Abstract
Tendinopathy is characterized histopathologically by lipid accumulation and tissue calcification. Adipogenic and osteogenic differentiation of tendon stem cells (TSCs) are believed to play key roles in these processes. The major inflammatory mediator prostaglandin E2 (PGE2) has been shown to induce osteogenic differentiation of TSCs via bone morphogenetic protein-2 (BMP-2), and BMP-2 has also been implicated in adipogenic differentiation of stem cells. We therefore examined the mechanisms responsible for PGE2-induced adipogenesis in rat TSCs in vitro. Insulin-like growth factor-1 (IGF-1) mRNA and protein were significantly up-regulated in PGE2-stimulated TSCs, measured by quantitative reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Incubation with specific inhibitors of cAMP, cAMP-dependent protein kinase A (PKA), and CCAAT/enhancer binding protein-δ (CEBPδ) demonstrated that IGF-1 up-regulation occurred via a cAMP/PKA/CEBPδ pathway. Furthermore, neither IGF-1 nor BMP-2 alone was able to mediate adipogenic differentiation of TSCs, but IGF-1 together with BMP-2 significantly increased adipogenesis, indicated by Oil Red O staining. Moreover, knock-down of endogenous IGF-1 and BMP2 abolished PGE2-induced adipogenic differentiation. Phosphorylation of CREB and Smad by IGF-1 and BMP-2, respectively, were required for induction of the adipogenesis-related peroxisome proliferator-activated receptor γ2 (PPARγ2) gene and for adipogenic differentiation. In conclusion, IGF-1 and BMP-2 together mediate PGE2-induced adipogenic differentiation of TSCs in vitro via a CREB- and Smad-dependent mechanism. This improved understanding of the mechanisms responsible for tendinopathies may help the development of more effective therapies.
Introduction
Tendons are constantly subjected to stress and mechanical loading, which can lead not only to acute tendon injuries, but also to chronic degenerative tendinopathies [1]. The Achilles, patella, elements of the rotator cuff, forearm extensors, biceps brachi and tibialis posterior tendons are most vulnerable to tendinopathies [2], which are a common clinical problem in both athletes and the general public. They involve degenerative changes exacerbated by overuse and mechanical loading [2], and are characterized histopathologically by lipid accumulation and tissue calcification [3], [4], [5], [6].
The presence of cells with multilineage differentiation potential, termed tendon stem cells (TSCs), has been demonstrated in humans [7], mice [7], [8], rabbits [9] and rats [10]. TSCs can differentiate into non-tenocyte lineages such as adipocytes, chondrocytes and osteocytes under suitable conditions [7], [9], [10], [11], [12], [13], providing a possible mechanism for the osteogenic and adipogenic changes associated with tendinopathies.
PGE2 is a major mediator of pain and acute inflammation [14]. Mechanical stretching of tendon fibroblasts (tenocytes) or tendon explants has been shown to increase the production of PGE2 in in vitro studies [15], [16], [17], [18], [19], [20]. PGE2 treatment may result in degenerative changes of the tendon characterized by lipid accumulation and tissue calcification, partly by inducing the differentiation of TSCs into non-tenocytes, including adipocytes and osteocytes [9], [11], [21].
We previously demonstrated that PGE2 induced BMP-2 production through phosphoinositide 3-kinase (PI3K)-Akt signalling [21], and BMP-2 has been shown to play a role in tendon calcification [22] and to mediate PGE2-induced osteogenic differentiation in TSCs [23]. Huang et al. found that the BMP signalling pathway was also required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage [24]. However, the role of BMP-2 in the adipogenic differentiation of TSCs remains unclear. Insulin-like growth factor 1 (IGF-1) is also known to promote adipogenic differentiation [25], [26], and was increased in tendons subjected to repetitive mechanical loading in vivo [26].
In this study, we investigated the roles of IGF-1 and BMP-2 in PGE2-induced adipogenic differentiation of cultured rat TSCs, and defined the roles of downstream cAMP response element-binding protein (CREB) and Smad signaling in the effects of IGF-1 and BMP-2. The findings of this study will help to clarify the nature of tendinopathies and so help in the development of future therapeutic strategies for these chronic conditions.
