Abstract
A functional mucus layer is a key requirement for gastrointestinal health as it serves as a barrier against bacterial invasion and subsequent inflammation. Recent findings suggest that mucus composition may pose an important selection pressure on the gut microbiota and that altered mucus thickness or properties such as glycosylation lead to intestinal inflammation dependent on bacteria. Here we used TM-IEC C1galt -/- mice, which carry an inducible deficiency of core 1-derived O-glycans in intestinal epithelial cells, to investigate the effects of mucus glycosylation on susceptibility to intestinal inflammation, gut microbial ecology and host physiology. We found that TM-IEC C1galt -/- mice did not develop spontaneous colitis, but they were more susceptible to dextran sodium sulphate-induced colitis. Furthermore, loss of core 1-derived O-glycans induced inverse shifts in the abundance of the phyla Bacteroidetes and Firmicutes. We also found that mucus glycosylation impacts intestinal architecture as TM-IEC C1galt-/- mice had an elongated gastrointestinal tract with deeper ileal crypts, a small increase in the number of proliferative epithelial cells and thicker circular muscle layers in both the ileum and colon. Alterations in the length of the gastrointestinal tract were partly dependent on the microbiota. Thus, the mucus layer plays a role in the regulation of gut microbiota composition, balancing intestinal inflammation, and affects gut architecture.
Introduction
The gastrointestinal tract harbours the most densely populated microbial ecosystem known encompassing more than 1014 bacteria, which outnumbers the cells of the human body by an order of magnitude. The gut microbiota resides in very close proximity to the host epithelium; however, despite this close association, the intestinal tract is normally healthy and free of inflammation primarily owing to a mechanical separation of host and microbial cells [1]–[3]. This separation is caused by the presence of several physical and biochemical barriers, the most important being gelatinous mucus, which covers the whole gastrointestinal tract [2]. Defects in the mucus layer are associated with intestinal inflammation [3], [4].
The mucus layer in the small intestine and colon is mainly made up of multimers of the mucin MUC2, a highly O-glycosylated protein of approximately 5200 amino acids and 2.5 MDa [5]. O-glycans contribute to about 80% of its mass and therefore mainly determine the physical mucus properties. O-glycosylation of MUC2 occurs post-translationally in the Golgi apparatus starting with the addition of the initial N-acetylgalactosamine (GalNAc) to the hydroxyl groups of serine and threonine in its two PTS domains (rich in proline, threonine and serine), where the larger is a stretch of about 2300 aa centrally located in MUC2 and rich in proline, threonine and serine [6]–[8]. The resulting GalNAcα-O-Ser/Thr structure is known as the Tn antigen and is normally not detectable as it is extended and branched by the action of other glycosyltransferases. The primary enzymes in this process are the core 1 β1,3-galactosyltransferase (C1galt1) in mice and core 3 β1,3-N-acetylglucosaminyltransferase (C3GnT) in humans [9], [10].
Mucus glycans not only protect the epithelial layer, but they also serve as an adhesion substrate for bacteria expressing adhesins and are a nutrient source for bacteria by hydrolysis through glycosidases [11], [12]. Thus, glycans could be an important factor in the selection of a beneficial microbiota and homeostasis. Indeed, glycosyltransferases have been shown to impact both intestinal inflammation and microbiota composition. In humans, loss of the α-1,2-fucosyltransferase FUT2, which is involved in the formation of ABO blood group antigens on the intestinal mucosa and in body fluids, also leads to an altered microbiota and increased susceptibility to infection and inflammatory disease such as Crohn's disease [13]. In mice, another blood-group-related glycosyltransferase β-1,4-N-acetylgalactosaminyltransferase 2 (B4galnt2) has been shown to affect gut microbiota composition [14] and thereby may affect susceptibility to intestinal inflammatory diseases.
Furthermore, mice fostered with milk of mothers deficient in the α2,3-sialyltransferase St3gal4 harbour an altered gut microbiota and are more resistant to dextran sodium sulphate (DSS)-induced colitis [15]. Finally, loss of core 3-derived O-glycans results in greater susceptibility to DSS-induced colitis [16] and loss of core 1-derived O-glycans has been reported to lead to spontaneous colitis [17]. Inflammation is in both models caused by altered mucus properties that abolish the separation of host epithelium and intestinal bacteria, thereby allowing bacterial penetration and overgrowth [4]. Disease development can be ameliorated by antibiotic treatment [17].
Together, these data indicate a causal role for the gut microbiota in the induction of colitis in susceptible hosts. However, it remains to be determined if the altered mucus in these models selects for a more colitogenic microbiota that then causes inflammation. Here we used mice that carry an inducible deficiency of core 1-derived O-glycans in intestinal epithelial cells to investigate if mucus glycosylation affects gut microbial ecology and thereby host physiology as well as susceptibility to intestinal inflammation.
