Abstract
Breath analysis is an attractive non-invasive method for diagnosis and therapeutic monitoring. It uses endogenously produced compounds and metabolites of isotopically labelled precursors. In order to make such tests clinically useful, it is important to have relatively small portable instruments detecting volatile compounds within short time. A particularly promising analytical technique is ion mobility spectrometry (IMS) coupled to a multicapillary column (MCC). The present paper focuses on demonstrating the suitability of breath analysis for pharmacokinetic applications using MCC-IMS with respect to practicability and reproducibility testing the model substrate eucalyptol. Validation of the MCC-IMS measurements were performed using proton transfer reaction mass spectrometry (PTR-MS) and resulted in an excellent correspondence of the time-dependent concentrations presented by the two different analytical techniques. Moreover, the good accordance in variance of kinetic parameters with repeated measures, and the determined inter-subject differences indicate the eligibility of the analysis method.
Keywords: breath analysis, ion mobility spectrometry, IMS, multi capillary column, MCC, proton-transfer-reaction mass spectrometry, PTR-MS, pharmacokinetics
Introduction
Pharmacokinetics deals with actions of drugs within the body including the processes of absorption, distribution, metabolism and excretion. Certain patient demographic, pathophysiological, and therapeutic features, such as body weight, age, excretory and metabolic functions, and the presence of other therapies, can alter dose-concentration relationships. Generally, for personalized medicine it is important to identify these measurable pathophysiologic factors that cause changes in the dose-concentration and dose-treatment efficiency relationship so that dosage can be appropriately modified.
Commonly, pharmacokinetic data, such as dose, elimination half-life and rate, etc. are determined from blood or serum. Due to the fact that blood sampling is generally not favoured by the patients and sample processing requires accredited lab and personnel trained in routine analytics, other possibilities for routine diagnostic in a non-invasive way could be of importance in the future. Exhaled breath analysis is a non-invasive diagnostic method that can be repeated frequently. Sampling can even be done from babies and from older or unconscious patients. Due to the development of trace gas detection techniques allowing considerable instrument size reduction with simultaneous increase of sensitivity and stability, breath analysis has the potential to be applied for point-of-care monitoring of selected volatile markers including its use by the patients themselves at home, allowing a more frequent control of drug concentration.
Currently, breath tests investigated in the clinical diagnostics use stable isotopically labelled substrates, mostly 13C-labelled compounds. Almost the only breath test applied routinely in hospital is the 13C-urea breath test [1] for detection of Helicobacter pylori causing most duodenal and stomach ulcers by chronic inflammation of the inner lining of the stomach. For the presently validated breath tests appropriate precursor compounds (such as 13C-urea, 13C-dextromethorphan or 13C-pantoprazol) are necessary to produce 13CO2 to be observed in exhaled breath. However, these tests are very attractive concerning the non-invasive procedure and low time-consumption but because of their limited dissemination in the routine clinical diagnostics the 13C-labelled substrates are still very expensive. Nevertheless13C-labelled compounds can be very interesting for real-time breath analysis to clear up metabolic pathways for research applications using mass spectrometric methods, like PTR-MS or PTR-TOF-MS.
On the other hand, several drugs end up during their metabolism in volatile products, which do not normally occur in human breath and therefore can be measured with modern analytical instruments without using labelled precursors. Thus, for their monitoring in routine clinical diagnostics small, portable and easy-to use analytical instruments for breath analysis are needed.
A promising technique for detection of selected volatile compounds in complex and humid gas samples like human breath is ion mobility spectrometry coupled to a multi capillary column (MCC). This technique was originally developed for the detection of chemical warfare agents, explosives and drugs [2-3]. However, in recent years different studies demonstrated its applicability for process analysis, as well as for quality control in environmental protection [4], pharmaceutical processes [5-6] or food production [7-11] and for urban search and rescue operations[12-13].
IMS in combination with gas chromatography was used in different biological applications e.g. to distinguish different fungi and bacteria species based on the IMS-fingerprints of the headspace VOC emission measured above cultures [14-15]. In some pilot studies the IMS-technique was applied in breath analysis for medical diagnostics in the case of patients with diabetes mellitus [14], with COPD [16], or with sarcoidosis [17], focusing on comparison of IMS-fingerprints of selected patient groups with healthy controls. Moreover, apart from application where a peak pattern analysis is sufficient for assignment of samples, IMS can be applied successfully to track selected known compounds, like for the monitoring of the anesthetic drug propofol during anaesthesia, which was carried out by Perl et al [18].
During the last decade, with reduction of the sensor size the activity of the ionization source could be scaled down intensely, reaching the exemption limit according to the European guidelines (e.g. 1GBq for Tritium (3H)- source) and below, which allows the overall use the IMS device, even in the clinical environment without trained personnel in radiation protection. Besides, alternative ionization sources, such as UV or Corona-discharge are also applied in diverse applications [19-20]. As shown above, the use of IMS as detector in combination with gas chromatographical separation facilitates the spread of the use of the technique in several fields due to its enhanced selectivity. Retention times combined with drift times allow the exact identification of previously determined compounds; nevertheless, due to the lack of a commercial available substance library, the identification of unknown substances is possible especially only in combination with other techniques such as mass spectrometry.
