Abstract
The selection of living kidney donors is based on a formal evaluation of the state of health. However, this spectrum of health includes subtle metabolic derangements that can cluster as metabolic syndrome. We studied the association of metabolic syndrome with kidney function and histology in 410 donors from 2005 to 2012, of whom 178 donors were systematically followed after donation since 2009. Metabolic syndrome was defined as per the NCEP ATPIII criteria, but using a BMI > 25 kg/m2 instead of waist circumference. Following donation, donors received counseling on lifestyle modification. Metabolic syndrome was present in 50 (12.2%) donors. Donors with metabolic syndrome were more likely to have chronic histological changes on implant biopsies than donors with no metabolic syndrome (29.0% vs. 9.3%, p < 0.001). This finding was associated with impaired kidney function recovery following donation. At last follow-up, reversal of metabolic syndrome was observed in 57.1% of donors with predonation metabolic syndrome, while only 10.8% of donors developed de novo metabolic syndrome (p < 0.001). In conclusion, metabolic syndrome in donors is associated with chronic histological changes, and nephrectomy in these donors was associated with subsequent protracted recovery of kidney function. Importantly, weight loss led to improvement of most abnormalities that define metabolic syndrome.
Keywords: Body mass index, kidney transplant, living donor, metabolic syndrome, nephrosclerosis, outcomes
Introduction
Living donor kidney transplantation is the best treatment for patients suffering from end-stage renal disease and accounted for approximately 34% of all kidney transplants in the United States in 2011 (1). Importantly, previous epidemiological studies on living kidney donors have suggested that long-term outcomes of these individuals are comparable with those of the general population (2–4). However, a small, but significant number of living donors have been reported to develop chronic kidney disease (CKD), hypertension and diabetes following donation (5). The prevalence of risk factors for CKD before and after donation is unclear but important to elucidate in order to determine the need for proactive preventive care in living donors.
The selection of prospective donors is based on a good state of general health and the absence of significant disease (6). However, the spectrum of health in donors may include subtle metabolic conditions and early or preclinical disease states that can portend future risk. In fact, contemporary donors are older, have a higher body mass index (BMI), and more risk factors for CKD than donors in earlier generations (7,8). In particular, metabolic syndrome is common among donors (9) and this condition is treatable with lifestyle interventions (10). Metabolic syndrome refers to a constellation of cardiovascular disease risk factors that confer a risk beyond the individual components, even when the individual risk factors are not severe (11). Furthermore, metabolic syndrome and each of its components are known to be independently associated in a dose-dependent manner with an increased risk of incident CKD (12,13). However, the relationship of metabolic syndrome with kidney function and underlying renal histology in otherwise healthy adults is unclear. Whether the presence of metabolic syndrome before donation is a risk factor for postdonation kidney dysfunction also remains unclear. Furthermore, whether the intense interface with the health care system during kidney donation has any impact in the adherence to lifestyle modification practices that reverse metabolic syndrome postdonation is also unknown.
The goals of the present study were: (1) to study the prevalence of metabolic syndrome in living kidney donors and its association with kidney function, renal mass and histology at the time of donation; (2) to study whether predonation metabolic syndrome associates with impaired postdonation renal function; and (3) to study the impact of postdonation body weight changes on metabolic syndrome and its components.
Materials and Methods
This study was approved by the Institutional Review Board at the Cleveland Clinic. A chart review was performed on 416 living donors aged 18 years and older from January 2005 to July 2012. We identified 410 donors with complete clinical data in whom we assessed metabolic risk factors prior to donation. In 2009 we initiated a dedicated donor wellness clinic where donors are followed at approximately 1, 6, 12 and 24 months postdonation. Through 2012, 178 donors have undergone monitoring of metabolic risk factors following donation [median (10th–90th percentile) of 428 days (196–801 days)]. In addition, 145 donors had their residual kidney function monitored approximately 1 year postdonation [median (10th–90th percentile) of 318 days (180–394 days)], with 110 donors having reached 2 years [median (10th–90th percentile) of 744 days (442–858 days)].
All living donors underwent a comprehensive evaluation prior to donation. This included anthropometric measures, office blood pressure, fasting blood glucose, serum creatinine, uric acid, lipid profile and the ratio of albumin to creatinine in a random urine sample. Kidney function was determined using radiolabeled iothalamate glomerular filtration rate (GFR) at the time of evaluation (14). Kidney size was measured by multidetector computed tomography and total kidney volume was calculated in cubic centimeters (cm3) and then adjusted for body surface area (cm3 per 1.73 m2) (15).
