Abstract
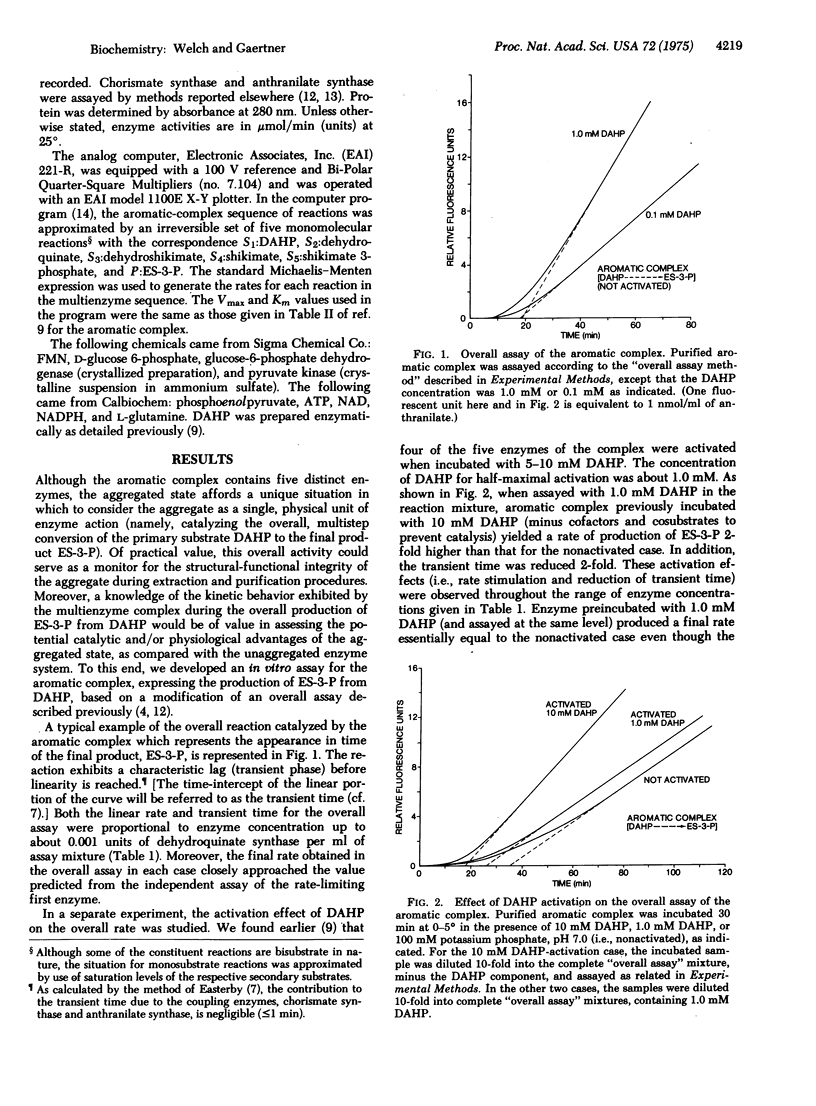
The aromatic complex of Neurospora crassa is an aggregated multienzyme system which catalyzes five consecutive reactions in the central pathway leading to the biosynthesis of the aromatic amino acids. In an attempt to understand the physiological importance of this complex in particular, as well as the importance of cellular organization of enzyme systems in general, we have isolated the complex and have begun to characterize its catalytic properties. Optimum conditions for the assay of the overall 5-step reaction catalyzed by the partially purified complex have been determined. An analog computer was programmed to represent an unaggregated system of five enzymes with rate constants identical to those found for the constituent enzymes of the complex. By direct comparison, it was shown that the lags (transient times) obtained for the overall reaction were 10-15 times longer for the hypothetical unaggregated system than for the complex. We conclude from these data that the aggregated multienzyme system compartmentalizes intermediate substrates during the course of the overall reaction. We suggest that, in addition to "channeling" intermediates of competing pathways, reduction of the transient time may be an important consequence of the containment of intermediates within a physically associated enzyme sequence. The fact that the aromatic complex exhibits a second catalytic property unique to aggregated enzyme systems, "coordinate activation" [Welch, G.R. & Gaertner, F.H. (1975) Arch. Biochem. Biophys., in press] indicates that the physical association of these enzymes may have more than one physiological function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlyn M. B., Giles N. H. Organization of enzymes in the polyaromatic synthetic pathway: separability in bacteria. J Bacteriol. 1969 Jul;99(1):222–230. doi: 10.1128/jb.99.1.222-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun G., Thomas D., Selegny E. Structured bienzymatical models formed by sequential enzymes bound into artificial supports: active glucose transport effect. J Membr Biol. 1972;8(4):313–332. doi: 10.1007/BF01868108. [DOI] [PubMed] [Google Scholar]
- Doy C. H. Anthranilate synthetase and the allosteric protein model. Biochim Biophys Acta. 1966 Apr 12;118(1):173–188. doi: 10.1016/s0926-6593(66)80154-6. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. Coupled enzyme assays: a general expression for the transient. Biochim Biophys Acta. 1973 Feb 15;293(2):552–558. doi: 10.1016/0005-2744(73)90362-8. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., Ericson M. C., DeMoss J. A. Catalytic facilitation in vitro by two multienyzme complexes from Neurospora crassa. J Biol Chem. 1970 Feb 10;245(3):595–600. [PubMed] [Google Scholar]
- Goldman R., Katchalski E. P. Kinetic behavior of a two-enzyme membrane carrying out a consecutive set of reactions. J Theor Biol. 1971 Aug;32(2):243–257. doi: 10.1016/0022-5193(71)90163-9. [DOI] [PubMed] [Google Scholar]
- Katchalski E., Silman I., Goldman R. Effect of the microenvironment on the mode of action of immobilized enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;34:445–536. doi: 10.1002/9780470122792.ch7. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., Roberts D. V. The behaviour of coupled enzyme systems in the transient and steady-state regions of the reaction. Biochim Biophys Acta. 1974 Oct 17;364(2):181–192. doi: 10.1016/0005-2744(74)90002-3. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Metabolic compartmentation at the molecular level: the function of a multienzyme aggregate in the pyrimidine pathway of yeast. Biochim Biophys Acta. 1970 Dec 16;220(3):365–372. doi: 10.1016/0005-2744(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Matchett W. H. Indole channeling by tryptophan synthase of neurospora. J Biol Chem. 1974 Jul 10;249(13):4041–4049. [PubMed] [Google Scholar]
- Mattiasson B., Mosbach K. Studies on a matrix-bound three-enzyme system. Biochim Biophys Acta. 1971 Apr 14;235(1):253–257. doi: 10.1016/0005-2744(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Mattiasson B., Mosbach K. An immobilized three-enzyme system: a model for microenvironmental compartmentation in mitochondria. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2534–2538. doi: 10.1073/pnas.70.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Broun G., Sélégny E. Monoenzymatic model membranes: diffusion-reaction kinetics and phenomena. Biochimie. 1972;54(2):229–244. doi: 10.1016/s0300-9084(72)80108-1. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Cole K. W., Gaertner F. H. Chorismate synthase of Neurospora crassa: a flavoprotein. Arch Biochem Biophys. 1974 Dec;165(2):505–518. doi: 10.1016/0003-9861(74)90276-8. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]



