Abstract
Exposure to bisphenol A (BPA) is implicated in many aspects of metabolic disease in humans and experimental animals. We fed pregnant CD-1 mice BPA at doses ranging from 5 to 50,000 μg/kg/day, spanning 10-fold below the reference dose to 10-fold above the currently predicted no adverse effect level (NOAEL). At BPA doses below the NOAEL that resulted in average unconjugated BPA between 2 and 200pg/ml in fetal serum (AUC0–24h),we observed significant effects in adult male offspring: an age-related change in food intake, an increase in body weight and liver weight, abdominal adipocyte mass, number and volume, and in serum leptin and insulin, but a decrease in serum adiponectin and in glucose tolerance. For most of these outcomes non-monotonic dose–response relationships were observed; the highest BPA dose did not produce a significant effect for any outcome. A 0.1-μg/kg/day dose of DES resulted in some but not all low-dose BPA outcomes.
Keywords: Metabolic syndrome, Adipocytes, Food intake, Glucose tolerance, Endocrine disruption
1. Introduction
Obesity and diabetes are epidemic in the United States and are two components of metabolic syndrome, which also includes hormonal and metabolic derangements such as glucose intolerance and dyslipidemia. While excess abdominal fat is recognized as a core component in the etiology of metabolic syndrome, aspects of metabolic syndrome such as type 2 diabetes can occur in the absence of obesity in a small percent of people [1]. The etiology of type 2 diabetes is thus complex, but occurs when people have elevated blood glucose and insulin resistance, and the pancreas is unable to compensate and maintain glucose homeostasis [2]. Metabolic diseases also significantly impact the brain, behavior and fertility as well as mortality [3].
Man-made chemicals have been identified which can disrupt the differentiation of adipocytes, pancreatic development, and metabolic and endocrine processes [4–6]. Developmental exposure to these endocrine disrupting chemicals (EDCs) can result in a permanent change in the regulation of insulin and glucose levels [2]. EDCs may also impact metabolism through effects on the neuroendocrine systems that control feeding as well as other behaviors [7–9]. Importantly, some neural and metabolic effects due to fetal exposure to EDCs are observed during specific periods during postnatal life, such as during early adolescence [10], adulthood [11] or middle age [12].
Adipose tissue is an endocrine organ with a substantial role in glucose and insulin homeostasis, and EDCs can disrupt signaling by regulatory hormones, such as adiponectin and leptin [13,14]. Studies with laboratory rats and mice, including the mouse model (CD-1) that we use, have reported increased body fat in animals exposed to exogenous drugs and chemicals, such as diethylstilbestrol (DES) and bisphenol A (BPA) during the fetal period of the differentiation of preadipocytes and early postnatal period of the differentiation of adipocytes [5,12,15,16]. The finding that exposure to BPA and other estrogenic chemicals during adipogenesis can lead to an increase in body fat later in life was not expected, given the opposite effect that estrogen has on body fat in adults [6,17]. However, there is also epidemiological evidence for a relationship in adults between BPA and obesity, as well as cardiovascular disease, insulin resistance, glucose intolerance and type 2 diabetes [18–20]; recent evidence also has also associated BPA exposure with obesity in children and teenagers [21].
We focus here on exposure to the endocrine disruptor BPA because of evidence that BPA exposure during critical periods in fetal developmental can cause permanent changes in tissue function that lead to disease onset later in life [22–24]. This is an example of the developmental origins of health and disease hypothesis [4,5]. BPA is the chemical monomer primarily used to make polycarbonate plastic, although BPA is also used in a variety of consumer goods, including epoxy linings for canned foods and beverages, cardboard and the coating of thermal receipt paper. At an estimated 10-billion pounds per year, BPA is one of the highest volume production chemicals in the world [25]. Because of the instability of BPA-based polymers, BPA leaches out of products under conditions of heat or either high or low pH, such that virtually all people that have been examined are continuously exposed to BPA [26,27]. Levels of bioactive (unconjugated) BPA found in human serum range from about 0.1 to 10ng/ml [28], and similar levels have been reported at term in woman and fetuses [29]. The median levels of BPA in humans based on biomonitoring studies are higher than those previously shown to alter body weight and fat homeostasis in mice and rats [6], based on measurement of unconjugated BPA in mouse and rat serum [30–32]. BPA is known to bind to estrogen receptors, and while BPA is generally considered to be an “environmental estrogen”, it also binds to other receptors that regulate genes important for differentiation and subsequent function of numerous tissues [24,33,34].
Prior research has shown that exposure of rats and mice to low doses of BPA during fetal–neonatal life increases postnatal growth rate [6,16,35–40]. However, not all studies find this relationship [22]; factors such as the dose administered, strain of animal examined, sex of animal examined, type of feed used, and other environmental factors may explain these different outcomes [9,41]. The fat content and source of protein (soy vs. casein) in feed are particularly important, since high-fat diets as well as soy-free diets lead to obesity in rats and mice independent of exposure to EDCs such as BPA, and the fat content of the feed interacts with BPA in terms of the outcomes observed [9,16,42,43]. We thus chose to maintain our mice after weaning on a soy-based feed with a relatively low (4%) fat content that allowed us to examine the effects of BPA on mice that were not already obese [43]. We also focused here on males. Fetal exposure of females to BPA leads to a different set of outcomes due to the effects of endogenous estradiol after puberty and the fact that developmental exposure to BPA alters the functioning of the female neuroendocrine system [23,44]; data from female siblings will be reported elsewhere.
In this study, our goal was to conduct a comprehensive examination of the effects of developmental exposure to BPA on outcomes related to metabolic disease, using doses that ranged from 5 to 50,000 μg/kg/day. We hypothesized that at least some effects of BPA would follow non-monotonic dose–response curves, and that the maximally effective dose of BPA would not be the same for all outcomes, due to variability in the sensitivity of different tissues to xenobiotics. We examined outcomes known to be components of metabolic disease: body weight and food intake as predisposing factors; fat pad weights, in addition to adipocyte size and number in those fat pads; glucose tolerance and insulin tolerance, and serum adiponectin, leptin and insulin concentrations. We also looked for abnormalities in the liver, because of the association of hepatic steatosis with obesity and metabolic disease [45], and in kidney weight, because the risk of chronic kidney disease has been linked to metabolic syndrome [46] and kidney size can be used to assess renal failure.
Prior studies examining developmental BPA exposure and metabolism have been criticized for only examining only one or two doses, which is insufficient to determine the dose–response relationship. The wide BPA dose range used here extends from 10-fold below the currently estimated reference dose (50 μg/kg/day) to 10-fold greater than the estimated no adverse effect level (NOAEL; 5000 μg/kg/day), which is wider and more detailed than other studies to date and allows for a more detailed assessment of variation in dose responses among the different outcomes. The dose range of BPA examined, as well as inclusion of 0.1 μg/kg/day DES as a low-dose estrogen positive control, was based on a unique approach for coordinating a number of studies examining different effects of BPA; this program was initiated and funded by the National Institute of Environmental Health Sciences (NIEHS) to increase the usefulness of the findings for risk assessment [47].
2. Materials and methods
2.1. Animal husbandry and dosing procedures
Three-month-old nulliparous female CD-1 mice were purchased from Charles River Laboratories (Raleigh, NC) and were housed in polypropylene cages with corncob bedding in a temperature- and humidity-controlled facility on a 12L:12D cycle at the University of Missouri (light on occurred at 0800 h). These are standard housing conditions for this colony, although corncob bedding has been reported to have some endocrine effects in mice [48,49]. After at least a 1-week acclimation period, females were time-mated to stud males, which were purchased from the same supplier. Gestation day (GD) 0 was determined by the presence of a vaginal plug. Vaginal plug positive females were housed 2–4 per cage and not handled again until GD 9. Pregnancy was confirmed by a significant gain in body weight on GD 9 relative to GD 0.
Pregnant females were randomly assigned to the following dosing groups: 0 (negative controls), 5,50,500,5000, and 50,000 μg/kg body weight/day BPA, or 0.1 μg/kg body weight/day DES (the positive control for low-dose estrogenic effects). We will refer to these groups as negative controls, BPA-5, BPA-50, BPA-500, BPA-5000, BPA-50,000, and DES-0.1; the number of litters per treatment group was 14, 9, 12, 12, 11, 14, and 9, respectively.
Dosing continued daily from GD 9 to 18 during the period of the differentiation of preadipocytes [50]. DES and BPA (≥99% pure; Sigma–Aldrich, St. Louis, MO) were fed to pregnant females one time each day using a micropipetter. DES and the different doses of BPA were dissolved in tocopherol-stripped corn oil (MP Biomedical, Santa Ana, CA), and the volume administered (∼30 μl) was adjusted daily to maintain a constant dose per kg body weight; negative controls received 30 μl oil with no added chemical. Mice readily consume these solutions, and we have used this procedure for all of our prior experiments with BPA, DES and other lipophilic chemicals. Allowing a pregnant female mouse to drink an oil solution from a pipetter is less stressful than gavage, and maternal stress can alter fetal growth, gonadal steroid levels and postnatal morphology and physiology in mice and rats [51]. Prior studies that use gavage administration of BPA or other chemicals are thus complicated by the potential interaction between chemical exposure and an elevated stress response [8,9].
