Abstract
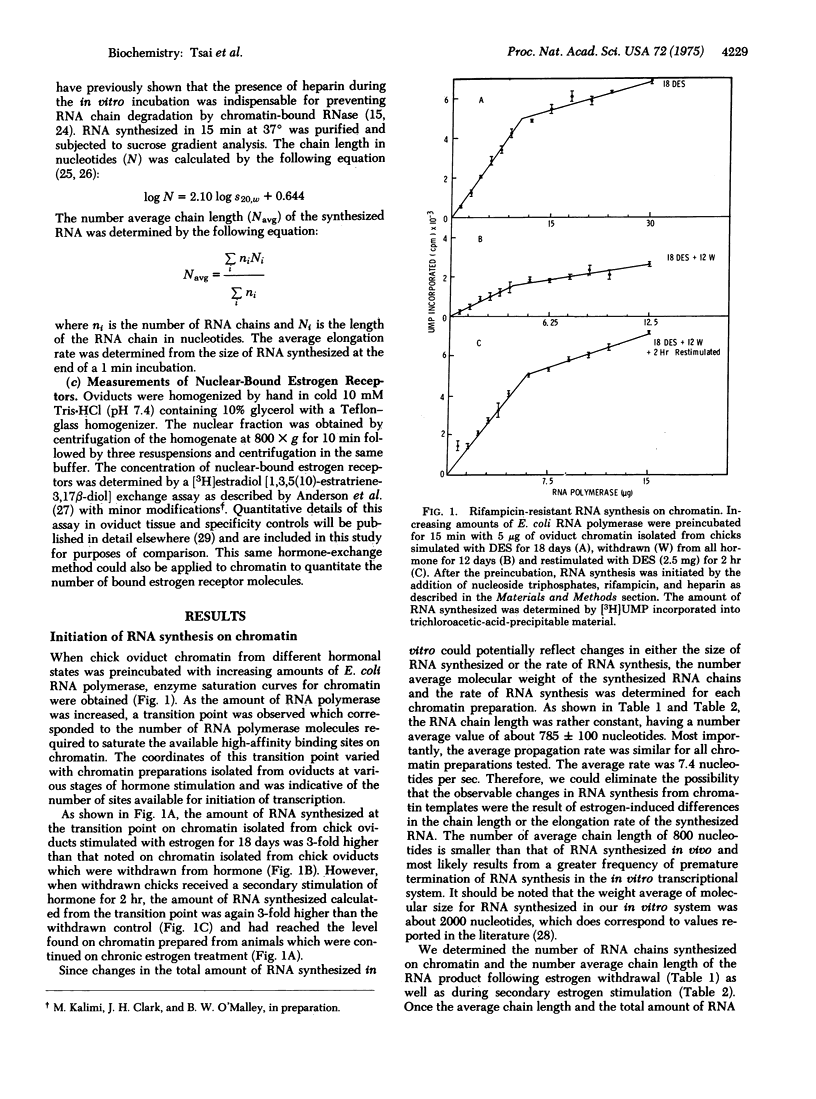
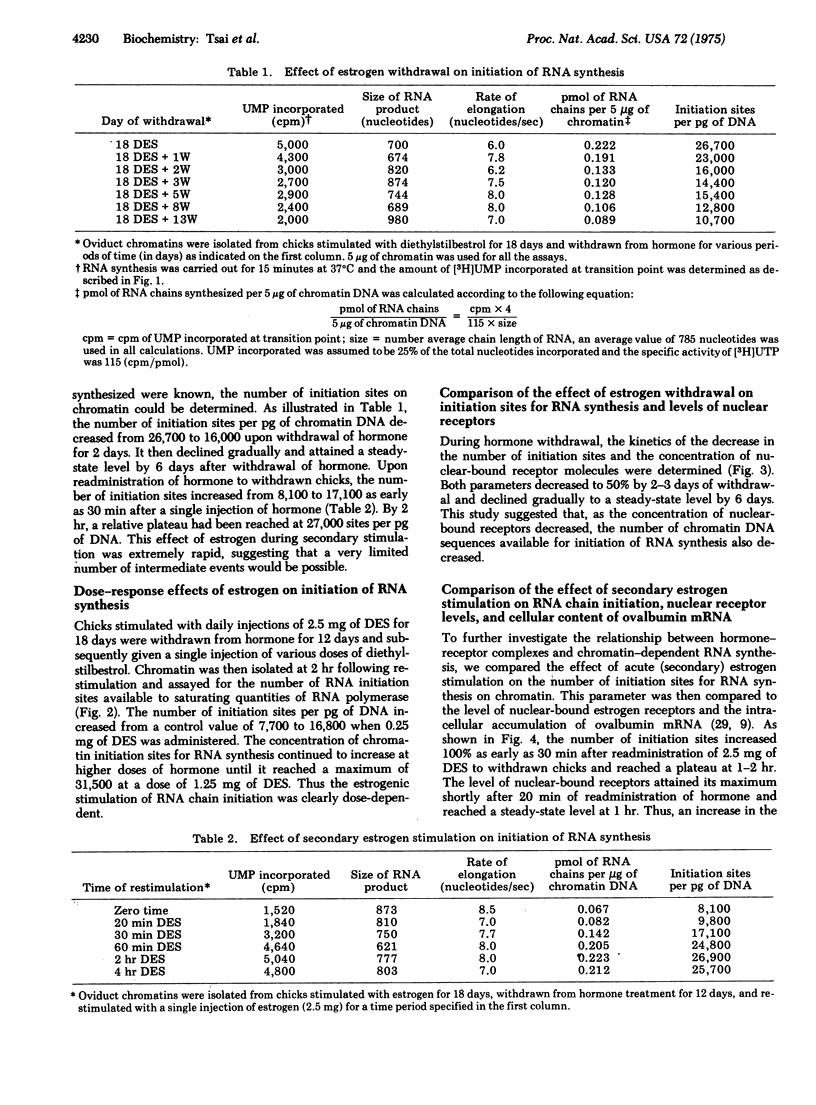
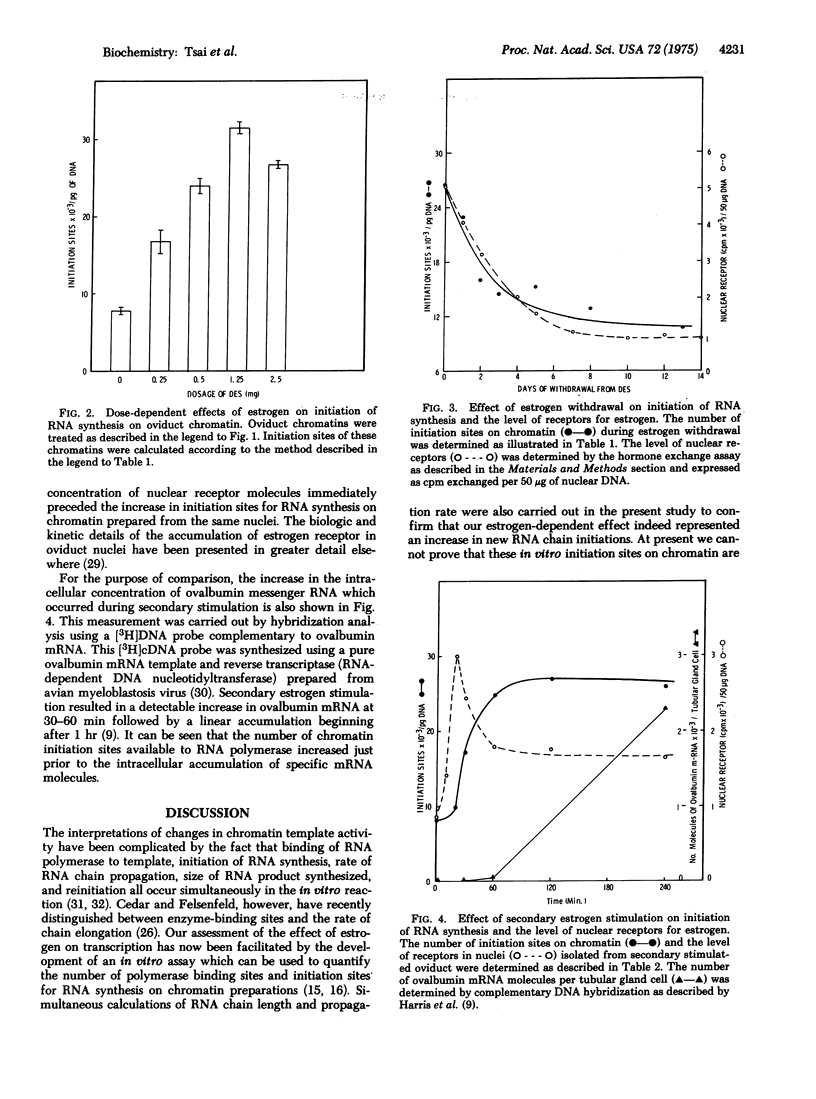
Estrogen (diethylstilbesterol) was administered in vivo to chicks for various time periods. Chromatin was then prepared from oviduct nuclei and assayed for its capacity to support initiation of RNA chain synthesis in vitro in the presence of saturating levels of Escherichia coli RNA polymerase (RNA nucleotidyltransferase; nucleosidetriphosphate:RNA nucleotidyltransferase; EC 2.7.7.6). These same nuclei were also assayed by a [3H]estradiol exchange assay for their endogenous receptor content. The number of available initiation sites for RNA synthesis on chromatin was shown to correlate with the endogenous levels of nuclear estrogen receptor. A decrease in the nuclear concentration of estrogen receptor molecules and the concentration of initiation sites for RNA synthesis occurred during withdrawal of estrogen from previously stimulated chicks. Both parameters declined with a similar half-life. When estrogen was readministered to withdrawn chicks, the number of initiation sites increased 2-fold as early as 30 min and approached a maximal level (3-fold) by 1 hr. During the same period of restimulation with estrogen, the number of estrogen receptor molecules bound to nuclei increased to a maximum at 20 min and then declined at 1 hr to a steady-state level 2-fold higher than the withdrawn chicks. Simultaneous measurements of RNA chain length and RNA chain propagation rate demonstrated that parameters remained relatively constant throughout estrogen withdrawal as well as secondary stimulation. The temporal correlation between changes in the levels of nuclear-bound estrogen receptor and the number of RNA chain initiation sites on chromatin prepared from these same nuclei strongly suggested that the hormone receptor complexes act on chromatin to mediate these changes in genetic transcriptional activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz E. K., Dunn J. J. DNA-dependent RNA polymerase from phage T4 infected E. coli: an enzyme missing a factor required for transcription of T4 DNA. Biochem Biophys Res Commun. 