Abstract
Background
Thymosin β4 (Tβ4) has been shown to enhance the survival of cultured cardiomyocytes. Here, we investigated whether the cytoprotective effects of Tβ4 can increase the effectiveness of transplanted swine mesenchymal stem cells (sMSCs) for cardiac repair in a rat model of myocardial infarction (MI).
Methods and Results
Under hypoxic conditions, cellular damage (lactate dehydrogenase leakage), apoptosis (TUNEL+ cells), and caspase-8 activity were significantly lower, while Bcl-XL protein expression was significantly higher, in sMSCs cultured with Tβ4 (1 µg/mL) than in sMSCs cultured without Tβ4, and Tβ4 also increased sMSC proliferation. For in-vivo experiments, animals were treated with basal medium (MI: n=6), a fibrin patch (Patch: n=6), a patch containing sMSCs (sMSC: n=9), or a patch containing sMSCs and Tβ4 (sMSC/Tβ4: n=11); Tβ4 was encapsulated in gelatin microspheres to extend Tβ4 delivery. Four weeks after treatment, echocardiographic assessments of left-ventricular ejection fraction (LVEF) and fractional shortening (FS) were significantly better (p<0.05) in sMSC/Tβ4 animals (LVEF=51.7±1.1%; FS=26.7±0.7%) than in animals from MI (39±3%; 19.5±1.7%) and Patch (43±1.4%; 21.6±0.9%) groups. Histological assessment of infarct wall thickness (TH) was significantly higher (p<0.05) in sMSC/Tβ4 animals (50%, (45%, 80%)) than in animals from MI (25%, (20%, 25%)) group. Measurements in sMSC (LVEF=45±2.6%; FS=22.9±1.6%; TH=43% (25%, 45%)), Patch, and MI animals were similar. Tβ4 administration also significantly increased vascular growth, the retention/survival of the transplanted sMSCs, and the recruitment of endogenous c-Kit+ progenitor cells to the infarcted region.
Conclusions
Extended-release Tβ4 administration improves the retention, survival, and regenerative potency of transplanted sMSCs after myocardial injury.
Keywords: angiogenesis, myocardial infarction, stem cell, tissue engineering, Microsphere
Introduction
Cell-based therapeutic approaches for improving recovery from myocardial injury have been extensively studied in both animals and humans1–4. Mesenchymal stem cells (MSCs) are among the most frequently investigated cellular populations1, 2, and the results from these studies suggest that when transplanted into the ischemic region, MSCs can improve cardiac functional recovery and prevent or reverse adverse cardiac remodeling by differentiating into cardiomyocytes and vascular cells, activating endogenous cardiac progenitor cells (CPCs), and secreting paracrine factors 1, 2. However, the proportion of transplanted cells that are retained and survive in the ischemic region is very low5, 6: less than 0.44% of human MSCs remained viable on the fourth day after transplantation in immunodeficient mice7, and the estimated rate of survival is less than 1% after autologous cell transplantation in patients8. This high rate of attrition is believed to limit the effectiveness of cell therapy and likely evolves from a variety of causes, including the immune and inflammatory response, the loss of trophic factors, and the limited supply of blood in the ischemic region9.
Thymosin β4 (Tβ4) is an actin-sequestering protein that participates in the cytoskeletal re-arrangements required for cell motility. It contributes to neurite outgrowth and neuron survival 10 and stimulates new hair growth by inducing the migration of hair-follicle stem cells11. The Tβ4 gene is located on the X chromosome and is likely expressed in megakaryocytes, while the release of Tβ4 protein from platelets has an important role in the protection, regeneration, and remodeling of injured or damaged tissue12, 13. Tβ4 promotes angiogenesis by stimulating endothelial-cell migration, adhesion, and tubule formation, as well as the sprouting of new vessels from aortic rings14, and can induce the differentiation of epicardial progenitor cells into endothelial cells15. It also impedes the inflammatory response, protects against apoptosis16–18, and reduces adverse cardiac remodeling by enhancing the survival, proliferation, and migration of cardiac cells19.
The studies described in this report are based on our hypothesis that Tβ4 can improve the potency of MSC transplantation for cardiac preservation and repair by increasing the retention and survival of the transplanted cells. We tested this hypothesis by determining whether Tβ4 protected cultured MSCs against hypoxic injury and by monitoring cardiac function, infarct size, perfusion, vascularity, and the survival of transplanted cells in rats that had been treated with MSCs alone or with both MSCs and Tβ4 after surgically induced myocardial infarction (MI).
Materials and Methods
Swine mesenchymal stem cells (sMSCs) and culture conditions
sMSCs were isolated as described previously 1, 20 and genetically engineered to express green fluorescent protein (GFP); then, the cell population was expanded for use in subsequent experiments by seeding the sMSCs (3000 cells/cm2) in a T-150 flask coated with 10 ng/mL fibronectin (Sigma-Aldrich, USA) and culturing the cells in growth medium consisting of 60% low-glucose DMEM (GIBCO-BRL, USA), 40% MCDB-201 (Sigma-Aldrich, USA), 1X insulin transferring selenium (Sigma-Aldrich, USA), 1X linoleic acid-bovine serum albumin (Sigma-Aldrich, USA), 0.05 µM dexamethasone (Sigma-Aldrich, USA), 0.1 mM ascorbic acid 2-phosphate (Sigma-Aldrich, USA), 2% FCS, 10 ng/mL PDGF (R&D Systems, USA), 10 ng/mL EGF (Invitrogen, USA), and 1X penicillin/streptomycin (Invitrogen, USA). Subcultures were performed every 3–4 days. For in-vitro experiments, normoxic culture conditions consisted of 5% CO2, 21% O2, and 74% N2, and hypoxic conditions consisted of 5% CO2, 1% O2, and 94% N2.
Tβ4-containing microspheres
Olive oil was heated to 45°C in a water bath, and 5 mL of 10% gelatin (type A, Sigma-Aldrich, USA) solution was heated to 50 °C; then, the gelatin was added to the olive oil, stirred, and cooled to 5 °C by adding ice to the water bath. Twenty-five minutes later, chilled (4 °C) acetone was added to the olive oil to induce microsphere formation, and the temperature was maintained at 5 °C for 1 hour. The microspheres were collected, washed 5 times to remove the olive oil, air-dried at 4 °C, and resuspended in 4 mL of chilled (4 °C) distilled H2O containing 0.25% glutaraldehyde (Sigma-Aldrich, USA) to induce cross-linking. The mixture was neutralized with glycine (Sigma-Aldrich, USA), and Tβ4 (Prospec, USA) was loaded into the microspheres by mixing 5 mg microspheres with 5 µL distilled H2O containing 25 µg Tβ4 and 0.1% bovine serum albumin for 30 minutes.
To determine the rate at which Tβ4 was released from the microspheres, 4 mL MEM and 5 mg microspheres containing 25 µg Tβ4 were added to each well of a 6-well plate; then, 1 mL of the conditioned medium was collected and replaced with fresh MEM each day for 15 days. The concentration of Tβ4 in the conditioned medium was determined with a Tβ4 ELISA kit (Bachem Americas, Inc., Torrance, CA, USA) as directed by the manufacturer’s instructions.
In-vitro assessments
Proliferation was determined with a CyQUANT® Cell Proliferation Assay Kit (Invitrogen, USA) as directed by the manufacturer’s instructions. Briefly, 1×104 sMSCs were cultured in a 24-well plate for 24 hours; then, fresh, Tβ4-containing medium was added, and the cells were incubated under the experimental conditions for an additional 48 hours, washed with PBS, and frozen at −80°C for at least 1 hour. RNA fluorescence was eliminated by adding CyQUANT® cell-lysis buffer (200 uL) containing DNase-free RNase (1.35 U/mL) to each well and culturing the cells at room temperature for 1 hour; then, 200 uL cell lysis buffer containing 2X solution of CyQUANT® GR dye was added to each well, and proliferation was evaluated 10 minutes later by measuring fluorescence intensity (wavelengths: 480-nm excitation and at 520-nm emission) with a Tecan fluorescence microplate reader (Tecan Infinite M200 microplate reader, LabX, Canada). All experiments were repeated three times and each sample was duplicated in each experiment.