Materials and Methods
Isolation and culture of rat TSCs
All experiments were approved by the Animal Research Ethics Committee, Third Military Medical University, China. Rat TSCs were isolated from Sprague-Dawley rats and cultured, as described previously [21]. To determine if PGE2 induced adipogenic differentiation, TSCs were seeded in six-well plates at a density of 6×104/well and were incubated with 0, 10, 50, 100 or 200 ng/ml PGE2 (Sigma-Aldrich, St. Louis, MO, USA) for 7 days, or with PGE2 100 ng/ml for 0, 3, 7 or 10 days. To determine the ability of BMP-2 to induce adipogenic differentiation, rat TSCs were incubated with 0, 10, 50, 100 or 200 ng/ml BMP-2 (Sigma-Aldrich) for 7 days, or with BMP-2 (100 ng/ml) for 0, 3, 7 or 10 days. The effects of IGF-1 on adipogenic differentiation of rat TSCs were determined by incubation with IGF-1 (Sigma-Aldrich) alone (10 nM), or with 0, 1, 5, 10 or 20 nM IGF-1 in combination with 100 ng/ml BMP-2. The cAMP synthesis inhibitor 2′,5′-dideoxyadenosine (ddA, 10 μmol/l, Sigma-Aldrich) and the PKA inhibitor H-89 (10 μM, Sigma-Aldrich) were added to PGE2-stimulated TSCs to determine the role of the cAMP/PKA/CEBPδ pathway.
RNA interference
Short-hairpin (shRNA) lentiviral particles targeting BMP-2, IGF-1, CEBPδ, CREB and Smad1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and used to transduct cells, according to the manufacturer's instructions.
Oil Red O Staining
Adipocytes were identified by staining with Oil Red O (Sigma-Aldrich), as described previously [27]. The area of the cells stained with Oil Red O was measured by ImagePro Plus software (Palmerton).
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
mRNA expression levels of IGF-1 and peroxisome proliferator-activated receptor γ (PPARγ) were determined by qRT-PCR. Total RNA was extracted from cells using TRIzol reagent following the protocol provided by the manufacturer (Invitrogen). cDNA was synthesized from total RNA using a Superscript III first-strand synthesis kit (Invitrogen). qRT-PCR was performed using a SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) and an ABI Prism 7900 Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA). Expression levels were calculated relative to expression of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences used in this research are listed in Table 1.
Table 1. Primers for PCR.
| Gene | Primer |
| PPARγ | F: 5′- ATGACCACTCCCATTCCTTT -3′ |
| R: 5′- TGATCGCACTTTGGTATTCTT -3′ | |
| PPARγ2 | F: 5′-CCCTTTACCACGGTTGATTTCTC-3′ |
| R: 5′- GCAGGCTCTACTTTGATCGCACT-3′ | |
| IGF-1 | F: 5′- GGCATTGTGGATGAGTGTTG-3′ |
| R: 5′- GCTGGGACTTCTGAGTCTTGG-3′ | |
| GAPDH | F: 5′-GGCAAGTTCAACGGCACAG-3′ |
| R: 5′- CGCCAGTAGACTCCACGAC-3′ |
F = forward; R = reverse; GAPDH = glyceraldehyde phosphate-3 dehydrogenase (internal control).
Measurement of IGF-1 and cAMP levels
IGF-1 protein levels were measured using a mouse IGF-1 enzyme-linked immunosorbent assay (ELISA) kit (EMI1001-1; AssayPro, Saint Charles, MO, USA). Intracellular cAMP levels were measured using a cAMP enzyme immunoassay (EIA) kit (Enzo Life Sciences, Lörrach, Germany) according to the manufacturers' protocols.
Protein extraction and western blotting
Cells were rinsed, harvested, and lysed in hypotonic buffer A (10 mM Hepes, 10 mM KCl, 2 mM MgCl2, 500 μM DTT, 1mM Na3VO4, 10 mM NaF, 1% Triton X-100) containing a mixture of protease inhibitors (Thermo Fisher Scientific Inc., Rockford, IL, USA). Lysates were centrifuged at 14000 rpm for 15 min and supernatants were collected as the cytosolic fraction. Pellets were resuspended in hypertonic buffer B (25% glycerol, 420 μM NaCl, 200 μM Na2EDTA, 500 μM DTT, 1 mM Na3VO4, 10 mM NaF, and phosphatase inhibitors) and shaken on ice for 30 min. Lysates were centrifuged at 12000 rpm for 5 min and the supernatants (nuclear extracts) were diluted in buffer C (20 mM Hepes, 20% glycerol, 50 mM KCl, 200 μM EDTA, 500 μM DTT, 1 mM PMSF). Protein concentrations were measured using a BCA protein assay kit (Thermo Fisher Scientific Inc.). Western blotting was carried out as described by Ji et al. [28], using the following antibodies: rabbit polyclonal anti-PKA C-α, anti-CREB, and anti-phosphorylated CREB (p-CREB) (Ser133), (all from Cell Signaling Technologies, Beverly, MA, USA); rabbit polyclonal anti-p-PKA IIα reg (Ser 96)-R, anti-CEBPδ, anti-pSmad1/5/8, anti-Smad, (all from Santa Cruz Biotechnology). Antibodies to β-actin and histone deacetylase (HDAC) (Santa Cruz Biotechnology) were used as loading controls. Results were visualized and images captured using a LiCoR Odyssey imager (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Data were expressed as mean ± SD. Multiple comparisons were made using one-way analysis of variance followed by Fisher's tests. A P value <0.05 was considered to be statistically significant.