It is our practice to set a BMI of <35 kg/m2 (ideally <32 kg/m2) and blood pressure of <140/85 mmHg in order to proceed with donation. Some donors with initial elevated blood pressure by office readings are then discovered to have normal blood pressure by 24-h ambulatory blood pressure monitoring. Occasionally, we accept donors with well-controlled hypertension with only one medication. Since 2005, we had 5 donors (1.2%) with BMI >35 kg/m2 and 24 donors (5.9%) with elevated office blood pressure but then donated because either blood pressure was controlled or proven to be normal. Our studied donor population closely resembles that of one of the US (7).
Definition of metabolic risk and metabolic syndrome
We characterized the metabolic risk of these donors by studying the prevalence of metabolic syndrome as defined by the National Cholesterol Education Program’s Adult Treatment Panel III criteria (16). Given that waist circumference is not routinely obtained in living donors at our institution, we used a BMI cutoff value of 25 kg/m2 as an indicator of overweight. On the basis of this definition, donors were considered to have metabolic syndrome if they showed evidence of at least three of the following five conditions: (1) BMI ≥25 kg/m2, (2) office systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg; (3) triglyceride levels ≥150 mg/dL; (4) high-density lipoprotein (HDL)cholesterol levels <40 mg/dLinmalesor <50 mg/dL in females and (5) fasting blood glucose ≥100 mg/dL. The presence of metabolic syndrome was used as an indicator of metabolic risk to understand its relationship with donor kidney function, anatomy and histology and its implications in terms of renal function outcome. In this regard, we also assessed the association between hyperuricemia and metabolic syndrome because hyperuricemia has been associated with hypertension, a component of metabolic syndrome. Hyperuricemia was defined as serum uric acid >7.0 mg/dL in males and >6.0 mg/dL in females according to population surveys.
Histological assessment of the donor kidney
Kidney histology was obtained from implant biopsies performed at the time of donation but prior to kidney reperfusion. As per protocol, implant needle (16–18-guage) biopsies have been routinely performed at our institution from living donors since 2005 and are reported by two renal pathologists. We analyzed 296 biopsy samples after the exclusion of inadequate samples (samples lacking glomeruli and arterioles or containing only renal medulla). The median number of sampled glomeruli was 23 (range 8–48 glomeruli). Specimens with <10 glomeruli were observed in 27 samples (9.1%). There were no significant difference in donor characteristics between donors with specimens with less than 10 glomeruli, therefore all samples were used for analysis. Chronic histological changes (sometimes described as nephrosclerosis or nephroarteriosclerosis) were characterized by the following: (1) >5% global glomerulosclerosis; (2) any interstitial fibrosis with tubular atrophy and (3) any arteriosclerosis. We then scored the number of any histological changes as 0, 1, 2 or 3, and nephrosclerosis was then defined as the presence of any two or more of these abnormalities (17).
Donor follow-up after surgery
Since 2009, all donors at our institution are encouraged to undergo a medical check-up at 2–4 weeks after donation, and then at approximately 6, 12 and 24 months after donation. At time of initial evaluation and at each follow-up visit, donors are counseled on any metabolic abnormality identified during the evaluation process and are then strongly encouraged to follow good lifestyle modification practices such as healthy diets, exercise and weight control.
During the follow-up visits donors undergo measurements of weight, blood pressure, serum creatinine, lipid profile and fasting blood glucose as well as a urine-analysis. For this study, postdonation GFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation (18). Serum creatinine levels were assayed by a standardized Isotope Dilution Mass Spectrometry traceable analyzer.
Statistical analyses
Data were statistically analyzed using JMP 9.0 software (SAS Institute Inc., Cary, NC). Statistical significance for the two groups was assessed using the Student’s t-test for continuous variables, the Pearson χ2 test for categorical variables, and the paired samples were analyzed using the matched-paired Student’s t-test. The measured values were expressed as means ± standard deviations and percentages. The correlation between variables was determined by the Pearson product-moment correlation coefficient. Logistic regression and linear regression were used to identify the associations of metabolic risk factors and metabolic syndrome with histology and to identify the associations between metabolic syndrome and histology with postdonation renal function. Explanatory variables that had a significant relationship (p < 0.10) with metabolic syndrome were analyzed using multivariate analysis to evaluate independent associations. The analyzed values were expressed as adjusted odds ratios for those explanatory variables. A p-value of <0.05 was considered statistically significant.