Materials and Methods
Mice
C1galt1 f/f;Villin-Cre-ERT2 (TM-IEC C1galt1 -/-) mice have been previously described [17] and were rederived as germ-free at Taconic. Germ-free mice were maintained in flexible film isolators. A conventional cohort was established by colonizing germ-free TM-IEC C1galt1 -/- mice with caecal flora of C57BL/6 mice and bred for at least three generations in our animal facility under specific pathogen-free conditions. All mice were housed under a 12-h light cycle and fed autoclaved chow diet ad libitum (Labdiet, St Louis, Missouri, USA). TM-IEC C1galt1 -/- mice were bred using heterozygous setup for Villin-Cre-ERT2 alleles to facilitate littermate controls. Excision of the C1galt1 gene was induced by i.p. injection of 1 mg tamoxifen for five consecutive days. Experiments were initiated after a further ten days. All experiments were performed using protocols approved by the Gothenburg Animal Ethics Committee (339-2012, 280-2012 and 281-2012).
Histology
For histological analyses, we used 8-9-week-old female mice that were killed by cervical dislocation. Intestinal specimens were harvested and fixed in methacarn (60% dry methanol, 30% dry chloroform, 10% glacial acetic acid) for 1-2 weeks at room temperature prior to paraffin embedding and sectioning. Sections were stained with haematoxylin/eosin (HE) for morphology, Alcian blue/periodic acid-Schiff (AB-PAS) stain for glycan composition, and Mab anti-Tn antibody (clone 5F4, [18]) with rat-anti-mouse-FITC antibody (BD Pharmingen) for detection of the Tn antigen. Muscle was visualized using rabbit-anti-smooth muscle actin antibody coupled to Cy3 fluorochrome (Sigma) and proliferative cells were stained using rabbit-anti-Ki-67 (Thermo Scientific) antibody and Vectastain Elite ABC kit (Rabbit IgG, Vector labs). HE and HOECHST were used as counterstaining for enzymatic and fluorescent detection, respectively. Villus length and crypt depth were assessed using HE-stained sections. Microscopy measurements were performed using Zeiss Axiovision LE v4.8 on sections of at least five mice per genotype with each 15 individual scores. Number of lamina propria cells was counted for 5–7 mice. For ileum five complete villi and for colon ten areas between two complete crypts per mouse were counted.
DSS treatment
Colonic inflammation was induced in 9–14-week-old male and female mice by administration of 3% DSS (TdB TdB Consultancy, Uppsala, Sweden) for five days in drinking water followed by a five-day recovery period before killing the mice by cervical dislocation [19]. Weight and faeces of the mice were monitored daily. Colitis severity was assessed by calculating the disease activity index (DAI) combining the scores of weight loss, stool consistency and faecal bleeding [20]. Briefly, the scoring system was as follows: weight loss: 0 = no loss, 1 = 1–5%, 2 = 5–10%, 3 = 10–20%, 4 = >20%; stool: 0 = normal, 2 = loose stool, 4 = diarrhoea; and bleeding: 0 = no blood, 2 = cryptic blood (Hemoccult positive, Hemoccult II; Beckman Coulter), and 4 = heavy bleeding. Interleukin (IL)-1β and tumor necrosis factor (TNF)α levels were measured in protein lysates from colon tissues of TM-IEC C1galt1 -/- and wild type mice using Mouse Basic Kit FlowCytomix with IL-1β and TNFα simplex (eBioscience) according to the manufacturer's instructions.
Microbiota analysis by 16S rDNA pyro-sequencing
Total genomic DNA was extracted from 100–200 mg snap frozen caecum of 8–9-week-old female mice as described previously [21]–[23]. The V2–V3 region of the 16S rDNA gene was amplified using barcoded primers. Purified amplicons were pooled and concentration was adjusted to 20 ng/µl for 454 pyrosequencing. Sequence data were analyzed using MacQIIME package v1.6 (http://www.wernerlab.org/software/macqiime, [24]). Briefly, sequencing reads were trimmed and mapped onto the different samples using the barcode information. Next, reads were assigned to operational taxonomic units (OTUs) using 97% identity, representative OTUs were picked and taxonomy assigned. Quality filtering was performed by removing chimeric sequences using ChimeraSlayer and by removing singletons. Relative abundance was calculated by dividing the number of reads for an OTU by the total number of sequences in the sample. Unifrac alpha and beta diversity were calculated and phylogeny constructed using UPGMA (Unweighted Pair Group Method with Arithmetic Mean). Significance of differences in abundances of various taxonomic units was calculated using t-test and false discovery-rate correction in R program.