Results
PGE2 induces adipogenic differentiation
To define the role of PGE2 in adipogenesis, rat TSCs were incubated with increasing doses of PGE2 for 7 d, or with 100 ng/ml PGE2 for different time. The presence of adipocytes was assessed by Oil Red O staining and mRNA expression of the adipogenic gene PPARγ. As shown in Fig. 1, adipocyte numbers and PPARγ expression were increased after PGE2 treatment in time- and dose-dependent manners, levelling off at a concentration of 100 ng/ml and duration of 7 days, respectively. These results indicated that PGE2 induced adipogenic differentiation of TSCs.
Figure 1. PGE2-induces adipogenic differentiation in rat TSCs.
Rat TSCs were incubated with 0, 10, 50, 100, or 200/ml PGE2 for 7 days. (A) Adipogenic differentiation detected by Oil Red O staining; (B) the Oil Red O staining areas in the cells were assessed as described in the text; (C) PPARγ mRNA levels determined by qRT-PCR. Rat TSCs were incubated with 100 ng/ml PGE2 for 0, 3, 7, or 10 days. (D) Adipogenic differentiation detected by Oil Red O staining; (E) the areas stained with Oil Red O in the cells were assessed; (F) PPARγ mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH. Results represent the mean ± SD. *P<0.05, **P<0.01 with respect to TSCs without PGE2.
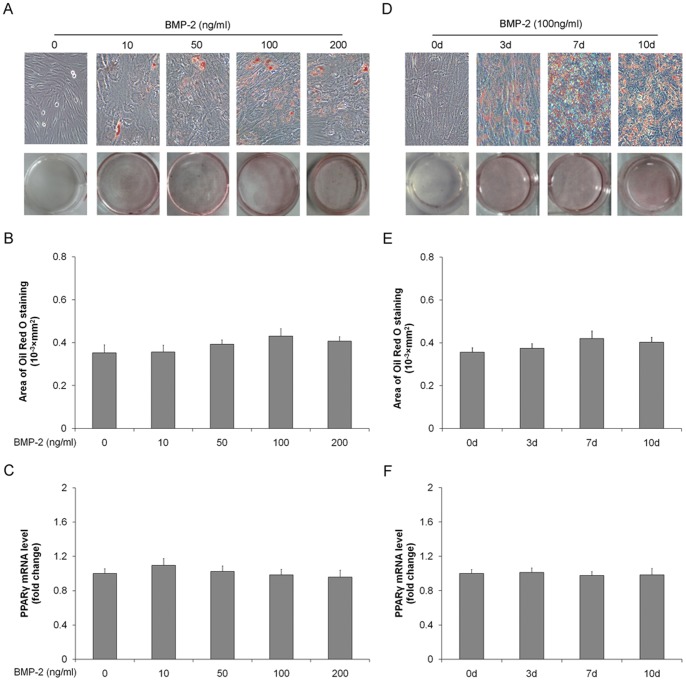
BMP-2 alone does not mediate PGE2-induced adipogenic differentiation
To determine if BMP was able to mediate PGE2-induced adipogenic differentiation directly, we assessed the differentiation of TSCs incubated with increasing concentrations of BMP-2, or with 100 ng/ml BMP-2 for the indicated days. BMP-2 had no significant effect on adipocyte number or on PPARγ expression (Fig. 2). This indicated that BMP-2 alone was unable to mediate PGE2-induced adipogenic differentiation, and suggested that additional factors are required.
