Abstract
Campylobacter fetus are important animal and human pathogens and the two major subspecies differ strikingly in pathogenicity. C. fetus subsp. venerealis is highly niche-adapted, mainly infecting the genital tract of cattle. C. fetus subsp. fetus has a wider host-range, colonizing the genital- and intestinal-tract of animals and humans. We report the complete genomic sequence of C. fetus subsp. venerealis 84-112 and comparisons to the genome of C. fetus subsp. fetus 82-40. Functional analysis of genes predicted to be involved in C. fetus virulence was performed. The two subspecies are highly syntenic with 92% sequence identity but C. fetus subsp. venerealis has a larger genome and an extra-chromosomal element. Aside from apparent gene transfer agents and hypothetical proteins, the unique genes in both subspecies comprise two known functional groups: lipopolysaccharide production, and type IV secretion machineries. Analyses of lipopolysaccharide-biosynthesis genes in C. fetus isolates showed linkage to particular pathotypes, and mutational inactivation demonstrated their roles in regulating virulence and host range. The comparative analysis presented here broadens knowledge of the genomic basis of C. fetus pathogenesis and host specificity. It further highlights the importance of surface-exposed structures to C. fetus pathogenicity and demonstrates how evolutionary forces optimize the fitness and host-adaptation of these pathogens.
Introduction
The ε-proteobacterial genus Campylobacter comprises bacteria with a high degree of niche adaptation and host tropism [1]. The species colonize mucosal surfaces and are animal and human pathogens [2]. The genomes of Campylobacter spp. are not large (≈1.5 Mbp) and show characteristics of genome decay typical for niche-adapted bacteria [3]. These features make Campylobacter species ideal model systems to study genetic contributions to niche specificity and virulence by comparative genome analysis [3]. Multi locus sequence typing (MLST) has shown that the two C. fetus subspecies, C. fetus subsp. fetus and C. fetus subsp. venerealis, have a clonal population structure [4] and differentiation of the taxa is only partially successful [5]. Both subspecies are important veterinary pathogens causing abortions and infertility in ruminants [6]. C. fetus subsp. venerealis is a bovine-adapted “clone” [7] causing venereal infections and epidemic abortion in cattle. Statutory preclusion of C. fetus subsp. venerealis infection underscores the importance of this veterinary pathogen [8], but human infections are rare [6]. In contrast the generalist subspecies, C. fetus subsp. fetus, colonizes the intestinal and the genital-tract of multiple hosts including sheep, cattle, birds and humans. It is an emerging human pathogen, leading to invasive infections and even death [9], [10]. Most bacteremic illnesses caused by Campylobacter are due to C. fetus [9], [11].
C. fetus displays two major (O-antigen based) sero-types, A and B, and a rare variant AB [12]. The sero-types correlate with the type of surface array protein (Sap) expressed by the bacterium [13] and differ in their lipopolysaccharide (LPS) composition [12], [14]. The Sap-layer (S-layer) creates a paracrystalline proteinaceous cover enabling C. fetus to resist serum bactericidal activity, and by phase variation to overcome immune recognition [11], [15], [16]. Sero-type A strains expressing SapA are more frequently isolated from human blood than sero-type B strains expressing SapB. The cattle-adapted C. fetus subsp. venerealis is exclusively sero−/sap-type A (type A). Four different Campylobacter clades were identified using MLST [4] and represent the genotypes (I) C. fetus subsp. venerealis type A, (II) C. fetus subsp. fetus type A or (III) type B and (IV) reptile C. fetus type A. The reptilian clade diverges most substantially from the other three closely related genotypes.
The evolutionary interplay between microbial pathogens and their hosts is a continual process of adaptation, manifested by genomic variation of host adaptation factors, and by the gain and loss of genes via horizontal gene transfer (HGT). The underlying hypothesis for this study was that genome reduction and acquisition of relatively few novel genes has enabled C. fetus to adopt distinct subspecies-specific lifestyles. To evaluate this, we performed comparative genetic analyses of C. fetus subsp. venerealis (type A) and C. fetus subsp. fetus (type A), and we compared regions of the two type A strains to type B and reptile C. fetus strains. To gain initial insights into the transcriptional organization of C. fetus, differential RNA-sequencing (dRNA-seq) was performed with the sequenced strains of both subspecies. The analyses revealed many of the molecular details involved in (sub)speciation and virulence of C. fetus and explain the strikingly different host tropism and clinical manifestations of these pathogens.
Results
Comparative Genomics of C. fetus Subspecies
The genome of the bovine strain C. fetus subsp. venerealis 84-112 (type A) was sequenced generating 216.8 Mbp sequence data (≈112-fold coverage). This strain harbors a single circular chromosome 1.93 Mbp in size with GC-content of 33.3% and a circular extra-chromosomal element of 61,141 bp with GC-content of 31.5%. Until now, the only other closed C. fetus genome publicly available was the human isolate C. fetus subsp. fetus 82-40 (type A). That 1.77 Mbp genome also has GC-content of 33.3%. Analysis of the two genomes revealed that they are highly syntenic with 92.9% overall sequence identity. The homologous regions exhibit 99.8% DNA identity. 180 kbp were unique for strain 84-112 and 35 kbp of unique sequences were identified in strain 82-40. Including the 73 extra-chromosomal element open reading frames (orfs), strain 84-112 harbors 204 unique orfs. Nearly all represent putative type IV secretion system (T4SS) components, transposons, or hypothetical proteins (File S4). The 25 orfs unique for strain 82-40 encode putative CRISPR associated (Cas)-proteins, LPS-biosynthetic enzymes, or hypothetical proteins (File S4). General genomic characteristics are summarized in Table 1 .
Table 1. C. fetus genome attributes, including the extra-chromosomal element.
| Attributes | Cff | Cfv | Cfv |
| strain 82-40 | strain 84-112 | ICE_84-112 | |
| *Genome size (bp) | 1,773,615 | 1,926,886 | 61,141 |
| *GC-content % | 33.31 | 33.34 | 31.54 |
| *coding DNA sequence (# of orfs) | 1,769 | 1,992 | 73 |
| *rRNA genes | 6 | 6 | – |
| *tRNA genes | 43 | 43 | – |
| Genomic Islands | 2 (FGI I–II) | 4 (VGI I–IV) | – |
| T4SS loci | |||
| tra-like gene cluster | 0 | 0 | 1 |
| vir-like gene cluster | 0 | 2 | 1 |
| Flexible gene pool | |||
| Integrase XERCD family | 1 | 1 | 0 |
| Integrases/recombinases | 1 | 2 | 0 |
| Insertion Elements (# of copies) | 0 | ISHa1152 (2) | ISHa1152 (3) |
| 0 | ISC1904 (3) | 0 | |
| Prophage-like gene clusters | 1 | 3 | 0 |
| CRISPR | |||
| Spacers (# of copies) | 21 (1) | 24 (1) | 0 |
| 26 (1) | |||
| cas-genes | cas1-6 | 0 | 0 |
according to RAST annotation.
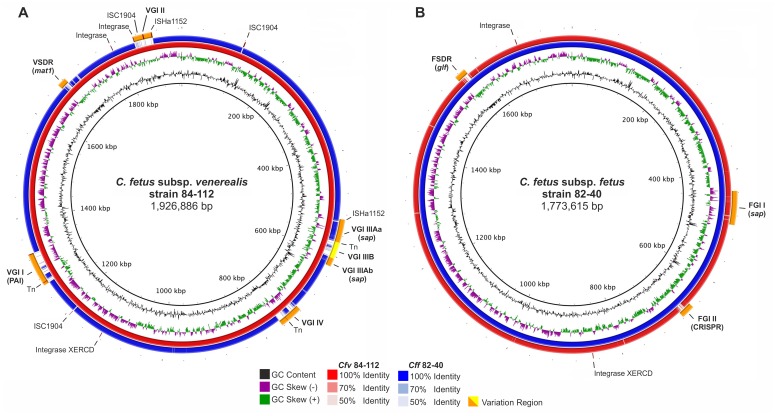
Comparative genome plots clearly illustrate that the unique DNA stretches are located in distinct genomic regions (termed variation regions, VR) scattered across the syntenic genomic core ( Figure 1 ). C. fetus subsp. venerealis 84-112 harbors 5 VRs and C. fetus subsp. fetus 82-40 harbors 3 VRs ( Figure 1 , Table S1 in File S5). All of these regions have features indicative of horizontal acquisition including a shift in %GC-content compared to the core genome, the presence of mobility-related genes (e.g. prophages, transposases) or proximity to tRNA genes, presumably marking their insertion sites into the chromosomal backbone. Two of the VRs of strain 84-112, Venerealis Genomic Island (VGI) I and VGI II, have no counterpart in strain 82-40. Two other VRs are shared between the two subspecies. VGI III of strain 84-112 corresponds to the position of Fetus Genomic Island (FGI) I of strain 82-40. The position of VGI IV corresponds to FGI II. The respective regions of variation are not identical between the two subspecies, but are highly similar, suggesting a common origin. Notably, the VRs of strain 84-112 carry additional blocks of genes, which are predominantly prophage-related. The extra insertions appear to interrupt functional gene modules, thus VGI III can be divided into three subsections designated VGI IIIA-1, VGI IIIA-2, and VGI IIIB (see below, Figure 2C , Table S1 in File S5). In strain 84-112, phage-related genes or transposases flank the VGIs. Similar genes are absent in strain 82-40 except for one area on FGI II containing prophage-like features (see below, Figure 2E ).