Results
Population characteristics at the time of donation
The population characteristics in relation to the presence or absence of metabolic syndrome are presented in Table 1. Metabolic syndrome was present in 50 (12.2%) donors, who were more likely to be older (44.6 ± 10.1 years vs. 41.0 ± 10.7 years, p = 0.024) and male (56.0% vs. 39.4%, p = 0.026). By definition, donors with metabolic syndrome were more likely to have higher blood pressure, fasting blood glucose, triglyceride and BMI and lower HDL levels. Furthermore, donors with metabolic syndrome were more likely to have hyperuricemia than donors without metabolic syndrome (34.0% vs. 10.1%, p < 0.001).
Table 1.
Sample characteristics by absence or presence of metabolic syndrome at the time of donation
| Donor characteristics | No metabolic syndrome (n = 360) | Metabolic syndrome (n = 50) | p-Value |
|---|---|---|---|
| Age, years | 41.0 ± 10.7 | 44.6 ± 10.1 | 0.024 |
| Male gender | 142 (39.4) | 28 (56.0) | 0.026 |
| Non African–American race | 320 (88.9) | 48 (96.0) | 0.120 |
| Height, cm | 170.8 ± 9.1 | 171.6 ± 9.6 | 0.598 |
| Weight, kg | 77.3 ± 15.2 | 87.2 ± 11.6 | <0.001 |
| Body surface area, m2 | 1.89 ± 0.22 | 2.00 ± 0.18 | <0.001 |
| Systolic BP, mmHg | 113.4 ± 11.6 | 124.4 ± 12.0 | <0.001 |
| Diastolic BP, mmHg | 72.0 ± 9.4 | 80.9 ± 8.8 | <0.001 |
| BMI ≥30 kg/m2, n (%) | 65 (18.1) | 20 (40.0) | <0.001 |
| BMI 25–29.9 kg/m2, n (%) | 155 (43.1) | 28 (56.0) | 0.085 |
| BP ≥130 or ≥85 mmHg, n (%) | 43 (11.9) | 26 (52.0) | <0.001 |
| Triglyceride ≥150 mg/dL, n (%) | 37 (10.3) | 36 (72.0) | <0.001 |
| HDL <40 mg/dL in males or <50 mg/dL in females, n (%) | 40 (11.1) | 29 (58.0) | <0.001 |
| Fasting blood glucose ≥100 mg/dL, n (%) | 17 (4.7) | 20 (40.0) | <0.001 |
| Iothalamate GFR, mL/min/1.73 m2 | 107 ± 17 | 103 ± 15 | 0.098 |
| Serum creatinine, mg/dL | 0.84 ± 0.15 | 0.90 ± 0.16 | 0.012 |
| eGFRCKD–EPI, mL/min per 1.73 m2 | 99 ± 15 | 93 ± 14 | 0.006 |
| UACR, mg/g | 3.4 ± 6.2 | 4.4 ± 7.6 | 0.579 |
| Adjusted kidney volume, cm3/1.73 m2 | 325 ± 48 | 324 ± 44 | 0.858 |
| Hyperuricemia, n (%) | 36 (10.1) | 17 (34.0) | <0.001 |
| Current Smoker, n (%) | 67 (19.9) | 5 (10.2) | 0.103 |
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; GFR, glomerular filtration rate; eGFRCKD–EPI, estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration equation; UACR, urinary albumin-to-creatinine ratio.
Association of metabolic syndrome with kidney function, kidney size and kidney histology
Donors with metabolic syndrome had higher serum creatinine and lower estimated GFR than those without metabolic syndrome (Table 1). There was a trend toward lower measured GFR in donors with metabolic syndrome compared to those with no metabolic syndrome (103 ± 15 mL/min vs. 107 ± 17 mL/min per 1.73 m2, p = 0.098). There were no significant differences in kidney volumes between groups.
The association of metabolic syndrome with kidney histological findings is presented in Table 2. Donors with metabolic syndrome were more likely to have glomerulosclerosis (31.6% vs. 15.5%, p = 0.015) and interstitial fibrosis/tubular atrophy (15.8% vs. 6.6%, p = 0.048). Two or more histological findings were present in 29.0% donors with metabolic syndrome in contrast with 9.3% of those with no metabolic syndrome (p < 0.001). Donors with metabolic syndrome were more likely to have a higher chronic histological score (p = 0.007; Figure 1).
Table 2.