For assessments of cytotoxicity and apoptosis, 4×104 sMSCs were cultured with basal medium in a 24-well plate for 24 hours, washed with MEM, and then cultured in Tβ4-containing medium or microsphere-conditioned medium under the experimental conditions for an additional 48 hours. Cytotoxicity was determined by measuring the intensity of lactate dehydrogenase (LDH) fluorescence in the supernatant via the CytoTox-One Homogenous membrane integrity assay (Promega, USA); the excitation and emission wavelengths were 560 nm and 590 nm, respectively. Apoptosis was evaluated with an In situ Cell Death Detection Kit (Roche Applied Science, Germany). Briefly, cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at 25 °C, washed with PBS for 30 minutes, incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 10 minutes at 4 °C, washed again, and then incubated with the reaction mixture for 1 hour at 37 °C in the dark; nuclei were counter-stained with DAPI, and apoptosis was evaluated by counting the number of TUNEL+ cells. All experiments were repeated three times and each sample was duplicated in each experiment.
Caspase activity was evaluated with a Caspase (-3 or -8) Fluorimetric Assay Kit (Sigma-Aldrich, USA). Briefly, cells were washed with PBS after removing culture medium. Cell would be lysed with lysis buffer on ice for 20 minutes. Then, the cell lysate would be collected and protein concentration was determined using Bradford reagent (Bio-Rad Laboratories, USA). 200 µg of total protein of each sample would be loaded into designated well of 96-well plate. 200 µL of 1X assay buffer (containing substrate) were added to each well. The plate was evaluated every minute for 60 minutes on a fluorescence plate reader (Synergy H1 hybrid reader, Biotek, USA) in kinetic mode at room temperature with excitation and emission wavelengths as specified in the manufacturer’s instructions. Caspase activity was determined by calculating the rate of change in fluorescence intensity per mL:
(FI)/min/ml= ΔFlt/(t × v),
where ΔFlt = difference in fluorescence intensity between time zero and time t min, t = reaction time in minute, and v = volume of sample in mL.
Western Blot analysis
Protein expression levels from treated and non-treated sMSCs were determined by western blot analysis as described21. Cell lysate was prepared using PhosphoSafe™ Extraction Reagent (Merck, Germany) and protein concentration was determined using Bradford reagent (Bio-Rad Laboratories, USA). Proteins were separated and electrophoretically blotted onto nitrocellulose membrane. After washing with 10 mM Tris-HCl wash buffer (pH 7.6) containing 0.05% Tween-20, the membrane was incubated in blocking buffer (5% non-fat dry milk, 10mM Tris pH 7.5, 100mM NaCl, 0.1% Tween-20) for 3 hours at room temperature. After that, the blots were incubated with diluted primary antibodies: Bcl-XL (1:500), and GAPDH (1:1,000) (all purchased from Santa Cruz Biotechnology, USA) at 4°C overnight. After that, anti-rabbit IgG conjugated with HRP (dilution: 1: 1, 000 and 1: 8,000) was used to detect the binding of antibodies. The binding of the specific antibody was visualized using the SuperSignal Chemiluminescent Substrate kit (Pierce, USA) and exposed to X-ray film (Pierce, USA). The concentration of each protein sample was normalized by GAPDH and expressed as percentage of GAPDH.
MI model and treatment
The experimental protocol was approved by the University of Minnesota Research Animal Resources Committee. All experimental and animal maintenance procedures were performed in accordance with the Animal Use Guidelines of the University of Minnesota and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No 85-23).
Myocardial infarction was surgically induced in female athymic nude rats (~160 g; Hsd: RH-Foxn1rnu/Foxn1+; Harlan Lab., USA) as described previously3. The induction of an inflammatory response was minimized by performing the experiments in immunocompromised athymic nude rats. The animals were anesthetized with Ketamine (100 mg/kg) and Xylazine (2.5 mg/kg), intubated, and mechanically ventilated with a rodent ventilator. A left-side thoracotomy was performed to expose the heart, the epicardium was removed, and the left arterial descending coronary artery was permanently ligated with a 6-0 prolene suture. Ten minutes later, animals were randomized into four experimental groups: sMSC/Tβ4 (n=11), sMSC (n=9), Patch (n=6), and MI (n=6). A fibrin patch containing 1×106 sMSCs and 2 mg gelatin microsphere containing 10 µg Tβ4 was positioned over the ligation site of animals in the sMSC/Tβ4 group, animals in the sMSC group received a patch containing sMSCs alone, and animals in the Patch group received a patch containing neither SMCs nor microspheres; animals in the MI group received 0.2 mL DMEM intramyocardial injection. After surgery, the animal was treated with Baytril for 5 days to prevent infection and with ketoprofen for pain control.
Fibrin patch administration
Immediately before transplantation, 1 million sMSCs were harvested freshly from cell culture and resuspended with Tβ4 (sMSC/Tβ4) or without Tβ4 (sMSC). 2 mg of gelatin microspheres containing 10 µg Tβ4 and cells were mixed in 0.1 mL bovine fibrinogen solution (25 mg/mL; F8630, Sigma-Aldrich Corp, USA); then, the fibrinogen solution was co-injected with 0.1 mL human thrombin solution (80 NIH units/mL; T7009, supplemented with 2 µL 400 mM CaCl2 and 200 mM ε-aminocaproic acid; Sigma Aldrich, USA) into a plastic ring that had been placed on the epicardium of the infarcted region to serve as a mold for the patch. The mixture usually solidified within 30 seconds to form a circular patch of 0.4-cm diameter.
Cardiac functional assessments
Cardiac functional parameters were performed via echocardiography as described previously3. Briefly, animals were anesthetized and placed in a supine position; then, left-ventricular end-systolic and end-diastolic internal diameters (LVIDes and LVIDed) were determined from M-mode images with a well-defined continuous interface between the septum and posterior wall. Images were obtained at a higher frame rate, and numeric acquisition was performed at the hard disc of the echocardiographic machine. LV ejection fraction (LVEF) and fractional shortening (LVFS) were calculated according to the following formulas: LVEF=1-(LVIDes/LVIDed)2; LVFS=1-(LVIDes/LVIDed).
Left ventricular free wall perfusion
Myocardial perfusion was measured via the injection of fluorescently labeled microspheres as described previously3. Briefly, saline (1 mL) containing 5×104 yellow-green fluorescent polystyrene microspheres (Life Sciences, USA) was injected into the left ventricle while a reference blood sample (1 mL) was withdrawn from the femoral artery. After sacrifice, heart samples and the reference blood sample were lysed and analyzed for fluorescence intensity. Perfusion was calculated according to the following formula:
Blood flow (mL/gram per min) = tissue fluorescence intensity/reference blood fluorescence intensity/weight of tissue × 1 mL/min.
Histochemical and immunohistochemical assessments
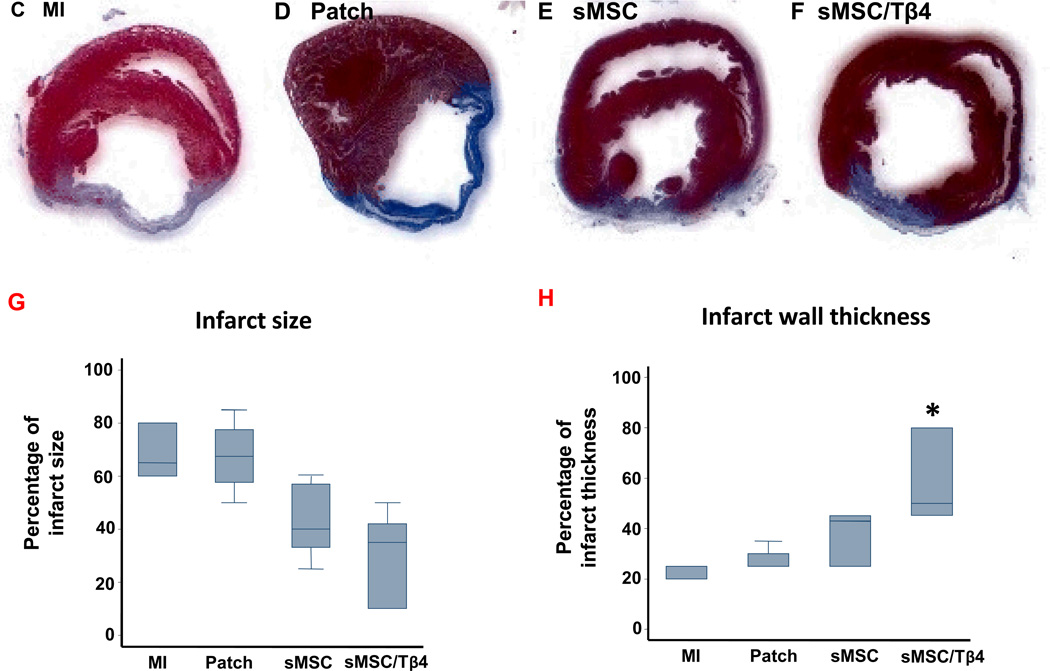
After sacrifice, hearts were explanted and cut into 7-µm cryosections or 5-µm paraffin-embedded sections. Fibrous tissue was identified by staining sections with an Accustain Trichrome Stains (Masson) kit (Sigma-Aldrich, USA); then, the infarct size and thickness of the fibrous region, the circumflexion lengths of the LV free wall, and the thickness of the septal wall were measured for sMSC/Tβ4 (n=7), sMSC (n=5), Patch (n=5), and MI (n=3) animals per experimental group. Infarct size was calculated as the ratio of the fibrous and left ventricular free wall circumflexion lengths, and infarct thickness was calculated as the ratio of the thicknesses of the fibrous region and the septal wall.