Results
Loss of core 1-derived O-glycans in adult mice increases susceptibility to DSS-induced intestinal inflammation
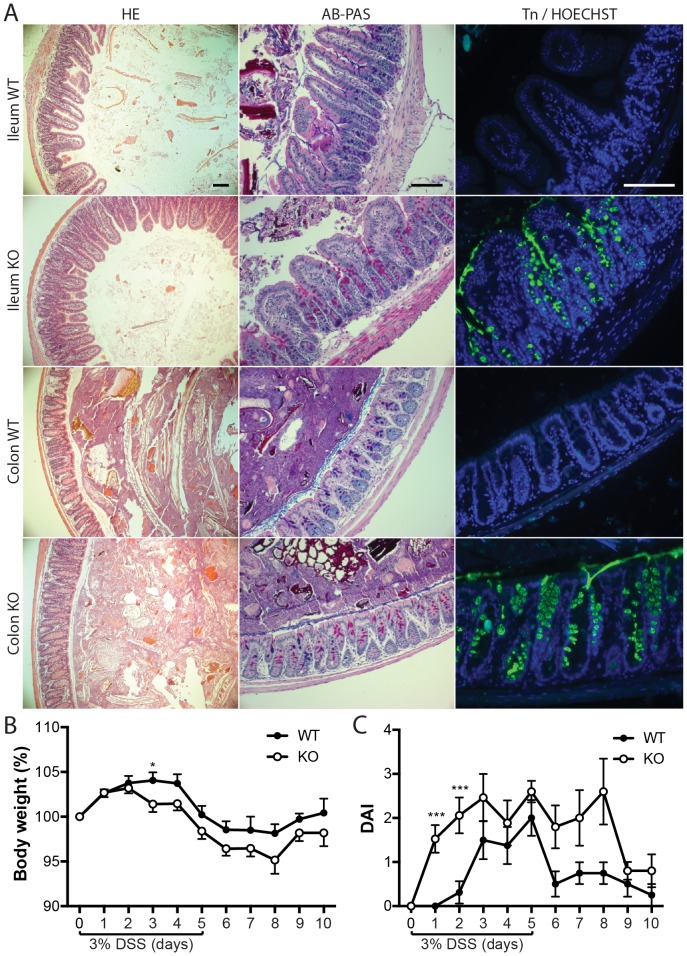
In several genetic mouse models used to study intestinal inflammation conditional transgenic mice are used. However, the mice are born with the respective defect causing inflammation, which may affect microbial composition per se, and thus this strategy is suboptimal to elucidate selection effects of host structures on the gut microbiota. Here we used recently developed TM-IEC C1galt -/- mice, which carry an inducible deficiency of core 1-derived O-glycans in intestinal epithelial cells in which the floxed C1galt1 gene is excised only after induction of the otherwise inert Cre recombinase by administration of tamoxifen. In contrast to a previous study in which TM-IEC C1galt -/- mice developed intestinal inflammation ten days after induced loss of core 1-derived O-glycans [17], TM-IEC C1galt -/- mice in our animal facility did not develop spontaneous colitis or show any signs of inflammation ten days (Fig. 1A, left panels) or even up to 26 weeks after knockout induction. This was not caused by an inability to induce the knockout allele since the TM-IEC C1galt -/- mice showed an increased ratio of cells producing neutral versus acidic carbohydrates (Fig. 1A, middle panels) and a clear removal of core 1-derived O-glycans exposing the Tn antigen after administration of tamoxifen (Fig. 1A, right panels). Furthermore, TM-IEC C1galt -/- mice displayed an altered mucus glycosylation pattern compared with the Cre-negative wild type littermates (data not shown, in agreement with a recent report on the constitutively deleted C1galt1 [10]).
Figure 1. TM-IEC C1galt -/- mice are not spontaneously colitic but more susceptible to DSS-induced intestinal inflammation.
(A) Histological analysis by HE, AB-PAS and Tn antibody staining of sections taken ten days post knockout induction from ileal and colonic tissue of wild type (WT) and TM-IEC C1galt -/- (KO) mice. Scale bars indicate 100 µm. (B) Body weight and (C) DAI during DSS treatment and recovery period (n = 16 per genotype for day 0, two mice sacrificed every day during DSS treatment; Data shows mean ± SEM; ** p<0.01, *** p<0.001).
Importantly, the TM-IEC C1galt -/- mice responded more quickly and with greater severity to treatment with DSS. After three days of DSS treatment, TM-IEC C1galt -/- mice had a significantly lower body weight than the wild type littermate controls and were unable to recover from this weight difference throughout the experiment (Fig. 1B). Furthermore, disease activity index (DAI; the combined score of weight loss, stool consistence and faecal bleeding) was higher in TM-IEC C1galt -/- mice on the first two days of the DSS treatment (Fig. 1C). Blood in the faeces was the main contributor to the DAI with only minor changes in faecal consistency. TM-IEC C1galt -/- mice seemed to recover more slowly from the DSS-induced colitis. Faecal blood could still be detected three days after the DSS treatment was stopped in TM-IEC C1galt -/- mice whereas the wild type controls recovered within one day (Fig. 1C). In addition, the colons of TM-IEC C1galt -/- mice had a slight but non-significant increase in IL-1β and a significantly elevated level of TNFα compared with those of wild type mice (Fig. S1). Taken together, these data indicate that although the TM-IEC C1galt -/- mice did not develop spontaneous colitis in our study, they were more susceptible to DSS-induced gut inflammation.