Figure 1. Genome comparisons of C. fetus subspecies.
Plots were generated using C. fetus subsp. venerealis 84-112 (Cfv) as a reference (A) or C. fetus subsp. fetus 82-40 (Cff) (B). Inside tracks represent GC-content (ring 1) and GC-skew (ring 2). Cff is shown in blue and Cfv in red. Variation regions (VR) relative to the reference genome are indicated in orange/yellow and named according to the corresponding Genomic Island (GI) or the subspecies definition region (SDR). (V) and (F) in the feature names designate the subspecies venerealis and fetus, respectively. Important genes or features are indicated in parenthesis. Positions of selected mobility genes are indicated.
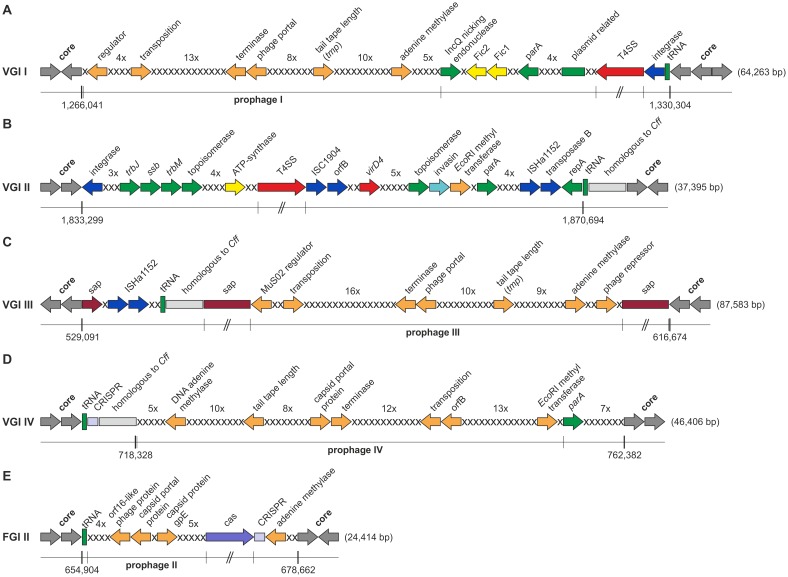
Figure 2. Comparative overview of Genomic Islands (GIs).
(A) VGI I (PAI) with the T4SS and putative prohage I, (B) VGI II with a vir-gene cluster and plasmid-related genes, (C) VGI III containing the surface array protein cluster and prohage III, (D) VGI IV containing the CRISPR-array and prophage IV and (E) FGI II with prophage-related genes (prophage II) and the CRISPR-cluster (array and cas-genes). The GI borders to genes shared between the subspecies (grey) are indicated with nucleotide position. Gene clusters are colored as follows: phage-related genes (orange), plasmid related genes (green), integrases and transposases (blue), T4SS (red), effector proteins (yellow), surface array proteins (purple), cas-genes (lavender), tRNAs (green boxes); Each x represents a hypothetical protein and their numbers in tandem are indicated above.
One VR region ( Figure 1 , Figure S1) that co-localizes in the genomes of both subspecies was designated as the Venerealis- or Fetus Subspecies Definition Region (VSDR/FSDR). These regions are marked by comparatively low GC-content (30.7% and 29.4%, respectively). They contain genes putatively involved in surface carbohydrate metabolism as analyzed below and differentiate the two subspecies.
Metabolic reconstruction based on the genome data and comparative analyses of metabolic pathways using RAST and SEED revealed only two differences between the genomes. C. fetus subsp. fetus 82-40 harbors two orfs putatively involved in thiamin (vitamin B1) biosynthesis, namely a phosphomethylpyrimidine kinase (EC 2.7.4.7) and the thiamin biosynthesis protein ThiC (peg.404), which are absent from the genome of C. fetus subsp. venerealis 84-112. ThiC does not appear to be specific for C. fetus subsp. fetus, however, since a thiC homolog is also present in the unfinished genome of C. fetus subsp. venerealis NCTC 10354. Also no other obvious differences in respiration systems, nutrient transporters and catabolic or anabolic pathways were identified. Whether more subtle genetic differences, like insertions, point mutations or variation in transcriptional control, which might influence metabolism, contribute to the different biology of C. fetus subspecies remains to be elucidated.
In summary, comparative genomics revealed that the two C. fetus subspecies are highly syntenic, but the chromosome of C. fetus subsp. venerealis 84-112 is about 9% larger. The genomic VRs distinguishing the two subspecies are located within a small number of hot-spots, displaying features typical for horizontally acquired DNA.
VGI I and II Contain T4SS-related Genes, Prophage- and Plasmid-like Features
We previously identified and characterized a pathogenicity island (PAI) in C. fetus subsp. venerealis that was absent in all 45 C. fetus subsp. fetus isolates tested [17]. The PAI contained a full set of virB/virD4 genes prototypical for a T4SS (for review see [18]). The T4SS of strains ATCC 19438 and 84-112 mediate conjugative DNA transfer as well as host interaction [17], [19]. This PAI is located in VGI I of strain 84-112 ( Figure 2A ). VGI I also harbors the putative prophage I encompassing a region of 33.7 kb (position: 1,266,041 to 1,299,761) with 47 orfs and a GC-content of 35.4%.
The gene organization of VGI II is less consistent, but with conserved functional modules ( Figure 2B ). Although T4SS-related genes are present, the system lacks virB5 and virB6 and may be non-functional (see below, and Figure S3). Under laboratory conditions, we did not detect transcription of these genes (data not shown). The gene for transposase ISHa1152 suggests a putative integration site for VGI.
FGI I and VGI III Contain the Sap-locus
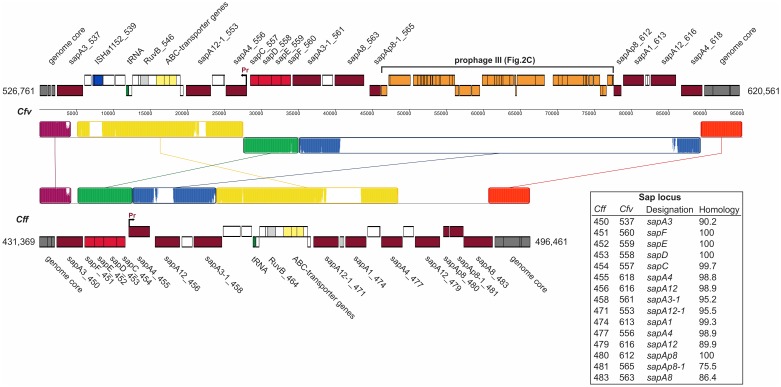
The sap-locus of C. fetus is present in both subspecies and represents the best-characterized C. fetus virulence attributes [11], [15], [16]. In C. fetus subsp. fetus 82-40 the sap genes are located on FGI I close to a tRNA and putative ABC-transporter genes ( Figure 3 ). In C. fetus subsp. venerealis 84-112, the comparable region of VGI III is highly similar to FGI I. However, a block of phage-related genes and a series of genes for hypothetical proteins indicate the presence of another prophage ( Figure 2C , Figure 3 ) apparently leading to rearrangement and separation of the sap genes that may affect S-layer variation of C. fetus subsp. venerealis 84-112. The transcriptome analysis indicates that the insertion of prophage III did not lead to inactivation or truncation of sapAb8_612 ( Figure 4 ). As in VGI I, the ISHa1152 transposase gene was detected, putatively marking a site for extra-chromosomal DNA insertion.
Figure 3. Schematic representation and structural comparison of VGI III and FGI I (sap region).
MAUVE was used to compare the VRs of both subspecies for visualization of rearrangements and insertions. Regions free of rearrangements are indicated by colored colinear blocks. White color within these blocks indicates insertions or non-homologous regions. Important orfs are colored and labeled. S-layer genes (purple) were identified in both C. fetus strains. The sap-promoter is indicated. In C. fetus subsp. venerealis 84-112, the sap genes were disrupted by an inserted prophage (orange). White boxes are mainly hypothetical proteins. Detailed annotation information can be found in File S1. Genes are labeled with RAST-peg numbers and the inset table lists homologous sap genes of the subspecies.