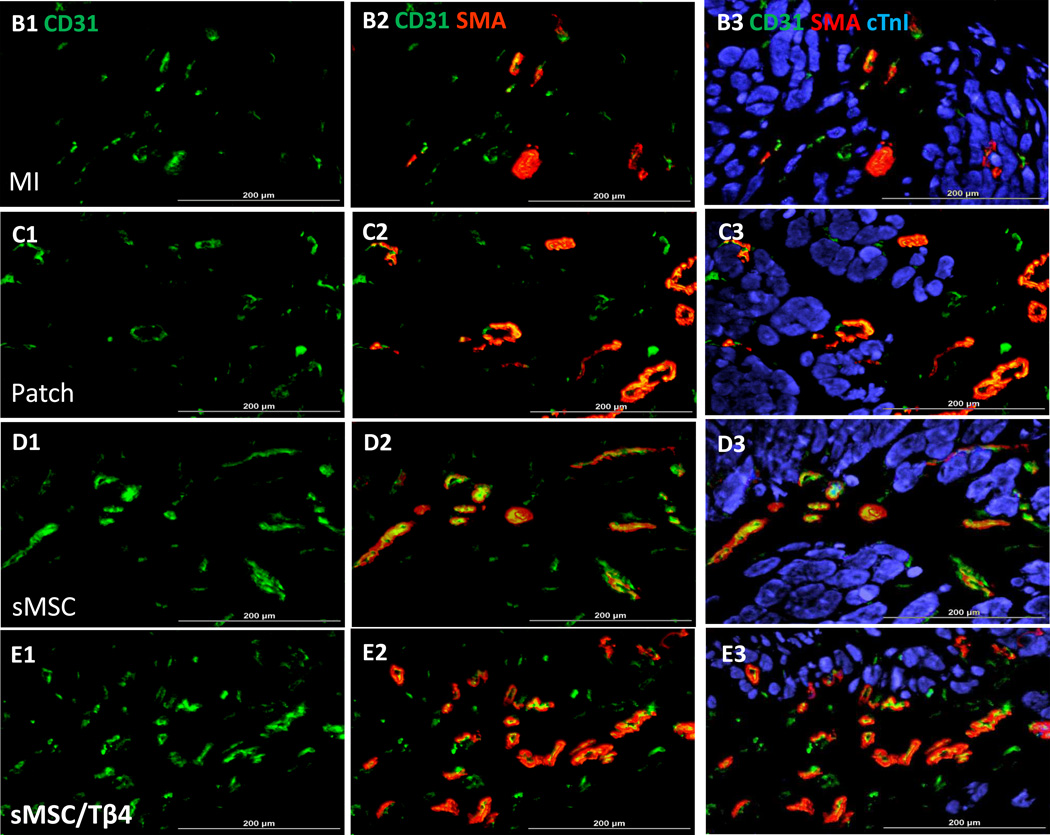
Vasculogenesis was evaluated by staining sections with rabbit anti-CD31 primary anti-bodies (1:100; Santa Cruz, USA), FITC-conjugated donkey anti-rabbit IgG secondary antibodies (JacksonImmuno Research, West Grove, PA, USA) and TRITC-conjugated anti-smooth-muscle actin antibodies (SMA, 1:500; Sigma-Aldrich, USA). Vascular structures positive for CD31 expression (i.e., FITC fluorescence), and for both CD31 and SMA expression (i.e., simultaneous FITC and TRITC fluorescence) were counted for 3–4 animals per group, in 5–6 slides per animal and 8–10 fields per slide.
The engraftment/survival of transplanted cells was evaluated by staining sections with fluorescent goat anti-GFP antibodies (Abcam, USA), the recruitment of endogenous c-Kit+ CPCs was evaluated by staining sections with goat anti-c-Kit antibody (R&D systems, USA), cardiac cells were identified by staining sections with rabbit antibodies against cardiac troponin I (cTnI, Abcam, USA), and hematopoietic cells were identified by staining sections with mouse anti-rat CD45 antibody (BD Pharmingen, USA). c-Kit+ cells were counted for 4–5 animals per experimental group, in 5–6 slides per animal and 4–5 fields per slide. To exclude the hematopoietic c-Kit+ cells, dual immunostaining for c-Kit+ and CD45+ expressions were performed in 6 slides from 3 animals.
Quantitative PCR (QPCR) analysis
The engraftment/survival rate of the transplanted sMSCs was also evaluated by measuring the DNA copy of swine coagulation factor IX (length=120bp) which is located on chromosome X via QPCR. Whole LV anterior wall will be collected and digested with 1 mL solution containing proteinase K for overnight. 100 µL of digested solution would be used to isolate total DNA to measure sMSCs number. Total DNA from 5×105 sMSCs would be used as standard after a serial dilution (4x). A standard curve would be plotted as cycle number of swine coagulation factor IX DNA against log of cell numbers. The cell number will be calculated based on the cycle number of experimental rat DNA after QPCR. Assessments were performed in 3 animals in sMSC and sMSC/Tβ4 groups with an SYBR Green kit (Fermentas, USA) and Eppendorf Realplex real-time PCR system (Eppendorf, USA); primer sequences were ATGGAGGCAGAGCTCCAAGAAACT (sense) and TGAAGAGGGCCTTTGAAGACACGA (anti-sense).