Chronic histological changes by absence or presence of metabolic syndrome at the time of donation
| No metabolic syndrome (n = 258) | Metabolic syndrome (n = 38) | p-Value | |
|---|---|---|---|
| >5% global glomerulosclerosis, n (%) | 40 (15.5) | 12 (31.6) | 0.015 |
| Any interstitial fibrosis/tubular atrophy, n (%) | 17 (6.6) | 6 (15.8) | 0.048 |
| Any arteriosclerosis, n (%) | 79 (30.6) | 14 (35.9) | 0.441 |
| Chronic histological changes1, n (%) | 24 (9.3%) | 11 (29.0%) | <0.001 |
Defined as at least two of the following: >5% glomerulosclerosis, any tubulointerstitial fibrosis and atrophy and any arteriosclerosis.
Figure 1. Frequencies of histological abnormalities (either glomerulosclerosis, interstitial fibrosis/tubular atrophy or arteriosclerosis) in donors with and without metabolic syndrome at the time of donation.
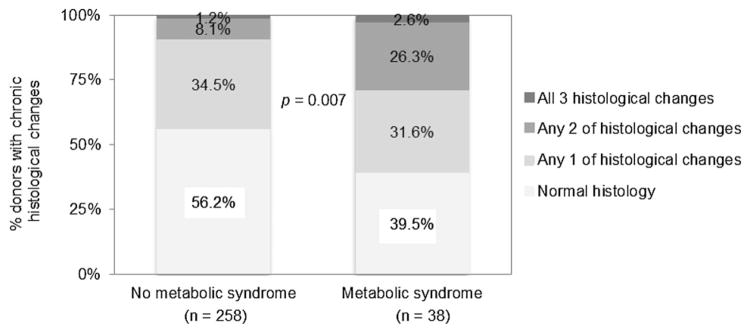
Chronic histological changes were characterized by the following: (1) >5% global glomerulosclerosis, (2) any interstitial fibrosis with tubular atrophy and (3) any arteriosclerosis, and scored the number of any histological changes as 0, 1, 2 and 3.
The associations of metabolic and demographic variables with chronic histological changes are presented in Table 3. Age and the presence of metabolic syndrome were associated with chronic histology in univariate analysis, whereas there were no significant associations between the individual metabolic risk factor components and renal pathology (not shown). In addition, measured GFR prior to donation and kidney size were not associated with chronic histological changes. In multivariate analysis, the presence of metabolic syndrome remained independently associated with chronic histological changes.
Table 3.
Independent factors associated with chronic histological changes on the implant kidney biopsy
| Univariate analysis
|
Multivariate analysis
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, per 10 years of age | 1.73 (1.24–2.45) | 0.002 | 1.79 (1.25–2.63) | 0.002 |
| Male gender | 0.59 (0.27–1.23) | 0.162 | ||
| African American race | 0.48 (0.11–2.13) | 0.213 | ||
| Presence of metabolic syndrome | 3.97 (1.71–8.88) | <0.001 | 5.21 (2.02–13.41) | <0.001 |
| Hyperuricemia | 0.80 (0.23–2.16) | 0.677 | ||
| Smoking | 0.67 (0.19–1.82) | 0.459 | ||
OR, odds ratio; CI, confidence interval.