This study aims at the detection and quantitative determination of eucalyptol in human breath after administration of Soledum®, a eucalyptol containing capsule concerning reproducibility of kinetics proving the suitability of ion mobility spectrometry for pharmacokinetic applications. Effects of the same capsule has been measured in breath using PTR-MS by Beauchamp et al [21] , however without the strict time schedule concerning capsule ingestion and breath sampling that was worked out and followed here for every test candidate. Eucalyptol was selected as model compound due to its beneficial physicochemical characteristics such as low water solubility, and moreover availability as pure substance in capsule forms. Eucalyptol (synonym: 1,8-cineol) is a monoterpenoid, a natural organic product with a menthol-like odour, which can be obtained by fractional distillation of eucalyptus oil gained by steam distillation from the leaves of selected Eucalyptus species. It is used in various pharmaceutical products to relieve the symptoms of colds, cough and sweats. Moreover, it is widely used in inflammatory airway diseases as a mucolytic agent [22]. Additionally, cineol has antimicrobial properties against many bacteria and immune-stimulatory, anti-inflammatory and antioxidant effects [23].
Materials and methods
Test protocol
Three volunteers (1female, 2 males, age: 34-42 years, all non-smokers) were recruited for the study, which was approved by the ethics committee of Innsbruck Medical University. Measurements were carried out on three days for each person, with a gap of at least 7 days between two measurements. Volunteers arrived in the morning after at least 12 hours overnight fasting. Before starting the test, breath baseline concentrations of cineol were measured in order to assure that exhaled air sample does not contain cineol arising from other sources (tooth paste, test before, etc.). Ion mobility spectrometry and a PTR-MS were used for the determination of the cineol content in exhaled air. The test started with the administration of Soledum® capsule (Casella-med GmbH Co. KG, Cologne, Germany) containing 100 mg cineol. Breath was measured before and then in 10-30 minute intervals after ingestion of the capsule during a total duration of 4-6 hours. In accordance with package insert, each volunteer consumed a breakfast 30 min after swallowing the capsule. After the breakfast volunteers washed their mouth using clean water to remove major VOC contaminations arising from food consumption. Additional breath measurements were carried out in case of two volunteers within the next 8 days after the test to monitor the cineol level. Room air was frequently controlled before and during the tests to eliminate effects of potential environmental contamination.
Breath analysis using ion mobility spectrometry
For the present study a commercial MCC-IMS (BreathSpec, GAS mbH, Dortmund, Germany) was used. The functionality of IMS is described elsewhere [2-3], therefore only a brief summary will be given in this paper.
The IMS is equipped with a radioactive ionization source (tritium- (3H), 300 MBq activity) for ion generation. A multi capillary column (OV-5, Novosibirsk, Russia) consisting of 1000 capillaries with an inner diameter of 40 μm and film thickness of 2 μm of each single capillary is coupled to the IMS for isothermal separation at 40°C. The length of the column is 20 cm.
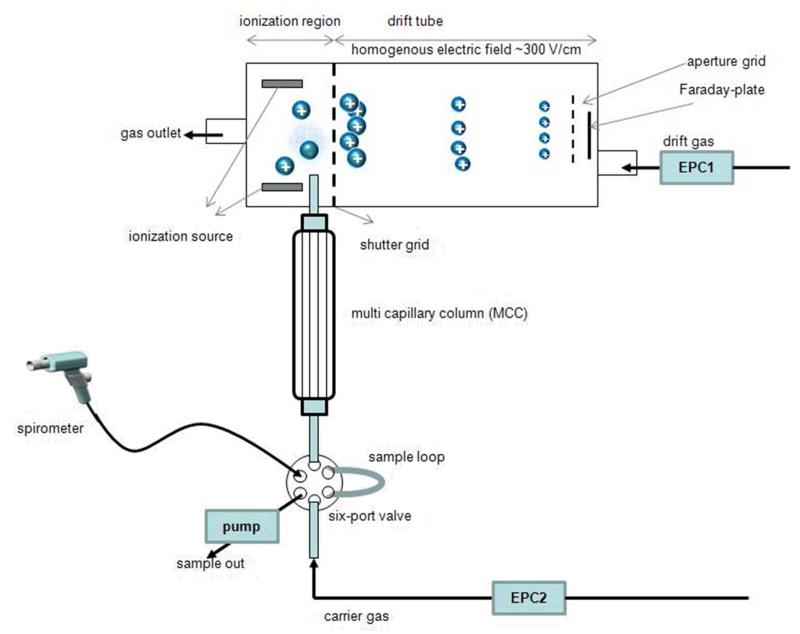
The analyte molecules separated by the MCC enter the ionization chamber, where they continually undergo a series of ion-molecule and ion-ion reactions, such as proton transfer reactions with the ionized drift gas (nitrogen) [3]. An ion swarm is injected into the drift tube (length: 50 mm) through a shutter grid periodically opened every 100 ms. The ions are drifting at ambient pressure under the influence of a uniform electric field (400 V/cm). Ions of different sizes achieve different velocities inversely related to their size (cross section), mass and charge. The collection of these ions on the detector (Faraday-plate) delivers a time-dependent signal, which corresponds to the mobility of the ions (Fig.1).
