Abstract
During activation of smooth muscle contraction, one myosin light chain kinase (MLCK) molecule rapidly phosphorylates many smooth muscle myosin (SMM) molecules, suggesting that muscle activation rates are influenced by the kinetics of MLCK-SMM interactions. To determine the rate-limiting step underlying activation of SMM by MLCK, we measured the kinetics of calcium-calmodulin (Ca2+-CaM)-MLCK-mediated SMM phosphorylation and the corresponding initiation of SMM-based F-actin motility in an in vitro system with SMM attached to a coverslip surface. Fitting the time course of SMM phosphorylation to a kinetic model gave an initial phosphorylation rate, kpo, of ~1.17 heads s−1·MLCK−1. Also we measured the dwell time of single QD-labeled MLCK molecules interacting with surface-attached SMM and phosphorylated SMM using total internal reflection fluorescence microscopy. From these data, the dissociation rate constant from phosphorylated SMM was 0.80 s−1, which was similar to kpo mentioned above and with rates measured in solution. This dissociation rate was essentially independent of the phosphorylation state of SMM. From calculations using our measured dissociation rates and Kds, and estimates of [SMM] and [MLCK] in muscle, we predict that the dissociation of MLCK from phosphorylated SMM is rate-limiting and that the rate of the phosphorylation step is faster than this dissociation rate. Also, association to SMM (11-46 s−1) would be much faster than to pSMM (<0.1-0.2 s−1). This suggests that the probability of MLCK interacting with unphosphorylated versus pSMM is 55-460 times greater. This would avoid sequestering MLCK to unproductive interactions with previously phosphorylated SMM, potentially leading to faster rates of phosphorylation in muscle.
Keywords: smooth muscle myosin, smooth muscle myosin light chain kinase, single molecule kinetics, total internal reflection microscopy, phosphorylation rate
When activated by Ca2+CaM, MLCK phosphorylates the SMM RLC at Ser19, which activates the actin-SMM ATPase leading to the cyclic interactions of SMM with actin required for smooth muscle contraction.1-3 In smooth muscle, MLCK is in low abundance relative to SMM with a ~1:20 molar ratio of MLCK to SMM and an even lower molar ratio of activated MLCK to SMM.4-11 Yet upon agonist activation of smooth muscle, Ca2+CaM-MLCK phosphorylates as much as 60% of the SMM in a relatively short (second) time.12-15 Clearly, determining the kinetics of i) SMM Ca2+CaM-MLCK activated phosphorylation, ii) MLCK dissociation from pSMM, iii) and selective rebinding of MLCK to SMM is central to understanding the rate-limiting mechanisms of smooth muscle activation, and the factors that may influence smooth muscle contractility.
MLCK is tightly associated with the contractile apparatus.16-18 It contains both myosin and actin binding sites and has been shown to bind both actin and myosin in solution. The C-terminal telokin domain of MLCK binds to the SMM heavy chain at the junction between the two head domains and the tail domain. This places the MLCK catalytic core close to the two RLC subunits bound to each head domain. The stoichiometry of binding is one MLCK per molecule of SMM.19
Little is known about the kinetics of SMM phosphorylation by Ca2+CaM-MLCK. Sellers and coworkers have shown that SMM phosphorylation significantly decreases the MLCK-SMM affinity in the absence of ATP (Kd from 0.8 μM to >100 μM).20 However, it is unclear whether SMM phosphorylation decreases the MLCK-SMM association rate or increases the MLCK-SMM dissociation rate. The specific kinetic steps (association, phosphorylation, or dissociation) that limit the SMM phosphorylation rate have not been identified. Since spectroscopic signals of association and dissociation that would be useful for standard transient kinetic studies in solution have not been developed, we have taken a non-spectroscopic approach. We have developed an in vitro model system in which smooth muscle proteins are attached to a coverslip surface in various configurations. This approach allows us to: (i) control the constituents of the system, (ii) measure the kinetics of SMM phosphorylation by MLCK and subsequent development of actin motion driven by surface-attached pSMM, and (iii) directly observe the interactions of single MLCK molecules with single SMM molecules under similar conditions using TIRF microscopy.
We previously characterized MLCK and CaM-MLCK complexes that co-purify with SMM from chicken gizzard muscle.18 With the CaM-MLCK-SMM complexes attached to a coverslip surface with SMM in the monomeric form, we showed that the MLCK was activated by Ca2+ to phosphorylate SMM, initiating actin filament sliding in an in vitro motility assay. Half-maximal actin-sliding velocity was observed at pCa50 6.1, similar to other in vitro and in vivo studies, suggesting that the CaM-MLCK was activated by Ca2+ in a physiological manner. We also showed that the MLCK reversibly interacted with the surface-attached SMM through its telokin domain, which is the known SMM-binding site on MLCK.
Here we use this in vitro system to correlate the rate of MLCK-induced SMM activation with the association and dissociation kinetics of single MLCK molecules interacting with surface-attached monomeric SMM (not filamentous). We found that the SMM phosphorylation rate of 1.17 s−1MLCK−1 is similar to the rate at which actin motility is activated and is comparable to the SMM-MLCK dissociation rate constant of 0.80 s−1 obtained from our single molecule experiments. We also show that MLCK dissociates from SMM and pSMM at essentially the same rate, implying that the decrease in SMM-MLCK affinity upon SMM phosphorylation 20 results from a decrease in the pSMM-MLCK association rate. This suggests that in the MLCK-SMM kinetic cycle, dissociation of MLCK from pSMM is rate-limiting and that the rate of the intrinsic phosphorylation step is faster than this dissociation step. Using our measured dissociation rates and Kds, and estimates of SMM concentrations in muscle, our measurements suggest that association to unphosphorylated SMM (11-46 s−1) would be much faster than to pSMM (0.1-0.2 s−1) in the muscle environment.
MATERIALS AND METHODS
Reagents
Tris-glycine gels (4-20% acrylamide, 1.0 mm thick, 10 or 12 wells) were from GE Heath Sciences (Carlsbad, CA). D-(+)-glucose, glucose oxidase, Type VII BSA (low biotin), cow brain CaM, methylcellulose, nitrocellulose and ATP were from Sigma-Aldrich (St. Louis, MO). Phalloidin was from Alexis Corp (San Diego, CA). ATPγS was from Roche Diagnostics (Indianapolis, IN). EZ-link Sulfo-NHS-LC-LC-Biotin was from Thermo Scientific Inc. (Rockford, IL). Microscope coverslips (22×30-1.5 mm) were from Fisher Scientific Company L.L.C. (Pittsburgh, PA). Qdot 525 (λ= 525 nm) ITK Streptavidin Conjugate Kit was from Invitrogen (Eugene, Oregon) and contains ~5-10 streptavidin conjugates per QD. DMCS-coated coverslips were prepared by treating with the following reagents for 20 s each: 0.1 M H2SO4, H2O, methanol, acetone, CHCl3, and 2.5% DMCS in CHCl3.21
Proteins
SMM was from frozen chicken gizzards as described 22 except that the last polymerization-depolymerization step was excluded. Based on sequences P10587, P02612, P02607-1, SMM is 530 kDa. SMM was thiophosphorylated in HMM buffer (10 mM MOPS, 50 mM NaCl, 0.2 mM EGTA, 2.0 mM MgCl2, 1mM DTT) with 3.0 mM CaCl2, 1 mM ATPγS, 10 μg/ml CaM, and 40 μg/ml MLCK at 25 °C for 0.5 h, followed by overnight incubation on ice.23 The extent of thiophosphorylation was quantified by urea-PAGE.24
MLCK was from frozen chicken gizzards as described 4 and was biotinylated by EZ-link Sulfo-NHS-LC-LC-Biotin (Thermo Scientific) according to the manufacturer’s protocol giving 2-5 biotins/MLCK. The kinase activity of biotinylated-MLCK was 3.76 μmol mg−1 min−1, comparable to MLCK without biotinylation (3.3 μmol mg−1 min−1) using RLC as the substrate. Biotinylated-MLCK and biotinylated-MLCK with attached QDs were able to fully phosphorylate SMM RLC as measured in a urea gel assay 24 (data not shown). Equilibrium binding studies of MLCK to SMM filaments (data not shown) showed that, relative to unmodified MLCK, biotinylated-MLCK had a similar affinity in the presence of Ca2+CaM but bound ~4-5 times more weakly in the absence of Ca2+CaM.20 Binding affinities were as described 20, except that the MLCK in the supernatant was measured by western blot.18
Actin (chicken pectoralis muscle 25) for motility assays was incubated with equimolar TRITC-labeled phalloidin overnight 26 and stored on ice at 4 °C. Tropomyosin was obtained from chicken gizzards.27 Protein concentrations were determined using the following extinction coefficients (1 mg ml−1): SMM (280 nm) = 0.56; MLCK (280 nm) = 1.14; F-actin (280 nm) = 1.1.