Figure 2. BMP-2 alone does not induce adipogenic differentiation in rat TSCs.
TSCs were incubated with (0, 10, 50, 100, or 200 ng/ml BMP-2 for 7 days. (A) Adipogenic differentiation detected by Oil Red O staining; (B) the areas stained with Oil Red O in the cells were assessed; (C) PPARγ mRNA levels determined by qRT-PCR. TSCs were incubated with 100 ng/ml BMP-2 for 0, 3, 7, or 10 days. (D) Adipogenic differentiation detected by Oil Red O staining; (E) the Oil Red O staining areas in the cells were assessed; (F) PPARγ mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH. Results represent the mean ± SD.
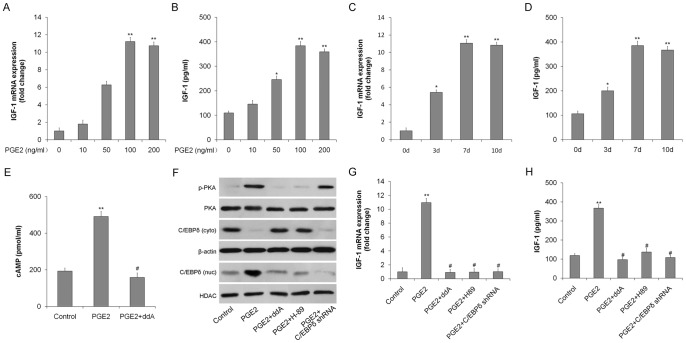
PGE2 upregulates IGF-1 via the cAMP/PKA/CEBPδ pathway
Rat TSCs were incubated with PGE2 (0–200 ng/ml) for 7 days to induce adipogenic differentiation. IGF-1 mRNA and protein levels, measured by qRT-PCR and ELISA, respectively, increased in dose-dependent manners in response to PGE2 treatment (Fig. 3A and B). PGE2 also upregulated IGF-1 mRNA expression and protein synthesis in a time-dependent manner (Fig. 3C and D).
Figure 3. IGF-1 expression in rat TSCs is upregulated by PGE2 via the cAMP/PKA/CEBPδ pathway.
TSCs were incubated with 0, 10, 50, 100, or 200/ml PGE2 for 7 days. (A) IGF-1 mRNA expression was determined by qRT-PCR; (B) IGF-1 protein levels were quantitated by ELISA. TSCs were incubated with 100 ng/ml PGE2 for 0, 3, 7, or 10 days. (C) IGF-1 mRNA expression was determined by qRT-PCR; (D) IGF-1 protein levels were quantitated by ELISA. (E) TSCs were incubated in medium containing PGE2 (100 ng/ml), with or without the cAMP inhibitor ddA (10 μmol/l) and intracellular cAMP levels were determined by EIA. TSCs were incubated in medium containing PGE2 (100 ng/ml) with or without the cAMP inhibitor ddA (10 μmol/l), the PKA inhibitor H-89 (10 μM), or after transduction with the CEBPδ shRNA. (F) p-PKA and total PKA, nuclear (nuc) and cytoplasmic (cyto) CEBPδ protein levels determined by western blotting. HDAC (nuc) and β-actin (cyto) were used as loading controls; (G) IGF-1 mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH; (H) IGF-1 protein levels determined by ELISA. Results represent the mean ± SD. *P<0.05, **P<0.01 with respect to TSCs without PGE2; #P<0.05 with respect to TSCs with PGE2.
To determine if PGE2 induced IGF-1 secretion via a cAMP/PKA/CEBPδ pathway, TSCs were incubated in medium containing PGE2 (100 ng/ml) for 7 days. PGE2 increased intracellular cAMP in TSCs (Fig. 3E), activated cAMP-dependent protein kinase PKA, and stimulated nuclear translocation of CEBPδ (Fig. 3F). Addition of the cAMP synthesis inhibitor ddA, the PKA inhibitor H-89, or CEBPδ shRNA markedly inhibited PGE2-induced IGF-1 production at both mRNA and protein levels (Fig. 3G and H), indicating that PGE2 stimulated IGF-1 expression through cAMP/PKA/CEBPδ pathway.