Figure 1.
Set-up of MCC-IMS device
The IMS was heated at a constant temperature of 45°C. Additionally, inlet tubing, 6-port-valve, sample loop were held at 40°C. Nitrogen (purity grade 99.9999%) was used as drift and carrier gas, with flow rates of 200 mL/min and 100 mL/min, respectively.
During breath sampling the volunteer was asked to exhale after a single deep breath into the spirometer, which controls the sampling according to the CO2 level in breath. As soon as the CO2 content exceeds 3%, the sampling starts automatically. The 5ml sample loop is filled (within 5 seconds) with exhaled air by a pump which generates a constant flow rate of ~120 ml/min. Subsequently the sample is injected into the MCC-column by a switch of the six-port-valve and data acquisition starts.
Breath analysis using PTR-MS
PTR-MS has been used in the past for off-line and on-line analysis of exhaled breath. It has been one of the most successful techniques for real-time analysis of exhaled breath [24-29]. The set-up of the high-sensitivity PTR-MS (Ionicon Analytik Gesellschaft mbH, Innsbruck, Austria) in combination with the BET (buffer end tidal sampler, Ionicon Analytik Gesellschaft mbH) used for this study is described by Herbig et al. [30]. The length of the drift tube of our PTR-MS is 9.3 cm, with an applied voltage of 600 V. The usual pressure in the drift tube was 2.3 mbar. The temperature of the BET system was kept constant at 60 °C during sampling. A gas flow of 50 mL/min was applied for carrying of exhaled air from the BET (40 ml sample volume) into the PRT-MS. By sucking the sample from the BET with such low flow rate mixing of the end tidal breath with room air can be avoided. During sampling candidates breathed deeply into the BET system at least 3 times. Acetone signal was monitored during exhalation and used for determination of the end-tidal points.
Data evaluation
MCC-IMS
Ion mobility spectra were recorded via an integrated computer (400 MHz X scale processor). 650 spectra were recorded during 5 min, each spectrum with 3000 sample points resulting in a matrix with 1.95 millions sample points. For data visualization and analysis the software LAV (version 1.5.1, GAS mbH, Dortmund, Germany) was used. In addition to correction of minimal variations in drift and retention time, the data were aligned using the LAV software. Peak volumes in defined areas of the identified compounds on the 3D topographic plots were calculated and used to determine calibration curves and compute concentrations.
Reduced ion mobilities of analytes were calculated from drift times using the normalisation factor FIMS according to the formula [31]:
whereby K0RIP: is reference reduced ion mobility for reaction ion peak (RIP) [cm2V−1s−1], tD RIP: drift time of RIP [ms], K0 analyte [cm2V−1s−1] and tD analyte : drift time of analyte.
The normalisation factor represents all variables that can change according to variations in environmental parameters, and thus was calculated for every measurement.
Reference reduced ion mobility for reaction ion peak (RIP) was calculated according to the following equation determined as 1.342 cm2V−1s−1.
, where:
tD: drift time [ms]
P: pressure of the drift gas [hPa]
P0: normal pressure = 1013.2 [hPa]
T: temperature of the drift gas [K]
T0: normal temperature = 273.2 [K]
PTR-MS
PTR-MS measurements were carried out in multiple ion detection mode. The count rate of primary ions (H3O+) was around 106 counts per second calculated from counts at m/z 21+. Beside H3O+ the first water cluster H2O H3O+ at m/z 37 was also measured and considered as primary ion. Previous studies from Beauchamp et al. observed fragmentation of cineol, thus additional to the molecule ions of acetone (m/z 59+) and cineol (m/z 155+) the following product ions were monitored: m/z 81+, m/z 137+, which are fragments of cineol. The dwell time was 0.5 s for each of the selected ions. Thus during one breath measurement spectra of approx. 60 cycles were recorded. The concentration of cineol was computed by calculating the averages of the maxima of the count rates belonging to the mass-to-charge ratios m/z155+, 81+ and 137+ with appropriate consideration of the counts of the primary ions H3O+ and H2O H3O+ for three exhalations.
Kinetics
To evaluate the correspondence of the time-concentration curves achieved by means of the different analytical instruments a quadratic distance measure was introduced based on 2-norm. Concentration patterns were calculated based on discrete measurements, for better comparison they were interpolated regularly spaced with 5 min steps. The so calculated values were specified as curves. Each curve can be represented by a point in an n-dimensional vector space (n= number of time points). The distance of the points achieved in this way was determined.
To compare the shape of the curves, they were normalized to and , where x is the distance of the separate points of the curve and i is the number of measurement points. The distance of two curves x and y corresponds then and results in a number >0. The closer this number is to 0 the higher is the similarity of the two functions.
In the case of normalized vectors the distance is equal to and we shall just use whose values are between 0 and 1. In case of a good similarity the computed values converge to 1.