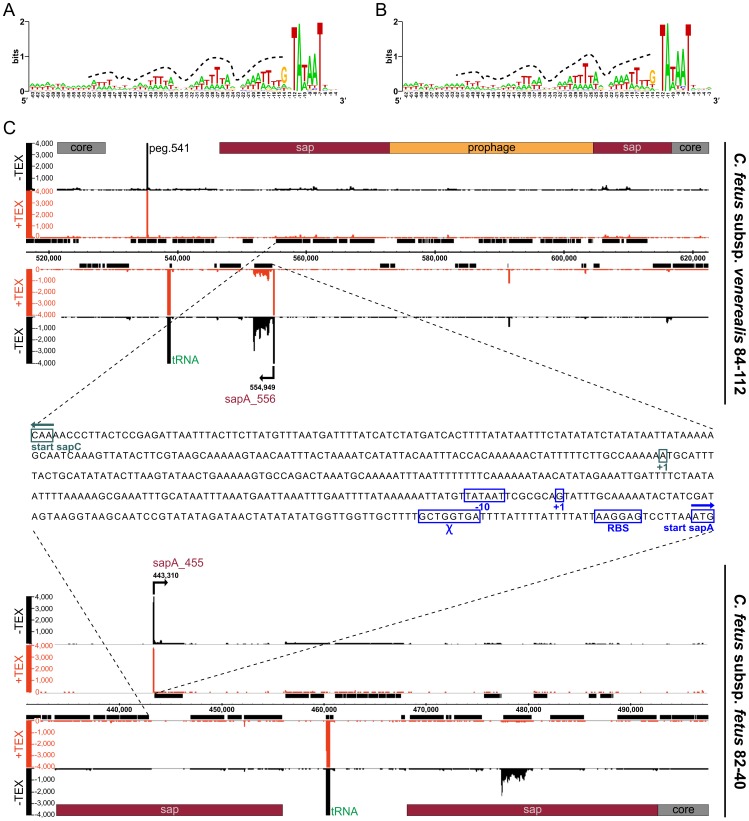
Figure 4. C. fetus promoter sequence and transcriptional organization of the sap-locus.
Promoter consensus sequence for (A) C. fetus subsp. venerealis 84-112 (Cfv) and (B) C. fetus subsp. fetus 82-40 (Cff). The promoter motif is defined by an extended Pribnow box (tgnTAtaAT) at the −10 position. The −35 motif is replaced by a periodic AT-rich signal upstream of position −14 (dotted line). (C) Transcriptional organization of Cfv VG III (top) and Cff FGI I (bottom), identical sap-promoter sequence of Cfv and Cff (middle).
FGI II and VGI IV Contain CRISPR Loci
We identified CRISPR-repeats on the genomes of both C. fetus subspecies (Figure S1). In C. fetus subsp. venerealis 84-112, a single locus (nt 684,618 to 686,228) (Cfv_CRISPR) displays the typical features of a CRISPR-array with 30-bp direct repeats (DR), separated by 21 different spacers. No cas-homologues were identified. Two CRISPR-arrays (nt 655,350 to 656,762 and nt 674,442 to 676,187) were identified in C. fetus subsp. fetus 82-40 (Cff_CRISPR_1 and Cff_CRISPR_2), but only Cff_CRISPR_2 is in close proximity to cas-gene homologues. The DRs and the leader sequence are identical in both subspecies. Some spacers are shared between Cfv_CRISPR and Cff_CRISPR_1, but Cff_CRISPR_2 has no homology to Cfv_CRISPR and Cff_CRISPR_1. Sequences homologous to the spacers of the CRISPR loci were not detected in public DNA databases, thus their putative DNA targets remain unknown.
Since Cas1 is a hallmark of dynamic CRISPR arrays, we screened 102 C. fetus strains for its presence. Cas1 was detected in 19 (47.5%) of 40 subsp. fetus subsp. fetus isolates but was absent in all 62 subsp. venerealis isolates (Odd ratio = 110, 95% CI: 6.3 to 1,897, p = 0.0012). In strain 84-112 another prophage-like gene cluster (prophage IV) is present instead of the cas-genes and the second CRISPR array ( Figure 2D , Figure S1). Interestingly, type B strains are more likely to carry the cas1 gene (14 of 15) compared to type A strains (5 of 24) (Odd ratio = 53.2, 95% CI: 5.6 to 507.4; p = 0.0006) (Table S6 in File S5).
The Extra-chromosomal Element of C. fetus subsp. Venerealis 84-112 Displays Features Typical for Integrative Conjugative Elements (ICE)
The extra-chromosomal element was designated as ICE_84-112 and is the first ICE described in C. fetus (physical map Figure S2; annotation details in File S3). Conjugative transfer (tra) and other genes of apparent plasmid origin were identified but autonomous replication features were lacking. The T4SS locus, termed ICE_trb/tra, most likely is involved in horizontal self-transfer, based on its close relation to the broadly disseminated RP4-like systems. Several phage-related genes and transposases, including the ISHa1152 transposase, could aid chromosomal integration and excision of the ICE ( Figure 1A , Figure 2BC). A region with structural homology to the PAI of VGI I was identified on ICE_84-112 (termed ICE_vir). ICE_84-112 also encodes proteins with a domain called filamentation-induced by cyclic AMP (Fic). This domain is similarly present in Fic1 and Fic2 expressed by the PAI of VGI I [17], [19]. We screened our C. fetus collection for the presence of ICE_84-112 using the ICE specific genes fic3 and fic4 as PCR targets. Of 62 C. fetus subsp. venerealis strains, 7 harbored the ICE-related genes (Table S6 in File S5). The target genes fic3 and fic4 were not detected in any of the 40 C. fetus subsp. fetus strains tested. Transcriptome analysis showed expression of the majority of genes on ICE_84-112.
ICE_84-112 may replicate extra-chromosomally via a conjugative transfer replication mode, as proposed for other ICEs [20], [21], since the obligatory features including a putative IncPnic-site, an origin of transfer-binding protein, a relaxase, a helicase and a nicking-endonuclease were identified (Figure S2). According to the classification of Barcillán-Barica et al. [22], the putative ICE_84-112 (CDS peg.24) relaxase belongs to the MPBP1 group (clade MOBP11) of relaxases, displaying the typical conserved sequence motifs. Most of the MPBP1 group of relaxases are linked to conjugative plasmids. Lee et al. [20] demonstrated that the chromosomally encoded Bacillus subtilis helicase PcrA associates with ICEBs1 during replication. ICEBs1 is defective for replication in pcrA-mutant strains and pcrA is necessary for ICEBs1 conjugation. PcrA orthologs, which could be recruited for replication and conjugation, are present in both C. fetus subspecies (84-112 CDS peg.56 & peg.1280 and 82-40 CDS peg.690 & peg.934).
dRNA-seq Identified Transcriptional Start Sites and the Typical Promoter Structure for Campylobacterales in Both Subspecies
Transcriptional start sites (TSS) annotation, performed computationally, allowed classification of TSS according to their location relative to the surrounding orfs. The analysis revealed a variety of transcripts with TSS located upstream and internal to their respective orf but also included antisense transcripts. Many TSS were simultaneously assigned to more than one category (Figure S5).
Sequences upstream of the annotated TSS were used to define C. fetus promoter motifs. C. fetus subsp. venerealis has more orfs than C. fetus subsp. fetus and we identified 797 promoter sequences in strain 84-112 and 575 promoter sequences in strain 82-40, with an extended Pribnow box (tgnTAtaAT) as the −10 motif in both subspecies. Consistent with other Campylobacterales [23], [24] the typical bacterial −35 motif is replaced by a periodic AT-rich signal upstream of position −14 (Figure 4AB). This also is evident in the sap-locus located on genomic islands VGI III and FGI I. The intragenic promoter region between sapC (component of the Sap-transporter) and a respective sap-homologue is 100% conserved between the subspecies and only the sap-homolog directly downstream of the promoter is transcribed ( Figure 4C ).
C. fetus subsp. Venerealis 84-112 Harbors T4SS-related Loci
C. fetus subsp. venerealis 84-112 harbors four regions showing homology to T4SS genes (Figure S3). Two are on the chromosome within VGI I (PAI) and II (Figure 2AB) and two are located on ICE_84-112 (Figure S2) annotated as ICE_trb/tra and ICE_vir. The ICE_trb/tra region differs from the other T4SS and shares homology to IncP plasmid RP4. For the ICE_vir region, blast searches and phylogenetic analyses using VirB4 and VirB11 [25] identified the PAI T4SS (Table S2 in File S5) and an as yet uncharacterized T4SS of Campylobacter hominis as their closest neighbor. The vir-genes located on VGI II did not share high homology with the vir-genes present on either VGI I or ICE_84-112. Instead the closest relative is a putative T4SS present in C. rectus RM3267, indicating a different origin. Finally, transcriptome analysis indicated that the VGI III T4SS components are not transcribed under laboratory conditions, whereas expression of the PAI T4SS (VGI I), ICE_vir and ICE_trb/tra was detected (data not shown, and [17]).
Genes Involved in LPS-biosynthesis Distinguish C. fetus Sero−/Sap-types
The subspecies definition regions contain unique genes putatively involved in LPS-biosynthesis. Although inserted at the same chromosomal position in both subspecies (Figure 1AB) the islands display only limited similarity (Figure S4). One obvious difference was that VSDR encodes a putative maltose O-acetyltransferase (mat1) (cd04647) and FSDR a putative UDP-galactopyranose mutase (glf) (EC 5.4.99.9) (Figure S4). Remarkable is the low GC-content of the VSDR and FSDR of 30.7% and 29.4%, respectively (Table S1 in File S5) and the absence of tRNA or apparent mobility genes.