Statistical analysis
For normally distributed data, values are presented as mean ± standard error of the mean (SEM). Overall differences between groups were tested for significance via one-way analysis of variance (ANOVA). When analysis of variance demonstrated a significant effect, post hoc analysis was performed using the Tukey Honestly Significant Difference (HSD) test. For non-normally distributed data, values are presented as median (25th, 75th percentile). Overall difference in distributions among multiple groups was determined using the Kruskal-Wallis test, while the post-hoc comparison was performed using Dunn’s test. The boxplot shows the median (line), and the 25th and 75th percentiles (box). The whiskers display the upper and lower values within 1.5 times the interquartile range beyond the 25th and 75th percentile. Analyses were performed with SPSS software (version 20) and a two-sided p-value of less than 0.05 was considered statistically significant. Box plots were created using Stata Version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Tβ4 (1 µg/mL) increases sMSC proliferation and protects sMSCs from hypoxic damage
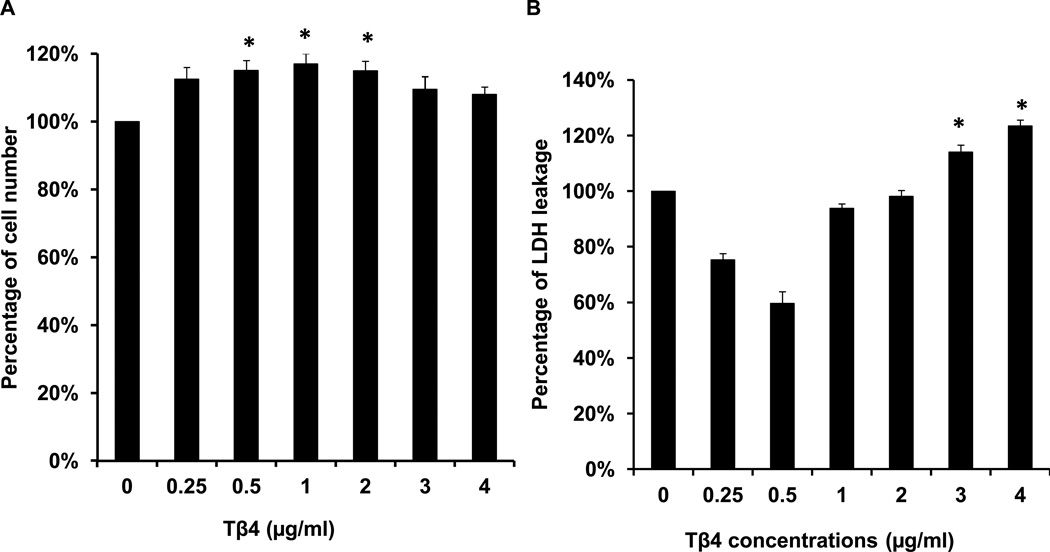
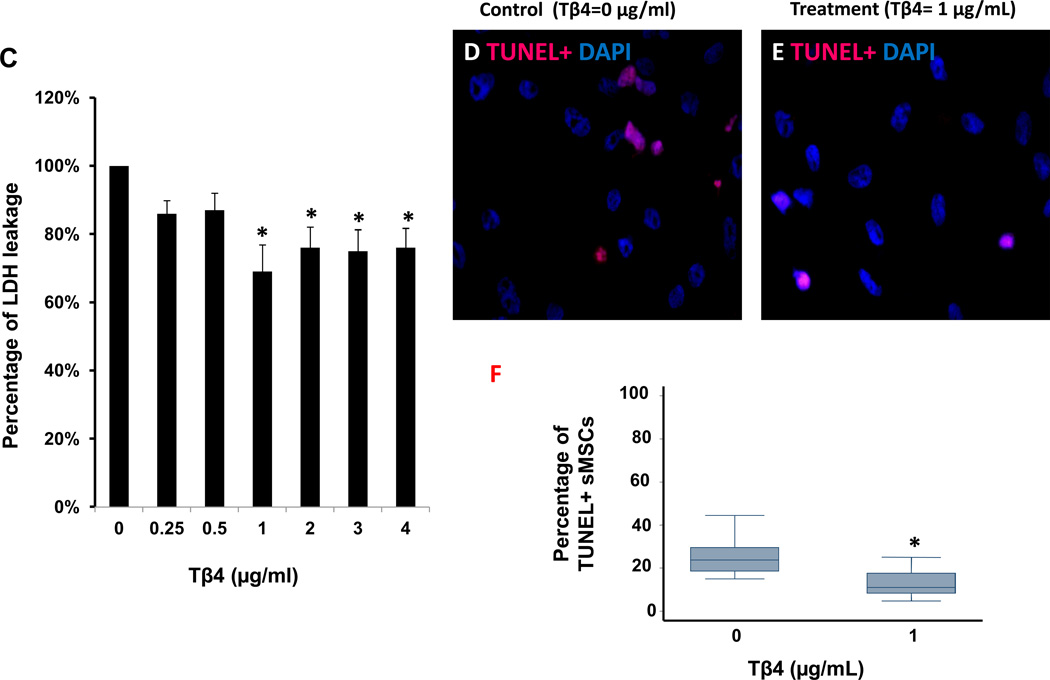
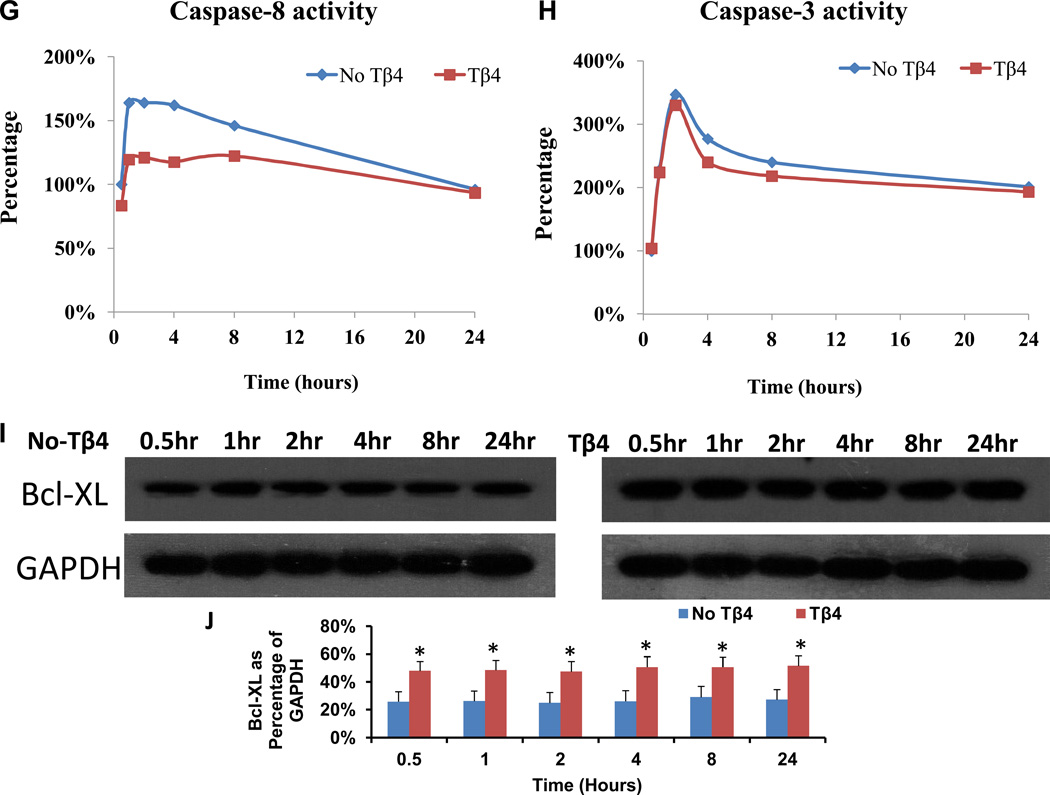
Proliferation was significantly greater in sMSCs that had been cultured with 0.5, 1 or 2 µg/mL Tβ4 than in sMSCs cultured in the absence of Tβ4 (Figure 1A), but was unaffected by higher Tβ4 concentrations, and concentrations of 3 µg/mL or greater were associated with significant increases in the amount of lactate dehydrogenase (LDH) in the culture medium (Figure 1B). Under hypoxic conditions, both LDH levels (Figure 1C) and the proportion of apoptotic (i.e., TUNEL+) sMSCs (Figure 1D–F) were significantly lower in cells cultured with 1 µg/mL Tβ4 than in cells cultured without Tβ4. Tβ4 also significantly reduced activity of the pro-apoptotic factor caspase-8 (Figure 1G), but not caspase-3 (Figure 1H), during the first 8 hours of hypoxic culture, and the expression of Bcl-XL, which promotes cell survival under hypoxia, was significantly elevated in Tβ4-cultured cells throughout the 24-hour experiment (Figure 1I&J). Collectively, these observations suggest that 1 µg/mL of Tβ4 promotes sMSC proliferation and protects sMSC against hypoxia-induced cellular damage and apoptosis; however, higher concentrations of Tβ4 may be cytotoxic.
Figure 1.
Tβ4 (1 µg/mL) promotes proliferation and protects against hypoxic injury in cultured sMSCs. sMSCs were cultured under (A, B) normoxic or (C–F) hypoxic conditions for 48 hours with the indicated concentrations of Tβ4. (A) Cell proliferation and (B–C) the concentration of LDH in the culture medium were measured and presented as a percentage of the measurements obtained in the absence of Tβ4. (D–F) Cells cultured with (D) 0 µg/mL or (E) 1 µg/mL Tβ4 were TUNEL-stained and counter-stained with DAPI (400X magnification), and then (F) apoptosis was quantified as the proportion of TUNEL+ cells. (G–I) sMSCs were cultured with 0 µg/mL or 1 µg/mL Tβ4 under hypoxic conditions, and then (G) caspase-8 activity, (H) caspase-3 activity, and (I–J) Bcl-XL protein levels were determined at the indicated time points. Caspase activitites were presented as a percentage of measurements obtained at 0 hr; (J) Bcl-XL levels were presented as a percentage of GAPDH protein levels. (Panels A–C, J: *p<0.05 vs. 0 µg/mL Tβ4; Panel F: *p<0.005 vs. 0 µg/mL Tβ4).
sMSC/Tβ4 administration improves cardiac functional recovery after MI
Because Tβ4 appeared to increase the proliferation and viability of cultured sMSCs, we investigated whether the potency of transplanted sMSCs for cardiac repair could be enhanced by co-treatment with Tβ4. Myocardial infarction was surgically induced in rats by permanently ligating the left-anterior descending branch of the coronary artery; then, animals in the sMSC group were treated with a fibrin patch containing 1×106 sMSCs, and animals in the sMSC/Tβ4 group were treated with both the sMSC-containing patch and Tβ4; control assessments were performed in animals treated with basal medium (i.e., the MI group) or a cell-free patch (the Patch group). The duration of Tβ4 delivery was extended by loading the Tβ4 into gelatin microspheres (15–50 µm) (Supplemental Figures 1A–B), which were subsequently added (with the sMSCs) to the patch. In culture, Tβ4 was continuously released from the microspheres for at least 15 days (Supplemental Figure 1C) at a rate of 1.44±1.02 µg/day, and when sMSCs were cultured under hypoxic conditions, the cytoprotective effects of media conditioned with the Tβ4-containing microspheres and of media prepared with fresh Tβ4 were similar (Supplemental Figure 1D).