1969 Jan 27;34(2):230–237. doi: 10.1016/0006-291x(69)90636-6. [DOI] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Carey N. H. Modification of the template capacity of chick-oviduct chromatin for form-B RNA polymerase by estradiol. Eur J Biochem. 1973 Feb 1;32(3):513–524. doi: 10.1111/j.1432-1033.1973.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Transcription of high-molecular-weight RNA from hen-oviduct chromatin by bacterial and endogenous form-B RNA polymerases. Eur J Biochem. 1973 Nov 1;39(1):49–61. doi: 10.1111/j.1432-1033.1973.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E., Bonner J. Increased template activity of liver chromatin, a result of hydrocortisone administration. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1370–1375. doi: 10.1073/pnas.54.5.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W., Aronow A., Bunting S. L., Peacock A. C., O'Malley B. W. Changes in chick oviduct ribonucleic acid following hormonal stimulation. Biochemistry. 1969 Feb;8(2):489–495. doi: 10.1021/bi00830a005. [DOI] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Means A. R., Mitchell W. M., O'Malley B. W. Synthesis of (3H)DNA complementary to ovalbumin messenger RNA: evidence for limited copies of the ovalbumin gene in chick oviduct. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3776–3780. doi: 10.1073/pnas.70.12.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Kohler P. O., Grimley P. M., O'Malley B. W. Estrogen-induced cytodifferentiation of the ovalbumin-secreting glands of the chick oviduct. J Cell Biol. 1969 Jan;40(1):8–27. doi: 10.1083/jcb.40.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. O., Grimley P. M., O'Malley B. W. Protein synthesis: differential stimulation of cell-specific proteins in epithelial cells of chick oviduct. Science. 1968 Apr 5;160(3823):86–87. doi: 10.1126/science.160.3823.86. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Adaptation of the rat liver tyrosine-alpha-ketoglutarate transaminase. Biochim Biophys Acta. 1957 Oct;26(1):85–88. doi: 10.1016/0006-3002(57)90057-4. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., O'Malley B. W. Ribonucleic acid polymerase activity of the chick oviduct during steroid-induced synthesis of a specific protein. Biochim Biophys Acta. 1968 Mar 18;157(1):187–194. doi: 10.1016/0005-2787(68)90277-3. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Korenman S. G. Estrogen stimulation of synthesis of specific proteins and RNA polymerase activity in the immature chick oviduct. Biochim Biophys Acta. 1967 Aug 22;145(1):204–207. doi: 10.1016/0005-2787(67)90679-x. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L. Studies on the mechanism of action of progesterone in regulation of the synthesis of specific protein. J Clin Invest. 1968 Mar;47(3):654–664. doi: 10.1172/JCI105761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L. Studies on the mechanism of estrogen-mediated tissue differentiation: regulation of nuclear transcription and induction of new RNA species. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1527–1534. doi: 10.1073/pnas.60.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Spelsberg T. C., Scharder W. T., Chytil F., Steggles A. W. Mechanisms of interaction of a hormone--receptor complex with the genome of a eukaryotic target cell. Nature. 1972 Jan 21;235(5334):141–144. doi: 10.1038/235141a0. [DOI] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. I. Antagonistic effect of progesterone on estrogen-induced proliferation and differentiation of tubular gland cells. J Cell Biol. 1969 Jun;41(3):816–831. doi: 10.1083/jcb.41.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Schwartz R. J., Tsai M-J, Tsai S. Y., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. V. Changes in the number of RNA polymerase binding and initiation sites in chromatin. J Biol Chem. 1975 Jul 10;250(13):5175–5182. [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Mitchell W. M., Chytil F., Wilson E. M., O'Malley B. W. Chromatin of the developing chick oviduct: changes in the acidic proteins. Biochim Biophys Acta. 1973 Jul 27;312(4):765–778. doi: 10.1016/0005-2787(73)90080-4. [DOI] [PubMed] [Google Scholar]
- Tsai M-J, Schwartz R. J., Tsai S. Y., O'Malley B. W. Effects of estrogen on gene expression in the chick oviduct. IV. Initiation of RNA synthesis on DNA and chromatin. J Biol Chem. 1975 Jul 10;250(13):5165–5174. [PubMed] [Google Scholar]



