Abstract
The rat retina contains dopaminergic interplexiform cells that send processes to the outer plexiform layer where dopamine is released in a light-dependent manner. We report herein that physiologically relevant concentrations of dopamine inhibited ouabain-sensitive photoreceptor oxygen consumption in dark- and light-adapted rat retinas and inhibited Na+,K+-ATPase specific activity (EC 3.6.1.37) in a rat rod outer-inner segment preparation. Experiments with the selective D1 agonist fenoldopam or D2 agonist quinpirole and experiments with dopamine plus either the D1 antagonist SCH23390 or D2/D4 antagonist clozapine showed that the inhibition of oxygen consumption and enzyme activity were mediated by D2/D4-like receptors. The amphetamine-induced release of dopamine, monitored by the inhibition of oxygen consumption, was blocked by L-2-amino-4-phosphonobutyric acid and kynurenic acid. Pharmacological and biochemical experiments determined that the IC50 values of ouabain for the alpha1-low and alpha3-high ouabain affinity isozymes of photoreceptor Na+,K+-ATPase were approximately 10(-5) and approximately 10(-7) M, respectively, and that the D2/D4-like mediated inhibition of Na+,K+-ATPase was exclusively selective for the alpha3 isozyme. The dopamine-mediated inhibition of alpha3 first occurred at 5 nM, was maximal at 100 microM (-47%), had an IC50 value of 382 +/- 23 nM, and exhibited negative cooperativity (Hill coefficient, 0.27). Prior homogenization of the rod outer-inner segment completely prevented the long-lasting inhibition, suggesting that the effect was coupled to a second messenger. Although the physiological significance of our findings to photoreceptor function is unknown, we hypothesize that these results may have relevance for the temporal tuning properties of rods.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990 Dec;259(6 Pt 2):F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- Biernbaum M. S., Bownds M. D. Influence of light and calcium on guanosine 5'-triphosphate in isolated frog rod outer segments. J Gen Physiol. 