Abstract
This longitudinal study was designed to investigate trajectories of nonverbal cognitive ability in adolescents with fragile X syndrome with respect to the relative influence of FMRP, autism symptom severity, and environmental factors on visualization and fluid reasoning abilities. Males and females with fragile X syndrome (N = 53; ages 10 - 16 years) were evaluated with the Leiter-R at up to four annual assessments. On average, IQ declined with age. FMRP levels predicted change in fluid reasoning, but not in visualization. The role of FMRP in the neural development that underlies the fragile X syndrome cognitive phenotype is discussed.
Keywords: fragile X syndrome, cognitive development, IQ, FMRP, autism, Leiter-R
In many neurodevelopmental disorders, including Williams syndrome (Fisch et al., 2010; but see Fisch et al., 2007) and neurofibromatosis type 1 (Fisch et al., 2007), intellectual ability is reported to be relatively stable with increasing age despite overall cognitive delays. In contrast, slowing in the rate of intellectual development (i.e., a decline in IQ over time) has been reported in other syndromes, such as chromosome 22q.11 deletion syndrome (Gothelf et al., 2005), and fragile X syndrome (FXS; Fisch et al., 2010; Fisch et al., 2007; Hall, Burns, Lightbody, & Reiss, 2008). Although the mechanisms underlying these distinct patterns of cognitive development are not well understood, the failure to keep pace with the rate of typical cognitive development seen in some populations likely results from interactions among multiple genetic, neurodevelopmental, behavioral, and environmental factors. In some cases, declines in IQ may be correlated with specific aspects of behavior or brain functioning. For example, slowing of cognitive development with age is associated with symptoms of schizophrenia in 22q deletion syndrome (Gothelf et al., 2005). It also seems reasonable to speculate that cognitive trajectories may be determined in part by etiology-specific variations in neural development at particular stages of life. The current study was designed to elucidate factors that influence developmental trajectories in neurodevelopmental disorders by examining the most common known inherited cause of intellectual disability, FXS (prevalence of 1/2500; Hagerman, 2008), during a pivotal period of neural development and learning.
FXS is caused by an expansion of a CGG sequence in the FMR1 gene of the X chromosome (Verkerk et al., 1991). The protein produced by the FMR1 gene, fragile X mental retardation protein (FMRP), is known to be critical for neural development (Bassell & Warren, 2008); however, production of FMRP is dramatically reduced in individuals with the full mutation (> 200 CGG repeats) associated with FXS. Across individuals with FXS, protein levels can vary due to mosaicism among males and differences in X-activation ratio among females (Tassone, Hagerman, Chamberlain, & Hagerman, 2000). Because FXS is a well-described single-gene cause of intellectual disability, it is an ideal disorder in which to examine the relationship between biological markers (e.g., production of FMRP) and behavioral outcomes, including the trajectory of cognitive development. Moreover, the role of behavioral and environmental factors can be considered relative to the contribution of FMRP to identify the most critical influences on intellectual development in this disorder.
Nonverbal Cognitive Development and Its Predictors in Fragile X Syndrome
Prospective studies of nonverbal cognitive development in males with FXS have shown that IQ declines with age, reflecting a widening gap from typically developing peers over time (Fisch et al., 2010; Skinner et al., 2005). Of particular interest is the fact that this relative slowing in rate of intellectual development tends to occur during later childhood and adolescence, although this does not preclude the possibility that absolute cognitive ability will continue to show growth (Roberts et al., 2009; Skinner et al., 2005). Several studies have examined how cognition and its rate of change in FXS may be impacted by factors such as expression of FMRP, autism symptoms, and environmental factors; however, the findings have been inconsistent (Bailey, Hatton, Skinner, & Mesibov, 2001; Loesch, Huggins, & Hagerman, 2004). The majority of studies have focused on males. Bailey et al. (2001) reported that FMRP was related to level of cognitive ability, but not its rate of change, for boys with FXS, whereas autism symptoms assessed with the Childhood Autism Rating Scale (CARS) were related to both cognitive level and rate of change. In contrast, Skinner and colleagues (2005) found that both FMRP and maternal education failed to account for variance in level or rate of change in either IQ or intellectual growth scores in males with FXS during childhood and early adolescence. In the latter study, autism symptoms reported on the CARS predicted level of ability on Leiter-R subtests assessing visual organization and disembedding (hereafter, visualization), suggesting that the impact of autism symptoms might be limited to certain cognitive domains.
Few studies have examined the course of cognitive development in females with FXS with respect to FMRP or autism symptoms. Fisch and colleagues (2010) reported that full-scale IQ scores declined over a period of two years in both males and females with FXS between the ages of 4 and 15 years, but did not examine predictors of change. Hatton and colleagues (2009) reported that CARS scores, but not FMRP, predicted growth in developmental age-equivalent scores for 15 young females with FXS. In contrast, Hall and colleagues (2008) found a significant positive correlation between FMRP and intellectual ability in males and females with FXS, but did not examine autism symptoms. Finally, in a cross-sectional study, Dyer-Friedman et al. (2002) reported that FMRP was associated with IQ for males, but not females, whereas the home environment was related to IQ for both males and females.