In vitro motility assays
Motility assays were performed using a Nikon TE2000 epifluorescence microscope (Nikon TE-2000U, Technical Instruments, Burlingame, CA) with a Roper Cascade 512B camera (Princeton Instruments, Trenton, NJ). The procedures described here relate to Figures 1-3, with additional details provided in the Figure Legends. Myosin buffer: 300 mM KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, 10 mM DTT, pH 7.0. Actin buffer: 50 mM KCl, 50 mM imidazole, 2 mM EGTA, 8 mM MgCl2, 10 mM DTT, pH 7.0. Motility buffer: 50 mM KCl, 50 mM imidazole, pH 7.0, 2 mM EGTA, 8 mM MgCl2, 10 mM DTT, 2 mM ATP, 0.5% methylcellulose and oxygen scavenger system (0.1 mg ml−1 glucose oxidase, 0.018 mg ml−1 catalase, 2.3 mg ml−1 glucose). Motility assays were performed as previously described 28 at 30 °C. SMM containing co-purified CaM-MLCK at the indicated concentrations in myosin buffer was applied to nitrocellulose-coated coverslips attached to glass slides using adhesive tape or plastic spacers (0.127 mm deep). This SMM preparation is described in our previous work 18. The ratio of MLCK:SMM was reproducible from preparation to preparation at 1:73 ± 9, or 1 MLCK:146 SMM heads. The actual amount of active MLCK present during the motility assay is estimated to be ~1:2600 SMM heads, considering that only 15-30% (22% on average) of the total MLCK was present as CaM-MLCK 18 and that ~75% would be expected to wash off the coverslip prior to the assay, given the affinities measured in our prior work. 18 After 1 min, the coverslip was washed with 2 × 40 μl myosin buffer. The nitrocellulose surface was blocked with 2 × 40 μl 0.5% BSA (w/v) in actin buffer for 1 min, followed by 2 × 40 μl 10 nM TRITC-actin in actin buffer for 1 min, and two washes with 40 μl actin buffer. Motility buffer (40 μl at [Ca2+] indicated) was loaded twice and 30 s image sequences of 1-5 fields were recorded. Data from these 1-5 fields constituted one (n = 1) experiment.
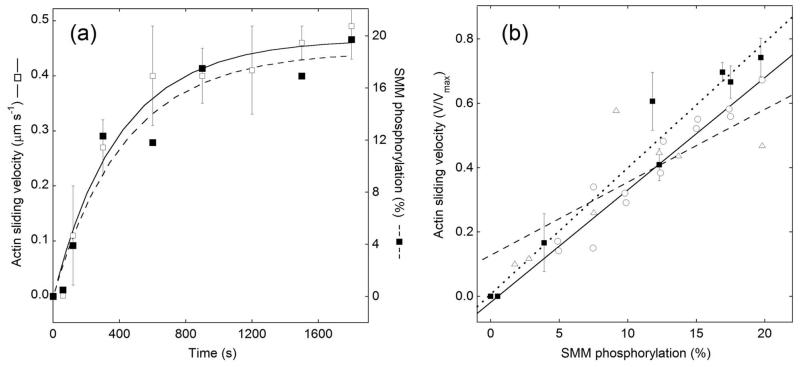
Figure 1.
Kinetics of MLCK-induced mechanical activation and phosphorylation of the SMM RLC. (a) Nitrocellulose-coated coverslips were treated as follows (see in vitro motility assays in Methods for further details): monomeric SMM (0.2 mg ml−1) containing co-purified CaM-MLCK, BSA block, and 10 nM TRITC-actin filaments. Actin filament velocities measured at different times after incubation with motility buffer at pCa 4 (open squares) increase as SMM is phosphorylated by MLCK. Velocity data indicate the mean ± s.d. from 4 SMM preparations and 4 independent measurements. For one experiment, after the motility assay was completed each coverslip was washed, blocked, and an on-coverslip ELISA assay (see Methods) was performed to measure the pRLC level and the percent phosphorylation (closed squares) by comparison to a standard curve 18. Experiments with other SMM preparations with fewer time points showed a similar time-course (not shown). (b) The velocity (V) and phosphorylation data from (a) along with a linear fit (closed squares, dotted line, slope = 0.039, R2 = 0.98) are plotted on the same graph with the data for known mixtures of SMM and pSMM (open circles, solid line, slope = 0.035, R2 = 0.96; 33) and for SMM phosphorylated in solution representing a random mixture of unphosphorylated, one-head phosphorylated and two-head phosphorylated species (open triangles, dashed line, slope = 0.023, R2 = 0.77; 32). Our maximal velocity (Vmax) was 0.66 μm s−1 (measured after adding exogenous CaM-MLCK), similar to the references quoted above (0.67 μm s−1).
Figure 3.
Effect of actin on phosphorylation of surface-attached SMM. (a) Effect of actin movement over surface-attached SMM. For step one, nitrocellulose-coated coverslips were treated as follows (see in vitro motility assay and on-coverslip ELISA sections in Methods for further details): 0.1 mg ml−1 SMM containing co-purified CaM-MLCK, washed, blocked, 10 nM TRITC-actin, incubation in motility buffer containing either 2 mM ATP or ATPγS at pCa 4 for 15 min at RT (sliding velocity not recorded). For motility data shown in grey bars, step two was as follows: washed, re-loaded 10 nM TRITC-actin, and motility assay was performed (with 2 mM ATP) at no added Ca2+. The free Ca2+ concentration at no added Ca2+ was estimated to be 1 nM. Data were collected immediately after loading the final motility buffer. The velocity values indicate mean ± s.d. Data are from 3 SMM preparations and 5 independent measurements. There was no significant difference between the two treatments (t-test; p<0.05). For pRLC quantitation shown in black bars, step two was an ELISA procedure. Values indicate the mean ± s.d. from 3 measurements with no significant difference (t-test; p<0.05). (b) Effect of actin or actin-Tm on SMM phosphorylation time-course. Nitrocellulose-coated coverslips were treated as follows: 0.2 mg ml−1 SMM containing co-purified CaM-MLCK, washed, blocked, 10 nM actin (grey bars), 100 nM actin (white bars), or 100 nM actin plus 50 nM Tm (black bars) in actin buffer or actin buffer alone (control), incubated with motility buffer with 2 mM ATP at pCa 4 for different times, followed by a wash, and ELISA for pRLC. Mean ± s.d. are from 3-7 repeats from 2 SMM preparations. Actin sliding velocities were ~ 0.15 - 0.5 μm s−1 (similar to Figure 1).
Quantification of percent of SMM phosphorylation by on-coverslip ELISA
We previously described our on-coverslip ELISA assay. 18 Standards of different percentages of SMM phosphorylation were mixtures of pre-phosphorylated SMM and SMM at different ratios at the same final concentrations used for Figure 1.
Direct visualization of QD-MLCK dynamically interacting with surface-attached SMM using TIRF imaging
Experimental
Flowcells were as described for motility assays. SMM (0.5 μg ml−1) in myosin buffer was applied to untreated glass coverslips for 2 min at 25 °C. Using the 4 lowest values in Table 1 from Harris et al 29 we estimate the average surface density to be 25 SMM molecules per μm2, giving ~200 - 230 nm between each SMM. Control surfaces without SMM were incubated for 2 min in 1% (w/v) BSA (Sigma; A3059) in myosin buffer. After 2 min, all surfaces were blocked with two 30 s washes of 1% BSA (w/v) in myosin buffer. Volumes of washes were typically 45 - 65 μl. QD-MLCK was prepared by mixing equal volumes of 1.2 nM streptavidin-QD (525 ITK, Invitrogen) in myosin buffer and 45 nM biotinylated-MLCK in myosin buffer containing 2% (w/v) methylcellulose and incubated for 5 min at 25 °C, producing a 0.6 nM QD, 22.5 nM MLCK mixture in 1% methylcellulose in myosin buffer. To prepare the CaM-MLCK complex, 1 μg ml−1 CaM, 2 mM CaCl2, and 1 mM ATP were incubated for 1 h with the biotinylated-MLCK. The QD-MLCK was applied to the coverslips and viewed immediately under TIRF conditions at 100× magnification using the same Nikon TE2000 epifluorescence microscope we used for the in vitro motility assays, but with a 488 nm excitation laser. Movies were captured with a Roper Cascade 512b (Princeton Instruments, Trenton, NJ) at exposure rates of 87.9 ms per frame in SimplePCI software. Typically, movies were captured for a little over 1 min and several movies were captured per slide. In several instances, flow cells were viewed continuously and intermittently for up to 30 min after preparation, but no obvious differences in the movies were detected. Over a dozen movies from at least three slides for the control, MLCK, and CaM-MLCK conditions were analyzed and quantified.