Mucus glycosylation has a minor influence on gut microbiota composition
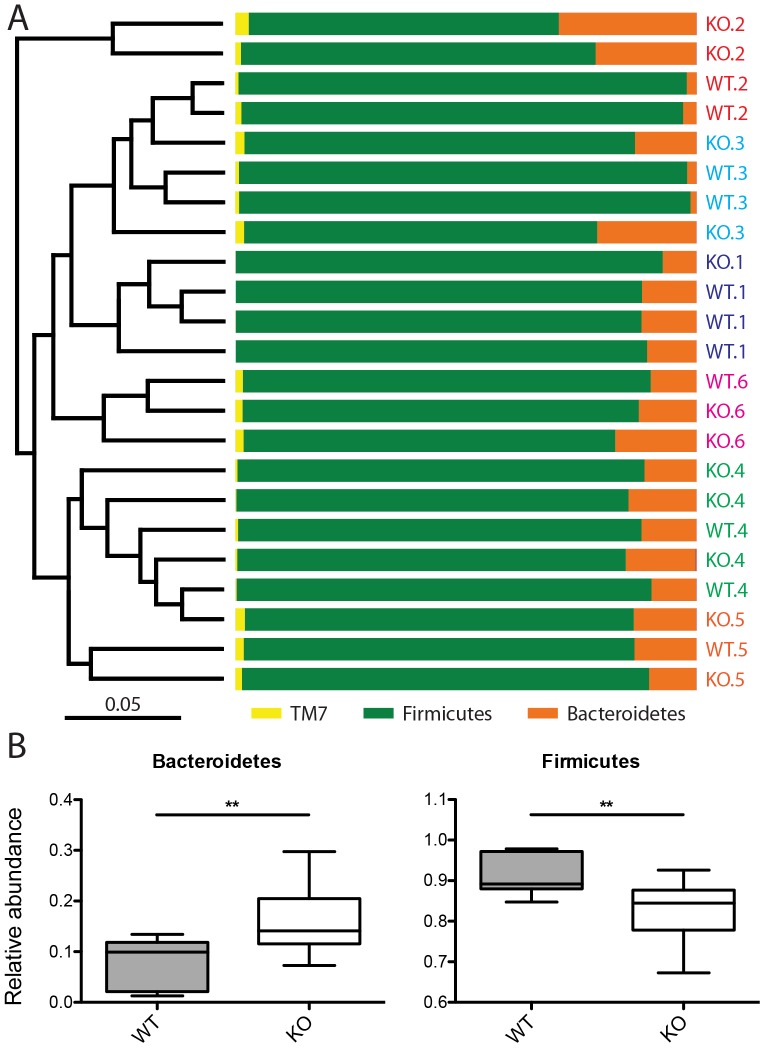
To test the hypothesis that mucus glycosylation plays a role in selecting the microbiota and maintaining homeostasis, we analyzed the microbial composition of TM-IEC C1galt -/- mice and their wild type littermates. When comparing the overall microbial composition, we found that the mice grouped primarily according to cage and within a cage the mice grouped secondarily according to genotype, indicating that loss of core 1-derived O-glycans induced subtle alterations in the microbiota (Fig. 2A). Abundance of the phylum Bacteroidetes was increased whereas that of Firmicutes was reduced in TM-IEC C1galt -/- mice (Fig. 2B). These phylum differences were reflected by lower taxonomic levels as well with, for example, classes bacteroidia and clostridia showing the same trends (Fig. S2, S3). However, on a species level, only one operational taxonomic unit (OTU) differed significantly among TM-IEC C1galt -/- and wild type mice (Fig. S2F), indicating rather broad but subtle differences in microbial abundance caused by loss of core 1-derived O-glycans. Moreover, no differences in alpha diversity (the number of microbial species in a sample) were detected (Fig. S4). In summary, in the absence of any detectable inflammation, loss of core 1-derived O-glycans seems to exert a subtle selection effect on the gut microbiota.
Figure 2. Mucus glycosylation influences gut microbiota composition.
(A) Microbiota composition and phylogenetic tree on phylum level. Labels indicate genotype and cage number. Samples are colour coded according to cage number. (B) Relative abundance of Bacteroidetes and Firmicutes in wild type (WT) and TM-IEC C1galt -/- (KO) mice (Data shows mean ± SEM; ** p≤0.01, *** p≤0.001).