Thus, previous research suggests that FMRP, autism symptoms, and aspects of the environment may be important predictors of the developmental trajectory of cognition in those with FXS; however, results are inconsistent across studies and across genders. Unfortunately, most studies have been constrained by exclusion of one gender, small sample sizes, large age ranges, and/or a small number of longitudinal assessments (e.g., only two time points). Finally, no study has utilized a gold-standard instrument of autism symptom severity (e.g., a diagnostic evaluation such as the Autism Diagnostic Observation Schedule; ADOS). Thus, an important step in resolving the discrepancies in previous research would be to simultaneously examine males and females over the course of a relatively narrow developmental period, while taking into account key biological, behavioral, and environmental factors. In addition, it would also be valuable to consider cognition within a neurodevelopmental framework, in which distinctions are made among cognitive abilities that rely differentially on brain regions that develop atypically in individuals with FXS.
Neural Substrates of Nonverbal Cognitive Development
In typical development, the acquisition of cognitive skills relies heavily upon the development of specific brain systems at particular stages of life. Adolescence is known to be an especially important time for the development of neural systems underlying logical problem solving, abstract reasoning, and formulation of rules (Wright, Matlen, Baym, Ferrer, & Bunge, 2008). Cattell and others (Carroll, 1993; Horn & Cattell, 1966) have referred to such skills as fluid reasoning abilities. Over the course of adolescence, changes in neural organization and functioning in typical development are mirrored by gains in fluid reasoning (Rubia et al., 2006). Neural development during adolescence is particularly significant within frontostriatal regions and prefrontal cortex, which are associated with cognitive abilities on which fluid reasoning relies, such as working memory and cognitive control (Rubia et al., 2006). Visualization has been distinguished from fluid reasoning (Horn & Cattell, 1966). Performance on visualization tasks requiring working memory and the neural substrates on which such performance relies no doubt also continue to develop during adolescence (Kwon, Reiss, & Menon, 2002). However, foundational visualization abilities (e.g., form and motion perception) supported by ventral and dorsal pathways reach adult-like function late in childhood or early in adolescence (i.e., by age 12; Parrish, Giaschi, Boden, & Dougherty, 2005).
Evidence from neuroscience and neuropsychology suggests that a reduction in FMRP is likely to affect various neural systems to different extents, resulting in some skills that are more severely impacted than others. For example, FXS is associated with atypical development of several specific neural structures, including an enlarged caudate nucleus and a reduced cerebellar vermis (Gothelf et al., 2008). Interestingly, size of the caudate and cerebellar vermis in FXS is associated both with level of FMRP expression and aspects of cognitive functioning (e.g., IQ and executive functioning, of which working memory is one commonly included component; Gothelf et al., 2008; Mostofsky et al., 1998). Particular cognitive weaknesses in FXS have also been associated with abnormal activation of specific brain regions. Performance on tasks of working memory and executive control, which are relative weaknesses for both males and females with FXS (Kirk, Mazzocco, & Kover, 2005; Lanfranchi, Cornoldi, Drigo, & Vianello, 2009), is related to frontostriatal activation, including the ventrolateral prefrontal cortex (Hoeft et al., 2007). Taken together, this literature suggests that aspects of nonverbal intellectual functioning that build upon working memory and executive function, such as fluid reasoning, may be particularly sensitive to variations in FMRP expression. This may be especially apparent during adolescence, which is when these skills develop most rapidly.
Research Aims
The purpose of the current study was to elucidate the nature of the slowing of cognitive development for adolescents with FXS during a crucial period of neural and intellectual development by separately examining two aspects of cognition: visualization and fluid reasoning. The distinction between visualization and fluid reasoning abilities is well supported by the Cattell-Horn-Carroll theory of intelligence, on which many batteries of cognitive assessment are based (Carroll, 1993; Horn & Cattell, 1966). Moreover, as noted, the distinction is supported by findings concerning differences in neural substrates and maturational timetables. Given that the neural underpinnings of fluid reasoning typically mature during adolescence and might be particularly sensitive to reduced FMRP, a characterization of such trajectories could substantially contribute to our understanding of the cognitive phenotype of FXS. In this study, we used a prospective longitudinal design with four annual assessments of nonverbal cognition to investigate (1) the trajectory of nonverbal intellectual development in males and females with the FXS full mutation in terms of absolute ability and ability relative to age expectations and (2) the separate trajectories of visualization and fluid reasoning abilities. In each case, we examined the extent to which biological, behavioral, and environmental variables account for variability in age-related trajectories among individuals with FXS. We hypothesized that higher levels of FMRP, lower levels of autism severity, and higher socioeconomic status would independently predict higher cognitive performance. Furthermore, we hypothesized that the relationship of FMRP to cognition would be stronger for fluid reasoning than for visualization abilities.
Method
Participants
Fifty-three adolescents with FXS (37 males; 16 females) participated in the current study. Participants ranged in age from 10 to 16 years at the time of initial enrollment in the study (M = 12.61, SD = 1.68). Families were recruited for this research through newspaper advertisements, nationwide radio announcements, a university registry of families with children who have developmental disabilities, and postings on internet sites, listservs and newsletters of developmental disability organizations. Parents of all study participants provided written informed consent. The study was approved by Institutional Review Boards (IRB) at the University of Wisconsin-Madison, the New York State Institute for Basic Research, and the University of California, Davis. The participants described here overlap with samples reported in other studies drawn from the same larger project (e.g., McDuffie et al., 2010).
Written documentation confirming an FMR1 full mutation based on molecular genetic testing was obtained for all participants. Of those from whom a blood sample was collected as described below, approximately 20% of males, despite having a full mutation, were mosaic for CGG repeat length or methylation (Brown et al., 1993). The remainder had a fully methylated full mutation. Table 1 displays descriptive statistics at Time 1 for all study participants, separated according to gender. The average number of annual assessments per participant was 3.6 (range: 1 - 4). The participant sample included 6 mixed-gender sibling pairs.