Table 1.
Summary of measured and calculated parameters for in vitro model system.
| Data source | Experimental Condition | ||
|---|---|---|---|
| MLCK | Ca2+CaM-MLCK + ATP | ||
| State of SMM | SMM | pSMM | |
| Initial phosphorylation rate (kpo) | Figure 1 | - | 0.045% s−1 or 1.17 SMM heads s−1 MLCK−1 |
| Rate of loss of MLCK (kloSt) | Figure 1 | - | 0.22% s−1 |
| Average lifetimes (τon) in s and distributions in % |
Figure 7 | 1.09 ± 0.17 (36 ± 2%) 8.23 ± 0.48 (64 ± 2%) |
1.25 ± 0.06 (49 ± 2%) 12.39 ± 0.48 (51 ± 2 %) |
| Dissociation rate constants (k−1 for SMM and k+2 for pSMM) in s−1 |
Figure 7 | 0.92 (36%) 0.12 (64%) |
0.80 (49%) 0.08 (51%) |
| Association rate constants (k+1) (estimated) |
Figure 7 and Kd(a) | 0.24 s−1μM−1 for SMM <0.01 s−1μM−1 for pSMM |
|
| Association rate for Figure 1 (estimated) |
Figure 1, Figure 7 and Kd |
2.3 – 4.6 s−1 for SMM | |
Kd measured in solution with myosin filaments (3.8 and >60 μM for SMM and pSMM, respectively).
Data analysis
The simple PCI files of image sequences were exported to TIFF format and viewed and analyzed in ImageJ 1.47b 64 bit and a demonstration version of Imaris 7.5.1 software (Bitplane, Zurich, Switzerland) on a OS × 10.7.5 Mac 3.4 Ghz Intel core i7. Imaris movies could be viewed as volumes (see Figure 6). Additional details of data analysis are in Supplemental Figure 1.
Figure 6.
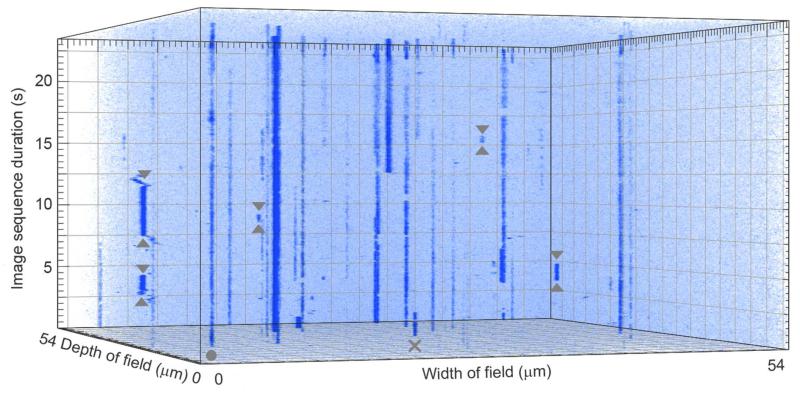
Three dimensional kymograph of a representative image sequence for the interaction of QD-MLCK with surface-attached SMM using TIRF. The field of view is 512 pixels by 512 pixels (54 μm by 54 μm). The total duration is 24.35 s or 277 frames. Vertical blue lines are kymographs of QD-MLCK molecules. For further details of the experiment see Materials and Methods and of the analysis method see Supplemental Figure 1. A few but not all instances of binding/detachment events that meet the criteria described in Methods are bracketed with up and down arrows. Examples of data not meeting the criteria are QD-MLCK binding that began before the initiation of the movie (X) and a QD-MLCK showing no binding event or dissociation event (filled circle).
QD blinking analysis
The Spots function in Imaris was used to analyze image sequences of control surfaces (without SMM; data not shown). Only non-specifically bound QDs that remained attached to the surface for the duration of the movies were observed. These data were used to statistically analyze QD blinking behaviors so that we could identify blinks with confidence in the sequences of the interaction of QD-MLCK with SMM. Using the Spots function, we exported data to create pivot tables in Excel to determine the durations of 895 dark events (blinks). A statistical analysis using JMP software (SAS, Cary, NC) showed that dark events greater than 6 frames (0.53 s) were outliers (greater than 1.5 times the interquartile length of the distribution of all off times). From this we defined a blink as a dark event lasting 6 frames or less with a statistical confidence of greater than p = 0.05. Review of the datasets showed that a more stringent cut-off of p = 0.01 would not have changed the scoring of the datasets.
Criteria for a true binding/detachment event
For image sequences of QD-MLCK interacting with surface-attached SMM, we set specific criteria that had to be satisfied before an event was included in the dataset to determine the duration of attachment (see Figure 7). We excluded from analysis: (i) QD-MLCK were bound for the duration of the image sequence, (ii) QD-MLCK that were bound prior to movie data collection, (iii) transient binding or collision with the surface lasting only 1 frame, and (iv) QD-MLCK that had traces in the exact same position after a dissociation event (or an QD dark event longer than a 6 frames to exclude blinks). We included QDs that clearly bound and detached (see Figure 6, up and down arrows) without returning to the same location, but dark events during this bound time had to last 6 frames or less (blink). QDs that were observed to bind and remained bound for the remainder of the image sequence were also counted.
Figure 7.
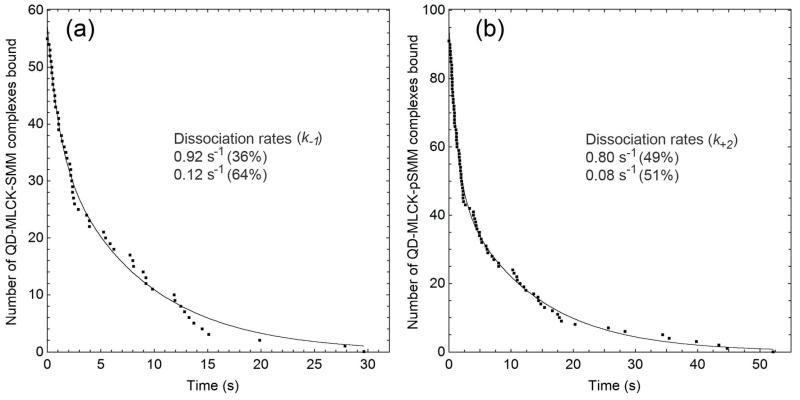
Determination of the dissociation rate of QD-MLCK from surface-attached SMM. Data taken from image sequences similar to those decribed in Figure 6 (also see Supplemental Figure 1) are plotted as survival curves. The durations of all binding events that meet the criteria (see Methods) are plotted on the X-axis and the Y-axis is the number of surviving QD-MLCK-SMM complexes or number of remaining bound at that time from the dataset. Conditions are (a) absence or (b) presence of ATP and Ca2+CaM in which the SMM on the surface is unphosphorylated or phosphorylated, respectively. For (a) event durations from 14 (~350 s) or for (b) 20 (~500 s) image sequences were compiled. Double exponential fits to the data are shown in solid lines. Dissociation rates and fractional contributions are summarized in the inset.
In general, independent of condition (QD, QD-MLCK, QD-MLCK-ATP, QD-MLCK-ATP-CaM, QD-MLCK-CaM) there were occasionally as few as 4 QD, rarely as many as 12 QD, but most often nearly 8 QD bound to the surface in a manner suggesting nonspecific binding. These traces rarely had blink durations long enough to suggest dissociation events. But even in a case where the trace had statistically unnaturally long dark periods, the trace would be disregarded because we did not observe its association event. For Figure 7 a double exponential fit to the survival curves was justified by comparing the sums of the mean-squared residuals for single and double exponential fits, which gave F-ratios of ~50, which are in excess of the confidence levels necessary. 30 The equation for fitting was y = A1e(−t/τon1) + A2e(−t/τon2) where A1+A2 = the total number of molecules analyzed in the dataset and A1 and A2 are the respective fractional contributions for each exponential. All fitting was done by the least-squares method 31 using Kaleidagraph (Synergy Software) and τon values are reported ± standard error. Further details of the method are in Supplemental Figure 1.
RESULTS AND DISCUSSION
Kinetics of phosphorylation and mechanical activation of surface-attached monomeric SMM by MLCK
We measured the kinetics of MLCK-induced phosphorylation of surface-attached monomeric SMM and the subsequent activation of actin filament sliding over the pSMM (Figure 1a). SMM containing co-purified CaM-MLCK 18 was attached to a nitrocellulose-coated coverslip in the absence of Ca2+ and ATP, and 10 nM fluorescent actin was added. No movement of actin was observed as expected because MLCK requires Ca2+ for enzymatic activity. Upon addition of motility buffer containing 100 μM free CaCl2 (pCa 4) to activate the co-purified CaM-MLCK and 2 mM ATP to provide a substrate for both SMM and MLCK, actin motility increased with time, reaching a maximum velocity of ~0.45 μm s−1 after 30 min (Figure 1a; open squares). This is lower than the maximal velocity (Vmax) of 0.66 μm s−1, which was observed for 100% pre-phosphorylated SMM, suggesting that the plateau in velocity in Figure 1a occurs before SMM is fully phosphorylated.