1979 Dec;74(6):649–669. doi: 10.1085/jgp.74.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bugnon O., Ofori S., Schorderet M. Okadaic acid modulates exocytotic and transporter-dependent release of dopamine in bovine retina in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1995 Jan;351(1):53–59. doi: 10.1007/BF00169064. [DOI] [PubMed] [Google Scholar]
- Chen C., Lokhandwala M. F. Inhibition of Na+,K(+)-ATPase in rat renal proximal tubules by dopamine involved DA-1 receptor activation. Naunyn Schmiedebergs Arch Pharmacol. 1993 Mar;347(3):289–295. doi: 10.1007/BF00167447. [DOI] [PubMed] [Google Scholar]
- Civelli O., Bunzow J. R., Grandy D. K. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990 Jan;4(1):43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., Todd R. D., Harmon S., O'Malley K. L. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Falardeau P., Shores C., Caron M. G. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol. 1991 Oct;11(5):437–453. doi: 10.1007/BF00734808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamgoz M. B., Wagner H. J. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int. 1992 Feb;20(2):139–191. doi: 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Neurotransmitter systems in the retina. Retina. 1982;2(4):305–321. [PubMed] [Google Scholar]
- Fox D. A., Chu L. W. Rods are selectively altered by lead: II. Ultrastructure and quantitative histology. Exp Eye Res. 1988 Apr;46(4):613–625. doi: 10.1016/s0014-4835(88)80017-4. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Rubinstein S. D. Age-related changes in retinal sensitivity, rhodopsin content and rod outer segment length in hooded rats following low-level lead exposure during development. Exp Eye Res. 1989 Feb;48(2):237–249. doi: 10.1016/s0014-4835(89)80073-9. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Rubinstein S. D., Hsu P. Developmental lead exposure inhibits adult rat retinal, but not kidney, Na+,K(+)-ATPase. Toxicol Appl Pharmacol. 