Table 1.
Participant Characteristics at Time 1
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| n | Mean | (SD) | Range | n | Mean | (SD) | Range | |
| Chronological age | 37 | 12.86 | (1.73) | 10 - 16 | 16 | 12.04 | (1.47) | 10 - 16 |
| Nonverbal IQa | 36 | 46.44 | (9.11) | 36 - 71 | 16 | 69.56 | (15.35) | 46 - 98 |
| Nonverbal growth scorea | 36 | 466.47 | (9.34) | 446 - 489 | 16 | 482.06 | (11.10) | 462 - 502 |
| % cells expressing FMRP | 30 | 4.08 | (7.60) | 0 - 30 | 14 | 48.32 | (4.51) | 34 - 51 |
| Autism symptom severityb | 36 | 5.53 | (3.02) | 1 - 10 | 14 | 3.14 | (2.77) | 1 - 9 |
Assessed with the Leiter-R Brief IQ subtests.
Assessed with the ADOS.
Assessment of Nonverbal Cognition
Nonverbal cognitive abilities were assessed using the Leiter International Performance Scale-Revised (Leiter-R) Brief IQ Screener (Roid & Miller, 1997). The Leiter-R was developed within the hierarchical model of cognitive abilities outlined by Carroll (1993; e.g., visualization, fluid reasoning) and the factor structure of the Leiter-R subtests reflects this. During adolescence, Figure Ground and Form Completion assess a domain of Visualization, and Fluid Reasoning is assessed with Repeated Patterns and Sequential Order (Roid & Miller, 1997). The four Leiter-R Brief IQ subtests were administered at an initial visit and at one-year intervals over the course of three years. In the Figure Ground subtest, the examinee identifies forms embedded in complex backgrounds. In the Form Completion subtest, the examinee must utilize fragments of an object or abstract design to determine the whole design, which is chosen from alternatives. The two fluid reasoning subtests, Sequential Order and Repeated Patterns, require identification of patterns or rules. In the Sequential Order subtest, the examinee must identify the stimulus or stimuli that complete a sequence. For Repeated Patterns, the examinee is shown repetitive sequences of pictures or figures that have missing elements and must determine how to order the missing elements to retain the pattern. These tasks are explained to the participant via simple pantomime and all scored responses from the participant are nonverbal.
Based on these four subtests, the Leiter-R yields a standardized nonverbal Brief IQ score. In addition, a growth score can be derived based on the participant’s raw scores on the Brief IQ subtests. Growth scores reflect the examinee’s absolute level of ability at a given time point, rather than ability relative to age norms. Leiter-R growth scores are well-suited for examining change over time because they are consistent across subtests and ages (Roid & Miller, 1997). Growth scores are anchored at 500, which is comparable to the abilities of a ten-year-old; scores range continuously from about 380 to 560 (Roid & Miller, 1997). The Leiter-R subtests used here have excellent psychometric properties: for 11 - 20 year-olds, internal-consistency reliability for the Brief IQ screener is reported as .89, test-rest reliability is reported as r =. 96, and concurrent validity is high between Brief IQ and WISC-III Full Scale IQ (r = .85; Roid & Miller, 1997).
The majority of participants completed all four subtests at each time point. For six participants, individual subtest data were missing for a single time point due to examiner error (n = 4) or non-compliance with the subtest (n = 2). For these cases, composite Brief IQ standard scores were prorated using the sum of the standard scores from the two or three subtests that were successfully completed. Similarly, growth scores were imputed for these six participants by averaging the growth scores obtained on the completed subtests. This method was only used when at least two subtests were completed in a valid manner. For one participant, two annual visits resulted in either no valid subtests or one valid subtest; Brief IQ standard scores and growth score data from these visits were not included in the present analyses.
To distinguish fluid reasoning and visualization abilities, composite scores were created by averaging the standard scores or growth scores from Figure Ground and Form Completion (visualization), and from Sequential Order and Repeated Patterns (fluid reasoning). Subtest standard scores have a mean of 10. In three cases, the composite score was comprised only of one subtest due to missing data at a particular time point.
Predictors of Nonverbal Cognition
We considered biological, behavioral, and environmental predictors of nonverbal cognitive development, all of which were assessed at the initial visit unless otherwise noted.
FMRP
Blood samples were obtained from 44 participants (30 males) to measure levels of FMRP expression. Employing the method of Willemsen et al. (1997), the proportion of cells that expressed the FMRP protein was determined for each participant (using a sample of 200 cells for males and 400 cells for females). The average percent of cells that expressed the protein was 4 for males (SD = 8, range = 0 - 30; 21 of 30 males expressed no FMRP) and 48 for females (SD = 5, range = 34 - 51). FMRP levels were not available for nine participants because samples were not collected due to participant or parent refusal to participate in a blood draw (n = 7) or because of logistical issues (n = 2). To examine whether there were differences on our primary cognitive and behavioral measures between those individuals for whom FMRP was collected and those with missing FMRP values, we conducted a series of t-tests to compare these groups. The groups did not differ significantly on Leiter-R Brief IQ standard scores and growth scores or autism symptom severity scores at Time 1 (ps > .25). Therefore, it was concluded that it would be appropriate to interpret the FMRP data from the participants from whom it was collected with minimal concern about systematic bias due to missing data. A sensitivity analysis described below further corroborated this conclusion.