Using separate slides for each time point after the actin motility was recorded, we performed an on-coverslip ELISA to determine the level of RLC phosphorylation (percent of total) using our previously described method. 18 Figure 1a shows that following the addition of Ca2+, SMM phosphorylation increased with time, reaching a maximum level of phosphorylation of ~20% after 30 min (Figure 1a; closed squares).
Figure 1b shows the correlation between actin sliding velocities expressed as V/Vmax, and phosphorylation % from Figure 1a (closed squares) with a linear fit (dotted line). Also plotted for reference are data and linear fits for randomly phosphorylated (triangles, dashed line; taken from 32) and for mixtures of unphosphorylated and fully phosphorylated SMM (circles, solid line; taken from 33). The three data sets are similar, showing a nearly linear relationship between V/Vmax and percent phosphorylation within this range of phosphorylation. The similarity of the three datasets is evidenced by the slopes to linear fits (0.039, 0.034, and 0.023 V/Vmax per % phosphorylation) and suggests three important points. First, our method for measuring the absolute levels of phosphorylation of surface-attached SMM by ELISA gives the expected levels of phosphorylation at a given actin filament velocity, demonstrating that this is a robust way to measure phosphorylation kinetics in this in vitro system. Second, actin-sliding velocities can be used to estimate phosphorylation rates within this range of phosphorylation. This is important because actin-sliding velocities are much less labor intensive to measure than phosphorylation. And third, we cannot determine from the data in Figure 1 whether or not the MLCK is generating two-head phosphorylated or a mixture of one-head and two-head phosphorylated SMM. It is likely that it is the latter, because it has been shown that MLCK phosphorylation kinetics in solution are consistent with a model in which phosphorylation of the two heads of SMM occurs randomly at equal rates.34 Therefore in Figure 1a we expect that most pSMM were phosphorylated on only one head because the level of maximal phosphorylation was only ~20%.
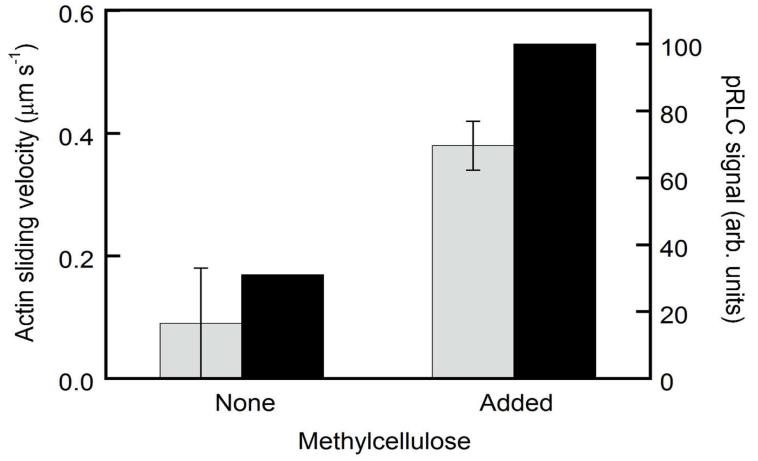
The observation that the rate of SMM phosphorylation approaches zero at less than 100% phosphorylation (Figure 1a) suggests a loss of available MLCK over time, presumably through diffusion away from the surface-attached SMM or by irreversibly binding to the coverslip surface. The former mechanism is supported by our observation that peak activation levels are more than three-fold higher in the presence than in the absence of methylcellulose (Figure 2). In a motility assay, methylcellulose holds actin filaments close to the surface, and it is possible that it does the same for MLCK. That is, it may decrease the probability that MLCK diffuses far from the SMM surface, effectively increasing the frequency of SMM-MLCK interactions. The low level of maximal activation does not result from contaminating phosphatase, because as shown below we observe similar sub-saturating levels of phosphorylation when ATPγS is used instead of ATP. Thiophosphorylated SMM, unlike pSMM, is resistant to dephosphorylation by the phosphatase.
Figure 2.
Effect of methylcellulose on MLCK-induced phosphorylation of SMM and on observed actin sliding velocities. Experimental conditions were as in Figure 1 except that the incubation with motility buffer was for 10 min and the motility buffer contained either no methylcellulose or 0.5% methylcellulose. Actin sliding velocity is shown by the grey bars and the error bars for motility represent the s.d. from 4 SMM preparations and 5 replicates. The pRLC signal is shown in black bars and data are from a single determination.
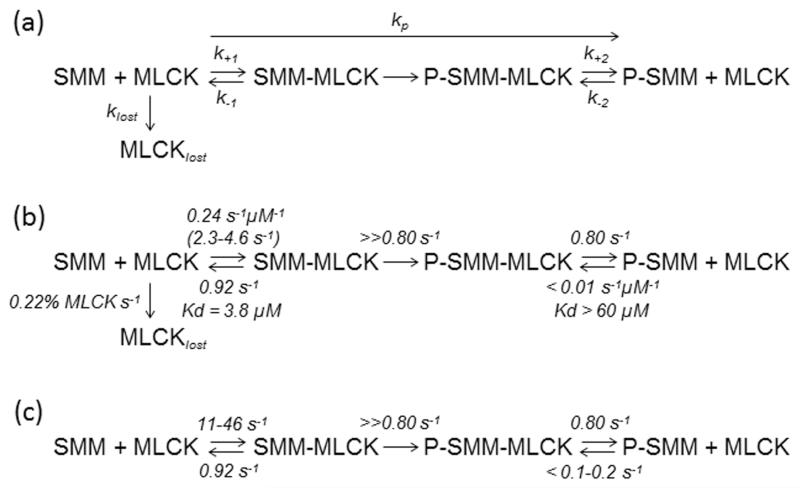
Scheme Ia accurately accounts for the data in Figure 1a (closed squares). Phosphorylation of SMM occurs with a pseudo first-order rate constant, kp, that varies linearly with [MLCK]. Because MLCK is depleted at a rate klost, kp decreases with time and kp = kpo·exp(−klost·t), where kpo is the initial phosphorylation rate at the MLCK concentration existing when t = 0. The dashed line in Figure 1a is a Matlab (Mathworks, Natick, MA) simulation based on Scheme Ia, which gives estimates for kpo and klost of 0.045% s−1 (of SMM heads) and 0.22%·s−1 (of MLCK; see Scheme Ib), respectively. We estimate that at t = 0 there is one active MLCK per 2600 SMM heads (see Methods), and so the initial activation rate, kpo, is ~ 1.17 heads s−1·MLCK−1 (Table 1). The solid line in Figure 1a shows the simulation of the velocity data, assuming that the fractional velocity increases linearly with time (see Figure 1b), and the maximal velocity occurs at 19% phosphorylation. The resulting simulation of velocity data gave kpo and klost of 0.05% s−1 (of SMM heads) and 0.23%·s−1 (of MLCK), respectively. Therefore the simulations of phosphorylation and velocity gave essentially the same rates.
Our estimated initial phosphorylation rate (kpo) of ~1.17 heads s−1 MLCK−1 is similar to the rate of phosphorylation measured in solution under pseudo first order conditions for the monomeric form of SMM of 1.14 heads s−1 MLCK−1.34 This is also similar to our previous estimates 18 of MLCK activity in solution of 1.5 s−1 MLCK−1. This implies that even with surface-attached SMM, the kinetics of phosphorylation by MLCK closely resembles the kinetics measured in solution studies.
Methylcellulose enhances the rate of MLCK-induced phosphorylation of SMM and MLCK-induced mechanical activation of SMM to move actin filaments
The experiments in Figure 1a were performed under standard motility assay conditions with methylcellulose in the buffer. Methylcellulose, an extended polymer, is used to limit the motion of the fluorescent actin to roughly two dimensions. In the absence of methylcellulose, we observed relatively slower actin sliding velocities and lower levels of SMM phosphorylation (Figure 2), suggesting that methylcellulose decreases klost (Scheme Ia) most likely by increasing the time the MLCK remains close to the surface-attached myosin. Also, methylcellulose may increase the association rate of MLCK to SMM, if this step is rate-limiting under these conditions (see below). Alternatively, since actin is more confined to the surface in the presence of methylcellulose, the closer proximity of actin and SMM may allow MLCK to attach to both actin and SMM through the respective binding sites on opposite ends of the molecule. Transient attachment to moving actin filaments may facilitate phosphorylation of SMM. This would involve MLCK binding to actin, which is then presented to SMM heads as the actin moves along, transporting the MLCK to the next SMM. The following section describes experiments designed to detect actin-mediated enhancement of motility and phosphorylation of SMM.