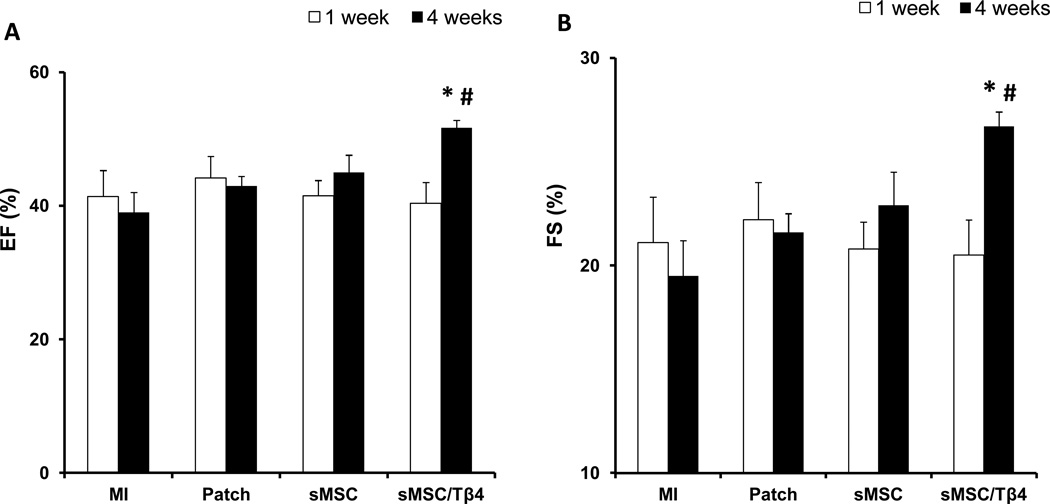
One week after myocardial injury and treatment, echocardiographic assessments of left-ventricular ejection fraction (LVEF) (Figure 2A) and fractional shortening (LVFS) (Figure 2B) did not differ significantly among treatment groups. At Week 4, measurements in the sMSC (LVEF: 45±2.6%, LVFS: 22.9±1.6%), MI (LVEF: 39±3%, LVFS: 19.5±1.7%), and Patch (LVEF: 43±1.4%, LVFS: 21.6±0.9%) groups remained similar, but both parameters were significantly greater in sMSC/Tβ4 animals (LVEF: 51.7±1.1%, LVFS: 26.7±0.7%) than in animals from the MI (p<0.005) or Patch (p<0.05) groups; measurements were also greater in the sMSC/Tβ4 group than in sMSC animals, but the differences did not reach statistical significance. sMSC/Tβ4 treatment was also associated with structural improvements at week-4 after injury. Though infarcts occupied a smaller proportion of the ventricular wall (35%, (10%, 42%)) in sMSC/Tβ4 animals than in animals from the MI (65% (60%,80%), Patch (67.5% (57.5%, 77.5%), and sMSC (40% (33%, 57%) groups, no significant difference was reached between any groups. The infarcted region of the wall was significantly thicker (50%, (45%, 80%)) in sMSC/Tβ4 animals than in animals from the MI (25% (20%, 25%), p<0.05) group, but was not significantly thicker as compared with Patch (25% (25%, 30%)) and sMSC (43% (25%, 45%)).
Figure 2.
Treatment with sMSCs and Tβ4 improves heart function and structure after MI. Rats were treated with culture medium (MI), a fibrin patch (Patch), a patch containing sMSCs (sMSC), or a patch containing sMSCs and microsphere-encapsulated Tβ4 (sMSC/Tβ4) after surgically induced MI. (A–B) Echocargiographic assessments of (A) left-ventricular ejection fractions and (B) fractional shortening were performed 1 and 4 weeks after injury and treatment (n=6–8 each group). At week 4, (C–H) sections of hearts from animals in the (C) MI (n=3), (D) Patch (n=5), (E) sMSC (n=5), and (F) sMSC/Tβ4 (n=7) groups were Masson’s trichrome–stained for histological assessments of (G) infarct size and (H) infarct wall thickness; infarct size was presented as a percentage of the left ventricular free wall circumflexion length, and infarct wall thickness was presented as a percentage of the thickness of septal wall. (Panels A-B: *p<0.005 vs. MI, # p<0.05 vs. Patch; Panels H: * p<0.05 vs. MI).
Tβ4 enhances the sMSC-induced vasculogenic response to MI
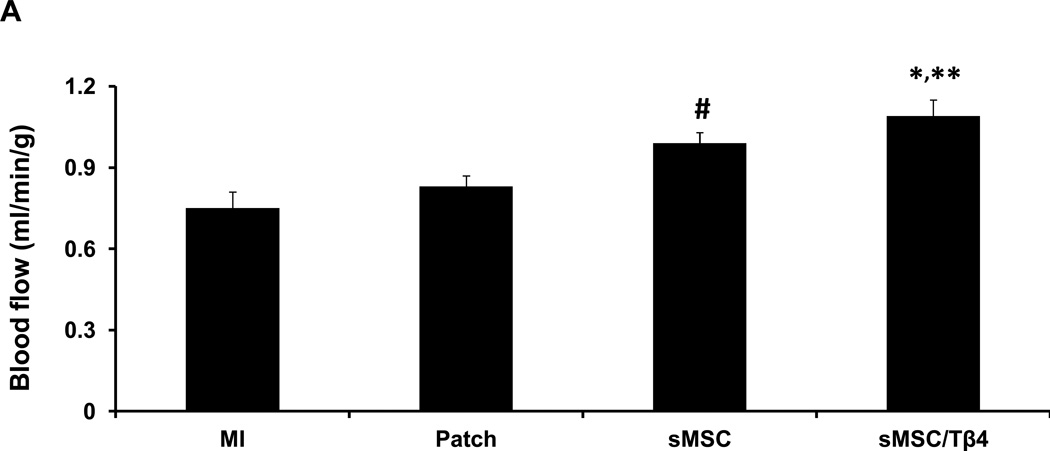
To confirm that the improvements in cardiac function and infarct size observed in sMSC/Tβ4 animals were accompanied by increases in blood flow, perfusion of the left ventricular anterior wall was evaluated by injecting fluorescent microspheres into the left ventricle of animals before sacrifice at week 4. Blood flow was significantly greater in the scar and at the border of the infarct in hearts from sMSC/Tβ4 animals (1.18±0.03 mL/min per g) than in the corresponding regions of hearts from the MI (0.75±0.11 mL/min per g, p<0.005) or Patch (0.83±0.08 mL/min per g, p<0.05) groups (Figure 3A). Measurements were also significantly greater in sMSC animals than in MI animals (0.91±0.08 mL/min per g, p<0.05), but not Patch group animals.
Figure 3.