1991 Jul;109(3):482–493. doi: 10.1016/0041-008x(91)90011-3. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hemrick-Luecke S. K., Ornstein P. L. Protection against amphetamine-induced neurotoxicity toward striatal dopamine neurons in rodents by LY274614, an excitatory amino acid antagonist. Neuropharmacology. 1992 Oct;31(10):1027–1032. doi: 10.1016/0028-3908(92)90104-w. [DOI] [PubMed] [Google Scholar]
- Gauchy C., Desban M., Glowinski J., Kemel M. L. NMDA regulation of dopamine release from proximal and distal dendrites in the cat substantia nigra. Brain Res. 1994 Jan 28;635(1-2):249–256. doi: 10.1016/0006-8993(94)91446-x. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Pressley T. A., Loeb J. N., Ismail-Beigi F. Ionic dependence of active Na-K transport: "clamping" of cellular Na+ with monensin. Am J Physiol. 1987 Jul;253(1 Pt 2):F26–F33. doi: 10.1152/ajprenal.1987.253.1.F26. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hemrick-Luecke S. K., Henderson M. G., Fuller R. W. MK801 antagonism of the prolonged depletion of striatal dopamine by amphetamine in iprindole-treated rats. Life Sci. 1992;50(6):PL31–PL33. doi: 10.1016/0024-3205(92)90383-z. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol. 1987 Sep;390:319–333. doi: 10.1113/jphysiol.1987.sp016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman S. R., Williams J. A. Sodium-potassium pump in guinea-pig parotid gland: secretagogue stimulation of ouabain binding to dispersed acini. J Physiol. 1985 Mar;360:121–134. doi: 10.1113/jphysiol.1985.sp015607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D., Witkovsky P. Effects of submicromolar concentrations of dopamine on photoreceptor to horizontal cell communication. Brain Res. 1993 Nov 5;627(1):122–128. doi: 10.1016/0006-8993(93)90755-c. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Lee R. H., Chase D. G., Racz E. Rod photoreceptor cells dissociated from mature mice retinas. Invest Ophthalmol Vis Sci. 1986 Mar;27(3):285–295. [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Marks M. J., Seeds N. W. A heterogeneous ouabain-ATPase interaction in mouse brain. Life Sci. 1978 Dec 31;23(27-28):2735–2744. doi: 10.1016/0024-3205(78)90654-9. [DOI] [PubMed] [Google Scholar]