Effect of actin on MLCK-induced mechanical activation and SMM phosphorylation
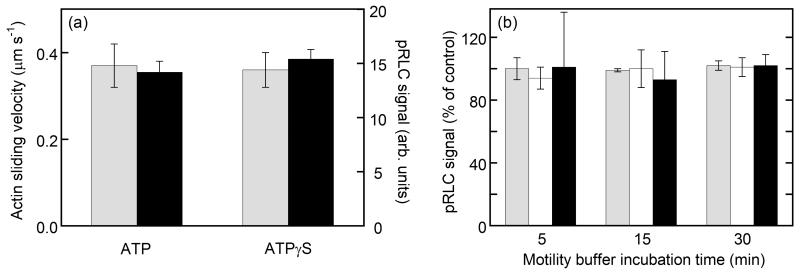
To examine the effects of actin movement on the rate of SMM activation (Figure 1), we performed a two-step experiment on coverslips coated with monomeric SMM containing co-purified MLCK. In the first step, to allow the SMM to be phosphorylated, the surface was incubated in standard motility assay buffer at pCa 4 in the presence of 10 nM TRITC-actin for 15 min but the actin sliding velocity was not recorded. The buffer either contained ATP or ATPγS. These nucleotides are used by MLCK to phosphorylate or thiophosphorylate SMM, respectively. On the other hand, SMM can use ATP but not ATPγS at an appreciable rate. Therefore actin moves in the presence of ATP but not ATPγS. In the second step, to determine the effects of the treatments from the first step, we washed out the motility assay buffers and re-added motility buffer (ATP) and TRITC-actin in the absence of added Ca2+ and immediately measured the actin sliding velocity. The grey bars in Figure 3a show that the actin-sliding velocities for the ATP and ATPγS treatments were not significantly different. An identical set of coverslips were treated in the same manner for the first step, but the pRLC signal was measured by ELISA in the second step. The black bars in Figure 3a show that the pRLC signals were very similar for the ATP and ATPγS treatments, consistent with the motility data. These data suggest that a similar amount of SMM phosphorylation and similar levels of actin motility occurred whether or not the actin was allowed to move over the surface-attached SMM during the phosphorylation reaction in step 1.
We next addressed whether or not actin was necessary for phosphorylation (Figure 1), and if increasing the actin concentration or substituting actin with actin-Tm enhanced the phosphorylation rate. Actin-Tm binds MLCK with ~4-fold higher affinity than actin alone.20 The protocols for the experiments in Figure 3b were similar to those for Figure 3a except that in step 1 we included 0 (control), 10 (grey bars), or 100 nM (white bars) actin, or 100 nM actin with 50 nM Tm (black bars). The incubation time in the presence of motility buffer containing Ca2+ and ATP was varied before measuring the pRLC signal by ELISA on the coverslip. Data are plotted as percent pRLC signal observed for the actin-treated coverslips relative to the pRLC signal obtained for coverslips treated with actin buffer alone (control). For all three incubation times, the pRLC signal obtained in the presence of actin was similar to that of the control. These data show that similar levels of SMM phosphorylation were attained over a 30 min time period at 0, 10 or 100 nM actin, and at 100 nM actin-Tm. In summary, under these conditions: (i) the presence of actin was not necessary to obtain similar phosphorylation kinetics to that shown in Figure 1, and (ii) adding higher actin concentrations or substituting actin with actin-Tm did not significantly affect phosphorylation kinetics. These results do not necessarily indicate or predict that actin-SMM interactions will not affect phosphorylation kinetics in muscle. Note that actin in Figure 1 is at a very low concentration, so that only a small fraction of the total surface-attached SMM is interacting with the actin at any point in time. This is true even under the higher actin concentrations of 100 nM used above.
Visualization of single QD-MLCK molecules statically bound to SMM and to SMM in SMM-F-actin rigor complexes using TIRF imaging
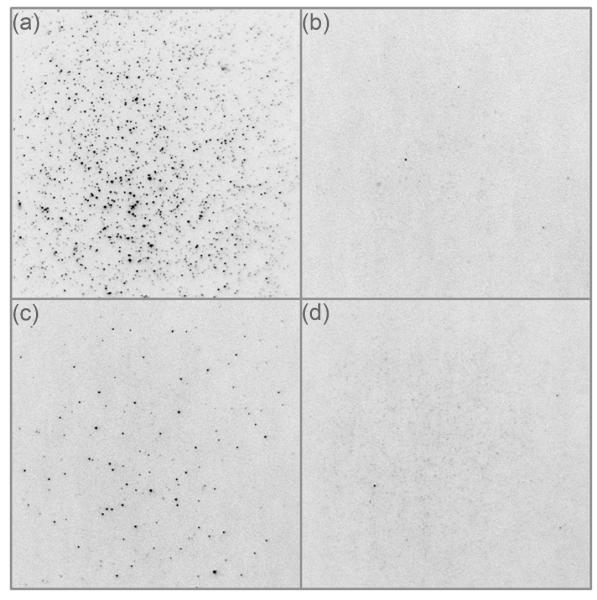
To visualize QD-labeled MLCK molecules bound to surface-attached monomeric SMM and to assess issues of non-specific binding we did the experiment in Figure 4a which shows a representative image of a DMCS-coated coverslip surface treated in sequence as follows (see Methods for details): (i) monomeric SMM, (ii) BSA block, (iii) biotinylated-MLCK in the presence of CaM at pCa 4, (iv) wash, (v) QDs, (vi) wash, and (vii) motility buffer without ATP. The image shows that the surface was coated with single QD (identified by blinking). The SMM and MLCK concentrations were purposefully high to increase the likelihood that a single QD was interacting simultaneously with more than one MLCK-SMM complex due to its multivalent streptavidin coating. Therefore the lifetimes of the interactions were very long (>10 min). This allowed us to easily count a significant number of QDs in each field of view, without observing attachment or detachment events. Most QDs were bound specifically to MLCK-SMM, since relatively few QDs were observed in the absence of either biotinylated-MLCK (Figure 4b) or SMM (Figure 4c), or to the blocked surface (Figure 4d). Note that under these conditions MLCK cannot phosphorylate the SMM on the surface (no ATP) and therefore the QD-MLCK is interacting with unphosphorylated SMM. In Figure 4 about ten-times more QDs were observed if SMM and a BSA block were applied prior to adding MLCK, versus if only MLCK was applied to a BSA-blocked surface. This directly shows that the non-specific binding of MLCK to a blocked surface is ~1/10 the binding observed for SMM.
Figure 4.
Visualization of single QD-MLCK molecules bound to SMM using TIRF microscopy. Instrumentation was as for motility assays except with a 488 nm excitation laser. Images are inverted so that QDs appear as dark spots. DMCS-coated coverslips were treated with the following reagents in order: (a) SMM (0.2 mg ml−1, ~0.4 μM) for 2 min in myosin buffer, blocked with 2% BSA for 2 h in actin buffer, a mixture of 0.2 μM biotinylated-MLCK, 0.2 μM CaM at pCa 4 for10 min, wash with actin buffer, 0.5 nM streptavidin-QDs in actin buffer for 1 min, wash, motility buffer without ATP and CaCl2. (b) Same as (a) except that MLCK and CaM were omitted. (c) Blocked with 2% BSA for 2 h, followed by a mixture of 0.2 μM biotinylated-MLCK, 0.2 μM CaM at pCa 4 for 10 min, wash, 0.5 nM QDs, wash, motility buffer without ATP and CaCl2. (d) Blocked with 2% BSA for 2 h, 0.5 nM streptavidin-QDs, wash, motility buffer without ATP and CaCl2. QD numbers in (a), (b), (c), (d) were measured using the particle analysis tool in ImageJ and found to be 1090, 3, 91and 6, respectively. The dimensions for all images are 54 μm by 54 μm.