Autism symptom severity
Participants were evaluated for severity of autism symptoms by a research-reliable examiner using the ADOS (Lord, Rutter, DiLavore, & Risi, 1999). In most cases (n = 43), the ADOS was administered at the first annual visit; however for 9 participants, scheduling difficulties required administration at the second visit. Autism symptom severity was assessed only at one visit because we were interested in the relationship between autism severity and subsequent cognitive development. Autism symptoms were quantified by calculating a severity score based on each participant’s performance on the ADOS. Severity scores were derived using an algorithm developed by Gotham, Pickles and Lord (2009). This algorithm yields a severity score between 1 and 10, and can be applied across ADOS Modules 1, 2 and 3. Severity scores were obtained using this standard method for all but four participants. One male with FXS had just passed the age range covered by the Module 1 norms (age at test administration = 15 years, 2 months); for this participant a severity score was derived based on Module 1 norms for the 6 - 14 year-old age group. A severity score could not be obtained for two female participants because they received Module 4 of the ADOS, and the severity algorithm cannot be applied to this module. Scores for these two individuals, along with a fourth participant to whom the ADOS was not administered due to logistical reasons, were treated as missing for all analyses that included this measure. According to Gotham et al. (2009) severity scores, 60% of males and 28% of females fell into the range of autism or autism spectrum.
Environmental factors
Three environmental measures were collected to index the family background of participants enrolled in the study: maternal IQ, parental education level, and family income. Maternal IQ was assessed at Time 1 using the Kaufmann Brief Intelligence Test, Second Edition (KBIT-2; Kaufman & Kaufman, 2005). Parental education levels and income were reported on a family background questionnaire administered at each time point. Education levels were measured separately for mothers and fathers on a scale ranging from 1 (< 9th grade education) up to 8 (advanced graduate degree). Parental education levels were calculated as the average of maternal and paternal values. In cases of single parent families, values for only the primary caregiver were used. Household income was measured in $10,000 increments on a scale of 1 (> $10,000) to 16 (> $150,000).
A composite measure indexing the environmental background of participants was created by taking the average of the z-scores for maternal IQ scores, parental education, and income. For those individuals with missing data for any contributing variable (n = 6), the average was based on the remaining variables.
Statistical Analysis
Hierarchical linear models (also known as multilevel models) were used to estimate change in nonverbal cognitive abilities over time, as well as individual differences in levels of and rate of change in nonverbal cognitive abilities. Analyses are reported according to adapted guidelines from Dedrick et al. (2009) and were conducted using the program HLM for Windows Version 6.06 (Raudenbush, Bryk, Cheong, Congdon, & du Toit, 2008). Slopes were estimated to reflect changes in individuals’ scores based on chronological age. A fixed quadratic term composed of chronological age squared was included to assess whether the rate of change in cognitive functioning varied during adolescence. For ease of interpretation, chronological age was anchored at 14 years (the overall mean age of participants over the course of the study) in all models. Autism symptom severity and the environmental composite were centered on the overall mean (i.e., grand-centered). We neither centered nor transformed FMRP to ensure that coefficients would be directly interpretable. (Note that results with log-transformed FMRP were comparable with the exceptions noted in Tables 2 and 3.) In consideration of the distributional assumptions of HLM, we used growth scores in addition to standard scores as dependent variables. Given our moderate number of participants, we report model-based standard errors; robust standard errors were generally smaller and the pattern of results was consistent with those reported.
Table 2.
Estimates for Predictors of Leiter-R Brief IQ Standard and Growth Scores (n = 41)
| IQ | Growth Scores | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Fixed Effect | Coefficient | (SE) | p | Coefficient | (SE) | p |
| Intercept | ||||||
| Intercept (mean) | 44.70 | 3.00 | < .001* | 467.26 | 2.32 | < .001* |
| Autism Severity | −1.22 | .81 | .139 | −1.02 | .62 | .107 |
| FMRP | 41.44 | 11.53 | .001* | 35.36 | 8.88 | .001* |
| Environment | −.91 | 2.58 | .725 | −.50 | 1.98 | .801 |
| Slope | ||||||
| Age (rate of change) | −1.38 | .50 | .010* | .73 | .46 | .119 |
| Autism Severity | −.10 | .13 | .423 | −.10 | .11 | .406 |
| FMRP | 2.60 | 1.98 | .197 | 2.34 | 1.81 | .206 |
| Environment | −.20 | .41 | .637 | .16 | .37 | .671 |
| Slope | ||||||
| Age Squared | .17 | .10 | .107 | −.11 | .10 | .288 |
Note. Intercept reflects a chronological age of 14 years. Models estimated with log-transformed FMRP yielded similar results, with the exception of the fact that the positive effect of age for growth scores was also significant, t(37) = 3.50, p = .002.
Table 3.