Preparation of gas standards
Test gases of cineol were prepared in zero air (hydrocarbon impurities <10 ppb) with 100% relative humidity at 20 °C by means of a gas generator (GasLab, Breitfuss Messtechnik GmbH, Harpstedt, Germany). 5 μL of cineol (99%, Sigma-Aldrich Handels Gmbh Vienna, Austria) was dissolved in 25 ml of distilled water. This solution was introduced into a vaporizer with determined flow rate and was evaporated at 100°C. The so produced vapours were then diluted with nitrogen by means of integrated mass flow controllers according to the desired concentrations in the range of 0,5-500 ppbv. All generated calibration samples of eucalyptol were measured in parallel with MCC-IMS and PTR-MS.
Calibrations
For PTR-MS the fragmentation pattern for cineol was determined from measurements with pure cineol (100ppb) with 100% relative humidity at 20°C in zero air. While in the case of MCC-IMS the molecule ions (monomer and dimer) for cineol are produced, in PTR-MS spectra the main mass-to-charge ratio found was at m/z 81+. The percentage abundance of m/z 137+ and m/z 155+ accounted for 51% and 1%, respectively, relative to m/z 81+. Because of the small abundance of the parent ion 155+ also the masses 137+ and 81+ were integrated to the quantification of cineol based on the calibration with the pure standard. Detection limit was determined at 2.8 ppb (with signal to noise ratio S/N = 3 with three repetitions).
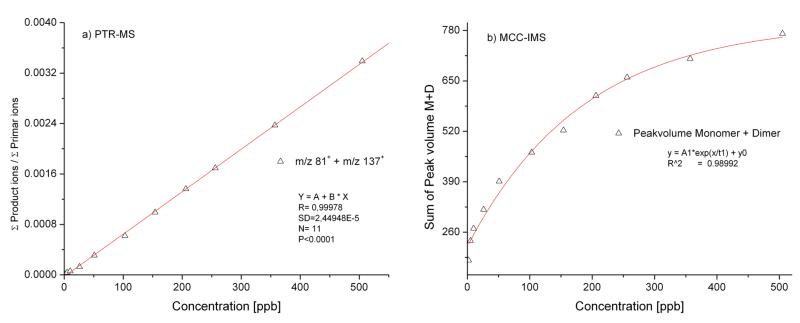
In case of MCC-IMS no fragmentation was observed for cineol. Calibration curve (fig 2/a) for cineol was calculated using the peak volumes in characteristic drift and retention time ranges for monomer and dimer peaks separately. LOD was evaluated from the calibration curves using t- distribution with 95% probability determined as 1.2 ppb with signal to noise ratio S/N = 3.
Figure 2.
a/b: Calibration graphs of eucalyptol using PTR-MS and MCC-IMS
Comparing the calibration results of the two techniques, for PTR-MS a linear calibration was achieved while for IMS a typical calibration curve had the shape of an exponential function. The different functions can be explained by the fact that in the case of PRT-MS there is an almost unlimited excess of primer H3O+ ions and clusters for the ionization of the analytes. The IMS technique uses water impurities existing in the drift and carrier gas (no additional supply of water), thus quantification is possible as long as reactant ions (i.e. water cluster ions) are available. If all of the reactant ions are consumed by the analyte molecules for the formation of product ions the upper quantification limit is reached.
Results
Breath cineol measured using MCC-IMS
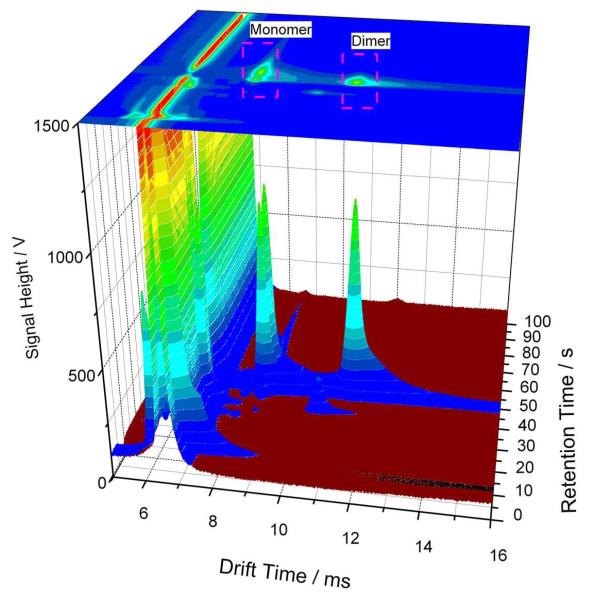
The combination of the ion mobilities, retention times and peak intensities leads to a 3D data set as shown in Fig. 3 illustrating the 3D-IMS-chromatogram of human breath spectra containing cineol. Two main peaks can be observed for cineol at 39.7s retention time. The monomer (AH+) appears at 8.61ms (K0M 1.094 cm2/ Vs) and, in case of higher concentration the dimer (A2H+) reaches its maximum at 11.40 ms (K0D 0.825 cm2/ Vs). No difference was observed between the peak characteristics of cineol peaks occurring in produced test gases and in breath after administration of Soledum® capsule. Table 1 summarizes the average retention times and calculated reduced mobility values of cineol with standard deviations (SD) for all measurements including breath samples and prepared test gases of cineol.
Figure 3.
3D-IMS- chromatogram of breath containing cineol. On this plot the drift time and the retention time are displayed on the X and Y- axis, respectively that are used for substance identification, while the peak height is presented on Z-axis, which serves for quantification.