On GD 18, pregnant females were individually housed in an AAALAC-accredited facility at the University of Missouri. All pregnant and lactating females were fed soy-based Purina 5008 rodent breeder chow (6% fat) ad libitum from GD 0 until postnatal day (PND) 22. Water was purified by ion exchange followed by a series of carbon filters and supplied in glass bottles ad libitum. Animal care conformed to the NIH Guide, and The Animal Care and Use Committee at the University of Missouri approved all procedures prior to conducting the study.
On PND 1, the sex and weight of mouse pups were determined. The pups were then not handled until weaning except to change cage bedding, and then only after the pups were at least 1 week old. Offspring were weaned on PND 22 and housed 2–4 of the same sex in polypropylene cages with corncob bedding. At this time animals were individually identified by an ear-notch pattern. After weaning animals were fed soy-based Purina 5001 rodent maintenance chow (4% fat) ad libitum; food was weighed every 3–4 days to monitor food intake per week, and body weight gain per week was also determined for the duration of the study, which ended when males were about 5-months old. We calculated metabolic energy consumed per week (for Purina 5001 chow, this is 3.04 kcal/g feed).
2.2. Glucose tolerance and insulin tolerance tests (GTT and ITT)
A subset of male mice (13–17 per treatment group) were given glucose and insulin tolerance tests (GTT and ITT, respectively), which were administered when animals were about 18 weeks old. No more than two animals per litter were used for this testing (see Section 2.7 for the method used to control for litter effects). For both GTT and ITT, males were only fasted for 4 h during the beginning of the light cycle when the mice would not normally be active and feeding. Preliminary studies had shown that an overnight (∼14h) fast resulted in a significant loss of body fat stores.
For GTT, mice were injected i.p. with 2g/kg glucose (Sigma–Aldrich) in 0.9% saline, with volume adjusted to have a constant dose per kg body weight (median bolus about 176 μl). Glucose was measured in blood obtained via a tail nick at 0 (baseline), 15, 30, 45, 60, and 120min after injection using an ACCU-CHEK Active glucometer (Roche, Indianapolis, IN). At least 4 days after the GTT, mice were given an ITT. ITT was performed in the same manner as the GTT, using 0.75 U/kg human insulin (Sigma) in 0.9% saline with volume adjusted to have a constant dose per kg body weight (median bolus of 155 (μl).
2.3. Necropsy
Animals (29–40 per treatment group) were weighed and then sacrificed 5–7 days following the ITT, when 19 weeks old. Sacrifice was performed between 2:00 and 5:00pm by CO2 asphyxiation followed by pneumothorax. Blood was collected in heparinized syringes with a 27-g needle, stored at 4 °C and allowed to clot until serum separation the following day. Tissues were quickly excised, weighed, flash-frozen in liquid nitrogen, and stored at −80°C.
2.4. Fat pad processing for adipocyte number and volume
No more than two animals per litter were used for cell count analysis. Excised gonadal and renal fat pads were weighed. A 40–60 mg piece of each of these tissues was placed into 1ml phosphate-buffered saline (PBS), lightly minced and digested for 1 h at 37 °C with 0.5 mg/ml collagenase in PBS supplemented with 1 mM CaCl2. The resulting adipocyte suspension was rinsed with 10 ml PBS and briefly centrifuged at 1000rpm for 30 s. The cells were rinsed again in PBS, briefly centrifuged, and 500 μl of the cell suspension was removed and diluted in 500 μl PBS. 100 μl of this cell suspension was added to 10 μl acridine orange solution (20-mg/ml acridine orange in deionized water used at 1:100 dilution) and counted in duplicate using a Nexcelom Cellometer (Nexcelom, Lawrence, MA). A minimum of 100 cells was counted for each animal for cell volume and cell number determinations. If 100 cells were not available for an animal, cell numbers of littermates were pooled to reach at least 100 cells. Adipocyte volumes were calculated using diameter measurements reported by the Nexcelom software. The total number of adipocytes was determined based on the weight of the entire fat pad.
2.5. Radioimmunoassays for serum hormones
One animal per litter was selected for these assays (6–7 per treatment group). Serum levels of insulin, leptin and adiponectin were measured using radioimmunoassay kits (Sensitive Rat Insulin SRI-13K; Mouse Leptin ML-82K, Mouse Adiponectin MADP-60HK, Millipore, Billerica, MA) according to the manufacturer's instructions. When possible, animals were selected based on gonadal fat pad and 19-week-old body weights within 1.5 standard deviations of the group means, with the objective being that the means for these sub-groups thus did not differ from the entire group. Each assay was performed once so only intra-assay CVs are reported here; these were 2.99%, 2.52% and 2.42% for insulin leptin and adiponectin, respectively.
2.6. Measurement of BPA in maternal and fetal serum
Using the same dosing method described above, from GD 11 to 17 we fed pregnant CD-1 mice one time per day a 20-μg/kg/day dose of unlabeled BPA (>99% pure; Sigma–Aldrich) mixed with 3H-BPA (15 Ci/mmol; Moravek Biochemicals, Brea, CA, USA), aiming for a 30-μl volume based on the average weight of these females during pregnancy; the final specific activity of the dosing solution was 0.51 Ci/mmol. Blood was collected after CO2 asphyxiation at 1, 3, 6, and 24 h after the last BPA administration on GD 17, with 2–4 pregnant females at each time point, and blood from all fetuses within each litter was pooled. Serum was separated by centrifugation at 4°C and then stored at −20°C. Unconjugated 3H-BPA was measured in serum by HPLC separation and scintillation counting using methods described previously [31,32].
The area under the curve (AUC) for the serum 3H-BPA levels over the 24 h after dosing (AUC0–24; (ngh)/ml) was calculated using the linear trapezoidal rule and the assumption that unconjugated 3H-BPA in serum just before administration (time 0) was zero. We then divided the AUC0–24 by 24 h to provide the average AUC0–24 (ng/ml) over the 24 h throughout GD 17–18 when the differentiation of preadipocytes and metabolic systems is occurring [6].
Based on prior findings that administered oral dose is linear with serum unconjugated BPA in mice [31,32], and also in rats [31,32,52], and that in adult female mice the linear range for serum unconjugated BPA (2-100,000 μg/kg/day; R2 = 0.9807) covers a wider dose range than the dose range in the present study (5–50,000 μg/kg/day) [32], we used the AUC0–24 values from the 20-μg/kg/day BPA dose to calculate the serum concentrations of unconjugated BPA in pregnant females and fetuses by interpolation. We also previously reported that administering adult female CD-1 mice 3H-BPA and measuring serum 3H-BPA by scintillation counting after HPLC separation resulted in virtually identical levels of unconjugated serum BPA over the 24 h after administration as were obtained after administration of authentic BPA and measuring serum BPA by LCMS [32].
2.7. Statistical analysis
We initially conducted one-way as well as repeated-measures ANOVA on all data using the PROC GLM procedure in SAS. Data were log-transformed where indicated based on the distribution to achieve homogeneity of variance. The LS means test in SAS, which includes Bonferroni correction, was used for group comparisons if the overall ANOVA was statistically significant (P<0.05). We adjusted for litter membership in all analyses in which we examined more than one animal per litter by including litter as a main effect variable and using litter within dose as the error term to calculate the F value. For some variables we also calculated Spearman correlation coefficients.
Since visual examination of the data for just the BPA doses (0, 5, 50, 500, 5000 and 50,000) suggested non-monotonic instead of linear dose–response patterns in many outcome variables, we also explored the statistical significance of these patterns by comparing ANOVA PROC GLM conducted on just the BPA data with two linear regression models. Model 1 tests for a linear trend of BPA dose vs. outcome, whereas model 2 adds the quadratic term to test for a U or inverted U curve – the simplest forms of non-monotonic dose–response. BPA dose was log transformed after adding 0.05 to all doses to enable taking the log of the control group dose (=0). Box-Cox transformations were used to normalize response variables. These analyses were performed within the R statistical system (R v2.14.2; RStudio vO.95.263). The Companion to Applied Regression (‘car’) R package was used for Box-Cox transformations (v2.0-12). Multilevel regression was performed using the ‘nlme’ package (v3.1-103).
Since the two models are nested (i.e., one completely contains the other), we used the ANOVA procedure to test whether the quadratic term provided a statistically significant improvement to the model. We accounted for litter effects in these regression models in two ways. Our primary analyses were conducted using the means, by litter, of the outcome variables. We then repeated the analyses using multilevel regression (individual animals nested within litter), which provides increased power over the litter means method. Comparisons of P values for just BPA dose effects (excluding the DES-0.1 group) using PROC GLM ANOVA and multilevel regression are presented in Table 2. For a more detailed explanation and application of this approach to a data set see Supplemental Materials in Do et al. [53].
Table 2.
