2.41) | 1.52 | (1.07–2.16) |
| Years 2–5 | 18 vs. 22 | 1.16 | (0.76–1.77) | 1.10 | (0.71–1.69) |
| Years >5 | 8 vs. 29 | 1.04 | (0.69–1.58) | 1.00 | (0.65–1.54) |
| Non-NHL overall | 48 vs. 181 | 1.41 | (1.03–1.94) | 1.22 | (0.87–1.71) |
| Year 1 | 33 vs. 117 | 1.58 | (0.84–2.98) | 1.34 | (0.71–2.52) |
| Years 2–5 | 8 vs. 29 | 1.47 | (0.91–2.39) | 1.27 | (0.77–2.08) |
| Years >5 | 7 vs. 35 | 1.22 | (0.68–2.17) | 1.05 | (0.55–1.99) |
HR, Hazard ratio; NHL, Non-Hodgkin lymphoma; HL, Hodgkin lymphoma, CLL, Chronic lymphatic leukemia; ALL, Acute lymphatic leukemia;
Non-NHL=Myeloma + Hodgkin lymphoma + Chronic lymphatic leukemia + Acute lymphatic leukemia.
For example, individuals with celiac disease + NHL were at increased risk (adjusted hazard ratio=1.23) to die from any cause compared with individuals with NHL only.
Adjustment, see methods for description
Similar HR patterns were observed for subtypes of LPM (Table 2). One hundred and fifty-two individuals with CD+NHL and 170 individuals with NHL only died during follow-up. The vast majority of NHL individuals who died did so in the first year after NHL diagnosis (CD: 82.9%; not CD: 70.0%). In comparison with NHL-only individuals, individuals with CD+NHL were at an increased risk of death (adjusted HR=1.23; 95% CI=0.97–1.56). CD individuals with LPM were however at no increased risk of death more than 1 year after LPM diagnosis (Table 2).
Because of the small numbers of “non-NHL” malignancies, mortality estimates for single subtypes were unstable (Table 2). Analyzing all non-NHL simultaneously, the overall HR for death in CD individuals with non-NHL LPM was 1.22 (95%CI=0.87–1.71), with a non-significantly increased risk in the first 5 years, but not thereafter (Table 2).
Death from LPM
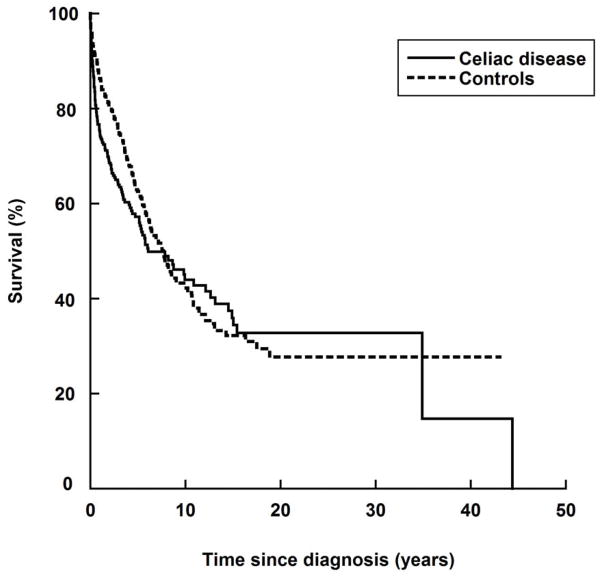
Out of 316 individuals with CD+LPM, some 140 died from LPM (LPM only: n=232 in 689 controls) (Table 3 and Figure 2). Some 82.1% (n=115) of LPM deaths in individuals with CD took place in the first year after LPM diagnosis (compared with 75.4% of individuals with no CD, n=175). When compared with individuals with LPM only, individuals with CD+LPM were at an increased risk of death from LPM (adjusted HR=1.22 (95% CI=0.97–1.52) (Table 3). This HR represented a two-fold increased risk of death in the first year after LPM, and no excess mortality thereafter (Table 3).
Table 3.