Lack of core 1 mucus glycosylation alters the intestinal architecture
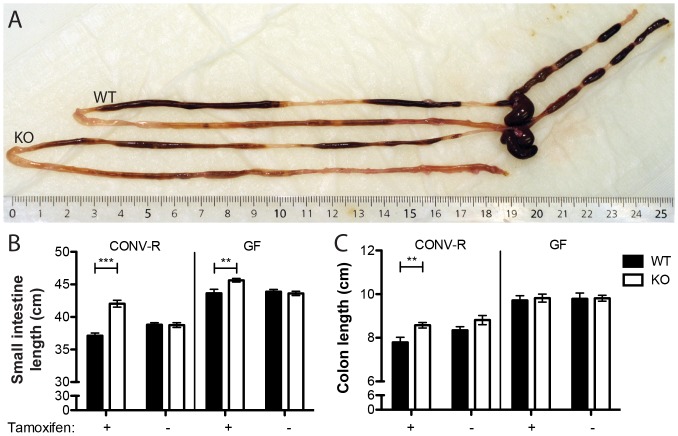
The most obvious phenotypic feature of TM-IEC C1galt -/- mice was an extension in the length of gastrointestinal tract. Both the small and large intestines of the TM-IEC C1galt -/- mice were about 10% longer than those of wild type controls (Fig. 3A–C). Notably, the effect on gut length was not seen in the absence of tamoxifen activation of the Cre recombinase and thus dependent on the core 1-derived O-glycans (Fig. 3B, C). Regardless of the genotype, the length of the gastrointestinal tract in germ-free mice was longer than that of conventional counterparts. Furthermore, in the small intestine the effect on gut length in the TM-IEC C1galt -/- mice seemed to be partly dependent on the microbiota as the difference in length between knockout and wild type mice decreased from 13.2% (p<0.0001) to 4.6% (p = 0.0039) in conventional and germ-free setting, respectively. In contrast, in the colon the effect on gut length seemed to be solely dependent on the microbes (decreased from 10.1%, p = 0.006 to 1.1%, p = 0.72 in the conventional and germ-free setting, respectively; Fig. 3C).
Figure 3. Lack of core 1-derived mucus O-glycosylation alters gut length.
(A) Representative gastrointestinal tracts of wild type (WT) and TM-IEC C1galt -/- (KO) mice. (B) Length of the small intestine and colon of wild type (WT) and TM-IEC C1galt -/- (KO) mice raised in the presence (CONV-R) and absence of bacteria (GF) as well as with or without injection of tamoxifen to induce Cre recombinase-mediated loss of core 1-derived mucus O-glycosylation (n = 7-16 mice per group; Data shows mean ± SEM; ** p<0.01, *** p<0.001).
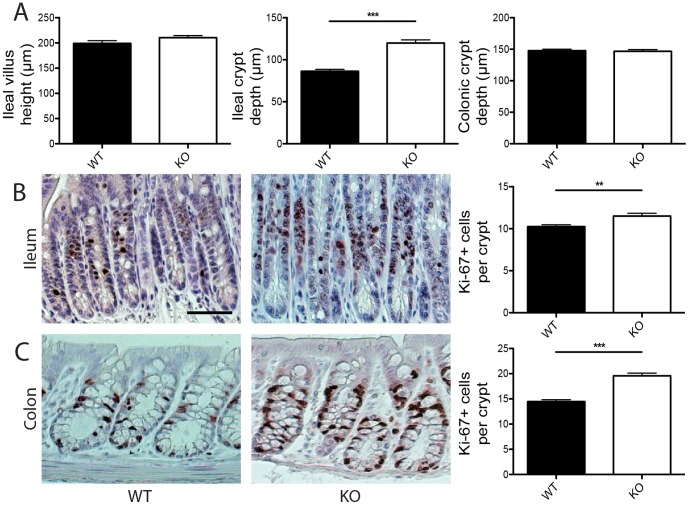
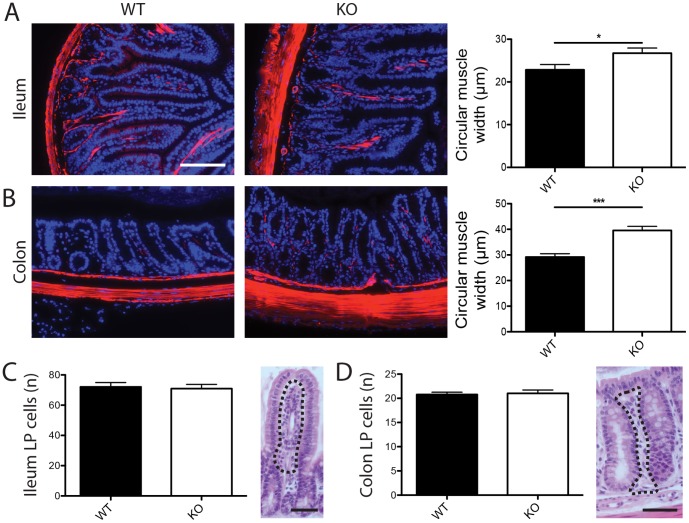
In addition to gut length, structural architecture of the gastrointestinal tract was also altered by loss of core 1-derived O-glycans. Although length of the ileal villi and depth of the colonic crypts did not differ between TM-IEC C1galt -/- and wild type mice, ileal crypts were deeper in TM-IEC C1galt -/- mice (Fig. 4A). We also observed small but significant increases in the number of Ki-67 positive cells both in ileal and colonic crypts of TM-IEC C1galt -/- mice (Fig. 4B, C), indicating slightly increased proliferation of intestinal epithelial cells. Furthermore, the circular muscle layers both in the ileum and colon were thicker in TM-IEC C1galt -/- mice (Fig. 5A, B). However, numbers of immune cells in the lamina propria were not altered in either the ileum or colon of TM-IEC C1galt -/- mice compared with wild type controls (Fig. 5C, D).