Table 1.
Retention time and reduced mobilities with SD for cineol in exhaled air and prepared test gas samples
| Retention Time [s] |
Reduced mobilities [cm2/Vs] | ||
|---|---|---|---|
| Monomer | Dimer | ||
| 1,8-Cineol | 39,6± 0,5 | 1,096±0,002 | 0,827±0,002 |
Breath cineol measured using PTR-MS
Initial measurements with the mass scan of breath after ingestion of the Soledum® capsule result in similar distribution of the product ions as in calibration measurements by working with test gases of cineol, thus in a percentage abundance of m/z 137+ and m/z 155+ corresponding to ~ 51% and ~1%, respectively, relative to m/z 81+ (100%). This result differs from the abundance of the selected masses observed by Beauchamp et al. which can be explained with the different operation parameters for the PTR-MS. We are aware that several terpenes, like limonene are constituents of foods and also as fragrance in cleaning agents, toothpastes can influence the quantification with the same mass-to-charge ratio of m/z 137+. To minimize such errors, room air values for 137+ and 81+ were subtracted from the detected breath values.
Kinetics of cineol containing capsule
Validation
Water solubility and partial pressure are probably the most important property of a compound affecting the mass transfer between aqueous and gas phase determining the transfer rate of VOCs from blood to alveolar breath. Cineol is poor water soluble, thus, after swallowing the capsule, cineol will be released in the intestinal tract, transferred to the blood stream and expected to be transferred within a short time into the exhaled air.
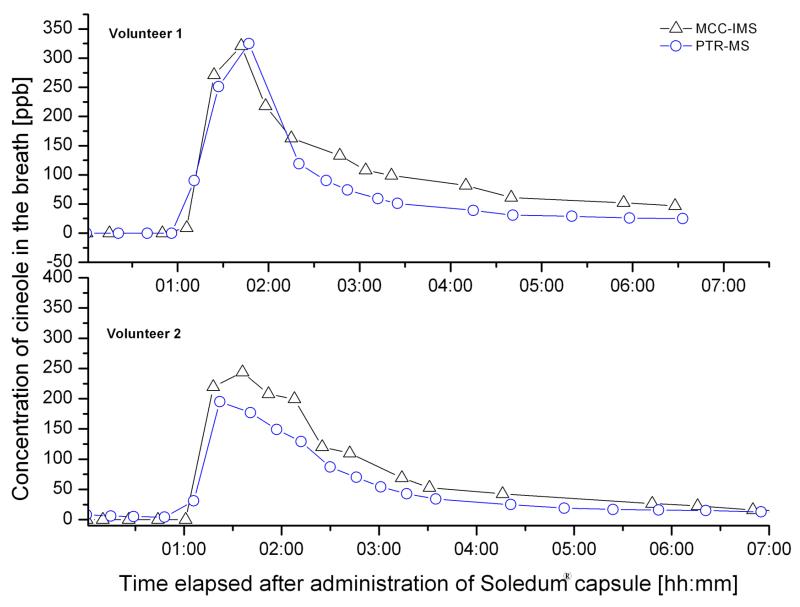
The change of cineol concentration in exhaled air of two volunteers after administration of the capsule is depicted on Fig 4. The two candidates were tested with MCC-IMS and PTR-MS according to the sampling protocol described above.
Figure 4.
Parallel investigated breath tests with Soledum® capsule using MCC-IMS and PTR-MS.
As assumed the concentration of cineol starts to increase already in the first hour after swallowing the capsule and reaches the maximal value rapidly, within two hours. The maximal concentrations of 322 ppb measured with IMS and 324 ppb determined with PTR-MS for Volunteer1 show an excellent consistency of 99%. For Volunteer2 the calculated values of 245 ppb with IMS and 195 ppb with PTR-MS match 80%. It has to be mentioned that measurements could be done in every 10-20 minutes, because the two volunteers performed the test in the same time using the two systems, so that probably no sampling occurred exactly at the time point when the real maximal concentration could have been detected. In the following 2-3 hours a decrease of breath cineol concentration was observed, which slows down at concentrations of 20-30 ppb. Levels between 5 and 20 ppb could be measured during the next 3-7 days.
The concentration patterns measured with the different devices shows similarity values of 0.968 for candidate 1 and 0.994 for candidate 2 based on the above described method for comparison of kinetic curves. Regarding this good similarity result it has to be concerned, that the experiment was carried out with different sampling and analysis methods and in various discrete points in time, respectively.