Cause-specific mortality according to time since diagnosis of lymphoproliferative malignancy in individuals with and without celiac disease
| Deaths in CD vs. deaths in controls | Crude | Adjusted | |||
|---|---|---|---|---|---|
| Follow-up | HR | CI | HR | CI | |
| Any overall | 140 vs. 232 | 1.33 | (1.08–1.65) | 1.22 | (0.97–1.52) |
| Year 1 | 115 vs. 175 | 2.11 | (1.53–2.91) | 1.94 | (1.39–2.70) |
| Years 2–5 | 18 vs. 40 | 0.98 | (0.67–1.43) | 0.94 | (0.64–1.38) |
| Years >5 | 7 vs. 17 | 0.91 | (0.58–1.42) | 0.77 | (0.49–1.21) |
| NHL* overall | 108 vs. 113 | 1.35 | (1.03–1.75) | 1.23 | (0.93–1.62) |
| Year 1 | 95 vs. 91 | 1.90 | (1.31–2.76) | 1.67 | (1.13–2.47) |
| Years 2–5 | 10 vs. 15 | 1.08 | (0.64–1.80) | 1.02 | (0.60–1.72) |
| Years >5 | 3 vs. 7 | 0.77 | (0.42–1.39) | 0.74 | (0.40–1.36) |
| Non-NHL# overall | 32 vs. 119 | 1.40 | (0.95–2.08) | 1.24 | (0.82–1.87) |
| Year 1 | 20 vs. 84 | 1.56 | (0.73–3.33) | 1.35 | (0.63–2.88) |
| Years 2–5 | 8 vs. 25 | 1.25 | (0.68–2.28) | 1.05 | (0.56–1.95) |
| Years >5 | 4 vs. 10 | 1.53 | (0.76–3.08) | 1.51 | (0.71–3.24) |
HR, Hazard ratio; NHL, Non-Hodgkin lymphoma; HL, Hodgkin lymphoma, CLL, Chronic lymphatic leukemia; ALL, Acute lymphatic leukemia;
Non-NHL=Myeloma + Hodgkin lymphoma + Chronic lymphatic leukemia + Acute lymphatic leukemia.
For example, individuals with celiac disease + NHL were at increased risk (adjusted hazard ratio=1.23) to die from NHL compared with individuals with NHL only.
Adjustment, see methods for description
Figure 2.
Survival from lymphoproliferative malignancy in individuals with lymphoproliferative malignancy in relation to celiac disease status.
Graphs were standardized to the distribution of covariates (see text).
Death from subtypes of LPM
Some 108 out of 243 (44.4%) individuals with CD and NHL died from NHL during follow-up, which can be compared with 113 of the 348 (32.5%) individuals with NHL only. Most of these deaths took place in the first year after LPM diagnosis (CD: n=95 (88.0%); not CD: n=91 (80.5%)). Individuals with CD+NHL were at a 23% increased risk of death from NHL compared with individuals with NHL only (Table 3). Only in the first year after NHL diagnosis was this risk increase statistically significant.
CD individuals with any LPM other than NHL (non-NHL) were at a small increased risk of death from “non-NHL”, but the risk estimate did not attain statistical significance (adjusted HR=1.24; 95% CI=0.82–1.87).
T- and B-cell NHL
Some 36/88 (40.9%) CD patients with reliable SnoMed information on NHL subtype had T-cell lymphoma, which can be compared with only 9/132 controls (6.8%). Restricting our analysis to individuals with available T- and B-NHL data, the HR for death in CD patients was 1.35 (95% CI; 0.88–2.08). Stratifying for subtype (T- or B), the HR decreased to 0.77 (95%CI=0.46–1.31), with the main effects showing that individuals with T-NHL were at a higher risk of death than individuals with B-cell lymphoma (HR=3.03; 95% CI=1.80–5.11). Additional data given in the appendix show that with stratification for NHL subtype, patients with CD and NHL were at no increased risk of death, even in the first year after NHL diagnosis.
Looking specifically at CD individuals with T-cell NHL, these individuals were at increased risk of death compared with controls with T-cell NHL. However, because of lack of power, the risk estimate failed to attain statistical significance (HR=4.10; 95% CI=0.51–32.8) and thus we refrained from calculating HRs according to follow-up.