Postdonation kidney function in donors with and without metabolic syndrome and histological changes
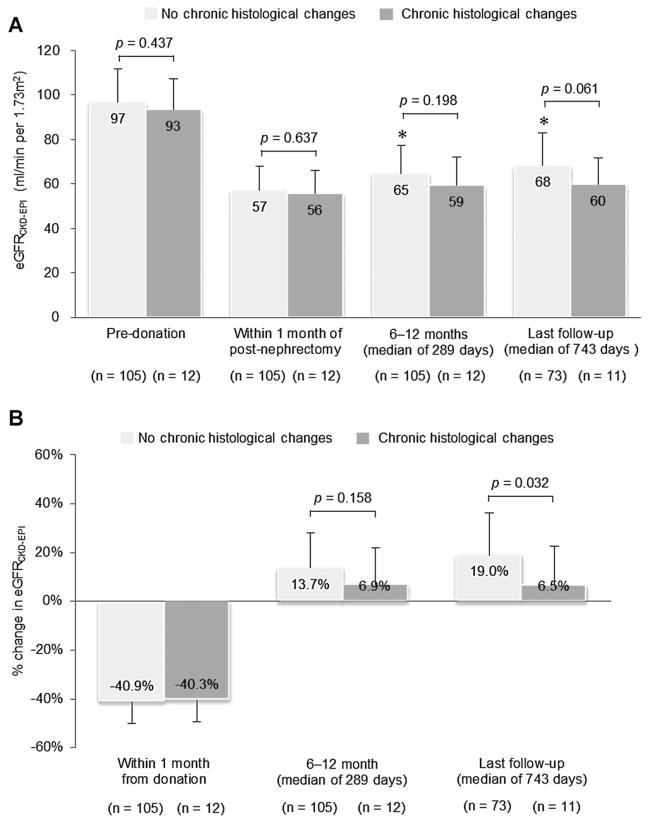
We then studied the impact of metabolic syndrome and chronic histological changes on residual kidney function following donation (Figures 2 and 3). The estimated GFR was lower following the first year after donation in those with metabolic syndrome than those with no metabolic syndrome (Figure 2A); however, this difference was less noticeable at last follow-up. Furthermore, the GFR decreased to a similar degree in both groups immediately postdonation (Figure 2B) but after the initial drop in GFR, renal function recovery was more pronounced in those without metabolic syndrome than in those with metabolic syndrome (14.1% vs. 7.9%, p = 0.050), albeit this difference was again less noticeable at later times postdonation (Figure 2A and B). When stratifying donors based on the presence or not of chronic histological changes on implant biopsy, there was no significant difference in the GFR levels within a year of donation (Figure 3A). However, renal function recovery following the first year was better preserved in those donors without chronic histological changes (19.0% vs. 6.5%, p = 0.032; Figure 3B). These differences remained statistically significant after adjusting for other factors in a multivariate analysis (Table 4).
Figure 2. Estimated GFR at each time point in donors with and without metabolic syndrome (A); and percent change in estimated GFR from baseline to 1 month postdonation, from within 1 month postdonation to within 12 months of follow-up and from within 1 month postdonation to 2 years postdonation in donors with and without metabolic syndrome at the time of donation (B).
eGFRCKD–EPI = estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration equation. *p < 0.001: compared to eGFRCKD–EPI at 1 month postnephrectomy in donors without metabolic syndrome by the matched-paired Student’s t-test. †p < 0.05: compared to eGFRCKD–EPI at 1 month postnephrectomy in donors with metabolic syndrome by the matched-paired Student’s t-test.
Figure 3. Estimated GFRCKD–EPI at each time point in donors with and without chronic histological changes on implant biopsy (A); and percent change in estimated GFRCKD–EPI from baseline to 1 month postdonation, from within 1 month postdonation to within 12 months of follow-up, and from within 1 month postdonation to 2 years postdonation in donors with and without chronic histological changes on implant biopsy (B).
eGFRCKD–EPI = estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration equation. *p < 0.001: compared to eGFRCKD–EPI at 1-month postnephrectomy in donors without chronic histological changes on implant biopsy by the matched-paired Student’s t-test.
Table 4.
Independent factors associated with percent change in estimated GFR at 2 years postdonation.
| Univariate analysis
|
Multivariate analysis1
|
|||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Age, per 10 years of age | −0.16 (−3.33–0.23) | 0.088 | −0.03 (−2.22–1.64) | 0.763 |
| Male gender | −0.14 (−3.61–0.50) | 0.137 | ||
| African American race | −0.01 (−4.13–3.65) | 0.902 | ||
| Adjusted kidney volume, per 10 cm3/1.73 m2 | 0.11 (−0.02–0.07) | 0.251 | ||
| Change of eGFR from baseline to immediate postnephrectomy (1 month) | 0.54 (0.48–0.89) | <0.001 | 0.56 (0.45–0.92) | <0.001 |
| Presence of metabolic syndrome | 0.05 (−2.38–4.09) | 0.603 | ||
| Presence of chronic histological changes | −0.18 (−6.68–−0.52) | 0.092 | −0.23 (−6.79–−0.81) | 0.013 |
| Smoking | 0.04 (−2.73–4.24) | 0.668 | ||
eGFRCKD–EPI, estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration equation; β, standardized regression coefficients; CI, confidence interval.
Factors associated with change in eGFR postdonation in univariate analysis (p < 0.10) were entered in the multivariable model. If change in serum creatinine was used as the dependent variable instead of estimated GFR, results remained similar (data not shown).