Acetyltransferases generally catalyze the CoA-dependent acetylation of the 6-hydroxyl group of sugar substrates. Maltose O-acetyltransferases exclusively acetylate maltose and glucose. C. fetus type A LPS contains 74.5% mannose as well as 6.5% D-glucose [26] and thus may serve as a substrate for Mat1. UDP-galactopyranose mutase (glf) drives the conversion of the ring form of galactose from pyranose to furanose. The latter isomer is specifically found in glycoconjugates (including LPS) of various prokaryotic and eukaryotic pathogens, and is essential for their physiology and virulence [27], [28]. To assess conservation of the subspecies-specific regions, a panel of 102 geographically and phenotypically diverse strains of C. fetus subspecies was screened for the presence or absence of mat1 and glf. Of 62 subsp. venerealis isolates (all type A), 58 (93.5%) were positive for mat1 and all were negative for glf. In contrast, only 16 (40%) of 40 subsp. fetus strains harbor mat1 but 25 (62.5%) were positive for glf. The 16 subsp. fetus strains positive for mat1 were all type B, whereas 24 of the 25 glf positive strains were type A (Table S3, Table S6 in File S5). The single exception, C. fetus subsp. fetus isolate F9, which was positive for both mat1 and glf, belongs to the rare group of type AB strains.
Our previous application of RDA (representational difference analysis) to C. fetus revealed that another LPS-biosynthesis gene (wcbK) encoding a putative GDP-mannose 4,6-dehydratase was exclusively present in C. fetus subsp. fetus strains [17]. In strain ATCC 27374 (type B), wcbK is flanked 3′ by wbbC, encoding a putative glycosyltransferase, and 5′ by a sap gene (data not shown). This region corresponds to FGI I in strain 82-40, which lacks wcbK. WcbK catalyzes the first step in the biosynthesis of GDP-D-rhamnose and GDP-L-fucose, and is involved in capsular polysaccharide or LPS-biosynthesis in bacteria such as Helicobacter pylori [29] and C. jejuni [30]. A PCR screen of the C. fetus panel confirmed that wcbK was not present in any of the C. fetus subsp. venerealis isolates but was exclusively detected in the 16 C. fetus subsp. fetus isolates, which were also positive for mat1. All of the wcbk+ mat1+ strains were type B. Thus, C. fetus subsp. fetus either carried glf alone in type A strains or mat1 in combination with wcbK in type B strains. C. fetus subsp. venerealis (type A) only carries mat1. C. fetus subsp. fetus strain F9 scored positively for mat1, wcbK and glf.
Another phylotype of C. fetus is represented by reptile C. fetus strains, which are type A, and may represent the ancestral C. fetus type [4], [31]. We screened four reptile isolates, which were all positive for mat1 but lacked glf, wcbK, virD4 and fic1-4 (Table S4 in File S5).
Finally, another enzyme of the LPS-biosynthetic pathway UDP-glucose 4-epimerase (GalE, EC 5.1.3.2) catalyzes the reversible conversion of UDP-glucose to UDP-galactose and is known to contribute to C. jejuni virulence [32]. Southern-blot and PCR screens of our collection showed that all 102 C. fetus isolates studied carried galE.
wcbK is Involved in LPS-biosynthesis and Accordingly should have an Impact on Acid Resistance and Serum Sensitivity in C. fetus subsp. Fetus Type B Strains
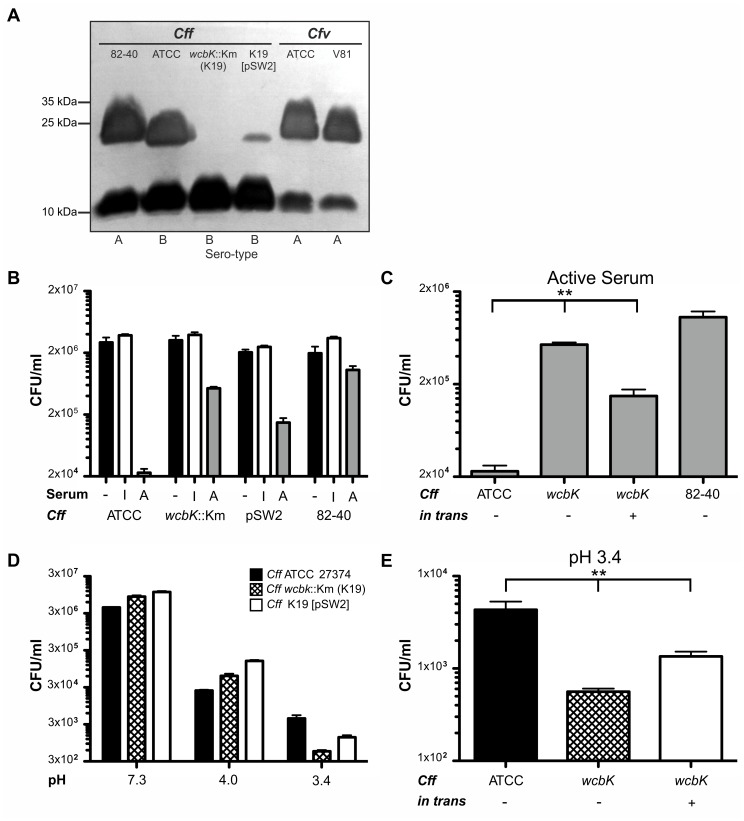
Type A strains are resistant to complement-mediated killing since C3b binding to the bacterial cell surface is inhibited by the presence of the S-layer [33], [34]. It is not known why type B strains are sensitive to non-immune serum [12], despite the presence of the surface array protein. We hypothesized that wcbK might be linked to the susceptibility of type B strains by generating O-specific side chains where the C3b binding site is not covered by the S-layer. To test this, we first screened C. fetus subsp. fetus type A and B strains with known serum resistance phenotypes for wcbK and glf (Table S5 in File S5). As hypothesized, wcbK was exclusively found in type B strains and correlated with serum susceptibility, whereas glf only was present in type A strains and correlated with serum resistance. Next we generated a non-polar wcbK mutant (K19) of C. fetus subsp. fetus ATCC 27374 (type B) that was deficient in LPS-production (Figure 5A). In Vibrio cholerae mutant strains it has been shown that providing genes in trans only partially restored LPS-production compared to wild type levels [35]. In our experiments, providing wcbK in trans also partially complemented LPS-production. Due to antibiotic selection throughout the experiment we can exclude the loss of the complementation vector. We next compared serum-susceptibility of mutant and wild type strains (Figure 5BC). As expected, C. fetus subsp. fetus ATCC 27374 did not survive serum treatment (log10 kill 2.23±0.06) whereas the isogenic wcbK mutant strain K19 had markedly increased serum-resistance (log10 kill 0.86±0.05). The phenotype was partially complemented (log10 kill 1.23±0.10) by providing wcbK in trans. The serum resistant strain 82-40 (type A) was used as a control (log10 kill 0.27±0.01).
Figure 5. WcbK is important for LPS-biosynthesis, attenuates survival in blood, and promotes acid resistance.
(A) SDS-PAGE pattern of purified LPS after silver staining. Samples were isolated from C. fetus. subsp. fetus (Cff) 82-40 (lane 1), Cff ATCC 27374 (type B) (lane 2), wcbK mutant K19 (wcbK::Km) (lane 3) and K19 [pSW2] (wcbK in trans) (lane 4); C. fetus subsp. venerealis (Cfv) ATCC 19438 (lane 5) and Cfv 84-112 (lane 6). (B) Cff serum resistance assays. Strains were incubated either with EMEM (-), heat-inactivated (I) or active (A) human serum and colony forming units (CFU) were counted. Results shown are for Cff ATCC 27374, K19 and K19 [pWS2]. Cff 82-40 served as a type A comparator. (C) Same as in (B) but for better visualization, CFU/ml obtained after treatment with active serum are displayed separately. **p<0.002 (D) Acid resistance assays. Cff were incubated in PBS pH range 7.3 to 3.4, plated and CFU determined. Survival after exposure to different pH of the wild type, K19 and K19 [pSW2] was compared. (E) For better visualization, CFU/ml for the three strains after treatment with pH 3.4 were plotted separately. **p<0.003.
Type A and type B C. fetus strains differ in the carbohydrate composition of their LPS [12], [26]. The O-antigen of type A strain has a higher molecular weight (Figure 5A) than that of type B strains. C. fetus strains 84-112, 82-40 and ATCC 27374 are similar in their resistance to acid (Figure 5 and results not shown). In H. pylori GDP-mannose 4,6-dehydratase (encoded by wbcJ) is important for the expression of O-antigen and for the bacterium to survive the acidic milieu of the stomach [29]. We hypothesized that the loss of LPS in the wcbK deficient C. fetus strain might result in increased acid sensitivity. Indeed, when incubated at low pH the wild type strain (ATCC 27374) survived significantly better than the wcbK mutant; this acid-sensitive phenotype was partially complemented by providing wcbK in trans (Figure 5CD).