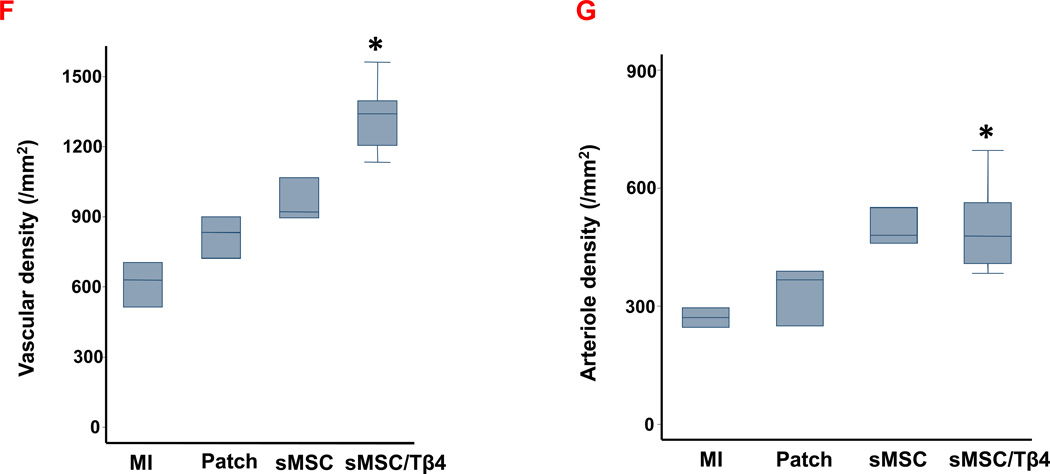
Tβ4 enhances sMSC-induced vessel growth after MI. Rats were treated with culture medium (MI), a fibrin patch (Patch), a patch containing sMSCs (sMSC) or a patch containing sMSCs and microsphere-encapsulated Tβ4 (sMSC/Tβ4) after surgically induced MI. Four weeks after injury and treatment, (A) left-ventricular perfusion was evaluated by injecting fluorescent microspheres into the hearts of animals immediately before sacrifice, and sections of hearts from (B1–B3) MI, (C1–C3) Patch, (D1–D3) sMSC, and (E1–E3) sMSC/Tβ4 animals were stained for the expression of (B1, C1, D1, E1) CD31, (B2, C2, D2, E2) SMA, and (B3, C3, D3, E3) cTnI. (Bar=200 µm). (F) Vascular density was quantified as the number of CD31+ vessels per mm2, and (G) arteriole density was quantified as the number of SMA+ vessels per mm2. (Panel A: *p<0.05 vs. Patch, ** p<0.005 vs. MI, # p<0.05 vs. MI; Panel F &G: * p<0.05 vs MI). (n=3 or 4 each group).
We corroborated these findings by measuring vascular density and arteriole density in tissue sections that had been harvested from the border zone of the infarct and stained for expression of the endothelial-cell marker CD31 and for the presence of alpha smooth-muscle actin (SMA) (Figure 3B–E). CD31+ vessel density was significantly greater in sections from the hearts of sMSC/Tβ4 animals (1340/mm2 (1204, 1395)) than in sections from the hearts of MI (629/mm2 (513, 705) p<0.05), but similar to Patch (833/mm2 (720, 900)) and sMSC (920/mm2 (893, 1067)) groups (Figure 3F). Arteriole density (i.e., the number of SMA+ vessels) was similar in sMSC/Tβ4 (478/mm2 (407, 563)), sMSC (480/mm2 (460, 550), Patch (367/mm2 (250, 389)), and MI (271/mm2 (247, 295) sections (Figure 3G). Thus, Tβ4 appears to enhance perfusion and the vasculogenic response to sMSC transplantation by increasing the growth of non-resistant vessels, but not arterioles.
Tβ4 increases the engraftment/survival of transplanted sMSCs
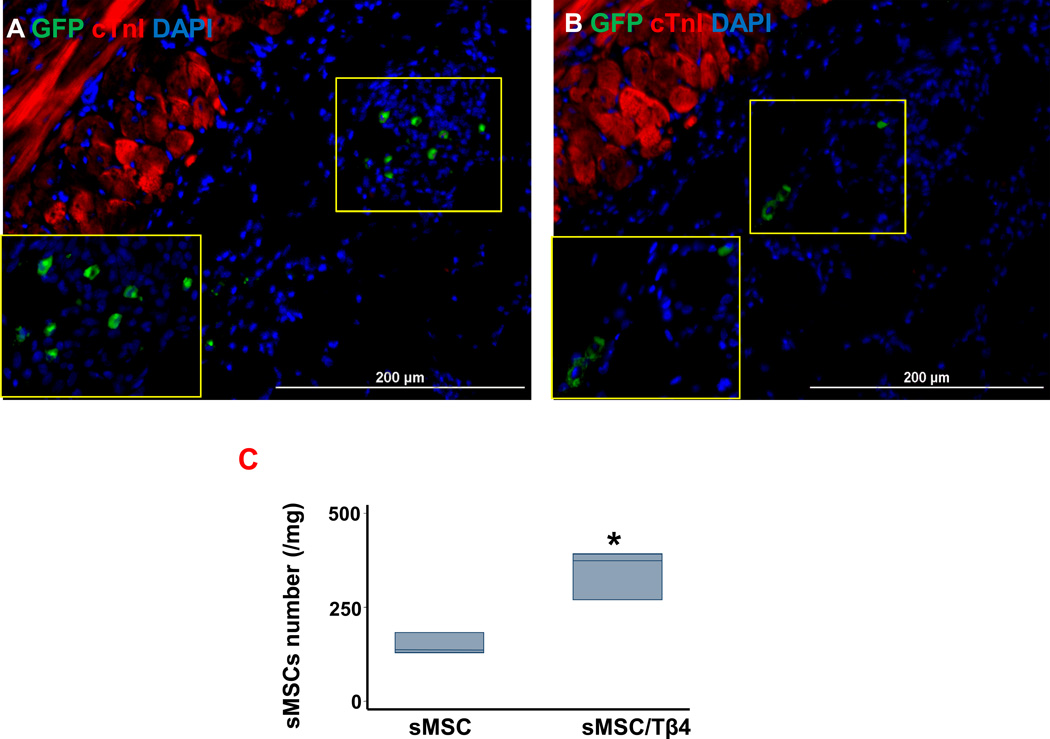
Because the transplanted cells had been isolated from swine and engineered to express GFP, we determined whether co-treatment with Tβ4 improved sMSC engraftment and survival by quantifying the expression of swine coagulation factor IX. sMSCs were significantly more common in the hearts of animals from the sMSC/Tβ4 group (374 cells/mg (269, 392)) than in the hearts of sMSC-treated animals (136 cells/mg (129, 183), p=0.05) (Figure 4A–C). Thus, Tβ4 appears to improve the survival of sMSCs both under hypoxic culture conditions (Figure 1C–F) and in infarcted myocardial tissue.
Figure 4.
Tβ4 increases the engraftment and/or survival of transplanted sMSCs. Rats were treated with a patch containing sMSCs (sMSC), or a patch containing sMSCs and microsphere-encapsulated Tβ4 (sMSC/Tβ4) after surgically induced MI. Four weeks after injury and treatment, sections of hearts from (A) sMSC and (B) sMSC/Tβ4 animals were stained for expression of GFP and cTnI, nuclei were counterstained with DAPI, and sMSC survival was evaluated by identifying GFP+ cells. (C) sMSC survival at week 4 was quantified by measuring the mRNA levels of swine coagulation factor 9 in the hearts of sMSC and sMSC/Tβ4 animals via qRT-PCR. (* p=0.05 vs. sMSC; n=3 per group). (Bar=200 µm).
Tβ4 increases the recruitment of endogenous CPCs after MI
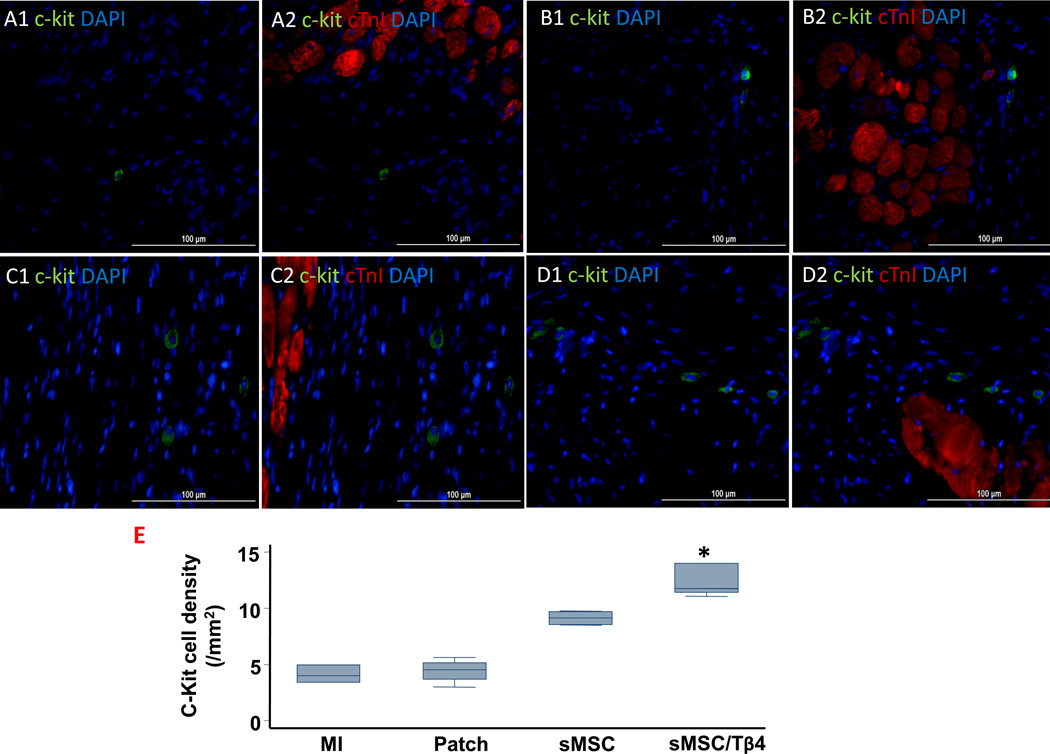
To determine whether the benefit associated with sMSC/Tβ4 treatment could have evolved, at least in part, from the enhanced activity of endogenous CPCs, expression of the progenitor-cell marker c-Kit was evaluated in the hearts of animals from all four experimental groups. c-Kit+ cell density in the infarct/peri-infarct region was significantly greater in sMSC/Tβ4 animals (11.8 cells/mm2 (11.4, 14)), than in animals from the MI (4 cells/mm2 (3.4, 5)), p<0.05) or Patch (4.5 cells/mm2 (3.7, 5.2), p<0.05) groups, and similar to sMSC animals (9 cells/mm2 (8.5, 9.7)) (Figure 5A–E).