Estimates for Predictors of Visualization and Fluid Reasoning Standard and Growth Scores (n = 41)
| IQ | Growth Scores | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Fixed Effect | Coefficient | (SE) | p | Coefficient | (SE) | p |
| Visualization Intercept | ||||||
| Intercept (mean) | 2.73 | .52 | < .001* | 474.21 | 2.70 | < .001* |
| Autism Severity | −.18 | .14 | .213 | −.87 | .73 | .236 |
| FMRP | 6.83 | 2.00 | .002* | 38.88 | 10.36 | .001* |
| Environment | −.18 | .45 | .693 | −.99 | 2.32 | .672 |
| Visualization Slope | ||||||
| Age (rate of change) | −.25 | .10 | .020* | .70 | .53 | .198 |
| Autism Severity | −.02 | .02 | .474 | −.12 | .13 | .380 |
| FMRP | .04 | .40 | .929 | .97 | 2.10 | .648 |
| Environment | −.07 | .08 | .405 | −.08 | .42 | .852 |
| Visualization Slope | ||||||
| Age Squared | .03 | .02 | .143 | −.07 | .12 | .542 |
|
| ||||||
| Fluid Reasoning Intercept | ||||||
| Intercept (mean) | 1.46 | .34 | < .001* | 460.73 | 2.70 | < .001* |
| Autism Severity | −.15 | .09 | .109 | −1.35 | .70 | .063 |
| FMRP | 4.94 | 1.30 | .001* | 38.82 | 10.19 | .001* |
| Environment | −.08 | .30 | .791 | −.23 | 2.25 | .919 |
| Fluid Reasoning Slope | ||||||
| Age (rate of change) | −.11 | .07 | .150 | .73 | .75 | .340 |
| Autism Severity | −.01 | .02 | .604 | −.07 | .19 | .692 |
| FMRP | .61 | .28 | .039* | 4.34 | 3.03 | .161 |
| Environment | .04 | .06 | .450 | .35 | .59 | .555 |
| Fluid Reasoning Slope | ||||||
| Age Squared | .01 | .02 | .564 | −.12 | .18 | .498 |
Note. Intercept reflects a chronological age of 14 years. Models estimated with log-transformed FMRP yielded similar results, with the exception of the fact that the positive effect of age for visualization, t(37) = 2.32, p = .026, and fluid reasoning, t(37) = 2.74, p = .010, growth scores was also significant.
Models were estimated based on two groups of participants. First, trajectories of nonverbal cognitive ability were estimated for the total sample of 53 participants for Leiter-R Brief IQ standard scores and growth scores. Second, the predictors of level and rate of change in abilities were estimated for the sample of 41 participants (29 males; 12 females) for whom data regarding both FMRP and autism symptom severity were available. Given the established relationship between autism and IQ among individuals with FXS (Loesch et al., 2007), autism symptom severity was entered into the model first. Second, FMRP was entered, having controlled for the effect of autism severity. The environmental predictor was added last to address whether socioeconomic factors could account for any remaining variance beyond the contributions of FMRP and autism severity.
Sensitivity analysis
To assess the potential impact of missing data within the models, we re-estimated the models for Brief IQ standard scores and growth scores after imputing FMRP, which had the greatest level of missing data. The nine missing values for FMRP were imputed, using regression imputation, based on the following predictors: gender, information about the FXS diagnosis provided by the parent at entry into the study, maternal IQ, and initial nonverbal IQ. This sensitivity analysis for the sample of 50 participants (41 with observed FMRP, 9 with imputed FMRP) yielded conclusions that were consistent with the observed data. Thus, results for predictors of nonverbal cognitive ability are only reported for the sample of 41 participants for whom they were observed.
Results
Trajectory of IQ and Growth Scores
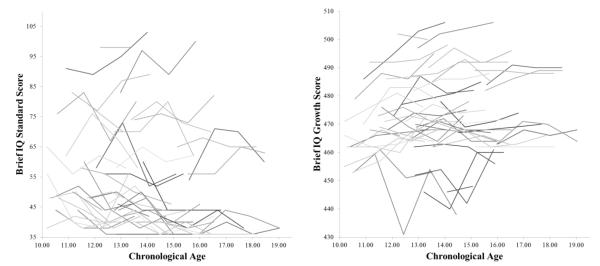
Across the full sample of 53 participants, there was a significant linear decline in IQ with age, with a loss of 1.04 points per year on average, t(52) = −3.16, p = .003. The quadratic term, which tested the possibility that the rate of decline in IQ changed during the course of adolescence, was not significant, p = .102. Figure 1 depicts IQ and growth scores over time and provides visual evidence of a floor effect for standard scores that might mask the true shape of the trajectory for IQ. Of the 193 assessments collected, 34 yielded the lowest possible Brief IQ standard score (i.e., a score of 36) and each of those assessments was contributed by a male. None of the 193 assessments resulted in a growth score at floor.
Figure 1.
Observed trajectories (N = 53) of Leiter-R Brief IQ standard scores (left panel) and growth scores (right panel) among adolescents with FXS. Individuals’ trajectories are shown in different grayscale shades for ease of differentiation.
There was a significant linear increase in growth scores with age, with an additional point gained per chronological year on average, t(52) = 3.43, p = .002. Again, the quadratic term for slope was not significant, p = .067. It should be noted that the variance components for chronological age were not significant (e.g., p = .139 for growth scores); however, deviance tests using full information maximum likelihood estimation comparing a model with and without random slopes (i.e., tests for the significance of the random slope variance and the covariance between random intercept and random slope) demonstrated that allowing random slopes for chronological age yielded a significantly better fit to the data for IQ and growth scores, χ2(2) = 6.44, p = .039 and χ2(2) = 6.44, p = .039, respectively. Given the significant improvement of model fit with random slopes and previous research suggesting that change in IQ might covary with FMRP, at least in terms of differences between males and females (Fisch et al., 2010), we chose to retain random slopes in subsequent analyses.