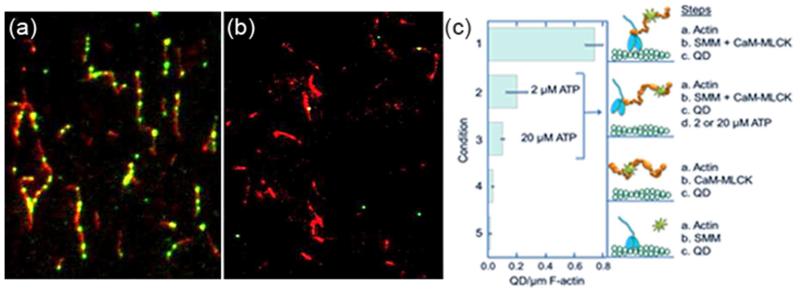
To further demonstrate specific MLCK-SMM interactions, we also observed single QD-MLCK molecules bound to SMM that was in a SMM-F-actin rigor complex. We treated a DMCS-coated coverslip surface with the following sequence of incubations: TRITC-labeled F-actin, BSA block, a mixture of SMM and biotinylated MLCK with CaM at pCa 4, wash, QDs, wash, and motility buffer without ATP (note that motility buffer contains EGTA and no added Ca2+). Again, SMM and MLCK concentrations were high to promote multivalent QD binding (see above). Using TIRF microscopy, both TRITC-actin (red) and QDs (green) were sequentially imaged in the same region of interest. Figure 5a is a representative combined image showing surface-attached actin (red) and QD-MLCK (green) with significant co-localization (yellow). Since MLCK is known to bind both SMM and actin, the QD-MLCK in Figure 5a could be bound either to actin filaments or to actin-bound SMM or both. To distinguish between these possibilities, we repeated the above experiments in the absence of SMM (Figure 5b) and observed relatively few QDs co-localized with F-actin (compare Figure 5a with 5b). This suggests that CaM-MLCK does not bind appreciably to actin filaments alone under these conditions. Note that we intentionally mixed MLCK with SMM in solution prior to a brief exposure to F-actin to minimize MLCK binding to F-actin under this condition. We have observed that QD-MLCK binds F-actin under conditions that more effectively promote their binding (data not shown; manuscript in preparation). Because SMM was required to observe QD co-localization on actin we conclude that MLCK first bound to monomeric SMM during the pre-incubation in solution, and the pre-formed MLCK-SMM complex then bound to surface-attached actin filaments through an acto-myosin rigor interaction.
Figure 5.
TIRF imaging demonstrating CaM-MLCK-SMM complexes bound to F-actin. (a) A DMCS-coated coverslip surface was treated in sequence as follows: 125 nM TRITC-actin for 20 min in actin buffer, block for 2 h with 2% BSA in actin buffer, a mixture of 0.4 μM (0.2 mg ml−1) SMM, 0.2 μM biotinylated-MLCK, 0.2 μM CaM at pCa 4 for 2 min, wash, 0.5 nM QDs for 1 min, wash, motility buffer without ATP. The cube on the microscope was switched to record both TRITC-actin (red) and QD (green) images in the same observing areas. Note strong colocalization of actin and QD-MLCK. (b) Representative image from an experiment identical to (a) except there was no SMM in the biotinylated-MLCK and CaM mixture. Note lack of significant colocalization of actin and QD-MLCK. (c) Number of QDs per μm actin was determined for Conditions 1-5. Values are the mean of data from 3 fields ± SEM (error bars). The total number of QDs counted for conditions 1-5 were 547, 117, 48, 13, and 9, respectively. The coverslip treatment sequences are summarized at the right and the details are the same as described for (a, b). In Condition 4, the SMM step was omitted and in Condition 5, the MLCK and CaM were omitted and no ATP was in the motility buffer. The corresponding cartoons depict our interpretation of the results. Actin is shown in green attached to the coverslip surface (blue line), SMM is blue, MLCK is orange and QD is green.
Figure 5c shows a comparison of the number of QDs/μm actin under various conditions. Conditions 1 and 4 correspond directly to those in Figure 5a and b, respectively. For Condition 1 with SMM, there were 0.75 QDs/μm actin, whereas in Condition 4 in the absence of SMM, there were only 0.03. In the absence of MLCK (Condition 5) QDs did not bind to the surface, suggesting that the QDs bound specifically to MLCK.
Since the QD-MLCK-SMM complexes in Figure 5a were bound to surface-attached actin in a rigor state (no ATP), addition of ATP to the final motility buffer should dissociate SMM (with bound QD-MLCK) from actin if the MLCK-SMM was specifically bound to actin but not if it was bound to the surface. Upon addition of MgATP most QDs dissociated from the surface. The addition of 2 and 20 μM MgATP (Conditions 2 and 3, respectively) decreased the number of QDs/μm actin filament in a concentration-dependent manner. This effect is quantified in Figure 5c. Figure 5c includes cartoons depicting our interpretation of the above results. The data in Figure 5 suggest that CaM-MLCK can bind tightly to monomeric SMM in solution, and that the CaM-MLCK-SMM complex can bind to F-actin through the actin-binding site on myosin. Importantly, as in Figure 4, the data in Figure 5 again demonstrate low non-specific binding to the surface.
Direct visualization of QD-MLCK dynamically interacting with surface-attached SMM using TIRF imaging
With non-specific binding issues having been addressed (Figures 4 and 5), we moved on to measure dynamic MLCK-SMM interactions to understand which processes may limit the rate of the phosphorylation reaction from Figure 1a. Our first objective was to quantify the lifetimes of QD-MLCK-SMM interactions (τon) to determine the dissociation rate of MLCK from SMM (k−1; Scheme 1a) or from pSMM (k+2; Scheme 1a). The data in Figures 6 and 7 were obtained under similar conditions to Figure 1 except that we used a much lower density of SMM (0.5 μg ml−1), resulting in a surface density of ~25 SMM molecules per μm2, 29 corresponding to ~200 - 230 nm spacing between each SMM assuming 2-D hexagonal packing. This low density was used to increase the probability that QD-MLCK bound to only one SMM at a time, while still allowing for a measurable number of events. Using QD (with streptavidin included, 20 nm diameter) and predicted MLCK dimensions,35 we estimated that a single QD-MLCK could not simultaneously bind to more than one SMM if they were more than 80 nm apart. After blocking, a mixture of 22.5 nM biotinylated-MLCK and 0.6 nM QD in motility buffer (1% methylcellulose) was applied to the surface and image sequences were recorded.
Figure 6 is a three dimensional kymograph of QD-MLCK interacting dynamically with a SMM-coated surface, showing QDs binding/detaching to the surface over time (a blue line is a single kymograph of one QD). The blinking of bound QDs appear as rapid fluctuations in the intensity of the blue lines (not indicated in the Figure). In contrast, a blue line starting and then ending indicates a QD has bound to and detached from the surface (see up and down arrows bracketing binding/detachment events). We excluded binding/detachment events that reoccurred at the same location, with the assumption that they may represent tethered molecules appearing in and out of the TIRF field. We assumed that MLCK had to detach from one SMM before it could reach another SMM and that the probability of any one SMM being visited more than once during a given experiment was low. Binding/detachment events were included in the datasets for Figure 7 only if they met the criteria described in Materials and Methods and in Supplemental Figure 1. Non-specifically bound QDs are also seen in Figure 6, an example of which is indicated with a closed circle. These QDs remain bound for the duration of the image sequence. In Figure 6 there are about 6 continuously bound traces, 5 unobserved association event traces and 12 traces of binding/detachment (not all indicated on the Figure for clarity). This was a typical ratio.
The durations of QD-MLCK-SMM binding events that met our criteria were plotted as survival curves for experiments performed both without (Figure 7a) and with (Figure 7b) ATP and Ca2+CaM. Under the latter condition, we estimated that SMM was fully phosphorylated by the time measurements were made (direct measurement of the phosphorylation was not possible at this low SMM density). Survival curves are best typically used to plot time until death or some other “time to event outcome variable” for a cohort of patients. However, this type of analysis can be used with any outcome variable that has a well-defined end point that can only happen once per subject or observation. In our case, the subject is an observable QD-MLCK-SMM complex and the “survival” refers to the time that each QD-MLCK-SMM “survives” or the duration of the QD-MLCK-SMM binding event. To generate the survival curve, the measured duration of all the events is plotted on the X-axis and the Y-axis is the number of surviving QD-MLCK-SMM complexes in the dataset at that time. The point at time zero represents the total number of events measured because all of those events are surviving at time zero.
We assume our events satisfy the ergodic theorem,36 meaning that we observe the behaviors of individual molecules for a long enough time so that when the event data are combined they approximate all the states that are seen in an infinite population in an instant in time. Therefore, the survival curves can be thought of as the equivalent of a first order decay in solution kinetics and can therefore be fit to an exponential function.37 This fit yields the average lifetime of the binding events or duration (τon) in s and the rate of detachment = 1/τon of the QD-MLCK from SMM in s−1. In our case double exponential fits were justified as described in Methods. For SMM (absence of Ca2+CaM and ATP; Figure 7a), the fit gave τons of 1.09 ± 0.17 s (36%) and 8.23 ± 0.48 s (64%), respectively. In the presence of Ca2+CaM and ATP (Figure 7b), the fit gave a very similar result, τons of 1.25 ± 0.06 s (49%) and 12.39 ± 0.48 s (51%), respectively.