Metabolic syndrome status postdonation and the importance of weight loss
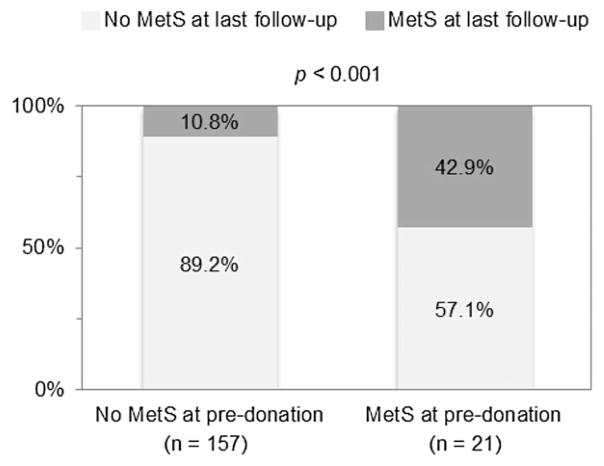
We then studied whether the status of metabolic syndrome varies during the donor evaluation and follow-up process. As shown in Figure 4 only 10.8% of donors developed metabolic syndrome postdonation, while more than half (57.1%) of those with metabolic syndrome prior to donation no longer had metabolic syndrome at last follow-up (p < 0.001). However, there was no significant improvement in GFR within the two years of follow-up in those donors reversing the metabolic syndrome status.
Figure 4. Proportion of donors who developed metabolic syndrome at follow up and donors who reversed the condition following donation.
MetS, metabolic syndrome.
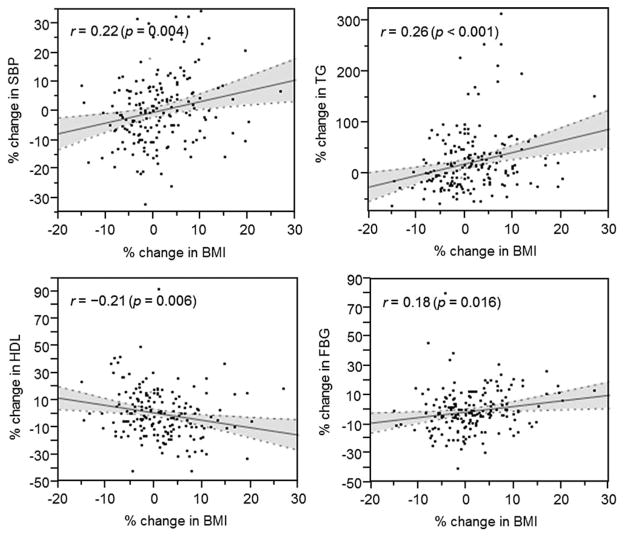
Our data further suggest that changes in BMI led to changes of each of the components of metabolic syndrome. Percent change in BMI showed small but positive correlation with changes in blood pressure, triglyceride and fasting blood glucose levels and had a negative correlation with change in HDL (Figure 5).
Figure 5. Scatter plots depicting the associations between BMI changes from pre- to postdonation and each of the metabolic syndrome components.
BMI, body mass index; SBP, systolic blood pressure; TG, triglyceride; HDL, high-density lipoprotein; FBG, fasting blood glucose.
Discussion
This study shows that metabolic syndrome is prevalent in living kidney donors, that its presence associates with various degrees of glomerulosclerosis, interstitial fibrosis/tubular atrophy and arteriosclerosis on implant biopsy, and that at least in the short to intermediate term, the recovery of kidney function following donation might be partially impaired when compared to the one from donors with no metabolic syndrome and normal histology. Importantly, our results suggest that an active follow-up program that encourages donors to follow healthy lifestyle modification practices leads to reversal of metabolic syndrome in the majority of donors. Furthermore, it appears from our results that weight loss is the main driver for the improvement of the metabolic syndrome components, therefore advising, promoting and supporting lifestyle practices including dietary and activity changes that promote weight loss at any stage of the donor evaluation process and follow-up is a modifiable risk factor that should be actively advocated.
Living kidney donors undergo a thorough evaluation to certify good health or the absence of a condition that could eventually lead to kidney disease. The majority of donors fare well in the long term; however, some develop chronic kidney disease (4,5). The criteria for donor selection used by some US programs has been less stringent in the past couple of decades and along with an increase in the incidence of obesity in the United States, the characteristics of the current prospective donor has also changed. Based on the United Network for Organ Sharing data, donors are now older and heavier than decades ago (7), similar to our study donor population (data not shown). Furthermore, while hypertension used to be a contraindication to donation, selected donors with well-controlled hypertension are now considered for donation (19). Consequently, it is not surprising that well-characterized cardiovascular and kidney disease risk factors, even at early stages, are commonly found in living donors. As such, increased body weight, blood pressure levels at the prehypertension stage, lipid and glucose abnormalities and increased uric acid levels are commonly found in this population. While each of these factors in the higher than normal range levels may independently carry risk for renal disease, the clustering of these conditions into the diagnosis of metabolic syndrome is believed to portend a greater and more definitive risk (11).