Personal and inter-individual variations
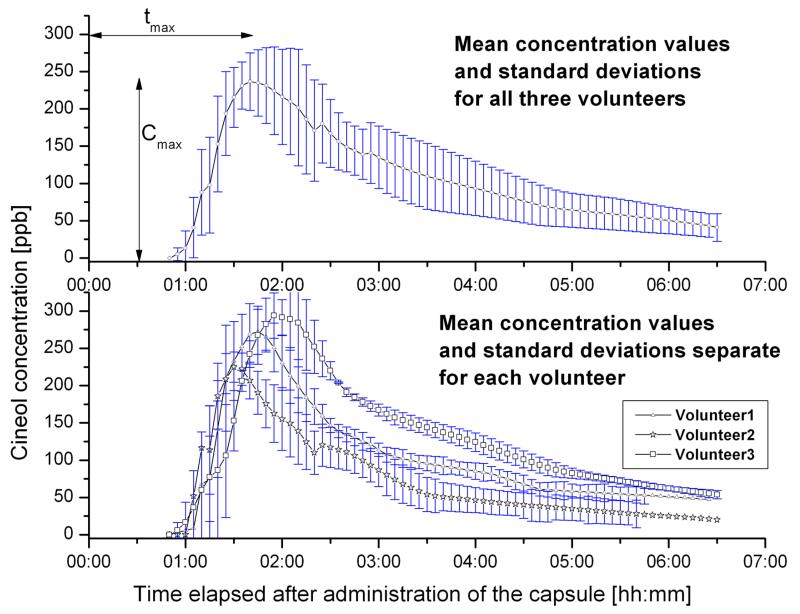
To achieve personal variations in kinetics, breath tests were performed three times for every volunteer using MCC-IMS following to the protocol. The characteristics of the mean curves determined for each volunteer confirm the results gained for the first measurements achieved by the different analytical techniques (Fig5/a.). Thus, cineol appeared in exhaled air approximately one hour after administration of the capsule and the maximal concentration values (Cmax) could be measured between 90 min (tmax_volunter2) as 201 ppb for Volunteer2 and 130 min (tmax_volunter3) as 350 ppb for Volunteer3. After this concentration peak, cineol concentration decreases first faster, and after 2-3 hours at a slower rate. Elimination half-time of the average curves were computed as 85 min for Volunteer3, 55 min for Volunteer1 and 65 min for Volunteer2.
Figure 5.
Mean kinetic curves for cineol (a) for all candidates (b) for every candidate separately
It must be mentioned, that this kinetic trend could be observed only with strict compliance to the protocol. As an example, when a volunteer was not holding the 8 hours fasting time prior the breath test, the release of maximal cineol concentration could take up to 18 hours after swallowing the capsule.
Concerning inter-individual variations (Fig5/b) of the calculated mean curves it can be observed that the difference between the maximal breath cineol concentrations of different persons is higher, than between the curves belonging to the same person. Maximal values of 293 ± 30 ppb were found for Volunteer3 with a dose rate of 1.54 mg/kg, while the smallest concentration values 225 ppb ± 20 ppb were measured in the breath of Volunteer2 with 1.18 mg/kg weight.
The highest area under the curve was found for Volunter3 (100%). Relative to this, 57% for Volunteer2 and 79% for Volunteer1 were calculated. All candidates administered the same dose of 100 mg, so it can be suggested that the exhaled cineol concentration depends also on the dose rate/body weight. However the examined group is too small to confirm this assumption.
Comparing the shape of the single curves as described before the correspondence of the concentration patterns of measurements belonging to the same person and between different individuals was obtained. From the correspondence values summarized in table 2 it can be recognized that curves belonging to one person are in better accordance, than curves achieved from different persons. Values for the same individual are above 0.95 which indicates a good reproducibility of the method. Regarding the inter subject variance a greater consistency between Volunteer1 and Volunteer2 can be observed, whereas curves for Volunteer3 exhibit the greatest difference. The above results show that breath analysis has the potential to become an attractive, non-invasive way for drug monitoring for the future. MCC-IMS seems to be considerable method to be applied in the routine diagnostic regarding size, portability and the speed of analysis. The analysis time of 5 minutes might be adequate for routine analysis, even if it does not ensure a real-time breath-to-breath resolution as e.g. PTR-MS, however the embedded CO2-sensor permits the monitoring of exhaled air samples directly from dead space (if required) and end-tidal volume as well, and room air measurements in separates runs. On the other hand, the fast chromatography can resolve contaminations and, besides, isomers can be separated, which is often not possible using on-line mass spectrometric methods.
Table 2.
Values for correspondence of the time-concentration curves measured with MCC-IMS achieved for the three candidates
| Volunteer1 | Volunteer2 | Volunteer3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meas1 | Meas2 | Meas3 | Meas1 | Meas2 | Meas3 | Meas1 | Meas2 | Meas3 | ||
| Volunteer1 | Meas1 | 1 | - | - | - | - | - | - | - | - |
| Meas2 | 0,987 | 1 | - | - | - | - | - | - | - | |
| Meas3 | 0,980 | 0,952 | 1 | - | - | - | - | - | - | |
| Volunteer2 | Meas1 | 0,887 | 0,919 | 0,818 | 1 | - | - | - | - | - |
| Meas2 | 0,896 | 0,923 | 0,863 | 0,986 | 1 | - | - | - | - | |
| Meas3 | 0,909 | 0,930 | 0,861 | 0,980 | 0,990 | 1 | - | - | - | |
| Volunteer3 | Meas1 | 0,612 | 0,615 | 0,615 | 0,636 | 0,672 | 0,654 | 1 | - | - |
| Meas2 | 0,637 | 0,608 | 0,666 | 0,610 | 0,660 | 0,647 | 0,960 | 1 | - | |
| Meas3 | 0,604 | 0,577 | 0,639 | 0,562 | 0,614 | 0,612 | 0,957 | 0,982 | 1 | |
The good repeatability of the test using the Soledum® capsule might encourage for examination of other pharmaceuticals ending up in volatile metabolites in the body. Regarding the overall usability of drug monitoring via exhaled air it must be considered, that breath analysis with the intention to calculate serum or blood concentrations is dependent on a fixed ratio between blood and exhaled air concentrations. This correlation might be disturbed in patients with a disturbed ventilation / perfusion ratio. Another theoretical limitation in this context is the potential changed distribution volume of pharmaceutical substances in patients. Therefore there is a need of further investigations.
