Abstract
Microvariant RNA, a small self-replicating molecule (114 nucleotides long), has been isolated from Qbeta replicase reactions incubated in the absence of exogenous template. Its complete nucleotide sequence has been determined. Comparison with MDV-1 RNA, a somewhat larger endogenous Qbeta replicase product (220 nucleotides long) that had previously been characterized, revealed no significant sequence similarity. Since Qbeta replicase can mediate the synthesis of both of these disparate RNA molecules, primary sequence cannot be the sole determining factor in the processes of enzyme recognition and replication. This implies that the key is to be found in the secondary or tertiary structures. The availability of two different replicating molecules of defined sequence should aid in identifying these critical structural features.
Full text
PDF

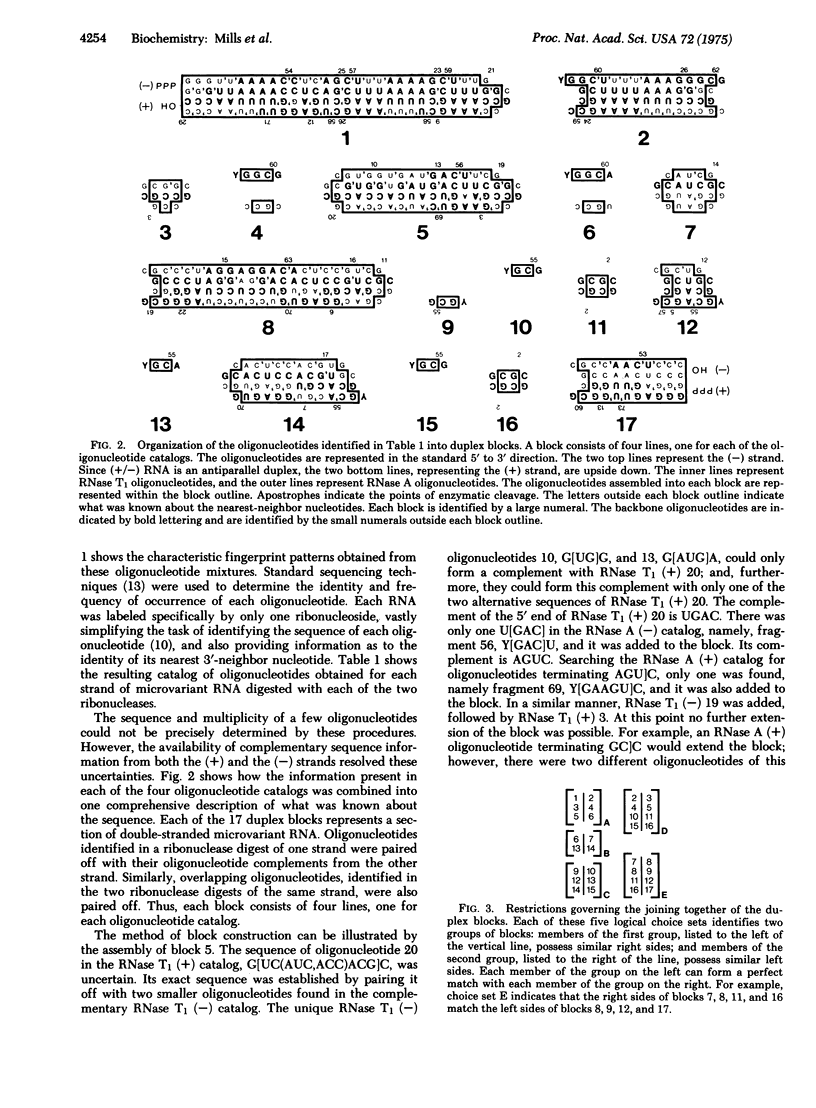
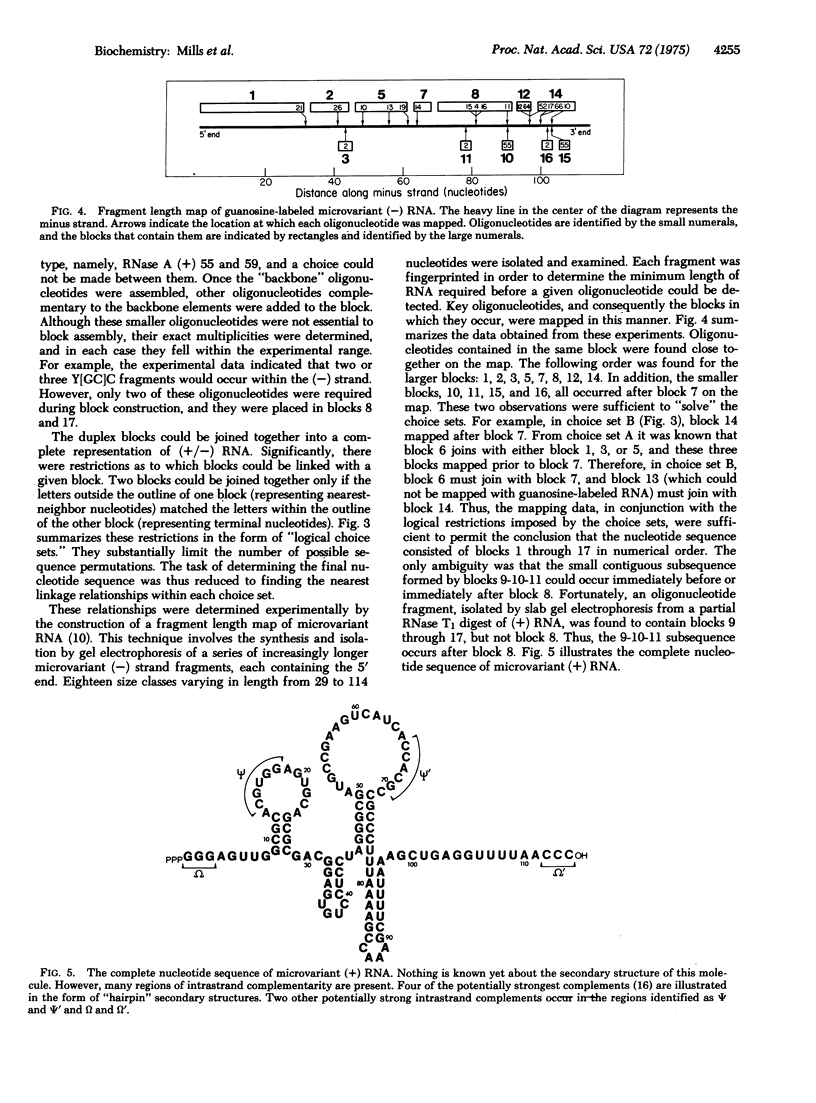

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Rensing U., August J. T. Replication of RNA viruses. X. Replication of a natural 6 s RNA by the Q-beta RNA polymerase. J Mol Biol. 1969 Oct 28;45(2):181–193. doi: 10.1016/0022-2836(69)90098-9. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Autocatalytic synthesis of a viral RNA in vitro. Science. 1965 Nov 12;150(3698):884–886. doi: 10.1126/science.150.3698.884. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Specific template requirments of RNA replicases. Proc Natl Acad Sci U S A. 1965 Aug;54(2):579–587. doi: 10.1073/pnas.54.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Mills D. R., Kramer F. R., Spiegelman S. A replicating RNA molecule suitable for a detailed analysis of extracellular evolution and replication. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3038–3042. doi: 10.1073/pnas.69.10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Mills D. R., Spiegelman S. The mechanism of Q replication: sequence at the 5' terminus of a 6-S RNA template. Biochim Biophys Acta. 1971 May 13;238(2):212–223. [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R., Cole P. E., Nishihara T., Spiegelman S. Evolution in vitro: sequence and phenotype of a mutant RNA resistant to ethidium bromide. J Mol Biol. 1974 Nov 15;89(4):719–736. doi: 10.1016/0022-2836(74)90047-3. [DOI] [PubMed] [Google Scholar]
- Levisohn R., Spiegelman S. Further extracellular Darwinian experiments with replicating RNA molecules: diverse variants isolated under different selective conditions. Proc Natl Acad Sci U S A. 1969 Jul;63(3):805–811. doi: 10.1073/pnas.63.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn R., Spiegelman S. The cloning of a self-replicating RNA molecule. Proc Natl Acad Sci U S A. 1968 Jul;60(3):866–872. doi: 10.1073/pnas.60.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Peterson R. L., Spiegelman S. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc Natl Acad Sci U S A. 1967 Jul;58(1):217–224. doi: 10.1073/pnas.58.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Spiegelman S. In vitro synthesis of an infectious mutant RNA with a normal RNA replicase. Science. 1966 Jul 1;153(3731):64–67. doi: 10.1126/science.153.3731.64. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Schneider-Bernloehr H., Orgel L. E., Spiegelman S. In vitro selection of bacteriophage Q-beta ribonucleic acid variants resistant to ethidium bromide. J Mol Biol. 1970 Aug;51(3):531–539. doi: 10.1016/0022-2836(70)90006-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Haruna I., Holland I. B., Beaudreau G., Mills D. The synthesis of a self-propagating and infectious nucleic acid with a purified enzyme. Proc Natl Acad Sci U S A. 1965 Sep;54(3):919–927. doi: 10.1073/pnas.54.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M., Luce R. Evidence for de novo production of self-replicating and environmentally adapted RNA structures by bacteriophage Qbeta replicase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):162–166. doi: 10.1073/pnas.72.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]