Figure 4. Intestinal architecture and proliferation are altered in TM-IEC C1galt -/- mice.
(A) Ileal villus height and ileal and colonic crypt depth of wild type (WT) and TM-IEC C1galt -/- (KO) mice. (B–C) Ki-67 stainings and quantification of sections from ileal (B) and colonic (C) tissue of wild type (WT) and TM-IEC C1galt -/- (KO) mice. For quantifications n = 5–7 mice were scored. Scale bar indicates 50 µm (B–C). Data shows mean ± SEM; ** p<0.01, *** p<0.001.
Figure 5. Intestinal muscle width is increased in TM-IEC C1galt -/- mice in absence of immune cell infiltration.
(A–B) Smooth muscle actin stainings and quantification of sections from ileal (A) and colonic (B) tissue of wild type (WT) and TM-IEC C1galt -/- (KO) mice. (C–D) Quantification and representative image of ileal (C) and colonic (D) lamina propria cells. LP - lamina propria. The dotted lines represent the areas used for scoring. For quantifications n = 5–7 mice were scored. Scale bars indicate 50 µm. Data shows mean ± SEM; * p<0.05, *** p<0.001.
Discussion
We aimed to investigate if mucus glycosylation has a selective effect on gut microbial ecology and thereby might impact susceptibility to intestinal inflammation and host physiology. Therefore, we used TM-IEC C1galt -/- mice which carry an inducible deficiency of core 1-derived O-glycans in intestinal epithelial cells as a model since it allows us to discriminate between selection effects of host structures in the adult mouse from those present when mice are born and raised with the respective defects. We found that TM-IEC C1galt -/- mice did not show any detectable signs of inflammation, but were more susceptible to DSS-induced colitis. This is in contrast to a previous study of TM-IEC C1galt -/- mice kept in another animal house where they spontaneously developed intestinal inflammation which was dependent on the microbiota ten days after induced loss of core 1-derived O-glycans [17]. Thus, the discrepancy between our and previous findings is probably due to different housing conditions and microbiota in the animal facilities. Presumably, the gut microbiota composition of the TM-IEC C1galt -/- mice rederived and bred in our facility has a flora that is less colitogenic. This is also observed for IL-10-/- mice that normally have spontaneous colitis, but in our animal house only show minor inflammation [4], [25]. An impact of housing on experimental phenotypes has been observed previously in other models. For example, C56BL/6 mice bred by Taconic Farms harbour more lamina propria lymphocytes than those bred by Jackson Laboratory and only caecal flora from Taconic mice is able to induce lamina propria lymphocyte formation in colonized germ-free mice [26]. Similarly, differential susceptibility to streptozotocin-induced diabetes in mice from Taconic Farms, Jackson Laboratory or Charles River Laboratories has been observed [27], [28]. Together, these findings highlight the importance of the microbiota in the development of several inflammatory or metabolic diseases and the need to keep experimental setups including housing conditions and microbiota as stable as possible to facilitate comparisons among studies performed in different animal facilities.
A number of recent publications highlighted the interaction between the intestinal mucus layer, its glycosylation and the microbiota for intestinal homeostasis [29]–[32]. Here, we made two observations that suggest a microbial role in intestinal homeostasis. (i) Loss of core 1-derived O-glycans, in the absence of inflammation, induced subtle gut microbial alterations, for example inverse shifts in the abundance of the phyla Bacteroidetes and Firmicutes, indicating that glycosylation of the intestinal mucus layer has a selective capacity on microbial ecology. Similarly, other immune components have previously been shown to modulate the microbiota [33]. (ii) The gastrointestinal tract was elongated in TM-IEC C1galt -/- mice and these alterations were partly microbially dependent. The small changes in intestinal architecture may thus potentially result from the altered microbial ecology.