Figure 5.
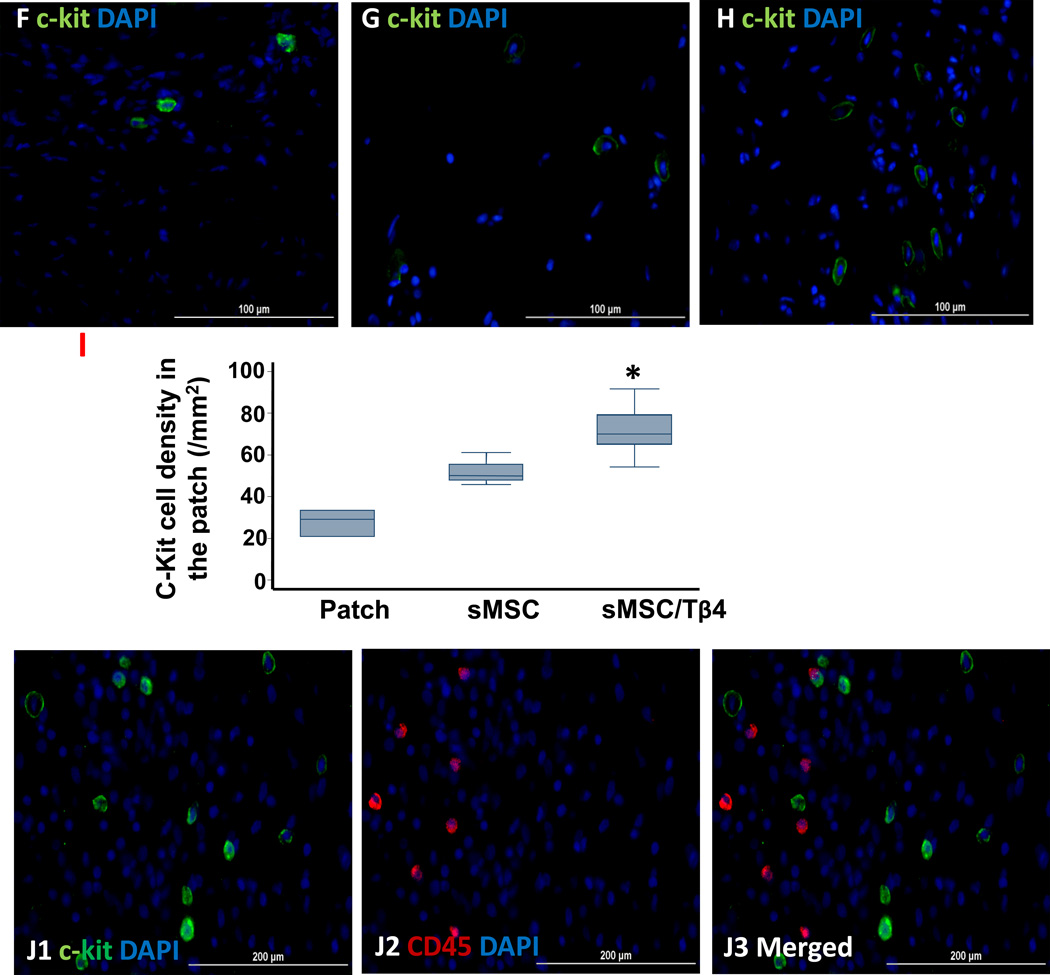
Tβ4 increases the sMSC-induced recruitment of endogenous CPCs. Rats were treated with culture medium (MI), a fibrin patch (Patch), a patch containing sMSCs (sMSC), or a patch containing sMSCs and microsphere-encapsulated Tβ4 (sMSC/Tβ4) after surgically induced MI, and then sacrificed four weeks later. (A–E) Sections from the infarct/peri-infarct region of hearts from (A1–A2) MI, (B1–B2) Patch, (C1–C2) sMSC, and (D1–D2) sMSC/Tβ4 animals were stained for the expression of c-Kit (A1, B1, C1, D1) or c-Kit and cTnI (A2, B2, C2, D2), nuclei were counterstained with DAPI. (Bar=100 µm). (E) The recruitment of endogenous CPCs was quantified as the number of c-Kit+ cells per mm2. (F–H) Sections from the patch region of hearts from (F) Patch, (G) sMSC, and (H) sMSC/Tβ4 animals were stained for the expression of c-Kit, nuclei were counterstained with DAPI (bar=100 µm), and (I) the recruitment of endogenous CPCs was quantified as the number of c-Kit+ cells per mm2. (J) Sections from the patch region of hearts from sMSC/Tβ4 animals were stained for the expression of (J1) c-Kit and (J2) CD45, nuclei were counterstained with DAPI, and (J3) the expression of c-Kit by inflammatory cells was evaluated by identifying c-Kit+/CD45+ cells (bar=200 µm). (Panel E: * p<0.05 vs. MI and Patch; Panel I: *p<0.05 vs. Patch). (Bar=200 µm). (n=4–5 each group).
Similar results were observed in the region of the patch (Patch: 29.2 cells/mm2 (20.8, 33.3); sMSC: 50 cells/mm2 (47.9, 55.6); sMSC/Tβ4: 70 cells/mm2 (65, 79.2), p<0.05 versus Patch) (Figure 5F–I), and only 2.4% of c-Kit+ cells also expressed CD45 (Figure 5J), which confirms that hematopoietic cells were not a significant source of c-Kit expression.
Discussion
MSCs are multipotent and secrete a wide variety of growth factors that can protect the myocardium from ischemic injury and induce new vessel growth1, 2; thus, the regenerative potency of these cells has been investigated in numerous preclinical and clinical studies. The results of these investigations suggest that MSC transplantation is safe and may improve contractile function in the hearts of patients with myocardial injury 22, 23; however, less than 1% of the transplanted cells survive5, 6, and this high rate of attrition is believed to be one of the primary barriers to the effectiveness of cell therapy. Here, we investigated whether the survival and potency of transplanted MSCs could be increased by co-treatment with Tβ4.
Tβ4 has been shown to enhance the survival of cultured cardiomyoctes19, and both intramyocardial (IM) and intraperitoneal (IP) injections of Tβ4 improved cardiac functional recovery after MI in mice19, however, measurements in animals that received IM injections, IP injections, or both IM and IP injections did not differ significantly, perhaps because the IM injections were administered just once, immediately after MI, whereas the IP injections were administered every three days until sacrifice. Collectively, these observations suggest that the benefit of local Tβ4 administration could be enhanced by increasing the duration of Tβ4 delivery.