In summary, wcbK is important for LPS-biosynthesis and SapB binding. Activity of this enzyme attenuates survival of the pathogen in blood, and also can provide effective protection from stomach acid en route to colonization of the intestinal niche.
Discussion
ε-Proteobacteria including Campylobacter and its close relative Helicobacter show evidence of genome reduction indicated by small genome size (≈1.5 to 2.5 Mbp) and the nearly complete absence of non-coding DNA. These features are typical for adaptation to a specific colonization niche and both species display strong host preference (“tropism”) [36], [37]. Among Campylobacters, C. fetus subspecies are an exceptional model system to study the molecular basis of pathogen-host adaptation since, despite a highly clonal structure, they display strikingly dissimilar host preferences and tissue tropism. To investigate the genetic basis underlying the distinct pathogenicity of C. fetus subspecies, we performed whole genome comparisons and transcriptome analyses of C. fetus subspecies, focusing on identifying differences that contribute to host and tissue tropism. We propose that the additional genome content of C. fetus subsp. venerealis was horizontally acquired (Table S1 in File S5). The observation that genes shared between the subspecies are nearly 100% identical on the nucleotide level supports the hypothesis that HGT and not mutation or genetic drift is the predominant factor in the evolution of C. fetus.
To gain insights to the genetic plasticity of C. fetus genomes, and particularly whether the identified variation regions are conserved we compared the VGI – IV of C. fetus subsp. venerealis 84-112 to the draft genome sequences of C. fetus subsp. venerealis NCTC 10354 (ATCC 19438) [38], C. fetus subsp. venerealis Azul-94 [39] and C. fetus subsp. venerealis biovar Intermedius INTA 99/541 [40]. We identified homologous sequences in all three strains with over 90% homology on the nucleotide level. These results indicate that the GIs are at least partially present in other venerealis strains. However, given that many of the remaining contig boundaries are located in the variable regions, to be able to perform more detailed analysis the draft genomes will need to be closed and the sequences verified.
We focus in the current study on the description of genomic regions and genes unique to each subspecies. Genome comparisons of C. fetus subspecies reported previously using the draft sequences of C. fetus subsp. venerealis strains [39], [41] focused mainly on the description of shared putative virulence factors or the identification of putative targets for diagnostics. Many of the genes putatively involved in adherence, invasion, motility, secretion and toxin production identified by Ali et al. [41] and Moolhuijzen et al. [39] were also present in strain 84-112 (File S1). Homologs to the antibiotic resistance gene cluster identified within a homologous genomic island in C. fetus subsp. fetus IMD 523-06 [42] were not present in C. fetus subsp. venerealis ATCC and 84-112.
Metabolic differences between C. fetus subspecies such as glycine tolerance, H2S production and selenite reduction have traditionally been used to discriminate the subspecies and are therefore intriguing features linked to niche adaptation. Nonetheless, metabolic modeling of the two genomes revealed no apparent subspecies differences, except a possible difference in thiamin (vitamin B1) biosynthesis. The overall metabolic capacity seems to be similar in both subspecies, consistent with our model that the described horizontally acquired genetic elements account for the different biology of C. fetus subspecies. However, it is important to note that subtle genetic differences, like point mutations, can inactivate genes or disrupt metabolic pathways. Therefore, nutrient utilization by the C. fetus subspecies remains an important priority for detailed study.
The extra-chromosomal element ICE_84-112 was identified. ICEs are plasmid-like self-transmissible mobile genetic elements, dependent on phages or transposons for inserting and excising from chromosomes, but carry their own transfer genes (tra-genes) for lateral transmission to other host cells. Notably the full repertoire of plasmid replication genes is typically absent. Some ICE replicate autonomously if they adopt a rolling-circle-like mechanism mediated by replication- or single-strand DNA transfer initiation factors [20], [21]. In Bacillus subtilis helicase PcrA associates with ICEBs1 during replication [20]. Candidate PcrA orthologs are present in both C. fetus subspecies (84-112 CDS peg.56 & peg.1280 (File S1) and 82-40 CDS peg.690 & peg.934 (File S2)). The surveyed fic3 and fic4 genes suggest that the distribution of ICE_84-112 is quite narrow. In that case important virulence-associated characteristics are unlikely to be carried by the element, but it may be a vehicle of interspecies gene exchange.
C. fetus subsp. fetus 82-40 mostly lacks phage- and plasmid-related genes and this might be due to the presence of an active CRISPR cluster, protecting from invasion of foreign DNA. Although there are six core cas-genes, cas1 may be of central importance in the acquisition of new spacers (for review see [43]). In contrast to C. fetus subsp. venerealis 84-112, we identified two CRISPR-arrays in strain 82-40. Since Cff_CRISPR_2 showed prototypical architecture, i.e., cas-genes and an AT-rich leader sequence followed by the DRs and the spacers, this CRISPR array may be functional. The presence of cas-genes in C. fetus subsp. fetus highlights another important subspecies difference. The occurrence of CRISPRs is linked to natural competence of bacteria [44]. That C. fetus subsp. fetus type B strains more frequently harbor putative functional CRISPRs than type A strains might have stabilized the type B phylotype and may explain why the type A clade later diverged [4] (Figure 6). All of the C. fetus strains that we and others have thus far tested are not naturally competent (unpublished data, [45], [46]) thus a possible connection between the presence of CRISPRs and natural competence of C. fetus subspecies remains unresolved.
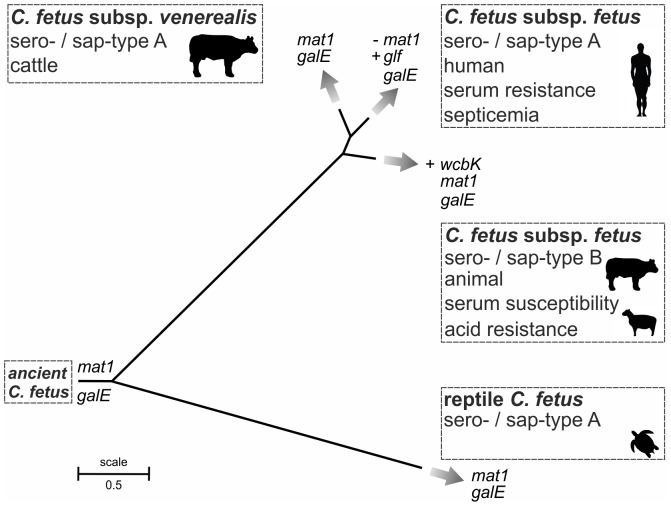
Figure 6. Phylogeny, niche specificity and virulence of C. fetus subspecies.
MLST tree showing the phylogeny of C. fetus, with original scale as reported [4]. Reptile C. fetus represent a distinct clade harboring mat1 and galE. Diversification of C. fetus subsp. fetus (Cff) type B happened prior to the diversification of Cff type A and C. fetus subsp. venerealis (Cfv) type A strains. Cff type B strains harbor galE, mat1 and wcbK. The latter gene provides protection from acid, and this genotype is associated with animal hosts. Cfv type A represents the bovine clone harboring mat1 and galE which is also prone to HGT. Cff type A have lost mat1 but acquired glf correlating with serum resistance in Cff.
The most important genetic differences between the subspecies are cell surface structures including the S-layer and LPS. The distribution of these genes across a panel of diverse C. fetus isolates indicates linkage to particular pathotypes. The distinct distribution patterns detected for wcbK, mat1, and glf among type A and B strains support the following model ( Figure 6 ). wcbK and glf are subsp. fetus-specific genes that have been acquired more recently than mat1 and galE, which represent “ancient” constituents of the C. fetus genome. These loci are similar in reptile C. fetus and C. fetus subsp. venerealis but MLST reveals that variation has emerged and that type B strains separated from type A prior to the division of C. fetus subsp. fetus and C. fetus subsp. venerealis [4]. We showed that type B strains maintained mat1 and galE but diversification of phylotypes led to acquisition of wcbK by C. fetus subsp. fetus type B. C. fetus subsp. venerealis also maintained mat1 and galE, but type A C. fetus subsp. fetus, the invasive pathotype often found in human infections, have lost mat1 and acquired glf. Extended analysis of C. fetus evolution will require analysis of more geographically and phenotypically diverse isolates. Moreover, analysis of the newly proposed subspecies/biovar intermedius [7] may provide a missing link in the subspecies divergence.