P value summary for analyses of effect of prenatal negative controls and BPA doses (0, 5, 50, 500, 5000, and 50000), not including the DES-0.1 group. Column one shows results of standard ANOVAs using PROC GLM in SAS. Column 2 gives P values for comparison of two nested linear regression models, with and without a [log(dose)]2 term using the R statistical system. A significant result (indicated in bold) provides evidence that a U or inverted-U curve fits the data better than a simple linear fit, and indicates a nonmonotonic dose–response function. More complex functions were not evaluated.
| Variable | Proc GLM ANOVA BPA doses | Multilevel regression Improvement of fit: quadratic over linear-only model |
|---|---|---|
| Body weight | ||
| Birth weight | =0.550 | =0.420 |
| Week 3 weight | =0.020 | =0.019 |
| Week 19 weight | =0.193 | =0.069 |
| Energy intake, weeks 3-4 | =0.358 | =0.033 |
| Energy intake, weeks 4-5 | =0.201 | =0.028 |
| Adipocytes | ||
| Gonadal fat weight | =0.017 | =0.022 |
| Renal fat weight | =0.019 | =0.076 |
| Total abdominal fat weight | =0.035 | =0.047 |
| Gonadal adipocyte number | =0.003 | =0.002 |
| Gonadal adipocyte volume | =0.006 | =0.052 |
| Renal adipocyte number | =0.011 | =0.250 |
| Renal adipocyte volume | =0.121 | =0.654 |
| Liver and kidney weight | ||
| Liver weight | =0.003 | <0.001 |
| Kidney weight | =0.137 | =0.947 |
| GTT and ITT | ||
| Glucose tolerance test | =0.062 | =0.008 |
| Insulin tolerance test | =0.144 | =0.137 |
| Serum hormones | ||
| Insulin | =0.008 | =0.004 |
| Leptin | =0.035 | =0.349 |
| Adiponectin | =0.031 | =0.099 |
Because of evidence for non-monotonic dose–response relationships, our focus is on comparisons of outcomes at low BPA doses that were different from negative controls and for the highest BPA dose group (BPA-50,000). Also of particular interest was whether the DES-0.1 males were significantly different from negative controls and the BPA-50,000 males. Prenatal treatment effects are considered statistically significant if P < 0.05.
3. Results
3.1. Day of birth, postnatal body weight and metabolic energy consumption per week
There was no difference due to prenatal treatment in sex ratio at birth or in the death of pups between birth and weaning. Between 1 and 5 pups died in a limited number of litters across all groups.
3.1.1. Body weight
We weighed animals on the day of birth and then each week beginning at weaning (Table 1). Across all treatment groups, body weight increased significantly from an average of 1.73 g on the day of birth to 13.6 g on postnatal day 22 (week 3), and then body weight increased by an average of 80% (by 10.9g) to 24.4 g by week 4, by an average of 23% (by 5.7 g) to 30.1 g between weeks 4 and 5, and by an average of 9% (by 2.7 g) to 32.8 g between weeks 5 and 6. Between weeks 6 and 13 the percent increase in body weight per week was: 5.1%, 3.5%, 3.1%, 2.1%, 2.6%, 1.7%, 2.1%, respectively, after which there was about a one percent increase in body weight per week until week 19 when the experiment ended. Thus, the time between weeks 3 and 5, which is the first 2 weeks after weaning, represents a period of rapid postnatal growth in the male CD-1 mouse (this is also true for females; data not shown).
Table 1.
Mean (±SEM) body weight at birth and then beginning at weaning (week 3) through week 19.
| Day of birth | Week 3 | Week 4 | Week 5 | Week 7 | Week 9 | Week 11 | Week 13 | Week 15 | Week 17 | Week 19 | Average weeks 7-19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=39) | 1.69 ± 0.04 | 13.26 ± 0.55 | 24.15 ± 0.79 | 30.07 ± 0.66 | 34.38 ± 0.67 | 36.90 ± 0.76 | 38.55 ± 0.79 | 39.45 ± 0.82 | 41.10 ± 0.92 | 42.04 ± 0.97 | 42.70 ± 0.93 | 39.08 ± 0.88 |
| BPA-5(n = 30) | 1.76 ± 0.04 | 13.88 ± 0.62 | 24.98 ± 0.89 | 30.01 ± 0.75 | 34.73 ± 0.75 | 37.04 ± 0.85 | 39.02 ± 0.89 | 40.08 ± 0.93 | 42.60 ± 0.98 | 43.30 ± 1.02 | 43.90 ± 0.98 | 40.14 ± 0.87 |
| BPA-50(n = 24) | 1.72 ± 0.04 | 14.12 ± 0.69 | 25.60 ± 0.98 | 31.13 ± 0.82 | 35.89 ± 0.90 | 37.94 ± 0.94 | 39.34 ± 0.99 | 41.29 ± 1.02 | 41.73 ± 1.08 | 42.60 ± 1.13 | 43.28 ± 1.49 | 41.33 ± 1.39 |
| BPA-500(n = 39) | 1.79†± 0.04 | 14.19 ± 0.53 | 25.78 ± 0.75 | 31.49 ± 0.63 | 35.59 ± 0.64 | 37.79 ± 0.72 | 39.67 ± 0.76 | 41.34†± 0.78 | 42.43 ± 0.82 | 43.53 ± 0.86 | 45.25†± 0.93 | 41.33†± 0.83 |
| BPA-5000(n = 33) | 1.70 ± 0.04 | 11.88* ± 0.57 | 22.78 ± 0.82 | 29.47 ± 0.69 | 34.02 ± 0.69 | 36.50 ± 0.79 | 38.23 ± 0.82 | 39.57 ± 0.85 | 40.51 ± 0.93 | 42.17 ± 1.07 | 42.30 ± 0.96 | 39.52 ± 0.98 |
| BPA-50,000(n = 38) | 1.75 ± 0.04 | 12.65 ± 0.53 | 23.44 ± 0.76 | 29.40 ± 0.64 | 33.46 ± 0.66 | 36.29 ± 0.73 | 37.72 ± 0.76 | 39.03 ± 0.79 | 40.80 ± 0.87 | 41.90 ± 0.87 | 42.20 ± 0.99 | 38.93 ± 0.95 |
| DES-0.1(n = 29) | 1.76 ± 0.05 | 14.38 ± 0.63 | 24.48 ± 0.89 | 29.25 ± 0.75 | 33.25 ± 0.76 | 35.36 ± 0.86 | 37.30 ± 0.90 | 38.60 ± 0.93 | 40.29 ± 0.98 | 41.81 ± 1.02 | 42.18 ±0.98 | 38.54 ± 0.88 |
P < 0.05.
P < 0.1 vs. controls.
The effect of prenatal treatment on body weight on the day of birth was not statistically significant based on linear ANOVA or regression analysis for non-monotonicity, although the BPA-500 males tended (P=0.08) to be heavier than negative controls. This trend continued throughout the experiment, and at every age examined, the mean body weight of BPA-500 males was higher than that of males in other groups (Table 1).
At weaning (3 weeks old), the overall ANOVA for the effect of prenatal treatment on body weight was statistically significant. In addition, the quadratic model fit the data significantly better (P=0.016) than the linear-only model, indicating that there was a significant non-monotonic dose–response relationship (Table 2). At weaning the BPA-5000 group had significantly decreased body weight compared to negative controls and DES-0.1 males (Table 1). Between weeks 7 and 19 when the study ended, although the effect of prenatal treatment was not statistically significant, the BPA-500 males tended to be heavier than negative controls (P=0.07) and were significantly heavier than BPA-50,000 males. There was no significant effect based on ANOVA of prenatal treatment on weight gain per week over the last 4 weeks of the experiment between weeks 15 and 19, since only the BPA-500 group showed a greater weight gain (2.82 ± 0.29 g) relative to negative controls (1.60 ± 0.18g) during this time. At week 19 (the end of the experiment) the regression analysis resulted in a significant difference between the groups as well as a better fit (P=0.034) relative to the linear-only model, which was not statistically significant (Table 2). The BPA-500 males tended to be heavier than negative controls (P=0.07) and were significantly heavier than BPA-5000 males (Table 1).
3.1.2. Food (metabolic energy) consumption
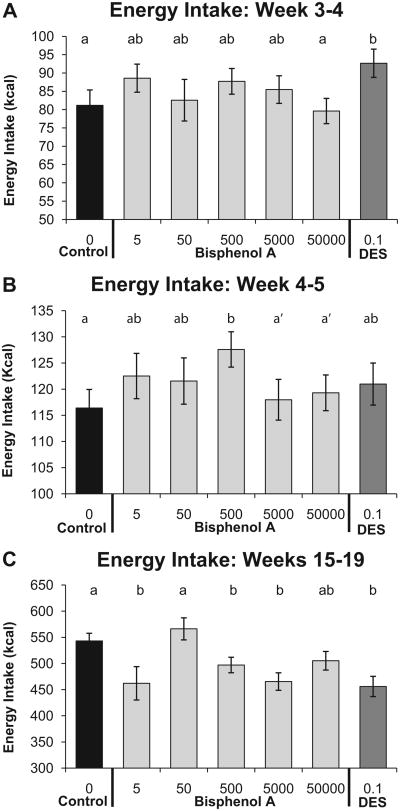
Food intake per week was converted to metabolic energy consumed in kcal. Between weeks 3 and 7, the linear ANOVA did not reveal significant differences, but between weeks 3 and 4 the quadratic model fit the data significantly better (P=0.028) than the linear-only model, revealing that there was a significant non-monotonic dose–response relationship for BPA (Fig. 1A; Table 2); the DES-0.1-treated animals exhibited the largest increase in metabolic energy intake relative to negative controls (P < 0.05). Between weeks 4 and 5 the quadratic model again fit the data better (P = 0.025) than the linear-only model (Fig. 1B; Table 2), and only the BPA-500 males exhibited a significant increase in metabolic energy intake relative to negative controls (P < 0.05). There were no treatment-related differences in metabolic energy intake between weeks 5 and 7.
Fig. 1.