Summary
The kinetics of release of cineol and its appearance in the breath after administration of cineol containing capsule was characterized using ion mobility spectrometry. In the selection of the appropriate technique for breath monitoring were criteria the comparatively small size and portability of the device, the excellent detection limit for the target compounds, and the short detection time.
Breath test were performed three times for every three candidates following the same protocol with regard to the ingestion of the capsule, breath sampling and analysis. For validation, a part of the tests were carried out also using PTR-MS, which resulted in a good accordance with cineol concentrations measured by MCC-IMS.
Time-concentration curves show good reproducibility with respect to the shape of the curves and detected maximal concentration values belonging to the same individual. Greater differences were experienced between different individuals, which indicate inter-subject variability in the release of cineol from the capsule and transfer to breath.
With the results gained by this study the applicability of exhaled air monitoring for pharmacokinetic purpose was presented which may extend the implementation of breath gas analysis for the screening of several other drugs.
Acknowledgments
Veronika Ruzsanyi gratefully acknowledges a Lise-Meitner fellowship from the Austrian Science Fund (FWF, Project number: M1213) and appreciates funding from the Austrian Agency for International Cooperation in Education and Research (OeAD-GmbH, project SPA 04/158 - FEM_PERS, in the program “Sparkling Science”). The generous support by the government of Vorarlberg (Austria) is greatly appreciated.
References
- [1].Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39(9):795–805. doi: 10.1016/j.dld.2007.06.012. [DOI] [PubMed] [Google Scholar]
- [2].Baumbach JI, Eiceman GA. Ion mobility spectrometry: Arriving on site and moving beyond a low profile. Applied Spectroscopy. 1999;53(9):338a–355a. doi: 10.1366/0003702991947847. [DOI] [PubMed] [Google Scholar]
- [3].Eiceman GAK, Z., editors. Ion mobility spectrometry. 2 edition CRC Press; 2004. [Google Scholar]
- [4].Ruzsanyi V, Sielemann S, Baumbach JI. Detection of sulfur-free odorants in natural gas using ion mobility spectrometry. Journal of Environmental Monitoring. 2007;9(1):61–65. doi: 10.1039/b613951e. [DOI] [PubMed] [Google Scholar]
- [5].O’Donnell RM, Sun X, Harrington P. Pharmaceutical applications of ion mobility spectrometry. Trends in Analytical Chemistry. 2002;27(1):44–53. [Google Scholar]
- [6].O’Donnell RM, Sun XB, Harrington PD. Pharmaceutical applications of ion mobility spectrometry. Trac-Trends in Analytical Chemistry. 2008;27(1):44–53. [Google Scholar]
- [7].Kolakowski BM, D’Agostino PA, Chenier C, Mester Z. Analysis of chemical warfare agents in food products by atmospheric pressure ionization-high field asymmetric waveform ion mobility spectrometry-mass spectrometry. Analytical Chemistry. 2007;79(21):8257–8265. doi: 10.1021/ac070816j. [DOI] [PubMed] [Google Scholar]
- [8].Vautz W, Zimmermann D, Hartmann M, Baumbach JI, Nolte J, Jung J. Ion mobility spectrometry for food quality and safety. Food Additives and Contaminants. 2006;23(11):1064–1073. doi: 10.1080/02652030600889590. [DOI] [PubMed] [Google Scholar]
- [9].Bota GM, Harrington PB. Direct detection of trimethylamine in meat food products using ion mobility spectrometry. Talanta. 2006;68(3):629–635. doi: 10.1016/j.talanta.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [10].Karpas Z, Tilman B, Gdalevsky R, Lorber A. Determination of volatile biogenic amines in muscle food products by ion mobility spectrometry. Analytica Chimica Acta. 2002;463(2):155–163. [Google Scholar]
- [11].Rauch PJ, Harrington PD, Davis DM. Ion mobility spectrometer measures food flavor freshness. Food Technology. 1996;50(6):83–85. [Google Scholar]
- [12].Agapiou A, Mochalski P, Schmid A, Amann A. Potential of volatile compounds in safety and security applications. In: Amann A, Smith D, editors. Volatile biomarkers: non-invasive diagnosis in physiology and medicine. Elsevier; Amsterdam: 2013. pp. 515–558. [Google Scholar]
- [13].Rudnicka J, Mochalski P, Agapiou A, Statheropoulos M, Amann A, Buszewski B. Application of ion mobility spectrometry for the detection of human urine. Anal Bioanal Chem. 2010;398(5):2031–8. doi: 10.1007/s00216-010-4147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vautz W, Baumbach JI. Analysis of bio-processes using ion mobility spectrometry. Engineering in Life Sciences. 2008;8(1):19–25. [Google Scholar]
