Abstract
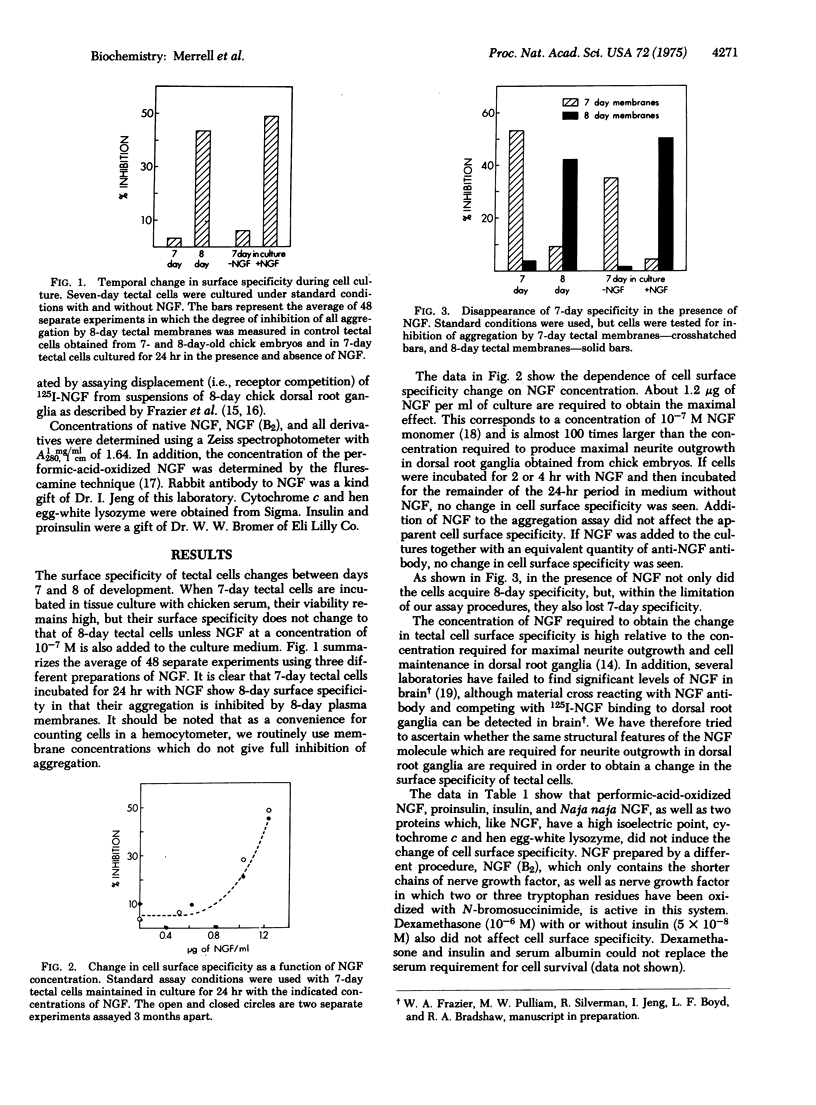
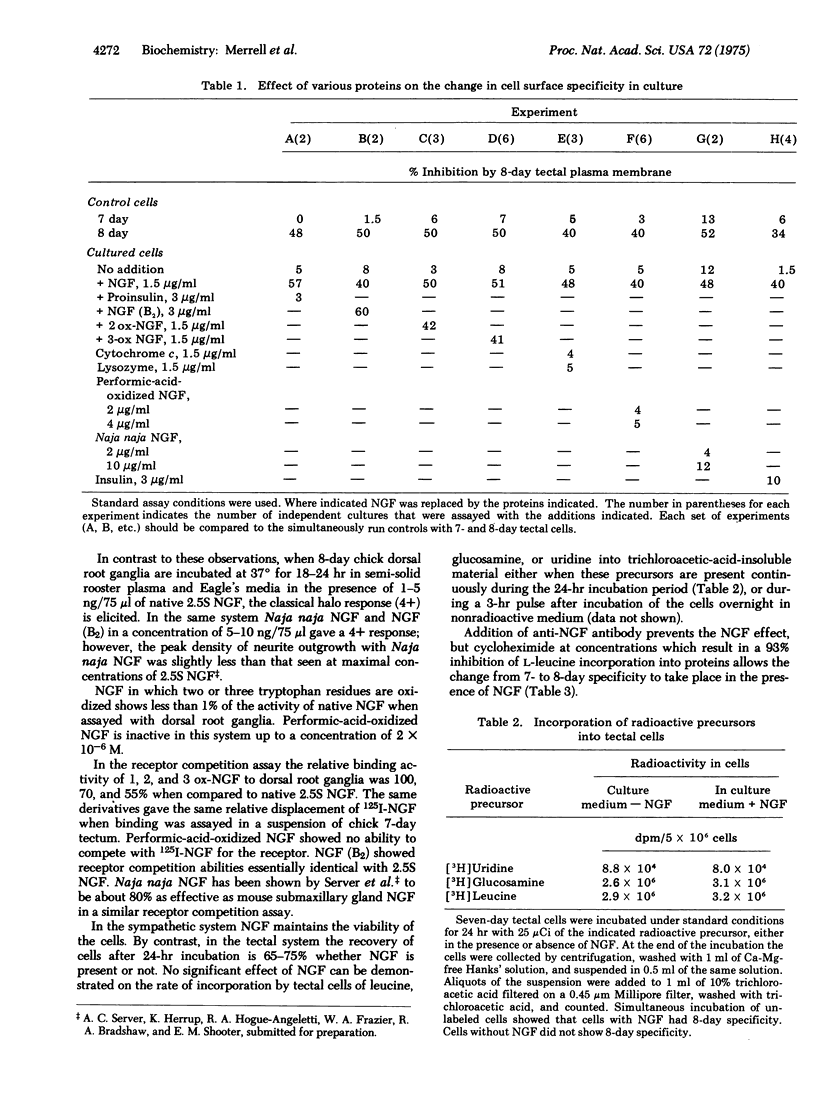
The change in cell surface adhesive specificity previously shown to occur between day 7 and 8 of development in the chick optic tectum ]Gottlieb et al. (1974), Proc. Nat. Acad. Sci. USA 71, 1800-1802] can be induced in rotating cultures of tectal cells by the addition of 10(-7) M mouse submaxillary gland nerve growth factor. Insulin, proinsulin, dexamethasone, and performic-acid-oxidized nerve growth factor are individually inactive in this system, but nerve growth factor in which the three tryptophan residues/subunit have been oxidized with N-bromosuccinimide is active. Thus, the specificity of this system for nerve growth factor is different than that observed with embryonic dorsal root or sympathetic ganglia, where the oxidized tryptophan derivative is inactive in stimulating neurite production. It is possible that in this system nerve growth factor serves as an analog of another specific trophic factor, presumably structurally related to nerve growth factor, may be active at much lower concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Hermodson M. A., Bradshaw R. A. Amino acid sequences of mouse 2.5S nerve growth factor. II. Isolation and characterization of the thermolytic and peptic peptides and the complete covalent structure. Biochemistry. 1973 Jan 2;12(1):100–115. doi: 10.1021/bi00725a018. [DOI] [PubMed] [Google Scholar]
- Angeletti R. H. Nerve growth factor from cobra venom. Proc Natl Acad Sci U S A. 1970 Mar;65(3):668–674. doi: 10.1073/pnas.65.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Pulliam M. W., Szutowicz A., Bradshaw R. A. Properties and specificity of binding sites for 125I-nerve growth factor in embryonic heart and brain. J Biol Chem. 1974 Sep 25;249(18):5918–5923. [PubMed] [Google Scholar]