Predictors of Level of Ability and Rate of Change
Models including predictors of IQ and growth scores were estimated for the 41 participants who had complete data for all predictors. Models were estimated with a linear and quadratic term for chronological age and the predictors of interest: autism symptom severity, FMRP, and the environmental composite. We first assessed the relationship between IQ and autism symptom severity. Autism severity did not predict rate of change in IQ, but was significantly associated with IQ level (i.e., intercept), t(39) = −2.86, p = .007, with an increase of one severity score point associated with a lower IQ of two points. FMRP was added to the model in the next step. FMRP expression was a significant positive predictor of IQ level, t(38) = 3.64, p = .001; a fifty percent increase in FMRP was associated with approximately 20 additional IQ points. After adding FMRP, autism severity was no longer a significant predictor of IQ. The environmental composite accounted for no additional variance. Despite the significant negative slope for IQ, none of the predictors of interest accounted for variance in the rate of change. Of the 152 assessments, 26 assessments (i.e., 25% of assessments from males) yielded the lowest possible standard score. Table 2 presents the final parameter estimates for each of the predictors of IQ and growth scores.
For growth scores, autism symptom severity was a significant negative predictor of level of ability, t(39) = −3.07, p = .004. After adding FMRP, the effect of autism symptoms was no longer significant and the average positive slope for growth scores was also no longer significant. However, FMRP was a significant predictor of level of ability, t(38) = 4.01, p < .001. A fifty percent increase in FMRP was associated with an increase of approximately 17 growth score points. The environmental composite had little effect on the model. As was the case for standard scores, no predictor significantly accounted for individual variability in the rate of change in growth scores. No assessments yielded the lowest possible growth score.
In exploratory analyses, we estimated these models again, but separately for the males (n = 29) and females (n = 12) for whom FMRP and autism symptom severity scores were available. For males, the average slope for IQ was significant and negative; however, the effects of the predictors were no longer detected. For growth scores for males, the average rate of change with age did not differ significantly from zero and no predictors reached significance. The wide variability in cognitive ability with notable floor effects and limited variability in FMRP among males with FXS might account for these null results. For females, no significant effects emerged for IQ and the only significant effect for growth scores was a quadratic slope, β = −.44, t(37) = −2.41, p = .021.
Visualization and fluid reasoning
Models were estimated separately for visualization and fluid reasoning as shown in Table 3. Of the 152 assessments, 27 for visualization (25% of those from males) and 77 for fluid reasoning (17% of those from females; 65% of those from males) yielded the lowest possible standard score. For standard scores, FMRP significantly predicted level of ability for both visualization, t(37) = 3.42, p = .002, and fluid reasoning, t(37) = 3.81, p = .001. An increase in FMRP of fifty percent was associated with an increase in approximately 3.5 standard score points in visualization and 2.5 standard score points in fluid reasoning. Only for visualization was the rate of change with age for standard scores significant, t(37) = −2.44, p = .020. The visualization standard score fell by one-quarter of a point per chronological year on average. No predictors of slope for visualization standard scores reached significance. In contrast, FMRP was a significant predictor of change in standard scores with age for fluid reasoning, t(37) = 2.14, p = .039.
For growth scores, FMRP significantly predicted growth score levels for visualization, t(37) = 3.75, p = .001, and fluid reasoning, t(37) = 3.81, p = .001. A fifty percent increase in FMRP was associated with an average of approximately 18 growth score points for visualization and fluid reasoning. As seen in Table 3, the rate of change for growth scores was not significant for visualization or fluid reasoning, p = .198 and p = .340, respectively, and there were no significant predictors of these slopes. One participant (male) had the lowest possible growth score for fluid reasoning.
Exploratory analyses with the predictors of interest were repeated separately for males and females. For males, no predictors reached significance; however, several significant results emerged for females. Autism symptom severity was negatively related to rate of change for visualization standard scores, t(8) = −2.63, p = .031, and visualization growth scores, t(8) = −2.50, p = .037. No effects were significant for fluid reasoning standard scores; however, FMRP significantly predicted level of fluid reasoning growth scores, t(8) = 2.36, p = .046, and a deceleration of slope was detected, t(37) = −2.45, p = .019. Complete results for these exploratory analyses are available from the authors.
Discussion
This study was designed to clarify the trajectory of nonverbal cognitive development in adolescents with FXS, in terms of absolute ability and relative to age expectations. We evaluated biological (FMRP), behavioral (autism severity), and environmental (family background) predictors of level and rate of change in nonverbal cognition, broadly defined, and two specific aspects of cognitive ability: visualization and fluid reasoning. Contrary to our hypotheses, neither autism severity nor family environment predicted nonverbal cognition. In contrast, the expected relationship between FMRP and cognitive ability was confirmed, with greater FMRP expression associated with higher standard scores and growth scores for the full Leiter-R Brief IQ battery. FMRP was also a significant predictor of variability in visualization and fluid reasoning. These results suggest that the reduction of FMRP has a critical impact on early cognitive development that cascades through adolescence, gradually widening the gap between cognitive achievements and age expectations (Cornish et al., 2004).
Neuropsychological research has established that individuals with FXS exhibit pervasive impairments on tasks that rely on frontal lobe functioning, such as those requiring working memory and executive function (Kirk et al., 2005; Lanfranchi et al., 2009). Based on this evidence, along with consistent neuroimaging findings of abnormal structure or function of prefrontal cortex and frontostriatal networks (Gothelf et al., 2008; Hoeft et al., 2007), we hypothesized that fluid reasoning would be particularly susceptible to the impact of reduced FMRP during adolescence. This hypothesis was supported. Indeed, we found that FMRP was a significant predictor of both level and rate of change in fluid reasoning standard scores during adolescence. The positive relationship between FMRP and fluid reasoning standard scores was present despite the fact that the average rate of growth for fluid reasoning across the participant sample was not significantly different than zero. Our findings are in line with the notion that FMRP plays an important role in frontostriatal and prefrontal development.