- [15].Perl T, Veldhoen S, Vautz W, Baumbach JI. Fast analysis of volatile metabolites by ion mobility spectrometry for characterization of bacteria. International Journal of Medical Microbiology. 2008;298:17–17. [Google Scholar]
- [16].Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L. Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. Journal of Chromatography A. 2005;1084(1-2):145–151. doi: 10.1016/j.chroma.2005.01.055. [DOI] [PubMed] [Google Scholar]
- [17].Westhoff M, Litterst P, Freitag L, Baumbach JI. Ion mobility spectrometry in the diagnosis of sarcoidosis: Results of a feasibility study. Journal of Physiology and Pharmacology. 2007;58:739–751. [PubMed] [Google Scholar]
- [18].Perl T, Carstens E, Hirn A, Quintel M, Vautz W, Nolte J, Junger M. Determination of serum propofol concentrations by breath analysis using ion mobility spectrometry. British Journal of Anaesthesia. 2009;103(6):822–827. doi: 10.1093/bja/aep312. [DOI] [PubMed] [Google Scholar]
- [19].Bahrami H, Tabrizchi M. Combined corona discharge and UV photoionization source for ion mobility spectrometry. Talanta. 2012;97:400–5. doi: 10.1016/j.talanta.2012.04.052. [DOI] [PubMed] [Google Scholar]
- [20].Mochalski P, Rudnicka J, Agapiou A, Statheropoulos M, Amann A, Buszewski B. Near real-time VOCs analysis using an aspiration ion mobility spectrometer. Journal of Breath Research. 2013;7(2):11. doi: 10.1088/1752-7155/7/2/026002. [DOI] [PubMed] [Google Scholar]
- [21].Beauchamp J, Kirsch F, Buettner A. Real-time breath gas analysis for pharmacokinetics: monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptol-containing capsules. Journal of Breath Research. 2010;4(2):12. doi: 10.1088/1752-7155/4/2/026006. [DOI] [PubMed] [Google Scholar]
- [22].Sadlon AE, Lamson DW. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern Med Rev. 2010;15(1):33–47. [PubMed] [Google Scholar]
- [23].Worth H, Schacher C, Dethlefsen U. Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: a placebo-controlled double-blind trial. Respir Res. 2009;10:69. doi: 10.1186/1465-9921-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].King JL, Unterkofler K, Teschl G, Teschl S, Koc H, Hinterhuber H, Amann A. A mathematical model for breath gas analysis of volatile organic compounds with special emphasis on acetone. Journal of Mathematical Biology. 2011;63(5):959–999. doi: 10.1007/s00285-010-0398-9. [DOI] [PubMed] [Google Scholar]
- [25].King J, Unterkofler K, Teschl G, Teschl S, Mochalski P, Koc H, Hinterhuber H, Amann A. A modeling-based evaluation of isothermal rebreathing for breath gas analyses of highly soluble volatile organic compounds. Journal of Breath Research. 2012;6(1) doi: 10.1088/1752-7155/6/1/016005. [DOI] [PubMed] [Google Scholar]
- [26].King J, Koc H, Unterkofler K, Mochalski P, Kupferthaler A, Teschl G, Teschl S, Hinterhuber H, Amann A. Physiological modeling of isoprene dynamics in exhaled breath. Journal of Theoretical Biology. 2010;267(4):626–637. doi: 10.1016/j.jtbi.2010.09.028. [DOI] [PubMed] [Google Scholar]
- [27].King J, Kupferthaler A, Frauscher B, Hackner H, Unterkofler K, Teschl G, Hinterhuber H, Amann A, Hogl B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiological Measurement. 2012;33(3):413–428. doi: 10.1088/0967-3334/33/3/413. [DOI] [PubMed] [Google Scholar]
- [28].King J, Kupferthaler A, Unterkofler K, Koc H, Teschl S, Teschl G, Miekisch W, Schubert J, Hinterhuber H, Amann A. Isoprene and acetone concentration profiles during exercise on an ergometer. Journal of Breath Research. 2009;3(2):027006. doi: 10.1088/1752-7155/3/2/027006. [DOI] [PubMed] [Google Scholar]
- [29].King J, Koc H, Unterkofler K, Teschl G, Teschl S, Amann A. Volatile biomarkers: non-invasive diagnosis in physiology and medicine. Elsevier; Amsterdam: 2013. Physiological modeling for analysis of exhaled breath; pp. 27–46. [Google Scholar]
- [30].Herbig J, Titzmann T, Beauchamp J, Kohl I, Hansel A. Buffered end-tidal (BET) sampling-a novel method for real-time breath-gas analysis. Journal of Breath Research. 2008;2(3) doi: 10.1088/1752-7155/2/3/037008. [DOI] [PubMed] [Google Scholar]
- [31].Vautz W, Bödeker B, Baumbach JI, Bader S, Westhoff M, Perl T. An implementable approach to obtain reproducible reduced ion mobility. Int J Ion Mobil Spec. 2009;12:47–57. [Google Scholar]