Effect of fetal BPA exposure on mean (±SEM) energy intake (in kcal) per week beginning after weaning (Panel A between weeks 3 and 4 and Panel B between week 4 and 5 of age) as well as during the last 4 weeks of the experiment (Panel C between weeks 15 and 19). Groups with different letters are significantly different from each other. Panel B: a′, P<0.1 vs. BPA-500.
During the last 4 weeks of the study (weeks 15–19), prenatal treatment was significantly different based on linear ANOVA for the total amount metabolic energy consumed over this 4-week period (P < 0.001). Specifically, the negative controls consumed significantly more food and thus a greater amount of metabolic energy relative to BPA-5, BPA-500, BPA-5000 and the DES-0.1-treated males (all comparisons: P < 0.05–0.001), while the BPA-50 males and BPA-50,000 males did not differ significantly from negative controls (Fig. 1C). Thus, the BPA-500 males gained more weight between weeks 15 and 19 while consuming less metabolic energy relative to negative controls, which is in marked contrast to the finding that the BPA-500 males gained more weight and also consumed a greater amount of food relative to negative controls between weaning and puberty at week 5.
3.2. Gonadal and renal fat pad weight, adipocyte number and adipocyte volume
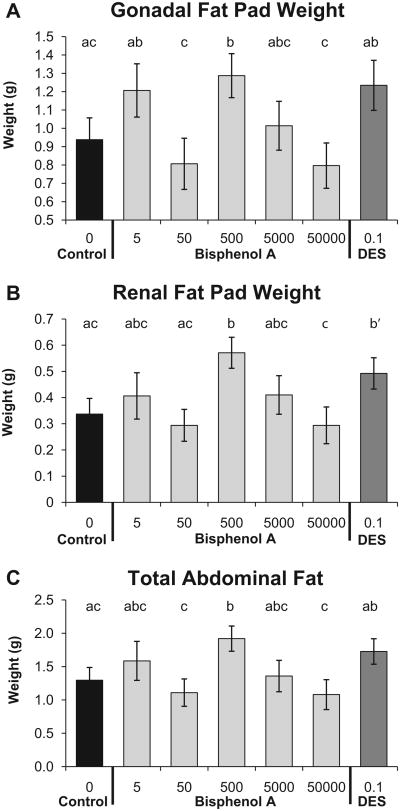
The overall ANOVA for gonadal fat pad weight was statistically significant, and addition of the quadratic term to the model provided a significantly better fit relative to the linear-only model (P=0.022; Table 2). Compared to the negative controls, gonadal fat pad weight was significantly greater in the BPA-500 group (P<0.05), and the BPA-500 males also were significantly different compared to the BPA-50,000 group (P < 0.01; Fig. 2A). Renal fat pad weight showed the same pattern across BPA doses and for DES as gonadal fat pad weight (Fig. 2B).The linear ANOVA for renal fat pad weight was statistically significant, and addition of the quadratic term to the model tended to result in a better fit relative to the linear-only model (P=0.076). Compared to the negative controls and the BPA-50,000 groups, the BPA-500 group had elevated renal fat pad weight (P <0.05 and P <0.01, respectively). The DES-0.1 males tended (P=0.07) to have heavier renal fat pads compared to negative controls. We combined the weights of the gonadal and renal fat pads collected for each animal as a measure of the total amount of abdominal fat (Fig. 2C).The linear ANOVA for total abdominal fat weight was statistically significant, and inclusion of the quadratic term resulted in a significantly better fit than the linear-only model (P=0.047). Compared to the negative controls and the BPA-50,000 groups, the BPA-500 group had more total abdominal fat (P < 0.05 and P < 0.01, respectively).
Fig. 2.
Effect of fetal exposure to BPA on mean (±SEM) abdominal fat pad weight in males from different prenatal treatment groups when 19 weeks old. Panel A: gonadal fat pad weight; Panel B: renal fat pad weight; Panel C: total abdominal fat pad weight consisting of the sum of the gonadal and renal fat pad weights. Groups with different letters are significantly different from each other. Panel B: b′, P= 0.07 vs. controls.
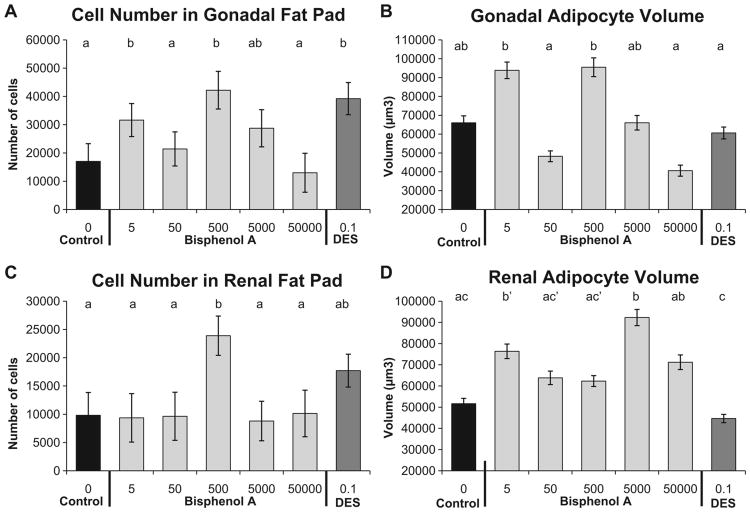
The linear ANOVA for gonadal fat cell number was statistically significant (P<0.01), and addition of the quadratic term resulted in a significantly better fit than the linear-only model (P=0.002). The BPA-5 and BPA-500 males had significantly more adipocytes compared to negative controls, the BPA-50, and BPA-50,000 males. The DES-0.1 males also had significantly more adipocytes than negative controls, BPA-50 and BPA-50,000 males (Fig. 3A). The linear ANOVA for gonadal fat cell volume was also statistically significant (P<0.01), and addition of the quadratic term tended to result in a better fit relative to the linear-only model (P=0.052). Although no group had significantly different adipocyte volume relative to negative controls, BPA-5 and BPA-500 males had significantly larger adipocytes relative to BPA-50, BPA-50,000 and DES-0.1 males (Fig. 3B).
Fig. 3.
Effect of fetal exposure to BPA on mean (±SEM) adipocyte number and volume in males from different prenatal treatment groups when 19 weeks old. Panel A: gonadal adipocyte number; Panel B: gonadal adipocyte volume; Panel C: renal adipocyte number Panel D: renal adipocyte volume. Groups with different letters are significantly different from each other. Panel D: b′, P= 0.055 vs. controls; c′, P= 0.07 vs. DES-0.1.
Within the renal fat pad, the linear ANOVA for renal fat cell number was statistically significant, and addition of the quadratic term in the regression analysis did not result in a statistically significant effect of BPA dose (Fig. 3C). The BPA-500 males had significantly more adipocytes in the renal fat pad compared to the negative controls and all other BPA dose groups. For renal adipocyte volume, the linear ANOVA was statistically significant, and addition of the quadratic term in the regression analysis did not result in a statistically significant effect of BPA dose (Fig. 3D). Compared to the negative controls, the BPA-5 (P=0.06) and BPA-5000 (P< 0.01) had larger renal adipocytes.
3.3. Liver and kidney weight and liver histopathology
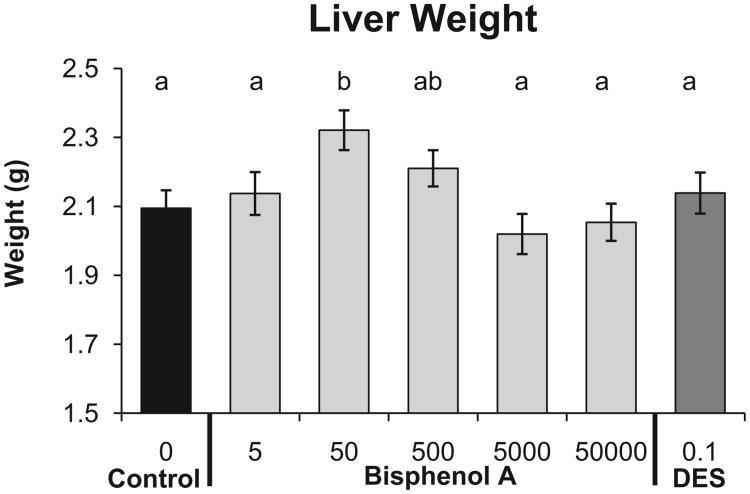
The linear ANOVA for liver weight (Fig. 4) was statistically significant (P = 0.01), and addition of the quadratic term in the regression analysis led to a significantly better fit compared to the linear-only model (P < 0.001). Compared to the negative controls, the BPA-50 group had significantly higher liver weight (P < 0.01). We also examined liver sections for evidence of steatosis (fatty liver) using previously published methods [54], and there was no significant relationship between prenatal treatment and steatosis (data not shown). The linear ANOVA for kidney weight was not statistically significant, and addition of the quadratic term in the regression analysis also was also not statistically significant (data not shown).
Fig. 4.
Effect of fetal exposure to BPA on mean (±SEM) liver weight in males from different prenatal treatment groups when 19 weeks old. Groups with different letters are significantly different from each other.