- McGrail Kevin M., Sweadner Kathleen J. Complex Expression Patterns for Na+,K+-ATPase Isoforms in Retina and Optic Nerve. Eur J Neurosci. 1990 Feb;2(2):170–176. doi: 10.1111/j.1460-9568.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
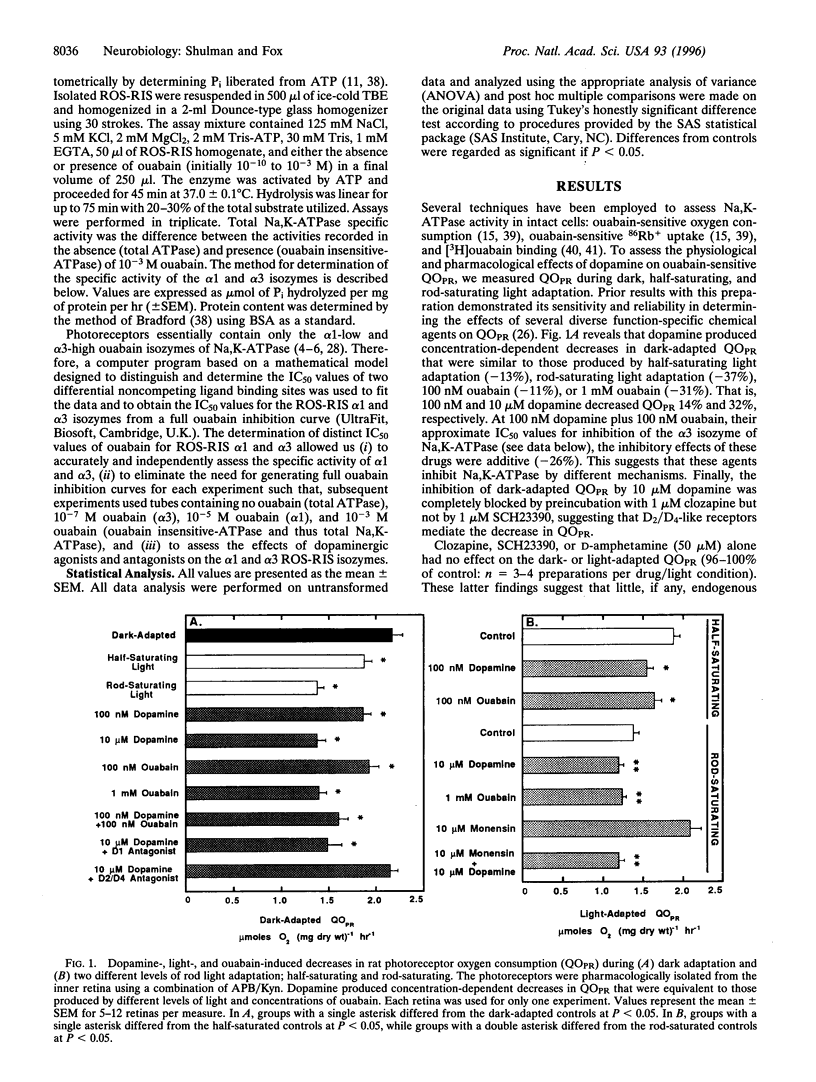
- Medrano C. J., Fox D. A. Oxygen consumption in the rat outer and inner retina: light- and pharmacologically-induced inhibition. Exp Eye Res. 1995 Sep;61(3):273–284. doi: 10.1016/s0014-4835(05)80122-8. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Tamura T., Yau K. W. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991 Mar;97(3):413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J., Moussafi F., Simon A. Sclerally directed processes of dopaminergic interplexiform cells reach the outer nuclear layer in rat and monkey retina. Vis Neurosci. 1990 Jun;4(6):547–553. doi: 10.1017/s0952523800005757. [DOI] [PubMed] [Google Scholar]
- Ottlecz A., Garcia C. A., Eichberg J., Fox D. A. Alterations in retinal Na+, K(+)-ATPase in diabetes: streptozotocin-induced and Zucker diabetic fatty rats. Curr Eye Res. 1993 Dec;12(12):1111–1121. doi: 10.3109/02713689309033509. [DOI] [PubMed] [Google Scholar]
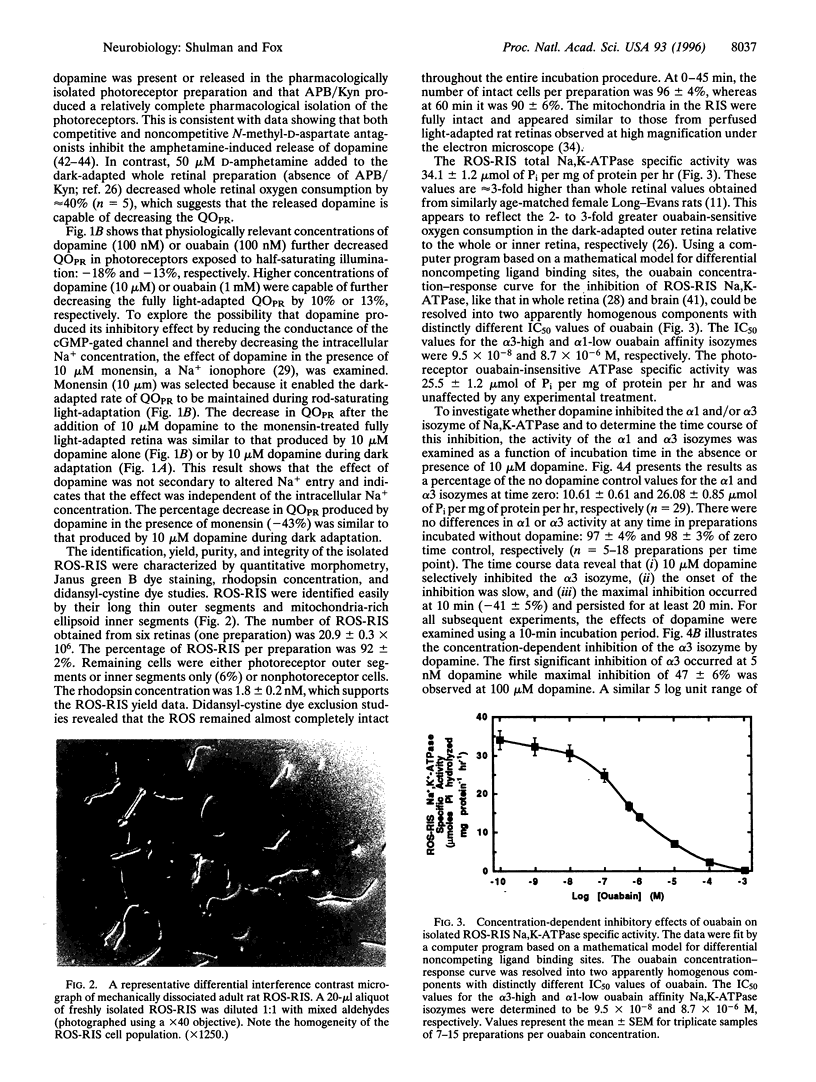
- Schneider B. G., Kraig E. Na+, K(+)-ATPase of the photoreceptor: selective expression of alpha 3 and beta 2 isoforms. Exp Eye Res. 1990 Nov;51(5):553–564. doi: 10.1016/0014-4835(90)90086-a. [DOI] [PubMed] [Google Scholar]
- Schneider B. G., Shyjan A. W., Levenson R. Co-localization and polarized distribution of Na,K-ATPase alpha 3 and beta 2 subunits in photoreceptor cells. J Histochem Cytochem. 1991 Apr;39(4):507–517. doi: 10.1177/39.4.1848572. [DOI] [PubMed] [Google Scholar]