CD individuals with B-cell lymphoma were at no increased risk of death (overall HR=0.71; 95% CI=0.38–1.34) compared with controls with B-cell lymphoma. HRs did not differ according to time since LPM diagnosis (first year: HR=0.70; 0.28–1.77); years 2–5: HR=0.96; 0.39–2.40); >5 years: HR=0.23; 0.03–1.99).
Restricting our analysis to individuals with CD diagnosed before LPM did not influence our risk estimates (Appendix).
Death from other causes than LPM
CD did not influence the risk of non-LPM death (overall death minus LPM) in individuals with LPM (data not shown).
Discussion
With the exception of a slight excess mortality in the first year after LPM diagnosis in individuals with CD, this study found no evidence that co-existing CD influences survival in individuals with LPM. A similar mortality pattern was noted in individuals with NHL. The excess risk in the first year after diagnosis was due to a high proportion of T-cell lymphoma in patients with CD+NHL; adjusting for subtype (T- or B-), we found that CD patients with NHL had a similar mortality to controls with NHL.
Previous research has shown that CD is associated with an increased relative risk of cancer, and several studies have suggested that individuals with CD[32 36–40] (as well as individuals with positive IgA transglutaminase antibodies[41]) are at increased risk of death from cancer (although exceptions exist[42]). However, none of these studies explored the role of CD for survival in LPM. We linked data on CD, cancer, and cause of death and were therefore able to show that concurrent CD does not modify the risk of death in individuals with LPM.
Although there was a small excess risk of death in the first year after LPM diagnosis in individuals with CD, the risk decreased after adjustment for confounders, including LPM subtype.
This study has some strengths and limitations. The main strength is the number of individuals with both CD and LPM (n=316), which allowed us to examine both overall and LPM-specific death. Another strength is our use of regional pathology registers to ascertain VA (equal to CD in this study).[24] More than 96% of Swedish pediatricians and gastroenterologists carry out a small intestinal biopsy in at least 9/10 individuals with suspected CD before diagnosis.[24] Hence, biopsy reports have a high sensitivity for diagnosed CD. Evaluating a subset of individuals using patient chart data, we found that VA has a positive predictive value of 95% for CD,[24] which is higher than having a physician-assigned diagnosis of CD in the Swedish Inpatient Register (88%)[9]. When two independent researchers manually reviewed more than 1500 biopsy reports, they found that other diagnoses than CD were very uncommon in individuals with VA (inflammatory bowel disease, IBD, was the most common disorder recorded in biopsy registers but only occurred in 0.3% of reports).[24] The histopathology examination was, on average, based on three tissue specimens, which should detect about 95% of all CD.
Many individuals with CD are never admitted to a hospital (and hence not recorded in the Inpatient Register), and in those admitted to a hospital the admission may be due to non-CD diagnoses and may have taken place years after CD diagnosis. Data on biopsy date instead allowed us to define an exact date of CD diagnosis and identify average individuals with CD and thereby minimizing bias regarding time of diagnosis and disease severity.
Our use of biopsy data, instead of hospital data, to ascertain CD may also explain why we found no increased risk of death in patients with CD+LPM, when others using hospital-based data have found a risk increase in LPM patients with other autoimmune diseases.[21 22]
The nationwide Swedish Cancer Register was established in 1958 and has virtually complete coverage of malignancies.[43] Approximately 99% of all malignancies are morphologically verified.[43] Each year, about 50,000 malignancies are first sent to six regional oncological centers where data are coded and recorded. These local centers also perform quality controls before the data are transferred to the Swedish Cancer Register.
Access to histopathology data in individuals diagnosed with NHL from 2000 and up allowed us to consider the distribution of T- and B-cell lymphoma in CD patients and controls with NHL. This adjustment was important because it revealed that the small excess mortality seen in CD+NHL individuals was due to a high proportion of T-cell NHL.