The shorter lifetimes give MLCK dissociation rate constants (1/τon) of 0.92 SMM s−1 (from SMM or k−1) and 0.80 SMM s−1 (from pSMM or k+2) (Table 1) indicating that MLCK dissociates from SMM and pSMM at similar rates. The value for MLCK dissociation from pSMM of 0.80 SMM s−1 is similar to the rate constant kpo of 1.17 SMM heads s−1 MLCK−1 estimated from Figure 1, suggesting the MLCK dissociation from pSMM is the step that is limiting the rate of phosphorylation seen in Figure 1. The longer lifetimes (slower dissociation rates) from the double exponential fits were much slower than kpo, suggesting a process that is not in effect in Figure 1a. Most likely, these slower dissociation rates are due to multivalent QD-MLCK interactions with more than one closely spaced SMM molecule (mentioned above), a situation that is possible even at the very low SMM densities used in the experiments.
Estimation of the MLCK-SMM association rate constants
It is known from equilibrium binding experiments 20 that the binding of MLCK to pSMM is weaker than to SMM. Thus, our data suggest that the association rate constant for MLCK binding to pSMM is slower than for MLCK binding to SMM. To directly measure the MLCK-SMM association rate constant at a single molecule level, a QD-MLCK molecule must be tracked in order to record the time it spends diffusing prior to binding to a SMM molecule. Unfortunately, the diffusion of a single QD is impossible to track. Thus, we estimate the MLCK-SMM association rate constant, k+1, as k+1 = k−1/Kd, where Kd is the dissociation constant (which we measured as ~3.8 μM in the presence of Ca2+CaM for SMM filaments using the same method as Sellers et al 20) and k−1 is the MLCK-SMM dissociation rate constant (which we estimate above to be 0.92 s−1). Thus, k+1 = 0.92 s−1 / 3.8 μM = 0.24 s−1μM−1 (Scheme Ib). For pSMM the corresponding Kd (with Ca2+CaM) is difficult to measure because it is very weak. Nevertheless, our measurements using the same method as mentioned above (20; data not shown) give a lower limit of Kd of 60 μM. The association rate constant, k−2, for pSMM is estimated by k+2 / Kd = 0.80 s−1 /60 μM, which is <0.01 s−1μM−1 (Scheme Ib). Therefore, MLCK associates at least 24 fold faster (0.24/.01) to SMM than to pSMM. The C-terminal end of the telokin domain of MLCK is important for SMM binding.20 This region is very negatively charged and likely is attracted to the positively charged region at the SMM head-tail junction where it is known to bind. Therefore, it makes molecular sense that adding negative charges on the pRLCs near this junction would slow of the rate of association.
In Figure 1, there are approximately 1150 SMM per μm2.29 If MLCK molecules diffuse within a methylcellulose-capped depth of 100-200 nm, there are 575-1150 SMM per 10−16 L, or roughly 9.5-19 μM SMM, giving an observed association rate of (0.24 s−1 μM−1)(9.5 - 19 μM) = 2.3-4.6 s−1 (Scheme Ib). This indicates that the rate at which MLCK phosphorylates SMM under the conditions of Figure 1 is likely limited by the rate of both the SMM-MLCK dissociation and association steps, because both rates are similar to the initial rate of phosphorylation (kpo) of 1.17 s−1 MLCK−1 determined from data in Figure 1.
Implications to kinetics of interactions of MLCK with SMM in smooth muscle
Scheme Ic is a summary of the observed rates in the MLCK-SMM kinetic cycle that we estimate to be in effect in smooth muscle based upon our measurements in the in vitro system. The calculated observed association rate (2.3-4.6 s−1) depends on the SMM concentration used on the coverslip, which is about 5 to 10-fold lower than found in smooth muscle. 5-7 Therefore, in muscle it is likely that the association rate would be 5 to 10-fold faster or 11-46 s−1. These values are much faster than the dissociation rate from pSMM measured here (k+2 = 0.80 s−1). Therefore, if the dissociation rate in muscle is similar to what we measure in the in vitro system, then dissociation of MLCK from pSMM would be the rate limiting step in the SMM phosphorylation kinetic cycle in muscle.
To use the above information to predict rates in muscle, we estimate that the concentration of pSMM is 20% of SMM at the initiation of contraction (9.5-19 μM). This gives a rate of association of MLCK to pSMM in muscle to be < (9.5-19 μM)(0.01 s−1 μM−1) or < 0.1 to 0.2 s−1 (Scheme Ic). This rate is 55-460 times slower than the rate of association to SMM (11-46 s−1) expected in the muscle. Therefore, an MLCK molecule that detaches from pSMM is ~55-460 times more likely to attach to a SMM than to a pSMM. This may be a mechanism to speed phosphorylation rates in the muscle, by kinetically partitioning the MLCK molecules, which are in limiting amounts and probably restricted in space, to productive interactions with only those SMM that have not yet been phosphorylated.
If the kinetics measured here accurately reflect what is happening in smooth muscle, we expect that the initial rate of SMM phosphorylation in situ would be of similar magnitude to our proposed rate-limiting step of dissociation of Ca2+CaM-MLCK from pSMM of 0.80 s−1. Injeti et al 7 measured this rate along with measurements of in situ MLCK and SMM concentrations in molar units and found that the in situ MLCK activity in adult sheep carotid arteries to be 1.4 heads s−1 MLCK−1, very similar to our value of 0.80 s−1. In other studies in which the MLCK:SMM ratio was not measured, the corresponding value can be calculated by estimating the MLCK:SMM ratio to be 1 MLCK to 40 unphosphorylated heads (20 SMM). For the trachealis muscle, values range from ~4.4-15 for neural stimulation12, 13 to ~0.27-0.46 heads s−1MLCK−1 for stimulation with a cholinergic agonist.14, 15 These values range across the value of our proposed rate-limiting step. Therefore, the kinetics measured in this in vitro system are consistent with the measured kinetics of SMM phosphorylation in smooth muscle.
In summary, we have developed an in vitro assay to measure the rate of surface-attached SMM phosphorylation and the rate of mechanical activation of SMM to move actin filaments. A major advantage of our system is that single molecule measurements, such as single molecule dissociation rates can also be made under very similar conditions. Since concentrations of the constituents in the assay are known with reasonable certainty, association rate constants can be estimated as well. Comparisons/correlations of different kinetic measurements can be applied to a simple kinetic model to make predictions about the rate limiting steps in the kinetic cycle of MLCK-SMM interactions in muscle. We are the first to show that SMM phosphorylation has an insignificant effect on MLCK-SMM dissociation rates, meaning that the weakening of the SMM:MLCK equilibrium binding affinity by phosphorylation is due to a decrease in the association rate constant. By comparing the rate of phosphorylation of SMM to the dissociation rate of MLCK from pSMM, and using these values to predict association rates in muscle, we conclude that dissociation of MLCK from pSMM would be the rate limiting step in the SMM phosphorylation kinetic cycle in muscle. We believe that this assay system has the potential to study effectors of SMM activation kinetics such as actin, phosphatase, small molecules, MLCK mutations, and filamentous SMM.
Supplementary Material
Scheme I.
Definition of model and kinetic parameters. (a) Rate constants defined for in vitro assay system and used for Matlab fit to data in Figure 1a. (b) Rate constants measured and calculated along with rates (in parentheses) predicted using protein concentrations in the in vitro assay system. (c) Predicted rates for same kinetic scheme as (b) but under condtions found in smooth muscle. Values are either directly measured (Table 1) or calculated from estimates of protein concentrations in smooth muscle (see text), using the rate and equilibrium constants from (b).
Acknowledgments
Funding Source Statement. This work was supported by DHHS-NIH-NHLBI grant 5R01HL110214 (to C.R.C., K.C.F., and J.E.B.), and NIH P20 grant RR018751-07 (to J.E.B.) and Canadian Institutes of Health Research Grant MOP-111262 (to M.P.W.). M.P.W. is an Alberta Innovates-Health Solutions Scientist and Canada Research Chair (Tier 1) in Vascular Smooth Muscle Research.
Abbreviations
- SMM
smooth muscle myosin purified from chicken gizzards (unphosphorylated unless indicated otherwise)
- p-SMM
phosphorylated SMM
- CaM
calmodulin and Ca2+CaM, with calcium bound
- MLCK
smooth muscle myosin light chain kinase
- RLC
smooth muscle myosin regulatory light chain
- pRLC
phosphorylated smooth muscle myosin regulatory light chain
- TRITC
tetramethylrhodamine isothiocyanate
- TRITC-actin
actin labeled with TRITC-phalloidin
- Tm
smooth muscle tropomyosin
- DMCS
dimethylchlorosilane
- TIRF
total internal reflection fluorescence
- ELISA
enzyme-linked immunosorbent assay
- QD
streptavidin-coated quantum dot
- ATPγS
adenosine 5′-[γ-thio]triphosphate
Footnotes
Supporting Information Available. This material is available free of charge via the Internet at http://pubs.acs.org. Figure S1 provides additional details about the method to determine rate of dissociation of QD-MLCK from surface-attached SMM and pSMM relating to Figures 6 and 7.