Little is known how C. fetus interacts with the host immunity, but LPS and the S-layer are important for TLR4-mediated recognition [47], [48]. The S-layer producing C. rectus induces TLR4 expression in the mouse placenta [49]. To avoid dysregulated inflammatory responses to LPS, the intestinal epithelium as well as placental tissue normally express no or low levels of TLR4 [48], [50], [51]. Low density of TLR4 may allow C. fetus to overcome the hosts’ immune response and subsequently invade the host cells. Type A and type B C. fetus strains are different in their LPS composition and S-layer proteins [12], [26]. The activity of WcbK and the putative functions of mat1 and glf are linked to the S-layer. C. fetus subsp, venerealis strains (wcbk−/glf−/mat1+) and C. fetus subsp. fetus type A strains (wcbK−/glf+/mat1-) are serum resistant, whereas C. fetus subsp. fetus type B strains (wcbK+/glf−/mat1+) are serum sensitive. We showed that wcbK is essential for LPS-biosynthesis in C. fetus subsp. fetus type B strains and that loss of wcbK leads to increased serum resistance. This data indicates that WcbK generated side chains are important for serum sensitivity. We propose that similar to wcbK, the products of mat1 and glf of C. fetus might be involved in LPS-biosynthesis by generating different O-antigen side chains, potentially influencing complement and antibody binding, acid resistance and TLR-4 recognition.
The bacterial transcriptome provides an additional reference to study genome composition as well as regulation of virulence. In the initial profile of C. fetus gene transcription, the characteristic ε-proteobacterial promoter signature was identified. We confirmed that the promoter region is 100% conserved between the subspecies, and that one sap gene is predominantly transcribed under laboratory conditions. This finding is intriguing since recombination and therefore exchange of sap-homologs occurs frequently in this region to enable phase variation of the pathogen [11]. It has been proposed that the sap-region belongs to the ancestral part of the C. fetus core genome and not a PAI [52]. That the region is shared between both subspecies confirms ancient presence of a horizontally acquired element. Based on the significance of the S-layer for immune evasion [11], [15], [16], the genome insertion can be considered as a classical PAI. To date, animal models of C. fetus infection are not readily available. Future analyses at the transcriptiome level should investigate C. fetus under in vitro conditions resembling their colonization niche or route of infection.
Whole-genome comparisons of related pathogens of distinct characteristics, such as those described in the presented work, lay the foundation for additional mutational, functional, and animal studies that will ultimately help elucidate the mechanisms underlying the emergence of new pathogens. This study broadens knowledge of the genomic basis of C. fetus pathogenesis and host specificity. The most interesting differences in the genetic repertoire of the subspecies relate to cell surface structures including the S-layer and LPS and distribution of these genes is associated with certain pathotypes. This emphasizes the importance of surface-exposed structures to C. fetus pathogenicity and demonstrates how evolutionary forces optimize the fitness and host adaptation of these pathogens. The presence of genes like glf is particularly interesting as the gene product is a promising drug target, as proposed for Leishmania [53], and relevant since glf is connected to type A strains, which are more often isolated from human blood. In any event, wcbK and glf are excellent candidates applicable for reliable subspecies differentiation.
Experimental Procedures
Bacterial Strains
Campylobacter and E. coli strains were grown as described [45]. Antibiotic selection applied concentrations of 100 µg ml−1 ampicillin, 75 µg ml−1 nalidixic acid, or kanamycin and chloramphenicol at 25 µg ml−1. Bacterial strains are listed in Table S6 and Table S7 in File S5. Only C. fetus strains typed definitively to the subspecies level were tested in PCR screens (n = 102). Subspecies were identified biochemically as described [17].
Gene Detection
Oligonucleotides are listed in Table S8 in File S5. PCR amplification for surveying gene prevalence used chromosomal DNA and the following primer pairs 1/2 for wcbK, 3/4 for glf and 5/6 for mat1. The sap-type was determined with primers 7/8 and 9/10, as described [54]. Southern blots were hybridized with radiolabeled DNA probes as described [17]. Probes for galE and cas1 were generated with primer pair 11/12 and 13/14 from chromosomal DNA of C. fetus subsp. fetus ATCC 27374, respectively. The same primers were used for PCR-screening for galE and cas1. fic3 and fic4 were amplified with primer pairs 15/16 and 17/18, respectively.
Genome Sequencing, Assembly and Annotation
A standard whole genome shot-gun and a 3-kb paired-end library were generated according to the manufacturer’s recommendations (Roche Diagnostics, Vienna, Austria) using 5 µg chromosomal DNA. For each library, high-throughput pyrosequencing was performed on a Genome Sequencer FLX system (Roche) producing 145 Mb and 62.2 Mb sequence data, respectively. Read assembly applied the Newbler assembly software, version 2.6 (Roche) and resulted in 89 contigs and 11 scaffolds. One scaffold represented the circular extra-chromosomal element and the remaining 10 were grouped into 3 super-scaffolds (SSc) using the information from the 3 kbp mate-pair library and the contig-graph generated by the Newbler assembler. Additionally, PCR and Sanger sequencing was used to determine the orientation and order of contigs and the SSc. Gaps in the extra-chromosomal element and the chromosome were closed in silico with a custom R script [55] and with PCR. Homopolymer uncertainties from the 454-reads were corrected through mapping of the Illumina reads derived from the C. fetus subsp. venerealis 84-112 RNA to the draft sequence using CLC Genomics Workbench 5.5 (CLC Bio; Arhus, Denmark). The resulting consensus sequences and C. fetus subsp. fetus strain 82-40 were annotated and compared with Rapid Annotations using Subsystem Technology version 4.0 (RAST) [56]. Annotation tables for each strain and the extra-chromosomal element are presented in Files S1–S3.
Differential RNA-sequencing
Library preparation for dRNA-seq was performed as reported [23]. In brief, RNA was isolated from bacterial cells grown on CBA plates for 24 h. To construct differential cDNA library pairs, aliquots of extracted RNA from each strain was treated with Terminator-5′-phosphate-dependent exonuclease (TEX; Epicentre) to deplete processed RNAs (denoted TEX+) in addition to untreated RNA (denoted TEX-). Construction of cDNA libraries was performed by vertis Biotechnology AG (Munich, Germany). Libraries were sequenced using cluster amplification with the TruSeq PE Cluster Kit v.5 on a cluster station. Each library was sequenced on a single HiSeq 2000 lane using TruSeq SBS 36 Cycle Kits v.5 (Illumina, San Diego, CA) and a 91 bp single-end protocol. Sequencing image files were processed with the Sequencing Control Software (SCS) Real Time Analysis (RTA) v2.6 and CASAVA v.1.7 (Illumina). Reads were mapped to the reference genomes using the CLC Genomics workbench (CLC Bio) with default settings. Information on transcriptional start site (TSS) and promoter annotation can be found in the supplement.
Lipopolysaccharide Analysis
C. fetus strains were grown for 24 h and resuspended in buffer (10% glycerine, 20% SDS, 5% β-mercaptoethanol, 62.5 mM Tris-HCl pH 6.8, bromophenol blue) for lysis at 100°C for 10 min. Proteinase K solution was added to 6 µg/µl and samples were incubated overnight at 55°C. LPS-preparations were electrophoretically on 15% polyacrylamide gels (running buffer: 86 mM glycine, 3,5 mM SDS and 25 mM Tris pH 8). Gels were fixed overnight (25% isopropanol, 7% acetic acid) under gentle shaking. LPS was oxidized with 100 ml fixative containing 4 mmol NaIO4 for 10 min. After three washing steps with H2O for 30 min each, the gels were stained (19 mM NaOH, 1.35% NH3, 20 mM AgNO3) for 10 min, then washed three times with H2O and immersed in developer (240 mM Na2CO3, preheated to 60°C, before addition of 30 µl 40% formaldehyde). The reaction was stopped with 50 mM EDTA (pH 8) for 1 h.
Serum and Acid Resistance Testing
Susceptibility of C. fetus strains to human serum was assessed as described [57]. All tests were performed in triplicate. Briefly, C. fetus was streaked on CBA plates 24 h prior to the assay and cell count was adjusted to 1×107 bacteria/ml, based on optical density in EMEM medium. The actual cell count was determined by plating serial dilutions. Heat-inactivated- (56°C for 30 min), or active- (thawed on ice) pooled human serum was added to the bacteria to a 10% final concentration and incubated for 1 h at 37°C. Surviving cells were counted on CBA plates after 48 h growth. For the acid resistance assays, C. fetus cells were harvested as described above, centrifuged, resuspended in PBS with different pH values and incubated at 37°C for 30 min. Cells were washed in PBS (pH 7.3) before the number of surviving bacteria was determined by plating serial dilutions.
Nucleotide Sequence Accession Numbers
The genome sequence of C. fetus subsp. venerealis 84-112 including the ICE element (ICE_84-112) has been deposited in EMBL Nucleotide Archive under accession numbers (HG004426 and HG004427). The genome of C. fetus subsp. fetus 82-40 used for comparative analyses has the GenBank accession number CP000487.1. dRNAseq data can be accessed via the EMBL-EBI short read archive under the accession number ERP002581.