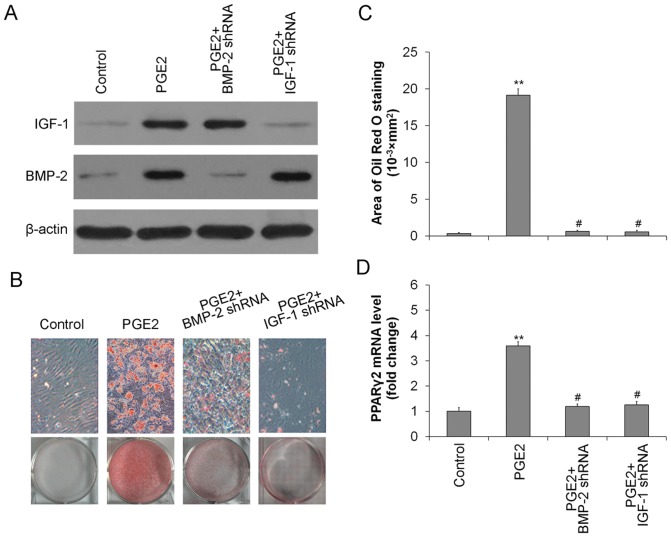
BMP2 and IGF-1 together mediate PGE2-induced adipogenic differentiation
IGF-1 in the absence of BMP-2 failed to induce adipogenic differentiation in TSCs. However, IGF-1 together with BMP-2 significantly induced adipogenic differentiation in TSCs, as demonstrated by Oil Red O staining (Fig. 4A). Both adipocyte numbers and PPARγ mRNA expression were enhanced in TSCs incubated with increasing concentrations of IGF-1 in the presence of BMP-2 (Fig. 4B and C). Adipogenic differentiation of TSCs in response to IGF-1+BMP-2 also occurred in a time-dependent manner (Fig. 4D, E and F). In order to further confirm the functions of endogenous IGF and BMP2, endogenous IGF-1 or BMP2 were knocked-down by shRNA, respectively. The BMP-2 and IGF-1 protein expression were significantly down-regulated by shRNA even in the presence of PGE2 (Fig. 5A). Moreover, BMP-2 shRNA or IGF-1 shRNA markedly reduced the adipocyte numbers (Fig. 5B and 5C), and also inhibited the PPARγ expression at the mRNA level (Fig. 5D). The results indicated that knock-down of either endogenous IGF-1 or BMP2 abolished PGE2-induced adipogenic differentiation.
Figure 4. IGF-1 and BMP-2 induce adipogenic differentiation of rat TSCs.
TSCs were incubated with 0, 1, 5, 10, or 20-1 and 100 ng/ml BMP-2 for 7 days. (A) Adipogenic differentiation detected by Oil Red O staining; (B) the Oil Red O staining areas in the cells were assessed; (C) PPARγ mRNA levels determined by qRT-PCR. TSCs were incubated with 10 nM IGF-1 plus 100 ng/ml BMP-2 for 0, 3, 7, or 10 days. (D) Adipogenic differentiation detected by Oil Red O staining; (E) the Oil Red O staining areas in the cells were assessed; (F) PPARγ mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH. Results represent the mean±SD. *P<0.05, **P<0.01 with respect to TSCs without PGE2.
Figure 5. Knock down endogenous IGF-1 and BMP2 abolished PGE2-induced adipogenic differentiation.
Rat TSCs were treated with PGE2 (100 ng/ml) with or without BMP-2 shRNA or IGF-1 shRNA. (A) Levels of IGF-1 and BMP2 were determined by western blotting. β-actin was used as loading controls. (B) Adipogenic differentiation detected by Oil Red O staining; (C) The Oil Red O staining areas in the cells were assessed; (D) PPARγ mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH. Results represent the mean ± SD. **P<0.01 with respect to TSCs without PGE2; #P<0.05 with respect to TSCs with PGE2.
IGF-1 and BMP-2 mediate PGE2-induced adipogenic differentiation through activation of CREB and Smad
Given the critical roles of Smad in BMP2 signalling and CREB in IGF-1 signalling, we next examined whether activation of Smad and CREB is invovled the induction of PPARγ2 and adipocytic differentiation of TSCs. As shown in Fig. 6A, CREB and Smad were activated by phosphorylation in the presence of PGE2 or IGF-1+BMP-2. PGE2 or IGF-1, not BMP-2, increased the phosphorylation of CREB, and the effect of IGF-1 was blocked by CREB shRNA. Similarly, the phosphorylation of Smad was activated only by PGE2 or BMP-2, and was blocked by Smad1 shRNA (Fig. 6A). Either CREB shRNA or Smad shRNA markedly inhibited IGF-1+BMP-2-induced adipogenic differentiation, as indicated by Oil Red O Staining (Fig. 6B and 6C). CREB shRNA and Smad shRNA also inhibited expression of PPARγ2 at the mRNA level (Fig. 6D). These results indicated that IGF-1-activated pCREB, together with BMP2-activated pSmad, subsequently up-regulated the expression of PPARγ2, thus enhancing the adipogenic differentiation of TSCs.