We also observed other intestinal changes in TM-IEC C1galt -/- mice compared with wild type controls, namely increased ileal crypt depth, a small increase in the number of proliferating epithelial cells, and a thicker circular muscle layer in the ileum and colon of TM-IEC C1galt -/- mice. This last observation might indicate differential muscle contraction in the TM-IEC C1galt -/- mice and could potentially contribute to the observed differences in length of the gastrointestinal tract of TM-IEC C1galt -/- mice.
Notably, it has previously been shown that the gut microbiota affects several aspects of host physiology within and outside of the gastrointestinal tract including organ morphogenesis and tissue homeostasis [1]. In Drosophila, the gut microbiota modulates length of the gastrointestinal tract along with proliferation and differentiation of intestinal epithelial cells [34]. In mice, proliferation of epithelial cells is reduced in the small intestine of germ-free compared to conventionally raised mice [35]–[37]. Furthermore, the gut microbiota influences intestinal architecture including villus and crypt morphology as well as remodelling of the intestinal vascular system [38], [39]. Altogether these observations suggest that the gut microbiota represents an important environmental factor in the regulation of intestinal homeostasis and physiology.
An altered intestinal architecture without major infiltration of immune cells is reminiscent of some of the features of the human disease irritable bowel syndrome (IBS) [40]. Furthermore, both hypertrophy of the jejunal muscle layer [41] and alterations in faecal microbiota composition were reported to be associated with IBS [42]. Further studies are required to determine if TM-IEC C1galt -/- mice could represent an animal model for IBS.
Taken together, mucus glycosylation seems to be important not only for protection and lubrication of the gastrointestinal tract, but also has selective effects on the composition of the resident gut microbiota and small effects on the structure of the intestine. The small alterations in mucus properties, microbiota composition and slightly increased epithelial proliferation in TM-IEC C1galt -/- mice could indicate a skewed intestinal homeostasis with altered regenerative response and intestinal architecture in the absence of inflammation. This could potentially suggest a number of physiological defence actions preceding an overall inflammatory response with infiltrating immune cells.
Supporting Information
Levels of pro-inflammatory cytokines IL-1β and TNFα in colon of TM-IEC C1galt-/- and wild type mice. Proteins were isolated from colonic tissue of wild type (WT) and TM-IEC C1galt -/- (KO) mice and IL-1β and TNFα measured with n = 4 mice per group. Data shows mean ± SEM; * p<0.05.
(TIF)
Taxa differentially abundant among wild type and TM-IEC C1galt -/- mice. Data shows mean ± SEM; * p<0.05, ** p<0.01.
(TIF)
Abundance overview of microbial phyla on class, order, family and genus level. Labels indicate genotype and cage number. Samples are colour coded according to cage number.
(TIF)
Microbial alpha-diversity in wild type and TM-IEC C1galt -/- mice.
(TIF)
Acknowledgments
We thank Caroline Jonstrand for measuring cytokines, Anna Hallén and Karin Ahlman for technical assistance and Rosie Perkins for editing the manuscript.
Funding Statement
This study was supported by the Swedish Research Council, Torsten and Ragnar Söderbergs' foundations, IngaBritt and Arne Lundberg's foundation, Swedish Foundation for Strategic Research - The Mucus-Bacteria-Colitis Center (MBC), Knut and Alice Wallenberg foundation, National Institute of Health (U01AI095473, R01DK085691), and the regional agreement on medical training and clinical research (ALF) between Region Västra Götaland and Sahlgrenska University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sommer F, Backhed F (2013) The gut microbiota - masters of host development and physiology. Nat Rev Microbiol 11: 227–238. [DOI] [PubMed] [Google Scholar]
- 2. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, et al. (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105: 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson ME, Sjovall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed]
- 4.Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, et al. (2013) Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. [DOI] [PMC free article] [PubMed]
- 5. Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, et al. (2011) Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68: 3635–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gum JR Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS (1994) Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem 269: 2440–2446. [PubMed] [Google Scholar]
- 7. Jensen PH, Kolarich D, Packer NH (2010) Mucin-type O-glycosylation—putting the pieces together. FEBS J 277: 81–94. [DOI] [PubMed] [Google Scholar]
- 8. Larsson JM, Karlsson H, Sjovall H, Hansson GC (2009) A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 19: 756–766. [DOI] [PubMed] [Google Scholar]
- 9. Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, et al. (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22: 736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomsson KA, Holmen-Larsson JM, Angstrom J, Johansson ME, Xia L, et al. (2012) Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology 22: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, et al. (2010) Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1: 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juge N (2012) Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20: 30–39. [DOI] [PubMed] [Google Scholar]