Chiu, et al.,15 extended Tβ4 delivery over 28 days by incorporating it into a collagen-chitosan hydrogel; however, the maximum amount Tβ4 that could be loaded into the gel was 1.5 µg, and the release rate was less than 0.1 µg/day. Although this rate may be sufficient to induce angiogenesis14, our findings suggest that concentrations of 0.5–1 µg/mL Tβ4 are required to protect cultured sMSCs from hypoxic injury and, consequently, that a substantially greater Tβ4 loading dose and release rate would be needed to maximize the benefit of sMSC/Tβ4 therapy. The microsphere-based method used here accommodated a loading dose of 25 µg and released Tβ4 for 15 days at a mean rate of 1.4 µg/day in vitro. Furthermore, both the microspheres and sMSCs were seeded in a fibrin patch positioned over the infarct site, thereby maximizing the exposure of the transplanted sMSCs and resident cardiac cells to the cytoprotective effects of Tβ4. The duration of Tβ4 delivery may also be extended by the addition of transglutaminase, which would crosslink Tβ4 to the fibrin matrix12, but whether this approach is compatible with our in-situ method for patch creation has yet to be determined.
sMSCs are known to release paracrine factors that protect the myocardium from ischemic injury 24 and to promote both vascular growth 25 and the migration/proliferation of progenitor cells 4. Furthermore, measurements of cardiac function, infarct size, wall thickness, and perfusion were better in sMSC/Tβ4 animals than in animals treated with sMSCs alone, but the between-group differences did not reach statistical significance. Thus, much of the benefit associated with sMSC/Tβ4 administration can likely be attributed to the transplanted sMSCs and to the Tβ4-induced enhancement of sMSC survival. Whether Tβ4-containing microspheres can also improve the survival of sMSCs that have been administered via direct intramyocardial injection, and the relative effectiveness of injected and patch-administered sMSC/Tβ4 treatments, should be investigated in future studies.
Our results also indicate that CD31+ vessel density and the number of c-Kit+ CPCs in both the infarct/peri-infarct region and the patch were significantly greater in the sMSC/Tβ4 group than in sMSC animals; however, SMA+ vessel density in the two groups was similar, which is consistent with previous reports demonstrating that Tβ4 alone can induce angiogenesis; endothelial-cell adhesion, migration, and tubule formation14, and the migration of epicardial progenitor cells26, but does not influence the migration or proliferation of smooth muscle cells27. Collectively, these observations suggest that Tβ4 and sMSCs can function both independently and cooperatively to enhance the growth of non-resistant vessels and c-Kit+ CPC migration.
Our in-vitro studies showed that Tβ4 significantly reduced apoptosis in hypoxia-cultured sMSCs, and the reduction was accompanied by increases in the expression of Bcl-XL, an anti-apoptotic member of the Bcl-2 protein family28, and by declines in caspace-8 activation. These observations are likely linked, because Bcl-XL is believed to disrupt formation of the death-inducing signal complex (DISC), which is required for caspace-8 activation29. Bcl-XL expression may also impede the apoptotic response to declines in the mitochondrial membrane potential by maintaining mitochondrial integrity and limiting cytochrome c release30.
MSCs are hypo-immunogenic, often lacking the expression of major histocompatibility complex-II and costimulatory molecules31; thus, allogeneic MSCs have been investigated as a potential “off-the-shelf” therapy for tissue repair and appeared to be safe and provided provisional evidence of efficacy in patients with MI22. Nevertheless, low engraftment rates are among the major problems encountered in all preclinical and clinical studies of cellular therapy for myocardial repair5–8, 32–34, even when autologous cells are used,34 which suggests that the survival of the transplanted cells is more likely to be limited by the ischemic environment of the infarct than by the induction of an immune response. The present study was performed in immunocompromised rats to reduce the likelihood of immune rejection and, consequently, the improvements in cardiac structure and function observed in sMSC/Tβ4-treated animals (Figures 2–5) likely evolved from the proliferative/cytoprotective effects of Tβ4 under hypoxic conditions. Whether these effects could also improve the survival of allogeneic MSCs has yet to be determined.
In conclusion, our findings demonstrate that the engraftment and survival of transplanted sMSCs in infarcted myocardium can be increased by continuous, localized co-treatment with Tβ4. Tβ4 administration also enhanced c-Kit+ CPC recruitment and the vasculogenic response to sMSC transplantation, and treatment with both sMSCs and Tβ4 significantly improved infarct size and cardiac functional recovery after MI.
Supplementary Material
Acknowledgements
The authors would like to thank W. Kevin Meisner, Ph.D., E.L.S., for his editorial assistance in preparation of this manuscript.
Funding Sources: This work was supported by U.S. Public Health Service Grants NIH RO1-114120 and UO1-HL100407.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Nakamura Y, Wang X, Xu C, Asakura A, Yoshiyama M, From AH, Zhang J. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:612–620. doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 2.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye L, Haider H, Tan R, Toh W, Law PK, Tan W, Su L, Zhang W, Ge R, Zhang Y, Lim Y, Sim EK. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation. 2007;116:I113–I120. doi: 10.1161/CIRCULATIONAHA.106.680124. [DOI] [PubMed] [Google Scholar]
- 4.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. The Journal of cell biology. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. Journal of the American College of Cardiology. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 9.Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of cd4+ and cd8+ cells or nk1.1+ cells. Cell Trans. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Cheng X, Yao Q, Li J, Ju G. The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating l1 expression. Neurochem Res. 2008;33:2269–2280. doi: 10.1007/s11064-008-9712-y. [DOI] [PubMed] [Google Scholar]
- 11.Philp D, Nguyen M, Scheremeta B, St-Surin S, Villa AM, Orgel A, Kleinman HK, Elkin M. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18:385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 12.Huff T, Otto AM, Muller CS, Meier M, Hannappel E. Thymosin beta4 is released from human blood platelets and attached by factor xiiia (transglutaminase) to fibrin and collagen. FASEB J. 2002;16:691–696. doi: 10.1096/fj.01-0713com. [DOI] [PubMed] [Google Scholar]
- 13.Huff T, Muller CS, Hannappel E. Thymosin beta4 is not always the main beta-thymosin in mammalian platelets. Annals N Y Acad Sci. 2007;1112:451–457. doi: 10.1196/annals.1415.029. [DOI] [PubMed] [Google Scholar]
- 14.Philp D, Huff T, Gho YS, Hannappel E, Kleinman HK. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB J. 2003;17:2103–2105. doi: 10.1096/fj.03-0121fje. [DOI] [PubMed] [Google Scholar]
- 15.Chiu LL, Radisic M. Controlled release of thymosin beta4 using collagen-chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. J Control Release. 2011;155:376–385. doi: 10.1016/j.jconrel.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Philp D, Scheremeta B, Sibliss K, Zhou M, Fine EL, Nguyen M, Wahl L, Hoffman MP, Kleinman HK. Thymosin beta4 promotes matrix metalloproteinase expression during wound repair. J Cell Physiol. 2006;208:195–200. doi: 10.1002/jcp.20650. [DOI] [PubMed] [Google Scholar]
- 17.Sosne G, Christopherson PL, Barrett RP, Fridman R. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. 2005;46:2388–2395. doi: 10.1167/iovs.04-1368. [DOI] [PubMed] [Google Scholar]
- 18.Qiu P, Wheater MK, Qiu Y, Sosne G. Thymosin beta4 inhibits tnf-alpha-induced nf-kappab activation, il-8 expression, and the sensitizing effects by its partners pinch-1 and ilk. FASEB J. 2011;25:1815–1826. doi: 10.1096/fj.10-167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Hu Q, Wang Z, Xu C, Wang X, Gong G, Mansoor A, Lee J, Hou M, Zeng L, Zhang JR, Jerosch-Herold M, Guo T, Bache RJ, Zhang J. Autologous stem cell transplantation for myocardial repair. Am J Physiol Heart Circ Physiol. 2004;287:H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 21.Ye L, Lee KO, Su LP, Toh WC, Haider HK, Law PK, Zhang W, Chan SP, Sim EK. Skeletal myoblast transplantation for attenuation of hyperglycaemia, hyperinsulinaemia and glucose intolerance in a mouse model of type 2 diabetes mellitus. Diabetologia. 2009;52:1925–1934. doi: 10.1007/s00125-009-1421-9. [DOI] [PubMed] [Google Scholar]
- 22.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 24.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation research. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 26.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 27.Rossdeutsch A, Smart N, Dube KN, Turner M, Riley PR. Essential role for thymosin beta4 in regulating vascular smooth muscle cell development and vessel wall stability. Circ Res. 2012;111:e89–e102. doi: 10.1161/CIRCRESAHA.111.259846. [DOI] [PubMed] [Google Scholar]
- 28.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Zhang J, Kim HP, Wang Y, Choi AM, Ryter SW. Bcl-xl disrupts death-inducing signal complex formation in plasma membrane induced by hypoxia/reoxygenation. FASEB J. 2004;18:1826–1833. doi: 10.1096/fj.04-2047com. [DOI] [PubMed] [Google Scholar]
- 30.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and bcl-xl promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 31.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/cd31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 33.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.