Within a developmental framework, it would be reasonable to expect that deficits in working memory and executive function could differentially impact certain cognitive domains during different periods of development (Cornish et al., 2004). Fluid aspects of intellectual ability might be particularly vulnerable to problems with executive function (Lanfranchi et al., 2009). The trajectories of nonverbal cognitive ability of male and female adolescents with FXS are consistent with the notion that deficits in frontal lobe functions (e.g., working memory) could be consequences of reduced FMRP (Loesch et al., 2004). These deficits, in turn, may limit acquisition of more complex skills, as reflected in our fluid reasoning composite. In contrast, FMRP expression did not distinguish among individuals’ rates of change in visualization over the study period. Future longitudinal research should test the direct contribution of working memory and executive function to specific aspects of nonverbal cognition.
Contrary to our hypothesis, the relationship of autism severity to nonverbal cognition was no longer significant after accounting for FMRP, except in an exploratory analysis of visualization ability for females. This finding suggests that reductions in FMRP may result in brain differences that account for both increased symptoms of autism and lower IQ, an interpretation that is supported by other research (Loesch et al., 2007; Loesch et al., 2004). Our results contrast with previous studies that have identified a relationship between autism severity and level or rate of change in IQ (or aspects of cognitive ability, such as visualization), even after controlling for FMRP (e.g., Skinner et al., 2005). The discrepancy between results from these studies and our own could be due to several factors. Other studies have excluded females, used different metrics of autism symptoms (i.e., the CARS vs. the ADOS), or investigated a different developmental period (e.g., childhood vs. adolescence; Roberts et al., 2009). The present study indicates that, independent of FMRP, autism symptoms do not significantly impact rates of cognitive development during adolescence when considering males and females together. Examining autism severity at only one time point, however, precluded us—as it has others (e.g., Roberts et al., 2009)—from detecting associations between cognition and changes in autism severity. Future research is needed to resolve this issue, given mixed evidence regarding autism symptom stability during adolescence.
Also contrary to our hypothesis, the family environment was not significantly predictive of nonverbal cognition when controlling for autism severity and FMRP. Family environment was indexed by a composite score that included maternal IQ; researchers might consider examining variables such as maternal IQ, parental education, and income independently in the future. It has been suggested that the relationship between parental IQ and child IQ might be weaker for individuals with FXS compared to individuals without reduced expression of FMRP (Reiss, Freund, Baumgardner, Abrams, & Denckla, 1995). It could be that our inclusion of some male-female sibling pairs affected our ability to detect an association between cognitive ability and family environment. It may also be that a focus on more proximal environmental variables, such as parental warmth and responsivity in interacting with their children, would yield a different conclusion. However, other studies of trajectories of cognitive development have also failed to identify a relationship between maternal IQ and cognitive ability (or rate of development) in boys with FXS (Roberts et al., 2009; Skinner et al., 2005). Taken together, evidence suggests that, for individuals with FXS, FMRP expression is more contributory to intellectual ability and growth during adolescence than behavioral and environmental factors.
Strengths, Limitations, and Implications for Future Research
The current study was the first to follow both male and female adolescents longitudinally within a well-defined age range over four annual assessments. In addition, we simultaneously examined FMRP levels, autism severity assessed with a gold-standard measure, and environmental factors as predictors of level of ability and rate of change. Several limitations should be noted. First, as Mervis (2005) has described, the use of standard scores for assessing individuals with intellectual disabilities has drawbacks. In particular, floor effects were present in our sample (especially among males, 25% of whom received the lowest possible standard score on the Leiter-R). This reinforces the importance of our decision to also examine growth scores, which were less subject to floor effects. Further, it highlights the need for standardized assessments with increased sensitivity below the mean of the norming sample (Mervis, 2005). Another limitation of the current study was that our sample of female participants was not large enough to allow for confident analysis of the potential predictors of trajectories in visualization and fluid reasoning. Additional longitudinal research on the cognitive development of females with FXS will be valuable, particularly because so many previous studies have only enrolled females without intellectual disability or excluded females altogether.
It is crucial to acknowledge that we favored examination of the effects of FMRP rather than gender, which is inherently confounded with FMRP expression. This approach followed from our theoretical emphasis on the role of FMRP, as well as the fact that gender is relevant in FXS primarily because it is associated with differences in FMRP. Our results indicated that the predictive effect of FMRP was diminished when females were excluded. This may reflect reduced statistical power due to the smaller sample size as well as reduced variability in FMRP (i.e., two thirds of males expressed no FMRP). Furthermore, within genders, the effects of FMRP on cognition may simply be more difficult to detect because of variable expression of FMRP across tissue types (e.g., leukocytes vs. the brain). Indeed, other studies have failed to identify a correlation between full scale IQ and FMRP within a single gender (Lightbody, Hall, & Reiss, 2006). Larger samples of males with FXS, in which distinctions can be made between fully methylated mutations, methylation mosaicism, and repeat-length mosaicism, might have the power to detect the subtle effects of FMRP expression based on peripheral samples. It may also be the case that other indices of biological affectedness, such as mRNA or, for females, activation ratios, would be preferable metrics for future studies. Nevertheless, within our sample of females with FXS, an effect of FMRP on fluid reasoning was detected, suggesting that this remains a useful metric for biological influences on development in this population.