REFERENCES
- 1.Sobieszek A. Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin. Eur. J. Biochem. 1977;73:477–483. doi: 10.1111/j.1432-1033.1977.tb11340.x. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu. Rev. Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- 3.Hartshorne DJ, Siemankowski RF. Regulation of smooth muscle actomyosin. Annu. Rev. Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- 4.Walsh MP, Hinkins S,, Dabrowska R, Hartshorne DJ. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- 5.Dabrowska R, Hinkins S, Walsh MP, Hartshorne DJ. The binding of smooth muscle myosin light chain kinase to actin. Biochem. Biophys. Res. Commun. 1982;107:1524–1531. doi: 10.1016/s0006-291x(82)80172-1. [DOI] [PubMed] [Google Scholar]
- 6.Ruegg JC. Calcium in Muscle Activation: A Comparative Approch. In: Burggren W, Ishii S, Johansen K, Langer H, Neuweiler G, Randall DJ, Farner DS, editors. Zoophysiology and Ecology. Springer-Verlag; Berlin: 1988. pp. 201–235. [Google Scholar]
- 7.Injeti ER, Sandoval RJ, Williams JM, Smolensky AV, Ford LE, Pearce WJ. Maximal stimulation-induced in situ myosin light chain kinase activity is upregulated in fetal compared with adult ovine carotid arteries. Am. J. Physiol. Heart. Circ. Physiol. 2008;295:H2289–H2298. doi: 10.1152/ajpheart.00606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc. Natl. Acad. Sci. U S A. 2004;101:6279–6284. doi: 10.1073/pnas.0308742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geguchadze R, Zhi G, Lau KS, Isotani E, Persechini A, Kamm KE, Stull JT. Quantitative measurements of Ca2+/calmodulin binding and activation of myosin light chain kinase in cells. FEBS Lett. 2004;557:121–124. doi: 10.1016/s0014-5793(03)01456-x. [DOI] [PubMed] [Google Scholar]
- 10.Hong F, Haldeman BD, Jackson D, Carter M, Baker JE, Cremo CR. Biochemistry of smooth muscle myosin light chain kinase. Arch. Biochem. Biophys. 2011;510:135–146. doi: 10.1016/j.abb.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J. Biol. Chem. 1994;269:9912–9920. [PubMed] [Google Scholar]
- 12.Kamm KE, Stull JT. Myosin phosphorylation, force, and maximal shortening velocity in neurally stimulated tracheal smooth muscle. Am. J. Physiol. 1985;249:C238–247. doi: 10.1152/ajpcell.1985.249.3.C238. [DOI] [PubMed] [Google Scholar]
- 13.Miller-Hance WC, Miller JR, Wells JN, Stull JT, Kamm KE. Biochemical events associated with activation of smooth muscle contraction. J. Biol. Chem. 1988;263:13979–13982. [PubMed] [Google Scholar]
- 14.de Lanerolle P, Stull JT. Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J. Biol. Chem. 1980;255:9993–10000. [PubMed] [Google Scholar]
- 15.Silver PJ, Stull JT. Phosphorylation of myosin light chain and phosphorylase in tracheal smooth muscle in response to KCl and carbachol. Mol. Pharmacol. 1984;25:267–274. [PubMed] [Google Scholar]
- 16.Adelstein RS, Klee CB. Purification of smooth muscle myosin light-chain kinase. Methods Enzymol. 1982;85(Pt B):298–308. doi: 10.1016/0076-6879(82)85029-5. [DOI] [PubMed] [Google Scholar]
- 17.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, De Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. J. Physiol. 2003;549:489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong F, Haldeman BD, John OA, Brewer PD, Wu YY, Ni SW, Wilson DP, Walsh MP, Baker JE, Cremo CR. Characterization of tightly - associated smooth muscle myosin - light chain kinase - calmodulin complexes. J. Mol. Biol. 2009;390:879–892. doi: 10.1016/j.jmb.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver DL, Vorotnikov AV, Watterson DM, Shirinsky VP, Sellers JR. Sites of interaction between kinase-related protein and smooth muscle myosin. J. Biol. Chem. 1997;272:25353–25359. doi: 10.1074/jbc.272.40.25353. [DOI] [PubMed] [Google Scholar]
- 20.Sellers JR, Pato MD. The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J. Biol. Chem. 1984;259:7740–7746. [PubMed] [Google Scholar]
- 21.Sundberg M, Rosengren JP, Bunk R, Lindahl J, Nicholls IA, Tågerud S, Omling P, Montelius L, Månsson A. Silanized surfaces for in vitro studies of actomyosin function and nanotechnology applications. Anal. Biochem. 2003;323:127–138. doi: 10.1016/j.ab.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Ikebe M, Hartshorne DJ. Effects of Ca2+ on the conformation and enzymatic activity of smooth muscle myosin. J. Biol. Chem. 1985;260:13146–13153. [PubMed] [Google Scholar]
- 23.Ellison PA, Sellers JR, Cremo CR. Kinetics of smooth muscle heavy meromyosin with one thiophosphorylated head. J. Biol. Chem. 2000;275:15142–15151. doi: 10.1074/jbc.275.20.15142. [DOI] [PubMed] [Google Scholar]
- 24.Wahlstrom JL, Randall MA, Jr., Lawson JD, Lyons DE, Siems WF, Crouch GJ, Barr R, Facemyer KC, Cremo CR. Structural model of the regulatory domain of smooth muscle heavy meromyosin. J. Biol. Chem. 2003;278:5123–5131. doi: 10.1074/jbc.M206963200. [DOI] [PubMed] [Google Scholar]
- 25.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 26.Warshaw DM, Desrosiers JM, Work SS, Trybus KM. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J. Cell Biol. 1990;111:453–463. doi: 10.1083/jcb.111.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smillie LB. Preparation and identification of alpha- and beta-tropomyosins. Methods Enzymol. 1982;85(Pt B):234–241. doi: 10.1016/0076-6879(82)85023-4. [DOI] [PubMed] [Google Scholar]
- 28.Hooft AM, Maki EJ, Cox KK, Baker JE. An accelerated state of myosin-based actin motility. Biochemistry. 2007;46:3513–3520. doi: 10.1021/bi0614840. [DOI] [PubMed] [Google Scholar]
- 29.Harris DE, Warshaw DM. Smooth and skeletal muscle myosin both exhibit low duty cycles at zero load in vitro. J. Biol. Chem. 1993;268:14764–14768. [PubMed] [Google Scholar]
- 30.Schlager G. Statistics for Biochemists. Madeline Press; Princeville, HI: 1999. [Google Scholar]
- 31.Mathews JH, Fink KK. Numerical Methods Using Matlab. 4th ed. Prentice-Hall Inc.; Upper Saddle River, New Jersey, USA: 2004. [Google Scholar]
- 32.Harris DE, Stromski CJ, Hayes E, Warshaw DM. Thiophosphorylation independently activates each head of smooth muscle myosin in vitro. Am. J. Physiol. 1995;269:C1160–1166. doi: 10.1152/ajpcell.1995.269.5.C1160. [DOI] [PubMed] [Google Scholar]
- 33.Harris DE, Work SS, Wright RK, Alpert NR, Warshaw DM. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. J. Muscle. Res. Cell Motil. 1994;15:11–19. doi: 10.1007/BF00123828. [DOI] [PubMed] [Google Scholar]
- 34.Sellers JR, Chock PB, Adelstein RS. The apparently negatively cooperative phosphorylation of smooth muscle myosin at low ionic strength is related to its filamentous state. J. Biol. Chem. 1983;258:14181–14188. [PubMed] [Google Scholar]
- 35.Mabuchi Y, Mabuchi K, Stafford WF, Grabarek Z. Modular structure of smooth muscle Myosin light chain kinase: hydrodynamic modeling and functional implications. Biochemistry. 2010;49:2903–2917. doi: 10.1021/bi901963e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkhoff GD. Proof of the Ergodic Theorem. Proc. Natl. Acad. Sci. USA. 1931;17:656–660. doi: 10.1073/pnas.17.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight AE, Veigel C, Chambers C, Molloy JE. Analysis of single-molecule mechanical recordings: application to acto-myosin interactions. Prog. Biophys. Mol. Biol. 2001;77:45–72. doi: 10.1016/s0079-6107(01)00010-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.