- 13. Rausch P, Rehman A, Kunzel S, Hasler R, Ott SJ, et al. (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A 108: 19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staubach F, Kunzel S, Baines AC, Yee A, McGee BM, et al. (2012) Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J 6: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, et al. (2010) Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med 207: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An G, Wei B, Xia B, McDaniel JM, Ju T, et al. (2007) Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204: 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu J, Wei B, Wen T, Johansson ME, Liu X, et al. (2011) Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest 121: 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandel U, Petersen OW, Sorensen H, Vedtofte P, Hakomori S, et al. (1991) Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J Invest Dermatol 97: 713–721. [DOI] [PubMed] [Google Scholar]
- 19. Johansson ME, Gustafsson JK, Sjoberg KE, Petersson J, Holm L, et al. (2010) Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 5: e12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, et al. (2013) Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol 190: 4795–4804. [DOI] [PubMed] [Google Scholar]
- 21. Yu Z, Morrison M (2004) Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36: 808–812. [DOI] [PubMed] [Google Scholar]
- 22. Salonen A, Nikkila J, Jalanka-Tuovinen J, Immonen O, Rajilic-Stojanovic M, et al. (2010) Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods 81: 127–134. [DOI] [PubMed] [Google Scholar]
- 23. Nylund L, Heilig HG, Salminen S, de Vos WM, Satokari R (2010) Semi-automated extraction of microbial DNA from feces for qPCR and phylogenetic microarray analysis. J Microbiol Methods 83: 231–235. [DOI] [PubMed] [Google Scholar]
- 24. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274. [DOI] [PubMed] [Google Scholar]
- 26. Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, et al. (2008) Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ (2011) The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med 61: 356–360. [PMC free article] [PubMed] [Google Scholar]
- 28. Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, et al. (2011) Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A 108: 11548–11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, et al. (2013) Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci U S A 110: 17059–17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wrzosek L, Miquel S, Noordine ML, Bouet S, Chevalier-Curt MJ, et al. (2013) Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kleiveland CR, Hult LT, Spetalen S, Kaldhusdal M, Christofferesen TE, et al. (2013) The noncommensal bacterium Methylococcus capsulatus (Bath) ameliorates dextran sulfate (Sodium Salt)-Induced Ulcerative Colitis by influencing mechanisms essential for maintenance of the colonic barrier function. Appl Environ Microbiol 79: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sperandio B, Fischer N, Chevalier-Curt MJ, Rossez Y, Roux P, et al. (2013) Virulent Shigella flexneri Affects Secretion, Expression, and Glycosylation of Gel-Forming Mucins in Mucus-Producing Cells. Infect Immun 81: 3632–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, et al. (2012) Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med 209: 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin SC, Kim SH, You H, Kim B, Kim AC, et al. (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334: 670–674. [DOI] [PubMed] [Google Scholar]
- 35. Abrams GD, Bauer H, Sprinz H (1963) Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest 12: 355–364. [PubMed] [Google Scholar]
- 36. Crawford PA, Gordon JI (2005) Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A 102: 13254–13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savage DC, Siegel JE, Snellen JE, Whitt DD (1981) Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Appl Environ Microbiol 42: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinhardt C, Bergentall M, Greiner TU, Schaffner F, Ostergren-Lunden G, et al. (2012) Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stappenbeck TS, Hooper LV, Gordon JI (2002) Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A 99: 15451–15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quigley EM, Abdel-Hamid H, Barbara G, Bhatia SJ, Boeckxstaens G, et al. (2012) A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on irritable bowel syndrome. J Clin Gastroenterol 46: 356–366. [DOI] [PubMed] [Google Scholar]
- 41. Tornblom H, Lindberg G, Nyberg B, Veress B (2002) Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 123: 1972–1979. [DOI] [PubMed] [Google Scholar]
- 42. Jeffery IB, O'Toole PW, Ohman L, Claesson MJ, Deane J, et al. (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61: 997–1006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of pro-inflammatory cytokines IL-1β and TNFα in colon of TM-IEC C1galt-/- and wild type mice. Proteins were isolated from colonic tissue of wild type (WT) and TM-IEC C1galt -/- (KO) mice and IL-1β and TNFα measured with n = 4 mice per group. Data shows mean ± SEM; * p<0.05.
(TIF)
Taxa differentially abundant among wild type and TM-IEC C1galt -/- mice. Data shows mean ± SEM; * p<0.05, ** p<0.01.
(TIF)
Abundance overview of microbial phyla on class, order, family and genus level. Labels indicate genotype and cage number. Samples are colour coded according to cage number.
(TIF)
Microbial alpha-diversity in wild type and TM-IEC C1galt -/- mice.
(TIF)