Supporting Information
Comparative maps of CRISPR-related genomic islands. (A) C. fetus subsp. venerealis 84-112 VGI IV harbors the direct repeats with spacers (CRISPR) but lacks CRISPR-associated (cas)-genes. Prophage-related genes (putative prophage IV) were identified (orange) adjacent to a region identical to C. fetus subsp. fetus 82-40 Downstream of these regions the core-genome continues with a chromosomal rearrangement between the two subspecies on the 3-prime end (striped boxes). A sequence region shared between the subspecies was identified (blue box). (B) C. fetus subsp. fetus 82-40 FGI I carries two regions of direct repeats and spacers. cas-genes precede the second CRISPR-array resulting in a putatively functional CRISPR-system. One region with a prophage-like structure (orange) was identified.
(TIF)
Physical map of the extra-chromosomal element ICE_84-112. Shown is the GC-content (circle 1), GC-skew (circle 2) and open reading frames (circle 3). The tra-region (red) comprises genes putatively involved in conjugative transfer of the ICE. The vir-region (orange) shows putative T4SS genes with homology to the chromosomal PAI on VGI I. Genes possibly involved in autonomous replication of the ICE are named individually and labeled (green and red). Genes of predicted plasmid origin (green); phage genes and transposons (blue); putative effector proteins or toxin-antitoxin system (yellow); hypothetical proteins (grey).
(TIF)
Schematic representation of the apparent T4SS identified in C. fetus subsp. venerealis 84-112. (A, B, C) Represent loci with homology to virB/virD4-genes. (A) The PAI T4SS is functional in virulence and conjugative DNA transfer [1], [2]. (B) ICE_vir displays a similar gene organization to VGI I but protein homologies are not strikingly high. virD4 is truncated compared to the functional PAI homologue. (C) A partial set of vir-genes. (D) ICE_trb/tra genes share homology to plasmid RP4 and are putatively involved in the conjugative transfer of ICE_84-112. Homologous genes (vir, tra) are indicated by color.
(TIF)
Comparative map of C. fetus subspecies variation regions VSDR and FSDR. (A) C. fetus subsp. venerealis 84-112 VSDR and (B) C. fetus subsp. fetus 82-40 FSDR. MAUVE was used to compare the regions to visualize rearrangements and insertions. Regions free of rearrangements are indicated by colored colinear blocks. White regions within these blocks symbolize insertions or non-homologous regions. Important open reading frames are colored and/or labeled accordingly. Genes unique to the subspecies, mat1 and glf, are highlighted in pink.
(TIF)
Venn diagram of annotated TSS. (A) C. fetus subsp. venerealis 84-112 and (B) C. fetus subsp. fetus 82-40. TSS were categorized according to the genomic context into five classes: primary (TSS having the most cDNAs within ≈500 bp upstream of annotated mRNA start codons), secondary (TSS associated with the same gene but with fewer cDNAs), internal (TSS within an annotated gene on the same strand), antisense (TSS situated inside or within ≈100 bp of the coding region of a gene encoded on the opposite strand), or orphan (TSS without annotated genes in proximity) [3]. Numbers in parentheses indicate the TSS, which associate with only one orf.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOC)
Acknowledgments
We thank B. Scherz and K. Gehmacher (Graz, Austria) for their contribution to this work and W. G. Miller (USDA, Atlanta, GA, USA) for providing sequence data of the wcbK/gmd region. We are grateful to C. M. Sharma and K. U. Förstner (Würzburg, Germany) for dRNA-seq and help with transcriptome data analysis.
Funding Statement
This study was supported by the Austrian Science Fund FWF grants P20479 (GG & ELZ) and P24016 (ELZ), the Hygiene Fund Young Scientist grant from the Medical University of Graz (SK), the Michael Saperstein Medical Scholars Program, New York School of Medicine (SK) and GM63270 from the National Institutes of Health (MJB), and the EU-FP7 COST Action SeqAhead, EC Grant BM1006 (GGT). The Austrian Centre of Industrial Biotechnology (ACIB) contribution was supported by FFG, The Federal Ministry of Economy, Family and Youth (BMWFJ), Federal Ministry for Transport, Innovation and TechnologyBMVIT, ZIT GmbH, Zukunftsstiftung Tirol and Land Steiermark within the Austrian COMET program FFG Grant 824186 (BH, GGT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. On SL (1996) Identification methods for Campylobacters, Helicobacters, and related organisms. Clin Microbiol Rev 9: 405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Man SM (2011) The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8: 669–685. [DOI] [PubMed] [Google Scholar]
- 3.Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, et al. (2005) Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol 3 72–85. [DOI] [PMC free article] [PubMed]
- 4. Dingle KE, Blaser MJ, Tu ZC, Pruckler J, Fitzgerald C, et al. (2010) Genetic relationships among reptile and mammalian Campylobacter fetus by Multilocus Sequence Typing. J Clin Microbiol 48: 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Bergen MA, Simons G, van der Graaf-van Bloois L, van Putten JP, Rombout J, et al. (2005) Amplified fragment length polymorphism based identification of genetic markers and novel PCR assay for differentiation of Campylobacter fetus subspecies. J Med Microbiol 54: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 6.Thompson SA, Blaser MJ (2000) Pathogenesis of Campylobacter fetus infections. In: Nachamkin I, Blaser MJ, editors. Campylobacter, 2nd Ed. Washington, D.C.: ASM Press. 321–347.
- 7. van Bergen MA, Dingle KE, Maiden MC, Newell DG, van der Graaf-Van Bloois L, et al. (2005) Clonal nature of Campylobacter fetus as defined by multilocus sequence typing. J Clin Microbiol 43: 5888–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Bergen MA, Linnane S, van Putten JP, Wagenaar JA (2005) Global detection and identification of Campylobacter fetus subsp. venerealis . Rev Sci Tech 24: 1017–1026. [PubMed] [Google Scholar]
- 9. Blaser MJ (1998) Campylobacter fetus–emerging infection and model system for bacterial pathogenesis at mucosal surfaces. Clin Infect Dis 27: 256–258. [DOI] [PubMed] [Google Scholar]
- 10.Skirrow MB, Blaser MJ (2000) Clinical aspects of Campylobacter infections. In: Nachamkin I, Blaser, M J., editor. Campylobacter, 2nd Ed. Washington, D. C.: American Society for Microbiology. 69–88.
- 11. Tu ZC, Gaudreau C, Blaser MJ (2005) Mechanisms underlying Campylobacter fetus pathogenesis in humans: surface-layer protein variation in relapsing infections. J Infect Dis 191: 2082–2089. [DOI] [PubMed] [Google Scholar]
- 12. Perez-Perez GI, Blaser MJ, Bryner JH (1986) Lipopolysaccharide structures of Campylobacter fetus are related to heat-stable serogroups. Infect Immun 51: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson SA (2002) Campylobacter surface-layers (S-layers) and immune evasion. Ann Periodontol 7: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran AP, O’Malley DT, Kosunen TU, Helander IM (1994) Biochemical characterization of Campylobacter fetus lipopolysaccharides. Infect Immun 62: 3922–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grogono-Thomas R, Blaser MJ, Ahmadi M, Newell DG (2003) Role of S-layer protein antigenic diversity in the immune responses of sheep experimentally challenged with Campylobacter fetus subsp. fetus . Infect Immun 71: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia MM, Lutze-Wallace CL, Denes AS, Eaglesome MD, Holst E, et al. (1995) Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J Bacteriol 177: 1976–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorkiewicz G, Kienesberger S, Schober C, Scheicher SR, Gully C, et al. (2010) A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis . J Bacteriol 192: 502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatty M, Laverde Gomez JA, Christie PJ (2013) The expanding bacterial type IV secretion lexicon. Res Microbiol. [DOI] [PMC free article] [PubMed]
- 19. Kienesberger S, Schober Trummler C, Fauster A, Lang S, Sprenger H, et al. (2011) Interbacterial macromolecular transfer by the Campylobacter fetus subsp. venerealis type IV secretion system. J Bacteriol 193: 744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CA, Babic A, Grossman AD (2010) Autonomous plasmid-like replication of a conjugative transposon. Mol Microbiol 75: 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. te Poele EM, Bolhuis H, Dijkhuizen L (2008) Actinomycete integrative and conjugative elements. Antonie Van Leeuwenhoek 94: 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcillan-Barcia MP, Francia MV, de la Cruz F (2009) The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 33: 657–687. [DOI] [PubMed] [Google Scholar]
- 23. Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, et al. (2010) The primary transcriptome of the major human pathogen Helicobacter pylori . Nature 464: 250–255. [DOI] [PubMed] [Google Scholar]
- 24. Petersen L, Larsen TS, Ussery DW, On SL, Krogh A (2003) RpoD promoters in Campylobacter jejuni exhibit a strong periodic signal instead of a −35 box. J Mol Biol 326: 1361–1372. [DOI] [PubMed] [Google Scholar]
- 25. Fernandez-Lopez R, Garcillan-Barcia MP, Revilla C, Lazaro M, Vielva L, et al. (2006) Dynamics of the IncW genetic backbone imply general trends in conjugative plasmid evolution. FEMS Microbiol Rev 30: 942–966. [DOI] [PubMed] [Google Scholar]