Patients with CD develop both T- and B-cell NHL in a similar way to those without CD.[18] In addition, patients with CD and B-cell NHL have a better prognosis than those with T-cell NHL[44], which is similar to the general non-celiac population. The increase in mortality found in those with CD-NHL in our study was due to a higher proportion of T-cell lymphoma, most likely enteropathy T-cell lymphoma (EATL), the very rare NHL that is specific to CD and that only rarely occurs in non-celiacs.[45] Few patients with EATL respond to therapy.[46 47]
We did not have access to dietary data in individuals with CD. A validation study of individuals with CD from our dataset found that 17% of individuals had evidence of poor adherence. [24] Although we cannot rule out that poor dietary adherence affects survival in individuals with CD+LPM, dietary adherence is unlikely to have more than a marginal effect on survival in individuals who have already received their diagnosis of LPM. Beyond 1 year of follow-up, CD did not influence the survival of LPM individuals; in fact, the adjusted HR for death more than 5 years after LPM diagnosis was below 1 (HR=0.90; 95% CI=0.64–1.26). A large proportion of individuals with CD are undiagnosed and therefore we cannot rule out that there are individuals with CD in our reference population. This potential misclassification, however, should not influence our risk estimates because the prevalence of CD in the general population is at most 2–3%.[48 49] Finally, despite the large number of individuals with LPM (n=1005), we had limited power to examine the influence of CD on the survival of non-NHL LPM. Overall, the relative risk of death in individuals with CD+non-NHL (adjusted HR=1.22) was very similar to that of CD+NHL (adjusted HR=1.23). Low statistical power also applies to NHL subtype analyses, and given that the upper 95%CI levels for e.g. disease-specific death in patients with CD and a subtype of NHL (Table 2) reached 1.71 in one analysis, we cannot rule out that CD is associated with a mortality excess risk of this size.
In conclusion, we found no evidence that CD influences survival in individuals with LPM. The increased mortality observed in the first year after LPM diagnosis in CD patients is related to the predominance of T-NHL in that population, but due to limited statistical power we cannot rule out some excess mortality in celiac patients with certain LPM subtypes.
Supplementary Material
Novelty & Impact Statements.
Earlier research suggests that individuals with celiac disease (CD) are at increased risk of lymphoproliferative malignancy (LPM) and overall death. In this nationwide cohort study, we were able to I) estimate the mortality in celiac patients with a diagnosis of LPM; II) show that the observed excess mortality in individuals with CD and LPM was due to a higher proportion of T-NHL in that population.
Acknowledgments
JFL: Örebro University Hospital, Karolinska Institutet, the Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195), the Swedish Celiac Society, the Fulbright commission.
BL: The American Scandinavian Foundation, the Celiac Sprue Association, and the National Center for Research Resources, a component of the National Institutes of Health (KL2 RR024157).
JAM: The National Institutes of Health – DK071003 and DK057892.
Abbreviations used in this article
- CD
Celiac disease
- CI
Confidence Interval
- HR
Hazard Ratio
- VA
Villous atrophy
Footnotes
Details of ethics approval: This project (2006/633-31/4) was approved by the Research Ethics Committee of the Karolinska Institute, Sweden on June 14th, 2006.
Conflicts of interest/Disclosure requirement
JAM: Grant support: Alba Therapeutics (>$50,000); Advisory board: Alvine Pharmaceuticals, Inc. (<$10,000), Nexpep (<$10,000), Consultant (none above 10,000 USD): Ironwood, Inc., Flamentera, Actogenix, Ferring Research Institute Inc., Bayer Healthcare Pharmaceuticals, Vysera Biomedical, 2G Pharma, Inc., ImmunosanT, Inc. and Shire US Inc.
The other authors declare that they have no conflicts of interest
Author contributions:
ICMJE criteria for authorship read and met: JFL, BL, ART, JAM, PHRG, AE, FG.
Agree with the manuscript’s results and conclusions: JFL, BL, ART, JAM, PHRG, AE, FG
Designed the experiments/the study: JFL, BL, ART, JAM, PHRG, AE, FG.
Collected data: JFL
Analyzed the data: FG
Wrote the first draft of the paper: JFL.
Contributed to study design, interpretation of data and writing: BL, ART, JAM, PHRG, AE, FG.