- Frazier W. A., Hogue-Angeletti R. A., Sherman R., Bradshaw R. A. Topography of mouse 2.5S nerve growth factor. Reactivity of tyrosine and tryptophan. Biochemistry. 1973 Aug 14;12(17):3281–3293. doi: 10.1021/bi00741a021. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Gottlieb D. I., Glaser L. A novel assay of neuronal cell adhesion. Biochem Biophys Res Commun. 1975 Apr 7;63(3):815–821. doi: 10.1016/s0006-291x(75)80456-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Merrell R., Glaser L. Temporal changes in embryonal cell surface recognition. Proc Natl Acad Sci U S A. 1974 May;71(5):1800–1802. doi: 10.1073/pnas.71.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin: interaction with membrane receprots and relationship to cyclic purine nucleotides and cell growth. Fed Proc. 1975 Jun;34(7):1556–1563. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Merrell R., Glaser L. Specific recognition of plasma membranes by embryonic cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2794–2798. doi: 10.1073/pnas.70.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai N., Lai C. Y., Horecker B. L. Use of fluorescamine in the chromatographic analysis of peptides from proteins. Anal Biochem. 1974 Apr;58(2):563–570. doi: 10.1016/0003-2697(74)90225-5. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. An assay for intercellular adhesive specificity. J Cell Biol. 1971 Nov;51(21):525–535. doi: 10.1083/jcb.51.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]