- 26. Senchenkova SN, Shashkov AS, Knirel YA, McGovern JJ, Moran AP (1997) The O-specific polysaccharide chain of Campylobacter fetus serotype A lipopolysaccharide is a partially O-acetylated 1,3-linked alpha-D-mannan. Eur J Biochem 245: 637–641. [DOI] [PubMed] [Google Scholar]
- 27. Oppenheimer M, Valenciano AL, Kizjakina K, Qi J, Sobrado P (2012) Chemical mechanism of UDP-galactopyranose mutase from Trypanosoma cruzi: a potential drug target against Chagas’ disease. PLoS One 7: e32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poulin MB, Nothaft H, Hug I, Feldman MF, Szymanski CM, et al. (2010) Characterization of a bifunctional pyranose-furanose mutase from Campylobacter jejuni 11168. J Biol Chem 285: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGowan CC, Necheva A, Thompson SA, Cover TL, Blaser MJ (1998) Acid-induced expression of an LPS-associated gene in Helicobacter pylori . Mol Microbiol 30: 19–31. [DOI] [PubMed] [Google Scholar]
- 30. McCallum M, Shaw GS, Creuzenet C (2011) Characterization of the dehydratase WcbK and the reductase WcaG involved in GDP-6-deoxy-manno-heptose biosynthesis in Campylobacter jejuni . Biochem J 439: 235–248. [DOI] [PubMed] [Google Scholar]
- 31. Tu ZC, Eisner W, Kreiswirth BN, Blaser MJ (2005) Genetic divergence of Campylobacter fetus strains of mammal and reptile origins. J Clin Microbiol 43: 3334–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fry BN, Feng S, Chen YY, Newell DG, Coloe PJ, et al. (2000) The galE gene of Campylobacter jejuni is involved in lipopolysaccharide synthesis and virulence. Infect Immun 68: 2594–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blaser MJ, Smith PF, Repine JE, Joiner KA (1988) Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Invest 81: 1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pei Z, Blaser MJ (1990) Pathogenesis of Campylobacter fetus infections. Role of surface array proteins in virulence in a mouse model. J Clin Invest 85: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nesper J, Kraiss A, Schild S, Blass J, Klose KE, et al. (2002) Comparative and genetic analyses of the putative Vibrio cholerae lipopolysaccharide core oligosaccharide biosynthesis (wav) gene cluster. Infect Immun 70: 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hofreuter D, Novik V, Galan JE (2008) Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 4: 425–433. [DOI] [PubMed] [Google Scholar]
- 37. Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, et al. (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori . Nature 388: 539–547. [DOI] [PubMed] [Google Scholar]
- 38. Stynen AP, Lage AP, Moore RJ, Rezende AM, de Resende VD, et al. (2011) Complete genome sequence of type strain Campylobacter fetus subsp. venerealis NCTC 10354T. J Bacteriol 193: 5871–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moolhuijzen PM, Lew-Tabor AE, Wlodek BM, Aguero FG, Comerci DJ, et al. (2009) Genomic analysis of Campylobacter fetus subspecies: identification of candidate virulence determinants and diagnostic assay targets. BMC Microbiol 9: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iraola G, Perez R, Naya H, Paolicchi F, Harris D, et al. (2013) Complete Genome Sequence of Campylobacter fetus subsp. venerealis Biovar Intermedius, Isolated from the Prepuce of a Bull. Genome Announc 1. [DOI] [PMC free article] [PubMed]
- 41.Ali A, Soares SC, Santos AR, Guimaraes LC, Barbosa E, et al. (2012) Campylobacter fetus subspecies: Comparative genomics and prediction of potential virulence targets. Gene. [DOI] [PubMed]
- 42. Abril C, Brodard I, Perreten V (2010) Two novel antibiotic resistance genes, tet(44) and ant(6)-Ib, are located within a transferable pathogenicity island in Campylobacter fetus subsp. fetus . Antimicrob Agents Chemother 54: 3052–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marraffini LA, Sontheimer EJ (2010) CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet 11: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorth P, Whiteley M (2012) An evolutionary link between natural transformation and CRISPR adaptive immunity. MBio 3. [DOI] [PMC free article] [PubMed]
- 45. Kienesberger S, Gorkiewicz G, Joainig MM, Scheicher SR, Leitner E, et al. (2007) Development of experimental genetic tools for Campylobacter fetus . Appl Environ Microbiol 73: 4619–4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tu ZC, Wassenaar TM, Thompson SA, Blaser MJ (2003) Structure and genotypic plasticity of the Campylobacter fetus sap locus. Mol Microbiol 48: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, et al. (2011) A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog 7: e1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, et al. (2001) Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 49. Arce RM, Caron KM, Barros SP, Offenbacher S (2012) Toll-like receptor 4 mediates intrauterine growth restriction after systemic Campylobacter rectus infection in mice. Mol Oral Microbiol 27: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trinchieri G, Sher A (2007) Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 7: 179–190. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez JM, Xu H, Ofori E, Elovitz MA (2007) Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am J Obstet Gynecol 197: 296 e291–296. [DOI] [PubMed]
- 52. Tu ZC, Dewhirst FE, Blaser MJ (2001) Evidence that the Campylobacter fetus sap locus is an ancient genomic constituent with origins before mammals and reptiles diverged. Infect Immun 69: 2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kleczka B, Lamerz AC, van Zandbergen G, Wenzel A, Gerardy-Schahn R, et al. (2007) Targeted gene deletion of Leishmania major UDP-galactopyranose mutase leads to attenuated virulence. J Biol Chem 282: 10498–10505. [DOI] [PubMed] [Google Scholar]
- 54. Dworkin J, Tummuru MK, Blaser MJ (1995) Segmental conservation of sapA sequences in type B Campylobacter fetus cells. J Biol Chem 270: 15093–15101. [DOI] [PubMed] [Google Scholar]
- 55.R_Core_Team (2012) R: A language and environment for statistical computing. In: Computing RFfS, editor. Vienna, Austria.
- 56. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blaser MJ, Smith PF, Kohler PF (1985) Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis 151: 227–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparative maps of CRISPR-related genomic islands. (A) C. fetus subsp. venerealis 84-112 VGI IV harbors the direct repeats with spacers (CRISPR) but lacks CRISPR-associated (cas)-genes. Prophage-related genes (putative prophage IV) were identified (orange) adjacent to a region identical to C. fetus subsp. fetus 82-40 Downstream of these regions the core-genome continues with a chromosomal rearrangement between the two subspecies on the 3-prime end (striped boxes). A sequence region shared between the subspecies was identified (blue box). (B) C. fetus subsp. fetus 82-40 FGI I carries two regions of direct repeats and spacers. cas-genes precede the second CRISPR-array resulting in a putatively functional CRISPR-system. One region with a prophage-like structure (orange) was identified.
(TIF)
Physical map of the extra-chromosomal element ICE_84-112. Shown is the GC-content (circle 1), GC-skew (circle 2) and open reading frames (circle 3). The tra-region (red) comprises genes putatively involved in conjugative transfer of the ICE. The vir-region (orange) shows putative T4SS genes with homology to the chromosomal PAI on VGI I. Genes possibly involved in autonomous replication of the ICE are named individually and labeled (green and red). Genes of predicted plasmid origin (green); phage genes and transposons (blue); putative effector proteins or toxin-antitoxin system (yellow); hypothetical proteins (grey).
(TIF)
Schematic representation of the apparent T4SS identified in C. fetus subsp. venerealis 84-112. (A, B, C) Represent loci with homology to virB/virD4-genes. (A) The PAI T4SS is functional in virulence and conjugative DNA transfer [1], [2]. (B) ICE_vir displays a similar gene organization to VGI I but protein homologies are not strikingly high. virD4 is truncated compared to the functional PAI homologue. (C) A partial set of vir-genes. (D) ICE_trb/tra genes share homology to plasmid RP4 and are putatively involved in the conjugative transfer of ICE_84-112. Homologous genes (vir, tra) are indicated by color.
(TIF)
Comparative map of C. fetus subspecies variation regions VSDR and FSDR. (A) C. fetus subsp. venerealis 84-112 VSDR and (B) C. fetus subsp. fetus 82-40 FSDR. MAUVE was used to compare the regions to visualize rearrangements and insertions. Regions free of rearrangements are indicated by colored colinear blocks. White regions within these blocks symbolize insertions or non-homologous regions. Important open reading frames are colored and/or labeled accordingly. Genes unique to the subspecies, mat1 and glf, are highlighted in pink.
(TIF)
Venn diagram of annotated TSS. (A) C. fetus subsp. venerealis 84-112 and (B) C. fetus subsp. fetus 82-40. TSS were categorized according to the genomic context into five classes: primary (TSS having the most cDNAs within ≈500 bp upstream of annotated mRNA start codons), secondary (TSS associated with the same gene but with fewer cDNAs), internal (TSS within an annotated gene on the same strand), antisense (TSS situated inside or within ≈100 bp of the coding region of a gene encoded on the opposite strand), or orphan (TSS without annotated genes in proximity) [3]. Numbers in parentheses indicate the TSS, which associate with only one orf.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOC)