Abstract
Tofacitinib (Tofa) is an inhibitor of Janus Kinase 3, developed for the treatment of autoimmune diseases and for the prevention of transplant rejection. Due to its selective action on proliferating cells, Tofa can offer a way to block T cell activation, without toxic effects on resting cells. However, few studies have investigated the effects of Tofa on lymphocyte activation in vitro. Our aim was to study the action of Tofa on different lymphocyte subsets after in vitro stimulation and to track the behaviour of treated cells after interruption of the treatment. Peripheral blood lymphocytes were stimulated in vitro with mitogen and treated with two concentrations of Tofa. After a first period in culture, cells were washed and further incubated for an additional time. Lymphocyte subsets, activation phenotype and proliferation were assessed at the different time frames. As expected, Tofa was able to reduce the activation and proliferation of lymphocytes in the first four days of treatment. In addition the drug led to a relative decrease of Natural Killer, B cells and CD8 T cells compared to CD4 T cells. However, treated cells were still viable after the first period in culture and begun to proliferate, strikingly, in a dose dependent manner when the drug was removed from the environment by replacing the culture medium. This novel data does not necessarily predict a similar behaviour in vivo, but can warn about the clinical use of this drug when a discontinuation of treatment with Tofa is considered for any reason.
Introduction
Tofacitinib (Tofa, alternative name CP-690550) is a small molecule inhibitor of Janus Kinase 3 (JAK3), a cytoplasmic tyrosine kinase associated with γc-containing cytokine receptors, involved in signal transduction of interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, IL-21. JAK3 is predominantly expressed in cells of the hematopoietic lineage and plays a key role in the regulation of lymphocyte differentiation, proliferation, survival and apoptosis [1]–[3]. The observation of a severe immunodeficiency in humans and mice with defects in JAK3 highlighted the importance of this signalling pathway in lymphocyte development and activation [4], [5]. An up-regulation of JAK3 has been on the contrary associated with rheumatoid arthritis both in animals and in humans [6]; for these reasons, JAK3 represents a good target for immunotherapy in severe autoimmune disorders.
Thanks to its potency, to its rather specific action and to its good bioavailability after oral administration, Tofa is considered a promising drug for T-cell mediated conditions such as rheumatoid arthritis, inflammatory bowel diseases, psoriasis and organ transplantation. However, available data are still insufficient to predict how Tofa will influence the balance between pathogenic and protective lymphocytes, which is a critical issue for T cell-targeted immunosuppressive therapies. In fact, few data are available on the immunosuppressive action of Tofa in vitro and the schedule of in vivo administration of the drug is largely empirical.
In this work, we evaluated the behaviour of lymphocytes after in vitro treatment with Tofa. We here demonstrated that Tofa strongly blocks T cell activation and proliferation, but, unexpectedly, treated lymphocytes display increased responsiveness to stimulation after the withdrawal of the drug from the culture. Although in vitro results do not necessarily predict a similar behaviour in vivo, we suggest that caution should be used when discontinuation of treatment with Tofa is considered for any reason. Furthermore, our data could help to develop novel strategies to synchronize and target the activation of pathogenic lymphocytes by exploiting the double-edged immunologic properties of Tofa.
Methods
Ethics statement
All the experiments were executed on peripheral blood mononuclear cells (PBMCs) obtained from heparinised blood of healthy donors after written informed consent. The study was approved by the Medical Ethics Review Board of the Institute for Maternal and Child Health – IRCCS “Burlo Garofolo”, Trieste (n.185/08, 19/08/2008) and by the Institutional Research Board (#28/09).
Drugs, chemicals and reagents
Tofacitinib (Tofa, CP-690550, Selleck Chemical LLC, TX, USA) was dissolved in dimethylsulfoxide (DMSO) and then diluted in the culture medium at a final concentration of 10 (Tofa10) or 100 μM (Tofa100), so that the final concentration of DMSO would not exceed 1∶320 v/v. These concentrations were chosen on the basis of a previous work [7] and did not induce unspecific cell toxicity, while permitting an effective reduction of proliferation.
Phytohaemagglutinin (PHA, Biochrom AG, Germany) was dissolved in water and then diluted 1∶200 in the culture medium at a final concentration of 1 μg/mL.
Oregon Green 488 carboxylic acid diacetate, succinimidyl ester (Carboxy-DFFDA, SE or CFSE, Invitrogen, Eugene, OR, USA) was dissolved at a concentration of 4.21 mM in DMSO and then used for proliferation assay at a final dye concentration of 5 μM.
7-aminoactinomycin D (7AAD) viability staining was from Immunostep (Salamanca, Spain).
All monoclonal antibodies for flow cytometric analysis were from Miltenyi Biotec (Bergisch Gladbach, Germany) except for CD25 APC-Cy7 (Biolegend, San Diego, CA, USA), HLA DR PE and CD19 FITC (Invitrogen, Carlsbad, CA, USA). 7AAD and monoclonal antibodies were used at concentrations indicated by the manufacturers.
Experimental plan
On day 0 peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient from heparinised blood of healthy donors and plated at a cell density of 1·106 per millilitre in X-VIVO 15 culture medium (Lonza, Belgium) containing 10% Human Serum AB (Sigma Aldrich, Germany). Cells were stimulated with 1 µg/ml PHA: preliminary experiments using either anti-CD3 and anti-CD28 coated beads or PHA as stimulus, showed that PHA can be a good model to examine Tofa's function on activated T cells. Tofa 10 or 100 µM was simultaneously added to the cell culture. After incubation, on day 4 cells were harvested. PHA and Tofa were washed out and cells were incubated in fresh medium for four additional days (day 4+4). On day 0, day 4 and day 4+4 the distribution of lymphocyte subsets was analysed and analysis of phosphorylation profile of Signal Transducer and Activator of Transcription (STAT) proteins was performed. On day 4 and day 4+4 culture supernatants were collected for cytokine determination and proliferation was assessed at the same time with the expression of the activation marker CD25 and 7AAD viability staining.
Cell proliferation
Cell proliferation was assessed by means of CFSE dilution after each cell division. CFSE staining was performed either at day 0 or day 4 to evaluate the relative proliferation in the first and second incubation time frame. Briefly, cells were suspended in PBS/BSA 0.5% with 5 µM CFSE, incubated for 10 min at 37°C and then washed three times with cold medium. Cells were then resuspended in complete medium and, after four days in culture, harvested and further stained with the following monoclonal antibodies, in different combination to assess the proliferation of each lymphocyte subpopulation: CD3 PerCP, CD4 APC, CD8 APC-Vio770, CD19 PE-Vio770, CD45 VioBlue, CD56 APC. Cells were also stained with CD25 APC-Cy7 and 7AAD to assess the activation and the viability of proliferated cells.
Data were acquired with Cyan ADP cytometer (Beckman Coulter, Fort Collins, Colorado, USA) and analysis was performed with FlowJo software v 7.6 (TreeStar, Ashland, OR, USA).
Lymphocyte subsets, activation and viability
At day 0, day 4 and day 4+4, cells were evaluated by flow cytometry to analyze the distribution of lymphocyte subsets. Cells were counted by microscopy using trypan blue viability staining, then 100,000 viable cells were resuspended in saline and stained with different combination of the following antibodies: CD3 FITC, CD4 APC, CD8 APC-Vio770, CD11c FITC, CD16 PE, CD19 PE-Vio770, CD25 APC-Cy7, CD56 PE, CD45 VioBlue, HLA DR PE. Cells were incubated with the antibody mix for 20 min at room temperature, then washed and fixed with FACS Lysing Solution (BD Bioscience, San Jose, CA, USA). T lymphocytes (CD3), B lymphocytes (CD19) and NK cells (CD16/56) were analysed after gating total lymphocytes based on CD45 expression and Side Scatter (SSC) (Figure S1). While this strategy can exclude quite well monocytes and dendritic cells on fresh blood samples, after PHA activation a small quote of monocytes can be included in the analysis. However, only a minor quote of monocytes (less than 3%) can be wrongly included in the natural killer gate, because of the expression of CD16. T helper (CD4) and cytotoxic T cells (CD8) were evaluated after gating on CD3 positive lymphocytes and then CD4/CD8 ratio was calculated. Activated T cells were evaluated by measuring the percentage of CD25 and HLA DR expression after gating on CD45 and CD3 lymphocytes. Activation of B cells was evaluated by measuring the Median Fluorescence Intensity (MFI) of HLA DR after gating on CD45 and CD19 positive cells. Activation of NK cells was evaluated by measuring the percentage of CD11c positive cells after gating on CD45 and CD16/56 cells.
To analyse the viability of each lymphocyte subpopulation, 100,000 cells were stained with CD3 FITC, CD45 VioBlue, CD4 APC, CD8 APC-Vio770 or CD19 FITC, CD45 VioBlue, CD56 APC for 15 min then 7AAD was added and cells were incubated for further 5 min before acquisition by flow cytometry.
The percentage of dead 7AAD positive cells was analysed after gating on CD45 positive lymphocytes and then on each subpopulation.
Data were acquired with Cyan ADP cytometer (Beckman Coulter, Fort Collins, Colorado, USA) and analysis was performed with FlowJo software v 7.6 (TreeStar, Ashland, OR, USA).
Cytokine profile
Cytokines release was evaluated on culture supernatants samples using a magnetic bead-based multiplex immunoassays (Bio-Plex®) (BIO-RAD Laboratories, Milano, Italy) following manufacturer's instructions. The analysis included 27 cytokines and chemokines: IL-1β, Il-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, Eotaxin, basic-FGF, G-CSF, GM-CSF, IP-10, IFN-γ, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, VEGF. Data were acquired using the Bio-Plex 200 reader, while a digital processor managed data output and Bio-Plex Manager® software presented data as Median Fluorescence Intensity (MFI) and concentration (ng/ml) as well (BIO-RAD Laboratories, Milano, Italy).
Analysis of phosphoproteins
Phosphorylation of STATs proteins was evaluated using MILLIPLEX MAP-5-Plex STAT panel (Millipore, Billerica, MA, USA), a bead-based multiplex assay. This assay was used to detect changes in phosphorylated STAT1 (Tyr701), STAT2 (Tyr690), STAT3 (Tyr705), STAT5A/B (Tyr694/Tyr699) and STAT6 (Tyr641) in cell lysates obtained from 4·106 PBMC derived cells.
At day 0, day 4 and day 4+4, cells were washed with ice cold PBS containing cOmplete protease inhibitors cocktail (Roche Diagnostic, Monza, Italy) and cell pellets were stored at −80°C until analysed. Before analysis, cells were lysed in lysis buffer containing 50 mM sodium fluoride and cOmplete protease inhibitor cocktail, then cellular debris were removed by filtration and protein content was measured with RC-DC Protein Assay (Bio-Rad Laboratories, Milano, Italy). The immunoassay was carried out following manufacturer's instructions. Data were acquired using the LumineX 200® reader (Millipore, Billerica, MA, USA), data output managed by X-Ponent® software (Millipore, Billerica, MA, USA) and data presented as Median Fluorescence Intensity (MFI).
Data analysis and statistics
PBMC from a total of eleven healthy donors were analysed in this study, allowing repeating most experiments three or four times.
All results are expressed as mean ± SD. Statistical significance was calculated using one-way analysis of variance (ANOVA) and Bonferroni post-test. Analysis was performed with GraphPad Prism software (version 5.0).
Results
Changes in lymphocyte subsets after in vitro Tofa treatment
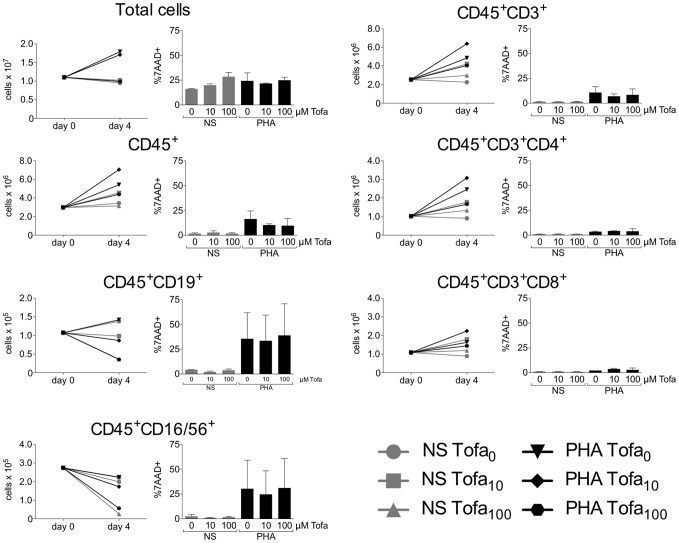
At day 4, the incubation with Tofa induced a relative decrease in the percentage of B cell (CD19) and NK cell (CD16/56) compared with baseline values (Table 1) both in resting cells and in PHA stimulated cells. This is probably due to an absolute reduction of the cell number because of cell death (Figure 1). In stimulated cells, this effect was even more evident after removal of the drug from culture (day 4+4), but this was likely due to the increase of proliferating CD3.
Table 1. Percentages of lymphocyte subsets after Tofa treatment.
| CD3 | CD4 | CD8 | CD4/CD8 ratio | CD19 | CD16/56 | ||
| day 0 | 70.4±6.2 | 63.2±8.3 | 29.0±6.1 | 2.3±0.7 | 7.5±3.1 | 17.1±10.0 | |
| day 4 | NS Tofa0 | 76.9±8.3 | 58.4±12.5 | 31.4±6.5 | 2.0±0.9 | 5.7±2.3 | 12.3±7.6 |
| NS Tofa10 | 80.6±5.0 | 60.8±12.2 | 29.4±5.7 | 2.2±0.7 | 5.5±2.3 | 9.6±4.2 | |
| NS Tofa100 | 87.6±3.8 | 52.3±23.8 | 25.7±9.7 | 2.6±2.0 | 1.9±0.8 | 3.6±1.8 | |
| PHA Tofa0 | 85.8±6.6 | 58.0±10.3 | 33.0±7.5 | 1.9±0.6 | 3.9±2.1 | 4.6±2.3 | |
| PHA Tofa10 | 83.5±7.4 | 68.7±9.2 | 23.6±7.3 | 3.2±1.1 | 2.0±1.1 | 3.1±1.5 | |
| PHA Tofa100 | 84.8±9.1 | 50.2±21.4 | 29.9±5.3 | 1.8±1.0 | 1.7±0.7 | 3.0±2.5 | |
| day 4+4 | NS Tofa0 | 85.5±1.5 | 61.0±8.6 | 30.4±4.6 | 2.1±0.6 | 5.0±2.3 | 2.1±1.3 |
| NS Tofa10 | 86.1±7.9 | 63.0±9.5 | 25.7±8.0 | 2.7±1.0 | 4.0±2.0 | 1.2±0.5 | |
| NS Tofa100 | 82.0±11.4 | 60.5±15.7 | 21.4±9.9 | 3.6±2.2 | 2.3±0.8 | 0.6±0.4 | |
| PHA Tofa0 | 87.7±1.1 | 50.1±5.9 | 43.5±4.0 | 1.2±0.2 | 3.5±0.6 | 5.1±1.7 | |
| PHA Tofa10 | 94.6±1.6 | 75.5±7.4 | 22.4±6.9 | 3.7±1.3 | 1.3±0.5 | 0.1±0.0 | |
| PHA Tofa100 | 91.7±4.0 | 73.5±9.7 | 22.9±9.7 | 3.7±1.6 | 0.9±0.3 | 0.2±0.1 |
Cells were analysed before treatment (day 0), after 4 days of incubation with Tofa (day 4) and then four days after the withdrawal of the drug (day 4+4). Percentage of CD3, CD19 and CD16/56 cells were evaluated on total lymphocytes, while CD4 cells, CD8 cells and CD4/CD8 ratio was evaluated on CD3 positive T lymphocytes. Data are expressed as mean ± SD of four independent experiments.
Figure 1. Absolute cell counts and viability.
The plots on the left represent the absolute number of cells. Histograms on the right report the percentage of dead cells, as 7AAD positive cells. Counts and viability are reported for each subpopulation of interest, before treatment (day 0) and after the first four days of incubation in presence or absence of PHA and Tofa (day 4). Data are expressed as mean ± SD of two independent experiments.
An increased CD4/CD8 T cell ratio was observed in cells stimulated with PHA and treated with Tofa, with higher values reached four days after the removal of the drug from culture. This was probably a result of an increase of CD4 absolute cell number, while CD8 absolute cell number was either unaffected or only slightly increased following Tofa treatment (Figure 1). Besides, a relative decrease of the percentage of CD8 cells rather than to an increase of CD4 cells was observed even in unstimulated (NS) cells treated with Tofa 100 µM (29.0±6.1% at day 0; 25.7±9.7% NS Tofa100 at day 4; 21.4±9.9% NS Tofa100 at day 4+4) (Table 1).
Both Tofa's concentrations reduced CD19 percentage in PHA stimulated cells, while in NS cells this reduction was evident only with the highest concentration. Moreover, considering cell viability in each lymphocyte subset (Table S1), PHA alone induced a high proportion of dead B and NK cells, which is not different in the presence of Tofa. Due to the small size of these populations, these data need further analyses.
Inhibition of lymphocyte proliferation during Tofa treatment is reversed with a paradoxical behaviour after removal of the drug
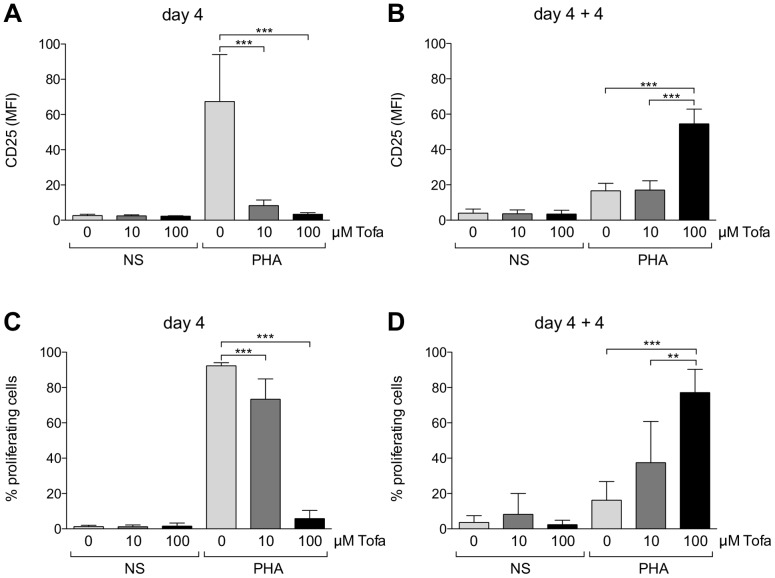
As expected, Tofa significantly reduced the proliferation of lymphocytes after PHA stimulation in a dose-dependent manner (92.3±1.7%, 73.4±11.5%, 5.8±4.7% of proliferating cells in Tofa0, Tofa10 and Tofa100 respectively; p<0.0001) (Table 2). The effect of the drug was also confirmed by a significantly reduced expression (p<0.0001) of the activation marker (CD25) on total lymphocytes (Figure 2).
Table 2. Lymphocytes activation and proliferation after Tofa treatment.
| % proliferating cells | CD25 MFI | ||
| day 4 | NS Tofa0 | 1.2±0.8 | 2.7±0.7 |
| NS Tofa10 | 1.2±1.0 | 2.5±0.6 | |
| NS Tofa100 | 1.5±1.8 | 2.2±0.2 | |
| PHA Tofa0 | 92.3±1.7 | 67.4±26.7 | |
| PHA Tofa10 | 73.4±11.5 | 8.2±3.2 | |
| PHA Tofa100 | 5.8±4.7 | 3.3±0.9 | |
| day4+4 | NS Tofa0 | 3.6±3.9 | 3.9±2.3 |
| NS Tofa10 | 8.2±11.8 | 3.6±2.1 | |
| NS Tofa100 | 2.4±2.5 | 3.5±2.2 | |
| PHA Tofa0 | 16.2±10.5 | 16.6±4.3 | |
| PHA Tofa10 | 37.4±23.3 | 17.0±5.3 | |
| PHA Tofa100 | 77.1±13.2 | 54.5±8.4 |
Cells were analysed after four days of incubation with Tofa (day 4) and four days after the withdrawal of the drug (day 4+4). The percentage of proliferating cells was assessed by measuring the level of CFSE fluorescence. Activation of cells is indicated as MFI of CD25. Data are expressed as mean ± SD of four independent experiments.
Figure 2. Proliferation and cell activation at the different time frames.
Cell activation at day 4 (A) and after four days after withdrawal of Tofa (B) is represented as mean fluorescence index (MFI) of CD25 ± SD of four independent experiments. Cell proliferation at day 4 (C) and after four days after withdrawal of Tofa (D) is represented as percentage of cells with reduced fluorescence of CFSE ± SD of four independent experiments. Statistical significance was calculated using one-way analysis of variance (ANOVA) and Bonferroni post-test. *** p<0.0001, **p<0.001.
In particular, Tofa treatment reduced the expression of activation markers induced by PHA at day 4 only on CD3 lymphocytes with a decreased percentage of CD25 positive (Figure 2, Table 2, Table S2), while the effects were not significant on CD19 (expression of HLA DR) and NK cells (percentage of CD11c positive cells) (Figure 2, Table 2, Table S2).
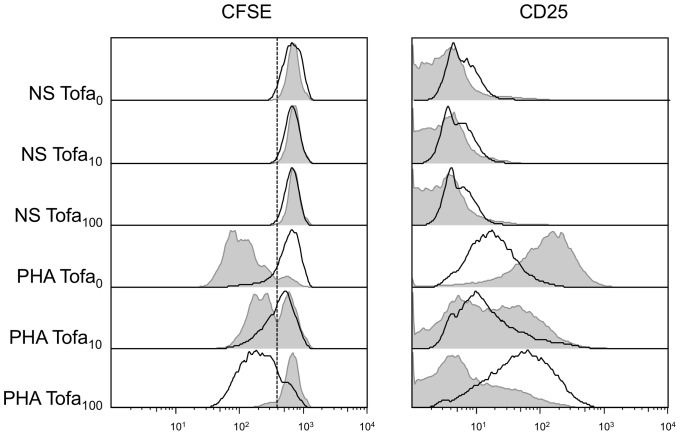
However, after removal of the drug from the culture medium (day 4+4), a rescue of lymphocyte proliferation was observed (Figure 2, Table 2), which was surprisingly more consistent in samples that have been previously treated with higher dose of Tofa (16.2±10.5%, 37.4±23.3%, 77.1±13.2% of proliferating cells in Tofa0, Tofa10 and Tofa100 respectively) (a representative experiment is reported in Figure 3) and was paralleled by an increase in activation markers (Figure 2, Table 2, Table S2). In contrast, in cells incubated for the first four days in medium containing PHA but without Tofa, a reduced residual proliferation was observed during the additional four days after medium wash out (4+4). Since cells where CFSE-stained after the first four days of incubation, the proliferation assay evaluated only the proliferation in the second time frame of culture. A subset of experiments, performed on cells stained at time 0, showed that cells treated with Tofa10 displayed a proliferation at day 4+4 similar to cells not treated with the drug, whilst a slight overall decrease of proliferation was achieved by cells treated with Tofa100 (data not shown). These results indicate that all the cells blocked during the first four days of Tofa administration are ready to proliferate when the drug is washed out.
Figure 3. Representative data of cell proliferation and activation.
Cells were incubated with PHA and the two concentrations of Tofa (grey histograms); after four days the medium was removed, and the incubation continued in drug-free medium for four additional days (black line histograms). The panel on the left represents the percentage of proliferating cells based on dilution of CFSE dye. On the right expression of CD25 is reported and evaluated as MFI.
Inhibition of cytokine release
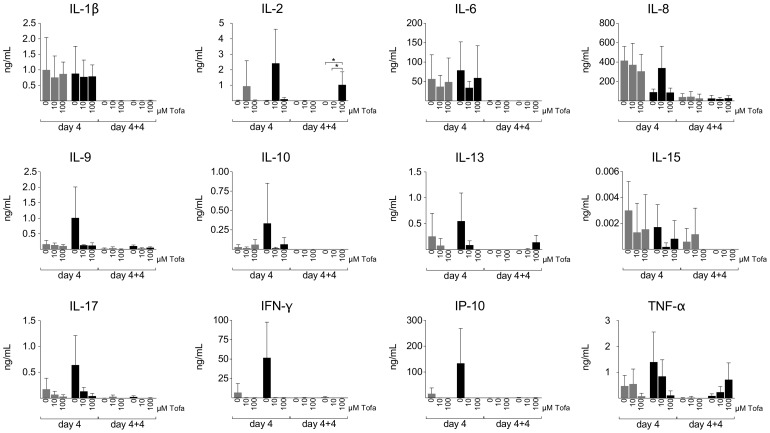
During the first four days of culture, Tofa treatment inhibited the secretion of IL-2, IL-9, IL-10, IL-13, IL-17, TNF-α in a dose-dependent manner. In contrast, at day 4+4, Tofa-treated cells showed an increased release only of IL-2 (p<0.05), TNF-α and IL-13 that was particularly evident in cells treated with Tofa100. Besides, a complete depletion of IFN-γ and IP-10 was seen despite the proliferation evidenced in PHA+Tofa100 treated cells (Figure 4). The reduced concentration of IFN-γ and its related product (IP-10) could be a result of the loss of the NK population.
Figure 4. Cytokine determination in culture supernatants at different time frames.
Cytokines were evaluated by magnetic bead-based multiplex immunoassays. The analysis was performed at day 4 and at day 4+4 on three series of culture supernatants. Data are expressed as mean concentration (ng/mL) ± SD. Only the histograms of the cytokines mainly involved in the effects evaluated in the study are reported in the figure. Grey bars: unstimulated cells; Black bars: PHA stimulated cells. Statistical significance was calculated using one-way analysis of variance (ANOVA) and Bonferroni post-test. *p<0.05.
Other cytokines, mainly secreted by monocytes, such as IL-1β, IL-6 and IL-8 were less influenced by the treatment in the first 4 days and decreased regardless of the treatment in the longer incubation time (day 4+4) (Figure 4).
Phosphorylation profile of STAT proteins
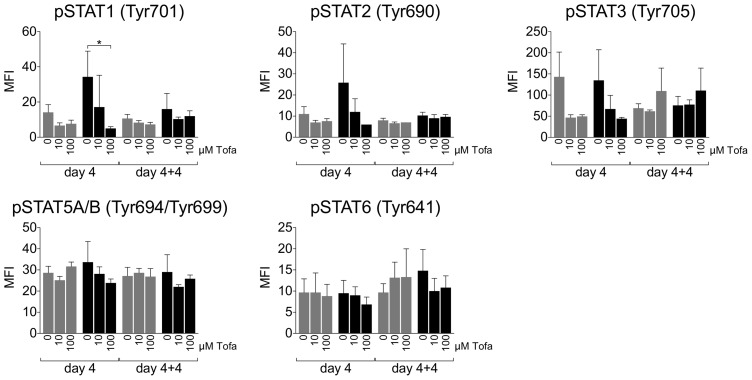
After the first four days of culture, STAT1 and STAT2 phosphorylation showed an activation-induced increase that was inhibited in a dose dependent manner by Tofa (significant for STAT1 p<0.05). Conversely, STAT3 phosphorylation seemed to be inhibited by Tofa both in NS and in PHA stimulated cells. Only a slight trend toward a lower STAT5 phosphorylation was observed in PHA stimulated cells after Tofa treatment, while STAT6 phosphorylation didn't show appreciable changes.
During the second period of incubation (day 4+4) the phosphorylation profiles of all the tested STAT proteins remained stable without any appreciable change regardless of the treatment of the cells (Figure 5).
Figure 5. Phosphorylation profile of STAT proteins.
Phosphorylation profile of STAT1, STAT2, STAT3, STAT5A/B, STAT6 was evaluated by magnetic bead-based multiplex immunoassays. Data are expressed as Median Intensity Fluorescence (MFI) ± SD of three independent experiments. Grey bars: unstimulated cells; Black bars: PHA stimulated cells. Statistical significance was calculated using one-way analysis of variance (ANOVA) and Bonferroni post-test. *p<0.05.
Discussion
Tofacitinib is a potent immunomodulatory drug belonging to the category of small molecules targeting kinase signalling [8]. Based on its mechanism of action, Tofa can fit into the tool box of drugs acting on lymphocyte activation, which already includes calcineurin inhibitors, mammalian target of rapamycin (mTOR) inhibitors and co-stimulation targeting biologics [9]. The potential field of clinical application is thus represented by disorders with inappropriate T cell response, such as rheumatoid arthritis, psoriasis, graft rejection, ulcerative colitis and graft versus host disease.
Compared to other drugs acting on lymphocyte activation, Tofa was intended to offer higher efficacy, due to its action on different lymphotropic cytokines, with a safer profile, being the expression of target kinases restricted to lymphoid cells [10].
However, the tuning of efficacy (suppression of pathogenic lymphocytes) and safety (suppression of protective immunity) remains an open issue. In fact, serious and sometimes fatal infections and immunosuppression-related malignancies have been reported in patients with rheumatoid arthritis receiving Tofa [11], [12]. Furthermore, the action of the drug in long term therapies, alone or in combination with other drugs, and the evolution of diseases after drug withdrawal, have not been thoroughly explored. Unexpectedly, whereas the drug has been widely used in animal models and has been already introduced into the clinics, only few studies had investigated its immunological potential in vitro.
Here we showed that Tofa exerts a rapid and strong effect on PHA stimulated lymphocytes, leading to a complete arrest in proliferation and to a strong down-regulation of activation markers. Notably, these results are achieved with a negligible toxicity on T lymphocyte viability assessed by 7AAD. However, the drug induces a redistribution of lymphocyte subsets by increasing the death of B cells and NK cells and by a stronger inhibition of CD8 compared to CD4 T cells. In other studies, anti-T cell receptor antibody-induced proliferation of naïve CD4 cells was not affected by the addition of Tofa at doses between 10 and 100 ng/ml, which are in the range of the pre-dose levels of the drug in peripheral blood of treated patients [18], [20], [21]. In contrast, we showed that a near complete block in proliferation can be obtained by using much higher doses of Tofa (100 µM), still without appreciable cytotoxicity.
What is completely new, is that, after the withdrawal of the drug, stimulated T lymphocytes resumed proliferation. Thus, transient treatment with Tofa doesn't lead to a relevant inhibition of final proliferation, but it still strongly affects the distribution of lymphocyte subsets, with a reduction of NK cells, B cells and CD8 cells.
Although in vitro PHA stimulus induces direct activation only on T cells, the bystander effects due to this activation clearly shows its side-effect also on other cell compartments. Besides, Tofa treatment could even impact the distribution of cell populations and this is coherent with the phenotype commonly found in JAK3-Severe Combined Immunedeficiency (SCID) in which a decrease of NK and CD8 is observed [13], [14]. More unexpected was the loss of B cells that is not a typical finding in vivo, both in T−B+NK− JAK3-SCID and in subjects treated with Tofa. Indeed, as reported by others [13]–[15] the survival of B cells in vivo may be ascribed to different mechanisms, such as an increased differentiation of mature B cells in the bone marrow, a compensatory IL-3-based mechanism [15]. Furthermore, B cells are dependent upon CD40L stimulation that, in vivo, can be provided not only by activated T cells but also by dendritic cells [16].
To the best of our knowledge, the finding that replenishment of Tofa from culture resulted in the rebound of suppressed T cell proliferation has not been previously described. Of course, these data may reflect just a particular experimental setting in vitro, but they may be relevant to answer unsolved questions about the immunomodulatory action of Tofa and not-infectious adverse events observed in the clinics [17]. Indeed, the lower dosage used in our study is close to the peak concentration occurring in patients' serum during treatment (Tofacitinib for Treatment of Rheumatoid Arthritis (NDA 203214) Advisory Committee Meeting).
Other authors evaluated the response to cytokine stimulation in the presence of Tofa, showing a variable impact on JAK signalling, as confirmed by changes in STAT proteins phosphorylation, accounting for a preferential action on Th1 and Th17 lineages [18], [19]. On the contrary, low dose Tofa led to the worsening of experimental encephalomyelitis in vivo and resulted in increased Th17 differentiation in vitro [20].
To evaluate the impact of Tofa and its replenishment on JAK signaling, we studied the production of different cytokines and the phosphorylation of different STAT proteins downstream JAK kinases.
Of note, while a Tofa-dependent reduction of activation-induced phosphorylation was evident for STAT1 and STAT3, a lower effect could be evidenced on STAT5, which is the major target of JAK3 signalling, confirming a broader effect of the drug on different JAK-STAT pathways. In contrast, the only STAT protein that showed a trend increase in phosphorylation after the second period of incubation was STAT3, suggesting a possible role of JAK1-STAT3 pathway in the proliferation rebound observed after withdrawal of the drug. However, STAT3-dependent cytokines such as IL-17 remained suppressed by the treatment with Tofa also after the second incubation time frame and lymphocytes rescued to proliferate only produced IL-2, IL-13 and TNF-α, raising the possibility of a modulation of their Th1/Th2 profile.
In other studies, responsiveness of lymphocytes to cytokines stimulation was shown to be restored after Tofa withdrawal, however the drug was used at lower dosages and the residual lymphocyte proliferation was not investigated [18].
Based on these data, we can presume that discontinuation of the drug after a short treatment may lead to reactivation of diseased lymphocytes also in vivo, possibly resulting in undesired effects of the drug. In addition, a reduction of NK cells has been already reported in clinical trials [11] and may contribute to the increased risk of infections and malignancies. To evaluate this possibility, a careful study of the expression of lymphocyte activation markers and of the distribution of lymphocyte subsets should be performed in all subjects after discontinuation of Tofa treatment. Moreover, we think that further studies are needed to evaluate the sensitivity of Tofa-treated lymphocytes to other immunosuppressive drugs: this would be useful to allow a safe discontinuation of the drug and to develop optimized schedules for using Tofa in combination with other drugs.
Supporting Information
Gating strategy to identify lymphocyte subpopulations. Lymphocytes were identified based on the side scatter and high CD45 expression (A); T lymphocytes were identified as CD3 positive cells (B and C, lower right); NK cells were identified as CD16/56 positive cells (B, upper left); B cells were identified as CD19 positive cells (C, upper left). Among CD3 T cells, CD4 positive (D, lower right) cells were separated from CD8 positive cells (D, upper left).