In line with previous research (Skinner et al., 2005), we detected a significant decline in overall IQ and a significant increase in growth scores, on average, over the period of 10 to 19 years of age. It is nonetheless important to note that change over time was generally small relative to individual differences. In fact, the average rate of skill acquisition was inconsistently distinguishable from zero, suggesting that, at the group level, patterns of positive change were unreliable. Although some individuals with FXS may make significant cognitive gains during adolescence, others may show improvement at a much slower rate. Identifying the complex factors that account for variability among individuals with FXS—and factors that moderate the effect of FMRP with age—will continue to be a primary goal of research.
The need for applying a developmental perspective to the study of the FXS phenotype has been long acknowledged (Cornish et al., 2004). The current findings further demonstrated the utility of longitudinal investigations of development, particularly ones in which specific aspects of cognitive functioning are distinguished. Extending a neurodevelopmental perspective to the study of other genetic and developmental disorders has great potential. Resources might be best utilized by examining specific aspects of cognition during targeted developmental periods, particularly for the study of genotype-phenotype relations. Ultimately, this type of research will be crucial for developing appropriate interventions for individuals with FXS and those with other neurodevelopmental disorders.
Acknowledgments
This research was supported by NIH grants R01 HD024356 and P30 HD003352 to Leonard Abbeduto and the Waisman Center, respectively, in addition to NIH F31 DC010959 awarded to the first author. We are especially grateful to the families who participated with such enthusiasm and patience.
Footnotes
Preliminary results were presented at the 11th International Fragile X Conference in St. Louis, MO in 2008.
References
- Bailey DB, Jr., Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Develompental Disorders. 2001;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. Cambridge University Press; New York: 1993. [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R. Annotation: Deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. Journal of Child Psycholology and Psychiatry. 2004;45(6):1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- Dedrick RF, Ferron JM, Hess MR, Hogarty KY, Kromrey JD, Lang TR, Niles JD, Lee RS. Multilevel modeling: A review of methodological issues and applications. Review of Educational Research. 2009;79(1):69–102. [Google Scholar]
- Dyer-Friedman, Glaser B, Hessl D, Johnston C, Huffman LC, Taylor A, Reiss AL. Genetic and environmental influences on the cognitive outcomes of children with fragile X syndrome. J Am Acad of Child Adolesc. 2002;41(3):237–244. doi: 10.1097/00004583-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, Simensen R. The course of cognitive-behavioral development in children with the FMR1 mutation, Williams-Beuren syndrome, and neurofibromatosis type 1: The effect of gender. American Journal of Medical Genetics. 2010;152A(6):1498–1509. doi: 10.1002/ajmg.a.33412. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, Simensen R, Nance W. Studies of age-correlated features of cognitive-behavioral development in children and adolescents with genetic disorders. American Journal of Medical Genetics. 2007;143A(20):2478–2489. doi: 10.1002/ajmg.a.31915. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8(11):1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annals of Neurology. 2008;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Gen. 2008;45(8):498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Burns D, Lightbody A, Reiss A. Longitudinal changes in intellectual development in children with fragile X. J Abnorm Child Psych. 2008;36(6):927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Wheeler A, Sideris J, Sullivan K, Reichardt A, Roberts J, Bailey DB. Developmental trajectories of young girls with fragile x syndrome. American Journal on Intellectual and Developmental Disabilities. 2009;114(3):161–171. doi: 10.1352/1944-7558-114.3.161. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Human Brain Mapping. 2007;28(6):543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Refinement and test of the theory of fluid and crystallized general intelligences. Journal of Educational Psychology. 1966;57(5):253–270. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd Edition American Guidance Service, Inc; Circle Pines, MN: 2005. [Google Scholar]
- Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT) Developmental Neuropsychology. 2005;28(3):755–777. doi: 10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Nat Acad of Sci. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working memory in individuals with fragile X syndrome. Child Neuropsychology. 2009;15(2):105–119. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Hall SS, Reiss AL. Chronological age, but not FMRP levels, predicts neuropsychological performance in girls with fragile X syndrome. American Journal of Medical Genetics. 2006;141B(5):468–472. doi: 10.1002/ajmg.b.30307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, Huggins RM. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience and Biobehavioral Reviews. 2007;31(3):315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch D, Huggins R, Hagerman R. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Res Rev. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 1999. [Google Scholar]
- Mervis CB. Designing measures for profiling and genotype/phenotype studies of individuals with genetic syndromes or developmental language disorders. Applied Psycholinguistics. 2005;26(1) [Google Scholar]
- Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50(1):121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vision Research. 2005;45(7):827–837. doi: 10.1016/j.visres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R, du Toit M. HLM 6: Hierarchical Linear and Nonlinear Modeling. Version 6.06 SSI; Lincolnwood, IL: 2008. [Google Scholar]
- Reiss A, Freund L, Baumgardner T, Abrams M, Denckla M. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nature Genetics. 1995;11(3):331–334. doi: 10.1038/ng1195-331. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Bailey DB., Jr. Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009;34(8):827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale-Revised. Stoelting; Wood Dale, IL: 1997. [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Robert J, Mirrett P, Schaaf J, Bailey DB. Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics. 2005;132A(1):25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman R, Chamberlain W, Hagerman P. Transcription of the FMR1 gene in individuals with fragile X. Am J Med Gen. 2000;97(3):195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Smits A, Oostra BA. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Human Genetics. 1997;99(3):308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- Wright SB, Matlen BJ, Baym CL, Ferrer E, Bunge SA. Neural correlates of fluid reasoning in children and adults. Frontiers in Human Neuroscience. 2008;1 doi: 10.3389/neuro.09.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]