- Seri I., Kone B. C., Gullans S. R., Aperia A., Brenner B. M., Ballermann B. J. Locally formed dopamine inhibits Na+-K+-ATPase activity in rat renal cortical tubule cells. Am J Physiol. 1988 Oct;255(4 Pt 2):F666–F673. doi: 10.1152/ajprenal.1988.255.4.F666. [DOI] [PubMed] [Google Scholar]
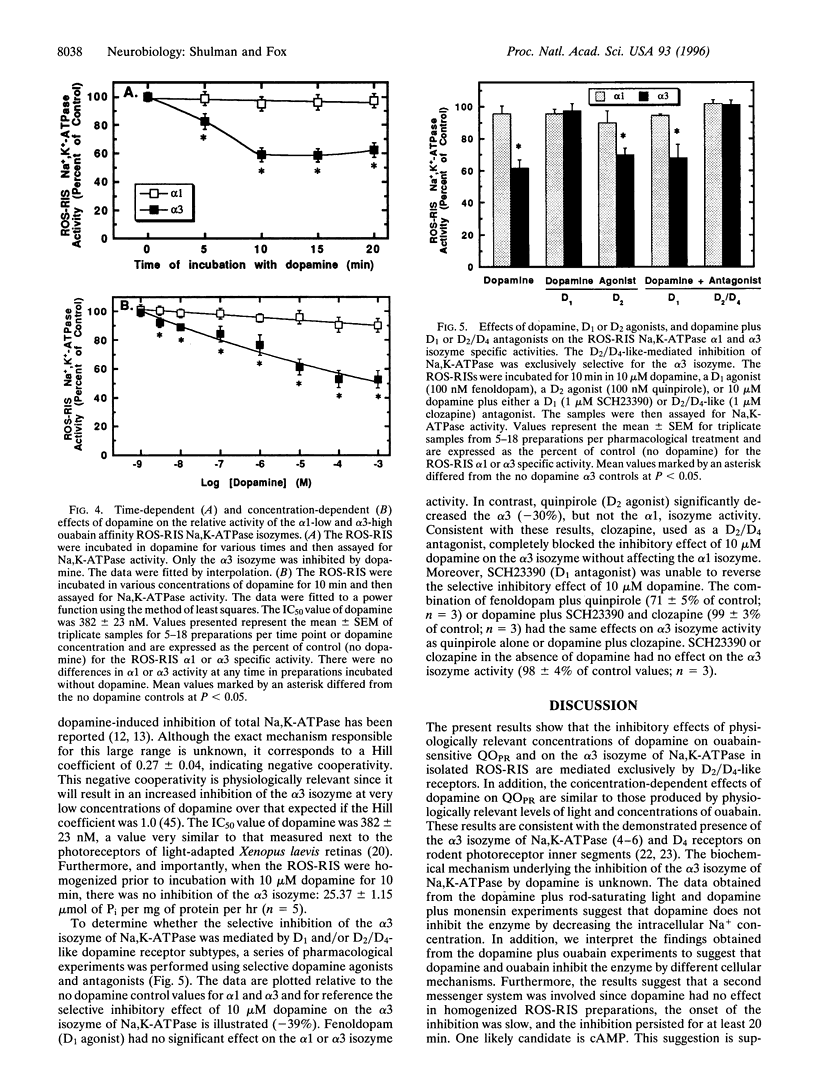
- Shimazaki H., Oakley B., 2nd Reaccumulation of [K+]o in the toad retina during maintained illumination. J Gen Physiol. 1984 Sep;84(3):475–504. doi: 10.1085/jgp.84.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H. J., Luo B. G., Ariano M. A., Sibley D. R., Stell W. K. Localization of D2 dopamine receptors in vertebrate retinae with anti-peptide antibodies. J Comp Neurol. 1993 May 22;331(4):469–481. doi: 10.1002/cne.903310404. [DOI] [PubMed] [Google Scholar]
- Winkler B. S., Riley M. V. Influence of calcium on retinal ATPases. Invest Ophthalmol Vis Sci. 1980 May;19(5):562–564. [PubMed] [Google Scholar]
- Winkler B. S., Riley M. V. Na+-K+ and HCO-3 ATPase activity in retina: dependence on calcium and sodium. Invest Ophthalmol Vis Sci. 1977 Dec;16(12):1151–1154. [PubMed] [Google Scholar]
- Witkovsky P., Nicholson C., Rice M. E., Bohmaker K., Meller E. Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5667–5671. doi: 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Shi X. P. Slow light and dark adaptation of horizontal cells in the Xenopus retina: a role for endogenous dopamine. Vis Neurosci. 1990 Oct;5(4):405–413. doi: 10.1017/s0952523800000493. [DOI] [PubMed] [Google Scholar]
- Xu X. J., Xu J., Huang B., Livsey C. T., Karwoski C. J. Comparison of pharmacological agents (aspartate vs. aminophosphonobutyric plus kynurenic acids) to block synaptic transmission from retinal photoreceptors in frog. Exp Eye Res. 1991 Jun;52(6):691–698. doi: 10.1016/0014-4835(91)90021-6. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., Robinson W. E., Hagins W. A. Topology of the outer segment membranes of retinal rods and cones revealed by a fluorescent probe. Science. 1974 Sep 27;185(4157):1176–1179. doi: 10.1126/science.185.4157.1176. [DOI] [PubMed] [Google Scholar]