Interpretation of data; approved the final version of the manuscript: JFL, BL, ART, JAM, PHRG, AE, FG
Responsible for data integrity: JFL.
Obtained funding: JFL.
References
- 1.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128(4 Suppl 1):S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of Genetic and Immunological Insights into a Model of Celiac Disease Pathogenesis. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-040210-092915. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson JF, Ludvigsson J, Ekbom A, Montgomery SM. Celiac Disease and Risk of Subsequent Type 1 Diabetes: A general population cohort study of children and adolescents. Diabetes Care. 2006;29(11):2483–8. doi: 10.2337/dc06-0794. [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129(2):454–63. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Sanders DS, Maeurer M, Jonsson J, Grunewald J, Wahlstrom J. Risk of tuberculosis in a large sample of patients with coeliac disease--a nationwide cohort study. Aliment Pharmacol Ther. 2011;33(6):689–96. doi: 10.1111/j.1365-2036.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- 6.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. Bmj. 2004;329(7468):716–9. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askling J, Linet M, Gridley G, Halstensen TS, Ekstrom K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123(5):1428–35. doi: 10.1053/gast.2002.36585. [DOI] [PubMed] [Google Scholar]
- 8.Elfstrom P, Granath F, Ekstrom Smedby K, Montgomery SM, Askling J, Ekbom A, Ludvigsson JF. Risk of Lymphoproliferative Malignancy in Relation to Small Intestinal Histopathology Among Patients With Celiac Disease. J Natl Cancer Inst. 2011;103(5):436–44. doi: 10.1093/jnci/djq564. [DOI] [PubMed] [Google Scholar]
- 9.Smedby KE, Akerman M, Hildebrand H, Glimelius B, Ekbom A, Askling J. Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut. 2005;54(1):54–9. doi: 10.1136/gut.2003.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card TR, West J, Holmes GK. Risk of malignancy in diagnosed coeliac disease: a 24-year prospective, population-based, cohort study. Aliment Pharmacol Ther. 2004;20(7):769–75. doi: 10.1111/j.1365-2036.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- 11.Grainge MJ, West J, Solaymani-Dodaran M, Card TR, Logan RF. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35(6):730–9. doi: 10.1111/j.1365-2036.2012.04998.x. [DOI] [PubMed] [Google Scholar]
- 12.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128(4 Suppl 1):S79–86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115(3):191–5. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 14.Mearin ML, Catassi C, Brousse N, Brand R, Collin P, Fabiani E, Schweizer JJ, Abuzakouk M, Szajewska H, Hallert C, Farre Masip C, Holmes GK. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol. 2006;18(2):187–94. doi: 10.1097/00042737-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Kristinsson SY, Goldin LR, Bjorkholm M, Caporaso NE, Landgren O. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136(1):91–8. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Jewell D. Cancer in patients with ulcerative colitis, Crohn’s disease and coeliac disease: record linkage study. Eur J Gastroenterol Hepatol. 2008;20(4):297–304. doi: 10.1097/MEG.0b013e3282f2a5e2. [DOI] [PubMed] [Google Scholar]
- 17.Elfstrom P, Granath F, Ye W, Ludvigsson JF. Low Risk of Gastrointestinal Cancer Among Patients With Celiac Disease, Inflammation, or Latent Celiac Disease. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Leslie LA, Lebwohl B, Neugut AI, Gregory Mears J, Bhagat G, Green PH. Incidence of lymphoproliferative disorders in patients with celiac disease. Am J Hematol. 2012;87(8):754–9. doi: 10.1002/ajh.23237. [DOI] [PubMed] [Google Scholar]
- 19.Smedby KE, Askling J, Mariette X, Baecklund E. Autoimmune and inflammatory disorders and risk of malignant lymphomas--an update. J Intern Med. 2008;264(6):514–27. doi: 10.1111/j.1365-2796.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 20.Mikuls TR, Endo JO, Puumala SE, Aoun PA, Black NA, O’Dell JR, Stoner JA, Boilesen EC, Bast MA, Bergman DA, Ristow KM, Ooi M, Armitage JO, Habermann TM. Prospective study of survival outcomes in Non-Hodgkin’s lymphoma patients with rheumatoid arthritis. J Clin Oncol. 2006;24(10):1597–602. doi: 10.1200/JCO.2005.04.6227. [DOI] [PubMed] [Google Scholar]
- 21.Ji J, Liu X, Sundquist K, Sundquist J. Survival of cancer in patients with rheumatoid arthritis: a follow-up study in Sweden of patients hospitalized with rheumatoid arthritis 1 year before diagnosis of cancer. Rheumatology (Oxford) 2011;50(8):1513–8. doi: 10.1093/rheumatology/ker143. [DOI] [PubMed] [Google Scholar]
- 22.Landgren O, Pfeiffer RM, Kristinsson SY, Bjorkholm M. Survival patterns in patients with Hodgkin’s lymphoma with a pre-existing hospital discharge diagnosis of autoimmune disease. J Clin Oncol. 2010;28(34):5081–7. doi: 10.1200/JCO.2010.29.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64(19):7073–7. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9(1):19. doi: 10.1186/1471-230X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346(3):180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 26.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56(10):1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136(1):99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Celigny P. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–62. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 29.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 30.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102(1):330–54. [PubMed] [Google Scholar]
- 31.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302(11):1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 33.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23(5):305–13. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 34.Norstrom F, Lindholm L, Sandstrom O, Nordyke K, Ivarsson A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011;11:118. doi: 10.1186/1471-230X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olen O, Bihagen E, Rasmussen F, Ludvigsson JF. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012;44(6):471–6. doi: 10.1016/j.dld.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Logan RF, Rifkind EA, Turner ID, Ferguson A. Mortality in celiac disease. Gastroenterology. 1989;97(2):265–71. doi: 10.1016/0016-5085(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 37.Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163(13):1566–72. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 38.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Guidetti CS, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358(9279):356–61. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 39.Solaymani-Dodaran M, West J, Logan RF. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population-based cohort study. Am J Gastroenterol. 2007;102(4):864–70. doi: 10.1111/j.1572-0241.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 40.Grainge MJ, West J, Card TR, Holmes GK. Causes of Death in People With Celiac Disease Spanning the Pre- and Post-Serology Era: A Population-Based Cohort Study From Derby, UK. Am J Gastroenterol. 2011 doi: 10.1038/ajg.2010.506. [DOI] [PubMed] [Google Scholar]
- 41.Metzger MH, Heier M, Maki M, Bravi E, Schneider A, Lowel H, Illig T, Schuppan D, Wichmann HE. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006;21(5):359–65. doi: 10.1007/s10654-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 42.Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38(6):374–80. doi: 10.1016/j.dld.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Socialstyrelsen. Statistics: Health and Diseases 2007. Vol. 3. Stockholm: 2007. Cancer incidence in Sweden 2005. [Google Scholar]
- 44.Halfdanarson TR, Rubio-Tapia A, Ristow KM, Habermann TM, Murray JA, Inwards DJ. Patients with celiac disease and B-cell lymphoma have a better prognosis than those with T-cell lymphoma. Clin Gastroenterol Hepatol. 2010;8(12):1042–7. doi: 10.1016/j.cgh.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973–2008. Cancer. 2012;118(15):3786–92. doi: 10.1002/cncr.26700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356(9225):203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 47.van de Water JM, Cillessen SA, Visser OJ, Verbeek WH, Meijer CJ, Mulder CJ. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24(1):43–56. doi: 10.1016/j.bpg.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D’Amato M, Lahr B, Talley NJ, Agreus L. Detection of Celiac Disease and Lymphocytic Enteropathy by Parallel Serology and Histopathology in a Population-Based Study. Gastroenterology. 2010;139(1):112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myleus A, Ivarsson A, Webb C, Danielsson L, Hernell O, Hogberg L, Karlsson E, Lagerqvist C, Norstrom F, Rosen A, Sandstrom O, Stenhammar L, Stenlund H, Wall S, Carlsson A. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J Pediatr Gastroenterol Nutr. 2009;49(2):170–6. doi: 10.1097/MPG.0b013e31818c52cc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.