(TIFF)
Percentage of dead cells in each lymphocyte subset. Dead cells was evaluated as percentage of 7AAD positive cells, before treatment (day 0), after stimulation with PHA and treatment with Tofa (day 4) and after withdrawal of the drug (day 4+4). Data represent the mean ± SD of two independent experiments.
(DOCX)
Evaluation of activation markers on the different lymphocyte subsets. Activation markers were evaluated at each time frames. Activation of CD3 cells was evaluated as percentage of CD25 and HLA DR positivity; activation of CD19 cells was evaluated as variation in Median Fluorescence Intensity (MFI); activation of NK cells was evaluated as percentage of CD11c positivity. Data report the mean ± SD of two independent experiments.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, et al. (1994) Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370: 151–153. [DOI] [PubMed] [Google Scholar]
- 2. Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen YQ, et al. (1994) Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A 91: 6374–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Shea JJ (2004) Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis 63 Suppl 2ii67–ii71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, et al. (1995) Defective lymphoid development in mice lacking Jak3. Science 270: 800–802. [DOI] [PubMed] [Google Scholar]
- 5. Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, et al. (1995) Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270: 797–800. [DOI] [PubMed] [Google Scholar]
- 6. Soto H, Hevezi P, Roth RB, Pahuja A, Alleva D, et al. (2008) Gene array analysis comparison between rat collagen-induced arthritis and human rheumatoid arthritis. Scand J Immunol 68: 43–57. [DOI] [PubMed] [Google Scholar]
- 7. Piscianz E, Cuzzoni E, De Iudicibus S, Valencic E, Decorti G, et al. (2011) Differential action of 3-hydroxyanthranilic acid on viability and activation of stimulated lymphocytes. Int Immunopharmacol 11: 2242–2245. [DOI] [PubMed] [Google Scholar]
- 8. Kyttaris VC (2012) Kinase inhibitors: a new class of antirheumatic drugs. Drug Des Devel Ther 6: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalluri HV, Hardinger KL (2012) Current state of renal transplant immunosuppression: Present and future. World J Transplant 2: 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, et al. (2010) Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem 53: 8468–8484. [DOI] [PubMed] [Google Scholar]
- 11. Vincenti F, Tedesco Silva H, Busque S, O'Connell P, Friedewald J, et al. (2012) Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant 12: 2446–2456. [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, et al. (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367: 495–507. [DOI] [PubMed] [Google Scholar]
- 13. Paniagua R, Si MS, Flores MG, Rousvoal G, Zhang S, et al. (2005) Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation 80: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 14. Conklyn M, Andresen C, Changelian P, Kudlacz E (2004) The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol 76: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 15. van Gurp EA, Schoordijk-Verschoor W, Klepper M, Korevaar SS, Chan G, et al. (2009) The effect of the JAK inhibitor CP-690,550 on peripheral immune parameters in stable kidney allograft patients. Transplantation 87: 79–86. [DOI] [PubMed] [Google Scholar]
- 16. Pinchuk LM, Klaus SJ, Magaletti DM, Pinchuk GV, Norsen JP, et al. (1996) Functional CD40 ligand expressed by human blood dendritic cells is up-regulated by CD40 ligation. J Immunol 157: 4363–4370. [PubMed] [Google Scholar]
- 17. van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, et al. (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367: 508–519. [DOI] [PubMed] [Google Scholar]
- 18. Vafadari R, Quaedackers ME, Kho MM, Mol WM, Chan G, et al. (2012) Pharmacodynamic analysis of tofacitinib and basiliximab in kidney allograft recipients. Transplantation 94: 465–472. [DOI] [PubMed] [Google Scholar]
- 19. Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, et al. (2011) Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 186: 4234–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshida H, Kimura A, Fukaya T, Sekiya T, Morita R, et al. (2012) Low dose CP-690,550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun 418: 234–240. [DOI] [PubMed] [Google Scholar]
- 21. Egli A, Kumar D, Broscheit C, O'Shea D, Humar A (2013) Comparison of the effect of standard and novel immunosuppressive drugs on CMV-specific T-cell cytokine profiling. Transplantation 3: 448–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy to identify lymphocyte subpopulations. Lymphocytes were identified based on the side scatter and high CD45 expression (A); T lymphocytes were identified as CD3 positive cells (B and C, lower right); NK cells were identified as CD16/56 positive cells (B, upper left); B cells were identified as CD19 positive cells (C, upper left). Among CD3 T cells, CD4 positive (D, lower right) cells were separated from CD8 positive cells (D, upper left).
(TIFF)
Percentage of dead cells in each lymphocyte subset. Dead cells was evaluated as percentage of 7AAD positive cells, before treatment (day 0), after stimulation with PHA and treatment with Tofa (day 4) and after withdrawal of the drug (day 4+4). Data represent the mean ± SD of two independent experiments.
(DOCX)
Evaluation of activation markers on the different lymphocyte subsets. Activation markers were evaluated at each time frames. Activation of CD3 cells was evaluated as percentage of CD25 and HLA DR positivity; activation of CD19 cells was evaluated as variation in Median Fluorescence Intensity (MFI); activation of NK cells was evaluated as percentage of CD11c positivity. Data report the mean ± SD of two independent experiments.
(DOCX)