Metabolic syndrome has been reported to be associated with glomerular hyperfiltration, glomerulomegaly and glomerulosclerosis, which can subsequently cause a decrease in GFR (20,21). Therefore, metabolic syndrome may have a negative impact on residual kidney function after donation due to impaired functional adaptation. In our study, donors with metabolic syndrome were more likely to be older and have more glomerulosclerosis, interstitial fibrosis and tubular atrophy. These findings may partially explain the higher predonation serum creatinine and lower GFR in these donors. This underlying parenchymal injury among donors with metabolic syndrome was expected to contribute to the lower functional reserve capacity and compensatory response as a result of the donation, especially in those with chronic histological changes. In our study the loss of renal mass due to donation led to a lower GFR postnephrectomy in the short term (up to 1 year) in those with metabolic syndrome and lower GFR recovery at 2 years in those with chronic histological changes on implant biopsy. After adjusting for donor age which was also associated with glomerulosclerosis, and interstitial fibrosis/atherosclerosis in our study, chronic histological changes but not metabolic syndrome remained as an independent risk factor for the relative loss in GFR after donation.
Two previous studies looked at the association between metabolic syndrome and renal histology. In a study of patients who underwent unilateral nephrectomies for renal cell carcinomas (22), subjects with metabolic syndrome had a greater prevalence of nephrosclerosis-related histological findings when compared with controls. In contrast, the studies by Rule et al. found that in kidney donors metabolic risk factors were associated with decreased glomerular density but only the hypertension component of metabolic syndrome associated with nephrosclerosis-related histological findings (9,17). A difference between our study and the one by Rule et al. was the definition of overweight (BMI > 30 kg/m2 by Rule et al.) and glomerulosclerosis >10% as nephrosclerosis-related histological finding. However, when we re-analyzed our data using these parameters the reported associations remain (data not shown). Regardless, the histological findings related to metabolic syndrome do not appear to always be accompanied by predonation kidney dysfunction in donors. In subjects with a relatively good state of health like in donors, compensatory responses of unaffected nephrons seem to preserve GFR and kidney size (23). More importantly is the fact that at this point it is unclear whether any chronic histological finding has any long-term implications to the health of the donor, and hence, these observations should be taken cautiously. Studies with long-term follow-up are needed to address this matter.
The continuing existence of metabolic syndrome or the new development of this entity in donors may lead to significant histological pathology over time with subsequent future risk for residual kidney dysfunction beyond 2 years of follow-up. It has been reported that donors with persistent metabolic syndrome following donation were more likely to have a lower estimated GFR compared to donors without metabolic syndrome after 5 years of follow-up (24). Current recommendations and obligations by transplant centers are to follow donors for at least 2 years postdonation. However, because renal dysfunction following nephrectomy in patients with metabolic syndrome might become clinically detectable more than 2 years postdonation, the need for longer follow-up periods warrant further consideration by the transplant community caring for these donors, activity that should also be supported by regulatory entities.
Importantly, metabolic syndrome is a medical condition that is potentially modifiable, because each of the components has either a pharmacologic treatment or can be managed by adhering to healthy lifestyle practices such as low fat diet, exercise and weight loss. Furthermore, living kidney donors are highly motivated subjects. To better understand how often the status of metabolic syndrome varies after donation, we studied how the metabolic risk components of the syndrome changed over time. We found that most donors without metabolic syndrome prior to donation, retained that status postdonation (89.2%), but importantly, more than 50% of donors with metabolic syndrome prior to donation lost weight, improved their metabolic risk profile and reversed the metabolic syndrome condition. Increased weight is associated with insulin resistance and an array of metabolic and hemodynamic disorders, including atherogenic blood lipid changes, hyperglycemia and elevated blood pressure (25). Furthermore, it has been reported that weight loss can improve these obesity-related risk factors (26–28), and the results of our study further confirms that this might also be true in kidney donors. This information is very important and encouraging because it implies that donation with appropriate follow-up is an opportunity for donors in general to initiate lifestyle modification behaviors that could lead to better health than prior to donation.
This study has several limitations. First, this is a single center study of a relatively homogeneous population and with limited follow-up; however, the comprehensive availability of information pertaining to kidney macro-anatomy, function and histology using gold standard methods permits these types of detailed analyses. Second, we recognize that BMI may be an inappropriate indicator of obesity; however, BMI has recently been shown in a large study to be independently associated with mortality risk (29). Moreover, some definitions of metabolic syndrome include BMI as a marker of obesity or insulin resistance (16,30), hence we used BMI as a substitute for waist-to-hip circumference ratio. Third, implant needle biopsy samples may not be optimal to thoroughly study renal histology due to sample error or insufficient tissue, therefore a sample bias could be present. Fourth, this study is also likely to be underpowered to confidently state that in the long-term kidney function is not different between those with and without metabolic syndrome, and importantly, our results could have been biased by the lack of follow up of all donors and the use of estimated GFR as an outcome measure. The loss-to-follow-up rates of this study were similar to those reported by other centers (31), a subject that should be a matter of future consideration. Finally, while all donors are equally encouraged to adhere to lifestyle modification practices, this was not a randomized controlled trial by design; however, the reported data support future studies in this area.
In conclusion, the presence of metabolic syndrome is associated with various degrees of glomerulosclerosis, interstitial fibrosis/tubular atrophy and arteriosclerosis in living kidney donors; and nephrectomy in these donors might be associated with worse kidney function in the short and intermediate term. Lastly, the subtle metabolic conditions commonly found in these complex donors are readily modifiable, and hence the findings of this study emphasize the importance of lifestyle modifications in all donors at any stage in the process.
Acknowledgments
Dr. Yasushi Ohashi is supported from a grant from the Toho University (Tokyo, Japan). This study was partially supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Abbreviations
- CKD
chronic kidney disease
- NCEP ATPIII
National Cholesterol Education Program Adult Treatment Panel III
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 annual data report: Kidney. Am J Transplant. 2013;13 (Suppl 1):11–46. doi: 10.1111/ajt.12019. [DOI] [PubMed] [Google Scholar]
- 2.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64:976–978. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 363:724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmonico FL, Dew MA. Living donor kidney transplantation in a global environment. Kidney Int. 2007;71:608–614. doi: 10.1038/sj.ki.5002125. [DOI] [PubMed] [Google Scholar]
- 7.Davis CL, Cooper M. The state of U.S. living kidney donors. Clin J Am Soc Nephrol. 5:1873–1880. doi: 10.2215/CJN.01510210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75:1979–1987. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rule AD, Semret MH, Amer H, et al. Association of kidney function and metabolic risk factors with density of glomeruli on renal biopsy samples from living donors. Mayo Clin Proc. 2011;86:282–290. doi: 10.4065/mcp.2010.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: The coronary artery risk development in young adults study. Circulation. 2007;115:1004–1011. doi: 10.1161/CIRCULATIONAHA.106.648642. [DOI] [PubMed] [Google Scholar]
- 11.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 12.Beddhu S, Kimmel PL, Ramkumar N, Cheung AK. Associations of metabolic syndrome with inflammation in CKD: Results from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2005;46:577–586. doi: 10.1053/j.ajkd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 15.Poggio ED, Hila S, Stephany B, et al. Donor kidney volume and outcomes following live donor kidney transplantation. Am J Transplant. 2006;6:616–624. doi: 10.1111/j.1600-6143.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78:276–282. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 20.Dengel DR, Goldberg AP, Mayuga RS, Kairis GM, Weir MR. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension. 1996;28:127–132. doi: 10.1161/01.hyp.28.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 22.Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK. Kidney pathological changes in metabolic syndrome: A cross-sectional study. Am J Kidney Dis. 2009;53:751–759. doi: 10.1053/j.ajkd.2009.01.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: With an emphasis on the glomeruli. Kidney Int. 82:270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuevas-Ramos D, Almeda-Valdes P, Arvizu M, et al. Association of the metabolic syndrome and long-term renal function in kidney donors. Transplant Proc. 43:1601–1606. doi: 10.1016/j.transproceed.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 28.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch Intern Med. 2008;168:571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 29.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 31.Waterman AD, Dew MA, Davis CL, et al. Living-donor follow-up attitudes and practices in U.S. kidney and liver donor programs. Transplantation. 2013;95:883–888. doi: 10.1097/TP.0b013e31828279fd. [DOI] [PubMed] [Google Scholar]