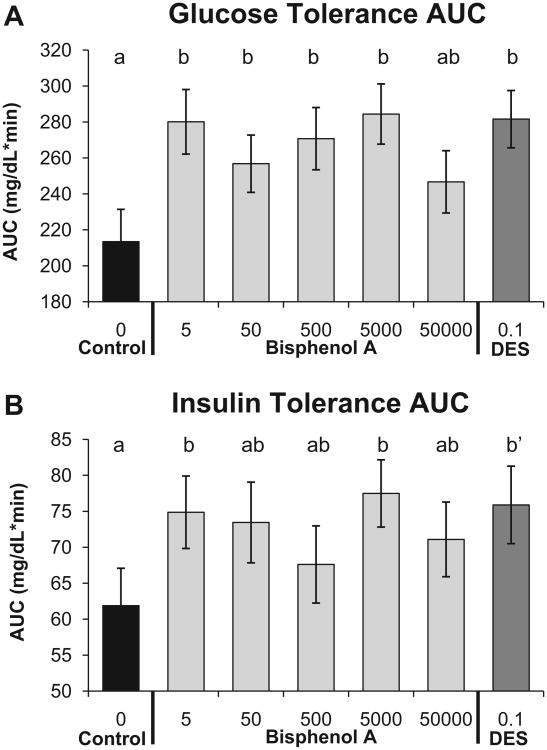
3.4. Glucose and insulin tolerance tests
Glucose tolerance tests (GTT) were conducted for adult male mice fasted for 4h to examine whether prenatal BPA exposure altered glucose homeostasis after an injection of glucose. The linear ANOVA using PROC GLM for the area under the curve (AUC) for blood glucose levels measured prior to and during the 2 h after the glucose injection showed a tendency to be different (P = 0.06; Fig. 5A). Addition of the quadratic term fit the data significantly better than the linear-only model (P = 0.008). Compared to the negative controls, with the exception of the BPA-50,000 group, all other BPA dose groups as well as the DES-0.1 group showed significantly increased AUC values (impaired glucose tolerance). The insulin tolerance test area under the curve (AUC) for blood glucose after administration of insulin was not statistically significant based on the linear ANOVA, and adding the quadratic term to the model did not result in a statistically significant difference (P = 0.137). While the overall ANOVA was not statistically significant, the BPA-5 and BPA-5000 groups had significantly greater insulin insensitivity, and the DES-0.1 males tended to have greater insulin insensitivity (P = 0.07) as displayed by a higher AUC value for blood glucose relative to negative controls (Fig. 5B).
Fig. 5.
Panel A: glucose tolerance test (mean±SEM) area under the curve (AUC) after a 4-h fast in males from different prenatal treatment groups when about 18 weeks old. Blood glucose was measured during the 2 h after i.p. injection of glucose. Panel B: insulin tolerance test area under the curve (AUC) for blood glucose after i.p. injection of insulin using the same approach as for the glucose tolerance test. Groups with different letters are significantly different from each other; b′, P= 0.07 vs. controls.
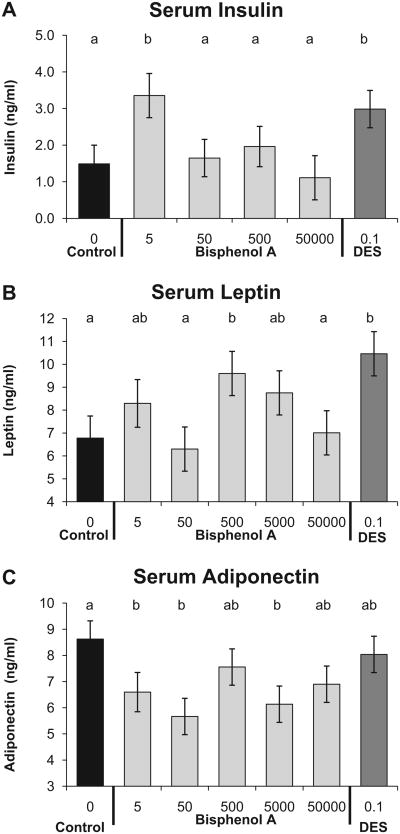
3.5. Serum hormones
We measured the levels of insulin, adiponectin, and leptin in blood collected at autopsy in fed animals. The animals were not food-deprived to ensure that there was no effect of even a short period of fasting on adipocyte number or volume. Insulin regulates blood glucose levels, while adiponectin and leptin are adipose tissue-derived hormones that tend to be deranged in cases of metabolic syndrome where obesity is a contributing symptom [6]. The linear ANOVA for the effect of prenatal treatment on serum insulin was significantly significant (P < 0.01), and addition of the quadratic term in the regression analysis resulted in a significantly better fit relative to the linear-only model (P = 0.004). The mean serum insulin level for the BPA-5 and DES-0.1 groups was significantly (about 2-fold) higher than the negative control and BPA-50,000 groups (Fig. 6A). The profile for the BPA dose effect on adult serum leptin (Fig. 3B) matched the total abdominal fat weight profile (Fig. 2A; Spearman correlation coefficient = 0.86; P < 0.001). This typically indicates normal leptin secretion, since leptin is secreted by adipose tissue in proportion to the mass of tissue [55]. The linear ANOVA for the effect of BPA dose on serum leptin was statistically significant, and addition of the quadratic term did not result in a significantly different fit relative to the linear-only model (P = 0.349). The BPA-500 and the DES-0.1 groups had significantly elevated serum leptin levels compared to the negative controls and BPA-50,000 males. A low serum adiponectin level is a biomarker for future onset of type 2 diabetes in human populations [56]. The linear ANOVA for serum adiponectin levels was statistically significant, and addition of the quadratic term in the regression analysis tended to result in a better fit (P = 0.099) relative to the linear-only model (Fig. 6C). Compared to negative controls, males in the BPA-5, BPA-50 and BPA-5000 groups had significantly lower serum adiponectin, with maximal reduction in the BPA-50 group.
Fig. 6.
Serum insulin, leptin, and adiponectin (mean ±SEM) concentrations in nonfasted males from different prenatal treatment groups when 19 weeks old. Panel A: serum insulin, Panel B: serum leptin, and Panel C: serum adiponectin. Groups with different letters are significantly different from each other.
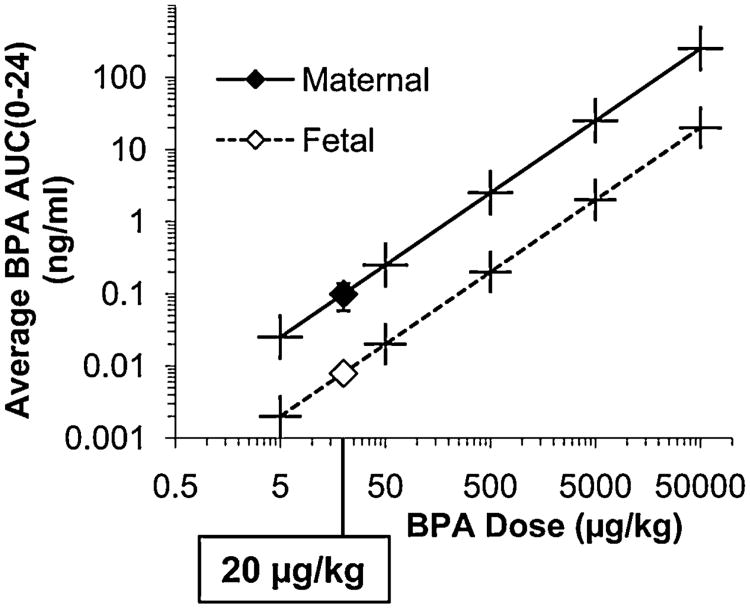
3.6. Maternal and fetal serum unconjugated BPA
Between GD 17 and 18 when differentiation of adipocytes, metabolic and neuroendocrine systems is occurring [6], the average maternal serum unconjugated BPA concentrations (average AUC0–24) over the 24 h collection period associated with the 5 doses (5, 50, 500, 5000, 50,000 μg/kg/day) used in this experiment were calculated based on interpolation from the results of administering 20 μg/kg; maternal serum unconjugated BPA was calculated to be: 0.025, 0.25, 2.50, 25 and 250ng/ml, respectively. The maternal average serum unconjugated BPA AUC0–24 values were 12.5-fold higher than the fetal values, which were: 0.002, 0.02, 0.20, 2 and 20 ng/ml, respectively (Fig. 7).
Fig. 7.
Average serum unconjugated BPA levels in maternal and fetal serum over the 24 h between GD 17 and 18 (AUC0–24/24 h) after the last oral administration of 3H-BPA on GD 17. Pregnant females were fed a 20 μg/kg mixture of BPA and 3H-BPA one time per day between GD 11 and 17. Data for each BPA dose are based on linear interpolation, since administered dose is linear with internal serum concentration over a wider range of doses than were examined in this study [32].
4. Discussion
Our major findings were that as a result of exposure of male fetuses to BPA via feeding pregnant mice doses of BPA at and below the current predicted NOAEL (5000 μg/kg/day) being used to estimate the “safe” daily intake dose by regulatory agencies in the USA and Europe, there was a significant increase in postnatal body weight gain, adipocyte number and volume and the overall amount of abdominal fat, altered food intake, serum insulin, adiponectin and leptin levels, and impaired glucose tolerance and insulin sensitivity. All of the changes that we observed in response to low doses of BPA are components of metabolic syndrome, and these findings thus have implications for metabolic diseases that are observed to be related to BPA exposure in humans [2,18,20,21]. The findings from the epidemiological studies referenced above indicate that there is sufficient exposure to BPA in the general population for BPA to be related to a variety of adverse metabolic outcomes in people. Our data here in mice show that all of these adverse effects occurred as a result of fetal exposure to BPA in the parts per trillion range of bioactive (unconjugated) BPA in fetal serum.
Importantly, while all of these outcomes occurred at doses of BPA at and below the presumed NOAEL, none of these outcomes was statistically different from negative controls for the highest dose of BPA that was administered (50,000 μg/kg/day). This finding, that “the dose does not make the poison” (i.e., that very high doses do not necessarily result in a greater response than very low doses) was confirmed for many of these outcomes by using regression analysis that included a quadratic term, which tests for a U or inverted U dose–response curve.
Our data here demonstrate the problem encountered when only one or two doses of BPA are examined, because it is understood that different tissues have different sensitivities to hormones [24,57]. The consequence is that we found maximum responses for various outcomes at doses ranging from 5 to 500 μg/kg/day. Also, for a number of outcomes the dose–response curve showed increases and decreases, and these types of multimodal dose–responses curves have been commonly reported in studies where effects of BPA have been mediated by non-classical (non-nuclear) receptors [58,59].
4.1. Concentration of unconjugated BPA in maternal and fetal serum
Of great importance with regard to the view held by some that BPA is too weak an estrogen to stimulate any effect within the dose range in which we observed the above statistically significant effects, the 5-μg/kg/day maternal oral BPA dose resulted in an average fetal serum BPA concentration (Fig. 7) of 2pg/ml (2ppt) over the 24 h between GD 17 and 18 (the day prior to parturition). This average AUC0–24 concentration over the 24 h after an oral bolus administration to the pregnant female is far below the median concentration of unconjugated BPA reported in published human biomonitoring studies [28,29]. These data suggest that there is exquisite sensitivity of fetuses to very low concentrations of BPA, since we found an increase in gonadal adipocyte number and volume (Fig. 3) and serum insulin (Fig. 6), a decrease in insulin sensitivity and glucose tolerance (Fig. 5), as well as serum adiponectin (Fig. 6) at the 5-μg/kg/day maternal dose of BPA.
Effects reported in the offspring of pregnant females fed low doses (5–500 μg/kg/day) of BPA that result in fetal serum unconjugated BPA in the range of 2–200 ppt have led to speculation that non-classical estrogen receptors associated with the cell membrane may be involved in mediating some responses at these very low concentrations [60]. However, while BPA is often characterized as a “weak estrogen” based on a lower affinity for nuclear estrogen receptors relative to estradiol, potency is not able to be predicted based solely on affinity of a hormone (or hormone-mimicking chemical) for the receptor, since tissue-specific co-regulators also significantly impact potency [24,57,61,62]. Also, the suggestion that nuclear estrogen receptors should not respond to concentrations of BPA in the part per trillion range is not consistent with the “spare receptor” hypothesis that detectable responses occur at very low receptor occupancy [33,63].
4.2. Low dose of DES as a positive control
A low (0.1 μg/kg/day) dose of the potent estrogenic drug DES resulted in some but not all of the outcomes observed in response to low doses of BPA, suggesting that classifying BPA only as an “environmental estrogen” does not capture the range of activities BPA has in vivo. However, testing a wide range of doses of DES would be required to adequately test this hypothesis. Specifically, the 0.1 μg/kg/day dose of DES led to significant differences compared to negative controls for increased gonadal adipocyte number (similar to the BPA-5 and BPA-500 groups), glucose tolerance (similar to BPA-5, BPA-50, BPA-500 and BPA-5000 groups), increased serum insulin (similar to the BPA-5 group), and increased serum leptin (similar to the BPA-500 group). Our prediction was that the 0.1 μg/kg/day DES would only be an appropriate positive control for the low (5, 50 and 500 μg/kg/day) doses of BPA [64]. This prediction was based on prior findings that for a number of outcomes caused by prenatal exposure to DES and BPA that are likely mediated by the nuclear estrogen receptor alpha, DES was between 100-and 1000-fold more potent than BPA [65–68].
Since each tissue and metabolic control system would be expected to exhibit a unique response profile to a range of doses of any regulatory hormone or endocrine disrupting chemical [24], it is not surprising that for different outcomes, the single dose of DES had an effect similar to different low doses of BPA. Importantly, the low 0.1 μg/kg/day dose of DES differed significantly from the high (50,000 μg/kg/day) BPA dose group for adult gonadal and renal fat pad weight, gonadal adipocyte number, and both serum insulin and leptin. These findings show that in addition to the finding that the BPA-50,000 group did not differ significantly from negative controls on any outcome, this high BPA dose also resulted in significant differences from a low dose of DES as well as from lower doses of BPA. Thus, our low dose of DES did not serve as a positive control for estrogenic activity of the high 50,000 μg/kg/day maternal dose of BPA in male offspring.
4.3. Altered adipose tissue characteristics resulting from fetal exposure to estrogenic chemicals
We examined the effect of fetal exposure to BPA on the overall weight, as well as number and volume of adipocytes in the two abdominal fat depots, the gonadal and renal fat pads, because in men, abdominal fat mass is correlated with metabolic disease [69]. Relative to negative controls, we found a significant increase in the weight of both the gonadal and the renal fat pads at 19 weeks of age in male offspring of dams fed the 500 μg/kg/day dose of BPA; the 0.1 μg/kg/day dose of DES showed a similar response (Fig. 2). The BPA-500 males also had significantly more gonadal and renal fat pad adipocytes, and gonadal adipocytes also had a significantly increased volume, compared with negative controls (Fig. 3). Although body weight followed a similar pattern as the abdominal fat pad weights, the effect of BPA on body weight was very small. However, body weight is not considered a sensitive outcome in mice [70], since even very large changes in abdominal fat pad weight have relatively small effects on body weight [43].
Similar to our findings here, in a study that did examine a wide range of doses (3–580,000 μg/kg/day) of BPA in CD-1 mice [71], body weight was increased in males at doses below 5000 μg/kg/day and decreased above this dose, although the authors did not conduct the appropriate non-monotonic statistical analysis, and it is thus not possible to determine whether there were statistically significant low-dose effects of BPA on body weight. Given the nonlinear dose–response curves often seen in endocrine physiology [24], we believe it essential to statistically assess data for non-monotonicity when visual inspection shows a clear non-monotonic trend.
A critical factor in assessing the impact of BPA on adipocyte development and subsequent function is the timing of exposure, and different effects can occur due to exposure during fetal life when preadipocyte differentiation from mesenchymal stem cells occurs as opposed to the first 2 weeks after birth in mice when differentiation of preadipocytes into adipocytes occurs [17,72]. Other chemicals, referred to as obesogens, such as organotins [73] and the fungicide trifumizole [74], have been reported to increase adipocyte proliferation in mice via activation of PPARγ, which is a major regulator of adipocyte differentiation and then subsequently adipocyte function [50]. However, BPA and BPA-diglycidyl ether (BADGE), used to line food and beverage cans, have been reported to stimulate adipocyte proliferation through PPARγ-independent mechanisms [75].
4.4. Altered glucose and insulin homeostasis
Homeostatic control of glucose was dysregulated in male mice exposed in utero to low doses of BPA. Specifically, impaired glucose tolerance, measured as the ability to return blood glucose levels to baseline after an injection of glucose, occurred at BPA-5, BPA-50, BPA-500 and BPA-5000 μg/kg/day (Fig. 5); however, not all of these doses impacted abdominal adipocyte weight, number or volume. These findings suggest that impaired glucose regulation is not just a consequence of an increase in central adiposity, which is consistent with prior findings [11]. There was also an impaired ability to lower blood glucose levels in response to an injection of insulin that was statistically significant for males in the BPA-5 and BPA-5000 exposure groups (Fig. 5), while males exposed to BPA-50 and BPA-500 did not show a statistically significant difference from controls on the insulin tolerance test.
4.5. Serum hormone levels are altered by fetal exposure to BPA
Adipose tissue is now recognized as an endocrine organ. As such, it secretes hormones such as adiponectin and leptin. Adiponectin levels were suppressed in adult male mice in most prenatal BPA treated groups (Fig. 6), but serum adiponectin was not significantly reduced in the males exposed to the 500-μg/kg/day dose of BPA that showed the greatest increase in adipocyte number and overall adipocyte weight. Adiponectin receptors are found in liver and skeletal muscle, and adiponectin signaling through these receptors increases insulin sensitivity [76]. Decreased adiponectin levels are associated with glucose intolerance and insulin resistance [77]. Ectopic fat deposition in liver often occurs with reduced adiponectin [78]. Consistent with this, we found maximally suppressed adiponectin (Fig. 6) and significantly elevated liver weight (Fig. 4) in the BPA-50 group, although we did not find evidence of steatosis associated with prenatal exposure to this dose of BPA. Other research has shown that BPA treatment of adipocytes from adult mice and humans inhibits the release of adiponectin [13]. Further research will be required to determine the mechanisms mediating effects of exposure to BPA and other estrogenic chemicals during fetal and neonatal life on the development of the complex systems regulating adipocyte hormones.
We found that serum insulin was significantly elevated in the BPA-5 and DES-0.1 groups compared with negative controls and the BPA-50,000 group. Since we measured serum insulin in fed animals, it is not appropriate to compare the insulin tolerance test AUC values (Fig. 5) with the serum insulin values (Fig. 6). However, Alonso-Magdalena and colleagues exposed pancreatic islets isolated from mice prenatally treated with BPA to stimulatory levels of glucose, which would be a more comparable situation to a fed animal. They found that pancreatic islets of animals prenatally exposed to 10-μg/kg/day BPA from GD 9 to 16 secreted more insulin than control islets [11]. Thus, in addition to its obesogenic actions on adipose tissue, BPA also affects other metabolic parameters independently of its effect on adipose tissue expansion, including disruption of glucose homeostasis, alteration of beta cell insulin content, increased leptin secretion, increased serum lipids, and decreased adiponectin secretion [6,11].
Bisphenol A can induce beta cell dysfunction whether the exposure is in adulthood or in utero [11,79]. For example, treatment of pregnant female CD-1 mice with a low dose of BPA(100 μg/kg/day) subsequently led to increased insulin resistance and glucose intolerance in the dams, a phenotype repeated (but at a 10-fold lower dose) in the adult male offspring that were only exposed in utero. Additionally, insulin secretion by mouse and human pancreatic islets is augmented by 1 nM (0.23 ng/ml) BPA in the presence of a stimulatory level of glucose [17,80–82]; this concentration of BPA is well within the range of internal serum levels of unconjugated (bioactive) BPA reported in humans [28,31,32]. The above studies investigating beta cell function after BPA exposure found that equimolar concentrations of estradiol gave the same response, providing evidence that BPA was as potent as estradiol and that signaling was through one or more of the estrogen receptor sub-types. Coincidentally, all tissues responsible for maintaining metabolic homeostasis express at least one known estrogen receptor sub-type [17,80-82].
We found significantly increased serum leptin levels in the BPA-500 group compared to both the negative controls and the BPA-50,000 group, and the DES-0.1 group also had significantly elevated circulating leptin (Fig. 6). As expected there was a high correlation (Spearman correlation coefficient = 0.86) between the combined weight of the gonadal and renal fat pads and serum leptin levels. Interestingly, while the BPA-500 males, that had elevated serum leptin, did consume significantly less food in adulthood between weeks 15 and 19 relative to negative controls (Fig. 1), they still gained more weight during this time relative to negative controls.
4.6. Opposite effects of fetal exposure to BPA on energy intake during puberty and adulthood
There is extensive evidence that developmental exposure to BPA can alter brain morphology and chemistry and impact neuroendocrine function and behavior [23,83]. Our study suggests that BPA may alter maintenance of energy balance, in that males in the 500-μg/kg/day BPA group exhibited increased food intake and body weight during the post weaning period in male mice, yet gained more weight while eating less than negative controls as adults between weeks 15 and 19 (Fig. 1). The regulation of energy intake and body weight is a homeostatic system, with neurons in the hypothalamus acting as the central coordinator [84]. Estradiol can affect neuronal circuits that control appetite and energy balance by acting on the hypothalamic nuclei that play a crucial role in sensing hormonal signals that reflect the overall availability of nutrients. Two neurochemically distinct populations of hypothalamic neurons located in the arcuate nucleus are critical for the integration of metabolic information. One neuron group expresses the potent orexigenic neuropeptide Y (NPY) [and Agouti related protein (AgRP)] and shows high concentrations of binding sites for many hormonal and metabolic signals such as insulin, leptin and ghrelin. An increase in NPY release results in increased food intake and decreased energy expenditure. Another set of arcuate neurons expresses the neuropeptide precursor propiomelanocortin (POMC), which is processed to melanocortin peptides; activation of these neurons decreases food intake and increases energy expenditure. These two populations of neurons thus exert opposite effects on energy intake and interact on several levels. The current hypothesis is that as adipose stores increase, both insulin and leptin levels increase along with POMC expression, while NPY synthesis and activity are inhibited and food intake is reduced. Conversely, when NPY synthesis and release are increased and POMC is decreased, the result is an increase in food intake [85–87]. Dysfunction of the NPY system has been implicated in obesity and type II diabetes in humans [88,89]. In summary, leptin and insulin levels, which are altered by fetal exposure to low doses of BPA, may play an important role in regulating the NPY system. However, the factors that lead to increased food intake during puberty and decreased food intake in adulthood due to low-dose fetal BPA exposure remain to be determined, as does the impact of a chemical such as BPA, with estrogenic and other modes of action, on the development of these systems in fetuses as opposed to activational effects in adults [90–92].
4.7. Implications for assumptions used in chemical risk assessments
Our study is the first to examine effects of BPA on traits related to metabolic syndrome due to fetal exposure to a wide range of doses, from below the reference dose to above the presumed no adverse effect dose or NOAEL The NIEHS funded this and related research as a coordinated set of projects that examined a wide dose range with the objective of providing data from NIH-funded studies that would be useful for regulatory agencies, since studies that only examine three or fewer doses of a chemical do not provide the data needed for establishing dose–response relationships that should be required for chemical risk assessments [47]. For the male mice exposed during fetal life to the 50,000 μg/kg/day dose of BPA, which is 10-fold higher than the NOAEL currently used by regulatory agencies, not one of the outcomes we examined was statistically different from negative controls, although statistically significant effects (both increases and decreases in responses) were observed at lower BPA doses in comparison to negative controls. We thus found evidence for non-monotonic dose–response relationships for many outcomes, based on comparing statistical models that examined for goodness of fit of the data using a linear-only model and a second model that contained a quadratic term that tested for non-linearity. The degree to which the data fit a non-monotonic rather than linear function for the variables we measured is presented in Table 2.
The current approach used throughout the world by regulatory agencies to assess the risks posed by all environmental chemicals, including EDCs, is to just examine a few (typically 3) very high doses, with the maximum tolerated dose (MTD) that is acutely toxic but not lethal being the reference point for establishing the relatively narrow range of very high doses that are examined; EDCs such as BPA are acutely toxic at very high doses (for BPA the MTD is greater than 1 g/kg body weight) [93]. This was the approach that initially led to the estimate that a daily oral BPA dose of 50 μg/kg/day would be safe for human consumption throughout the lifespan [93,94]. This approach used to assess the risks of EDCs has been previously challenged as invalid [24,57,63,95,96], and our current findings provide additional evidence that the core assumption in chemical risk assessments, namely that dose–response relationships are always monotonic, is invalid. Our results clearly demonstrate that the hazards posed by low doses of BPA cannot be predicted by examining a dose of BPA (50,000 μg/kg/day) ten-fold higher than the presumed NOAEL.
5. Conclusions
For a number of the endpoints we examined in male CD-1 mice: body weight, energy intake, gonadal and renal fat pad weights, adipocyte number and volume, glucose tolerance and serum concentrations of adiponectin, leptin and insulin, we observed long-term effects due to maternal oral exposure to BPA doses below the assumed NOAEL of 5000 μg/kg/day. These effects were not predicted by responses to the highest dose of BPA that we examined, which was 50,000 μg/kg/day. Without testing doses below the current NOAEL, it is likely that we would have found no effect of BPA on any of these outcomes related to metabolic syndrome. This study thus provides additional evidence that important cell-receptor-mediated activity can occur far below a high dose range for endocrine disrupting chemicals. Such evidence invalidates the crucial assumption of the current risk assessment paradigm that all dose–response relationships are monotonic and “the dose makes the poison”. Our findings demonstrate the urgent need for development of strategies for assessing the hazards posed by EDCs that are consistent with current endocrinological principles [57].
Acknowledgments
Support was provided by grants ES018764 and ES021394 to FvS from NIEHS.
Footnotes
The authors declare they have no competing financial interests.
Contributor Information
Brittany M. Angle, Email: brittanyangle@mail.missouri.edu.
Rylee Phuong Do, Email: pbd6v8@mail.missouri.edu.
Davide Ponzi, Email: depr29@mail.missouri.edu.
Richard W. Stahlhut, Email: richard.stahlhut@me.com.
Bertram E. Drury, Email: bed25c@health.missouri.edu.
Susan C. Nagel, Email: nagels@health.missouri.edu.
Wade V. Welshons, Email: welshonsw@missouri.edu.
Cynthia L Besch-Williford, Email: Cindy-Besch-Williford@idexx.com.
Paola Palanza, Email: paola.palanza@unipr.it.
Stefano Parmigiani, Email: stefano.parmigiani@unipr.it.
Frederick S. vom Saal, Email: vomsaalf@missouri.edu.
Julia A. Taylor, Email: taylorja@missouri.edu.
References
- 1.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. British Journal of Radiology. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nature Reviews Endocrinology. 2011;7:346–53. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 3.Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. Journal of Andrology. 2008;29:251–9. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 4.Heindel JJ, vom Saal FS. Overview of obesity and the role of developmental nutrition and environmental chemical exposures. Molecular and Cellular Endocrinology. 2009;304:90–6. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. International Journal of Andrology. 2012;35:437–48. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Molecular and Cellular Endocrinology. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, et al. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environmental Health Perspectives. 2013;121:586–93. doi: 10.1289/ehp.1205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, et al. Prenatal bisphenol A (BPA) exposure alters sex specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicological Sciences. 2013 doi: 10.1093/toxsci/kft035. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152:3049–61. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- 10.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, et al. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environmental Health Perspectives. 2010;118:1243–50. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Research A: Clinical and Molecular Teratology. 2005;73:478–80. doi: 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- 13.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environmental Health Perspectives. 2008;116:1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marroqui L, Gonzalez A, Neco P, Caballero-Garrido E, Vieira E, Ripoll C, et al. Role of leptin in the pancreatic beta-cell: effects and signaling pathways. Journal of Molecular Endocrinology. 2012;49:R9–17. doi: 10.1530/JME-12-0025. [DOI] [PubMed] [Google Scholar]
- 15.Somm E, Schwitzgebel VM, Vauthay DM, Camm EJ, Chen CY, Giacobino JP, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149:6289–99. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 16.Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environmental Health Perspectives. 2009;117:1549–55. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Experimental Biology and Medicine (Maywood, NJ) 2004;229:1127–35. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 18.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults: evidence from NHANES 2003/4. JAMA. 2008;300:1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 19.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS ONE. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–90. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 21.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–21. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 22.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin BS, Bisphenol A. An endocrine disruptor with widespread exposure and multiple effects. Journal of Steroid Biochemistry and Molecular Biology. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine Reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailin PD, Byrne M, Lewis S, Liroff R. [accessed 20.04.11];Public awareness drives market for safer alternatives: bisphenol A market analysis report. 2008 http://www.iehn.org/publications.reports.bpa.php.
- 26.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental Health Perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahlhut RW, Welshons WV, Bisphenol Swan SH. A data in NHANES suggest longerthan expected half-life, substantial nonfood exposure, or both. Environmental Health Perspectives. 2009;117:784–9. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental Health Perspectives. 2010;118:1055–70. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in human maternal-fetal-placental unit. Environmental Health Perspectives. 2002;110:A703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reproductive Toxicology. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor JA, Welshons WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reproductive Toxicology. 2008;25:169–76. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environmental Health Perspectives. 2011;119:422–30. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 34.Aghajanova L, Giudice LC. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reproductive Biomedicine Online. 2011;22:249–56. doi: 10.1016/j.rbmo.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 36.Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochemical and Biophysical Research Communications. 2000;270:918–21. doi: 10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- 37.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environmental Health Perspectives. 2001;109:675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 39.Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol - a exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2010;32:38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, et al. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reproductive Toxicology. 2004;18:803–11. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Heindel JJ, vom Saal FS. Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets - importance and approaches for laboratory animal research. Environmental Health Perspectives. 2008;116:389–93. doi: 10.1289/ehp.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cederroth CR, Vinciguerra M, Kühne F, Madani R, Klein M, James RW, et al. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environmental Health Perspectives. 2007;115:1467–73. doi: 10.1289/ehp.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, et al. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environmental Health Perspectives. 2008;116:322–8. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Research Reviews. 2008;57:352–62. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Gill HK, Wu GY. Non-alcoholic fatty liver disease and the metabolic syndrome: effects of weight loss and a review of populardiets. Are low carbohydrate diets the answer? World Journal of Gastroenterology. 2006;12:345–53. doi: 10.3748/wjg.v12.i3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clinical Journal of the American Society of Nephrology. 2011;6:2364–73. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birnbaum LS, Bucher JR, Collman GW, Zeldin DC, Johnson AF, Schug TT, et al. Consortium-based science: the NIEHS's multipronged, collaborative approach to assessing the health effects of bisphenol A. Environmental Health Perspectives. 2012;120:1640–4. doi: 10.1289/ehp.1205330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markaverich BM, Crowley JR, Alejandro MA, Shoulars K, Casajuna N, Mani S, et al. Leukotoxin diols from ground corncob bedding disrupt estrous cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environmental Health Perspectives. 2005;113:1698–704. doi: 10.1289/ehp.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thigpen JE, Setchell KD, Kissling GE, Locklear J, Caviness GF, Whiteside T, et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. Journal of the American Association for Laboratory Animal Science. 2013;52:130–41. [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nature Reviews Molecular Cell Biology. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 51.vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S. Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biology of Reproduction. 1990;43:751–61. doi: 10.1095/biolreprod43.5.751. [DOI] [PubMed] [Google Scholar]
- 52.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicology and Applied Pharmacology. 2010;247:158–65. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Nonmonotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reproductive Toxicology. 2012;34:614–21. doi: 10.1016/j.reprotox.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 55.Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–80. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 57.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kochukov MY, Jeng YJ, Watson CS. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environmental Health Perspectives. 2009;117:723–30. doi: 10.1289/ehp.0800182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–96. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 60.Watson CS, Jeng YJ, Kochukov MY. Nongenomic signaling pathways of estrogen toxicity. Toxicological Sciences. 2010;115:1–11. doi: 10.1093/toxsci/kfp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. Journal of Biological Chemistry. 2000;275:35986–93. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 62.Tai H, Kubota N, Kato S. Involvement of nuclear receptor coactivator SRC-1 in estrogen-dependent cell growth of MCF-7 cells. Biochemical and Biophysical Research Communications. 2000;267:311–6. doi: 10.1006/bbrc.1999.1954. [DOI] [PubMed] [Google Scholar]
- 63.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environmental Health Perspectives. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 65.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proceedings of the National Academy of Sciences of the United States of America; 1997; pp. 2056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proceedings of the Society for Experimental Biology and Medicine; 2000; pp. 61–8. [DOI] [PubMed] [Google Scholar]
- 67.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reproductive Toxicology. 2002;16:117–22. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 68.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proceedings of the National Academy of Sciences of the United States of America; 2005; pp. 7014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 2007;15:1061–7. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 70.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environmental Health Perspectives. 2012;120:779–89. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tyl RW, Myers C, Marr M, Sloan CS, Castillo N, Veselica MM, et al. Two-generation reproductive toxicity study of dietary bisphenol A (BPA) in CD-1® (Swiss) mice. Toxicological Sciences. 2008;102:362–84. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 72.Blumberg B. Obesogens, stem cells and the maternal programming of obesity. Journal of Developmental Origins of Health and Disease. 2011;2:3–8. doi: 10.1017/S2040174410000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. Journal of Steroid Biochemistry and Molecular Biology. 2011;127:9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Pham HT, Janesick AS, Blumberg B. Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARgamma) Environmental Health Perspectives. 2012;120:1720–6. doi: 10.1289/ehp.1205383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chamorro-Garcia R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, et al. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptorgamma-independent mechanism. Environmental Health Perspectives. 2012;120:984–9. doi: 10.1289/ehp.1205063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Molecular and Cellular Endocrinology. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Wasim H, Al-Daghri NM, Chetty R, McTernan PG, Barnett AH, Kumar S. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovascular Diabetology. 2006;5:10. doi: 10.1186/1475-2840-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine Reviews. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 79.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environmental Health Perspectives. 2006;114:106–12. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barros RP, Morani A, Moriscot A, Machado UF. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Molecular and Cellular Endocrinology. 2008;295:24–31. doi: 10.1016/j.mce.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. Journal of Steroid Biochemistry and Molecular Biology. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Soriano S, Alonso-Magdalena P, Garcia-Arevalo M, Novials A, Muhammed SJ, Salehi A, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor beta. PLoS ONE. 2012;7:e31109. doi: 10.1371/journal.pone.0031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broberger C. Brain regulation of food intake and appetite: molecules and networks. Journal of Internal Medicine. 2005;258:301–27. doi: 10.1111/j.1365-2796.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 85.Inui A. Transgenic study of energy homeostasis equation: implications and confounding influences. FASEB Journal. 2000;14:2158–70. doi: 10.1096/fj.00-0291rev. [DOI] [PubMed] [Google Scholar]
- 86.Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Frontiers in Neuroendocrinology. 2006;27:308–39. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Bertocchi I, Oberto A, Longo A, Mele P, Sabetta M, Bartolomucci A, et al. Regulatory functions of limbic Y1 receptors in body weight and anxiety uncovered by conditional knockout and maternal care. Proceedings of the National Academy of Sciences of the United States of America; 2012; pp. 19395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding B, Kull B, Liu Z, Mottagui-Tabar S, Thonberg H, Gu HF, et al. Human neuropeptide Y signal peptide gain-of-function polymorphism is associated with increased body mass index: possible mode of function. Regulatory Peptides. 2005;127:45–53. doi: 10.1016/j.regpep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Ukkola O, Kesaniemi YA. Leu7Pro polymorphism of PreproNPY associated with an increased risk for type II diabetes in middle-aged subjects. European Journal of Clinical Nutrition. 2007;61:1102–5. doi: 10.1038/sj.ejcn.1602621. [DOI] [PubMed] [Google Scholar]
- 90.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. American Journal of Physiology, Endocrinology and Metabolism. 2008;294:E817–26. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- 91.Martini M, Miceli D, Gotti S, Viglietti-Panzica C, Fissore E, Palanza P, et al. Effects of perinatal administration of Bisphenol A on the neuronal nitric oxide synthase expressing system in the hypothalamus and limbic system of CD1 mice. Journal of Neuroendocrinology. 2010;22:1004–12. doi: 10.1111/j.1365-2826.2010.02043.x. [DOI] [PubMed] [Google Scholar]
- 92.Vianna CR, Coppari R. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152:11–8. doi: 10.1210/en.2010-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA. The developmental toxicity of bisphenol A in rats and mice. Fundamental and Applied Toxicology. 1987;8:571–82. doi: 10.1016/0272-0590(87)90142-4. [DOI] [PubMed] [Google Scholar]
- 94.IRIS. Bisphenol A (CASRN 80–05–7); US-EPA Integrated Risk Information System Substance File. 1988. [Google Scholar]
- 95.vom Saal FS, Sheehan DM. Challenging risk assessment. Forum for Applied Research and Public Policy. 1998;13:11–8. [Google Scholar]
- 96.Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environmental Health Perspectives. 2009;117:1652–5. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]