Figure 6. CREB and Smad are phosphorylated by IGF-1 and BMP-2, respectively, in PGE2-induced adipogenic differentiation.
Rat TSCs were treated with PGE2 (100 ng/ml), IGF-1 (10 nM), BMP-2 (100 ng/ml), or IGF-1 (10 nM) + BMP-2 (100 ng/ml) with or without CREB shRNA or Smad shRNA. (A) Levels of total and phosphorylated CREB and Smad were determined by western blotting. β-actin was used as loading controls. TSCs were incubated with PGE2 (100 ng/ml), or IGF-1 (10 nM) + BMP-2 (100 ng/ml), with or without CREB shRNA or Smad shRNA. (B) Adipogenic differentiation detected by Oil Red O staining; (C) the Oil Red O staining areas in the cells were assessed; (D) PPARγ2 mRNA levels determined by qRT-PCR. The mRNA levels were normalized using GAPDH. Results represent the mean ± SD. **P<0.01 with respect to TSCs without PGE2; #P<0.05 with respect to TSCs with PGE2.
Discussion
The results of the current study confirmed that PGE2 induced the adipogenic differentiation of TSCs in vitro. Both IGF-1 and BMP-2 were implicated in the adipogenic differentiation of TSCs [24], [25], [26], and we also demonstrated that PGE2 induced IGF-1 gene and protein expression via cAMP/PKA/CEBPδ signalling pathway. However, neither IGF-1 nor BMP-2 alone was sufficient to induce adipogenic differentiation. Adipogenesis was significantly increased by treatment of TSCs with IGF-1 plus BMP-2. PGE2 also increased the phosphorylation of CREB and Smad via IGF-1 and BMP-2, respectively.
The degenerative changes seen in chronic tendinopathies are associated with mechanical stress, and the mechanisms responsible for chronic overuse tendon injuries may differ from those involved in acute tendon damage [29]. Although the role of inflammation in tendinopathies remains controversial, the inflammatory mediator PGE2 was increased in stretched tenocytes or tendons in vitro [15], [16], [17], [18], [19], [20], suggesting that it might be involved in the pathological changes associated with tendon overuse, including osteogenic and adipogenic changes. PGE2 was previously shown to induce BMP-2 [21], which in turn mediated osteogenic differentiation [23] and calcification [22]. The current study confirmed that PGE2 was also able to induce the adipogenic differentiation of TSCs.
BMPs are multifunctional growth factors with strong chondro-osteogenic effects. BMP-2 has been shown to mediate PGE2-induced osteogenic differentiation of human TSCs [23]. However, recent studies have shown that BMP-2 also exert adipogenic effects [30], [31], [32], and the BMP signalling pathway was required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage [24]. It is possible that the involvements of BMP-2 in the osteogenic and adipogenic differentiation of TSCs are mediated by different BMP receptors [33], or may depend on BMP concentration [34], [35] and/or the presence of other intracellular and extracellular factors However, the results of the current study demonstrated that BMP-2 was necessary, but not sufficient, for inducing adipogenic differentiation of TSCs.
IGF-1 is also known to stimulate adipogenesis [25], [26]. IGF-1 attaches to its receptors and up-regulates phosphorylation of CREB via the PI3K/Akt pathway [25]. Activated CREB then increases the expression of PPARγ2, which acts as a crucial factor in adipogenic differentiation by controlling the expression of specific genes for adipocytes, such as phosphoenolpyruvate carboxykinase (PEPCK) and aP2 [25], [36]. This study showed that PGE2 stimulated IGF-1 synthesis via the cAMP/PKA/CEBPδ signalling pathway. However, as with BMP-2, IGF-1 alone was unable to induce PPARγ or adipogenic differentiation, and the presence of BMP-2 was also required. Knock-down of either IGF-1 or BMP2 abolished PGE2-induced adipogenic differentiation, further confirming the necessity of both IGF-1 and BMP2 in PGE2-induced adipogenic differentiation of TSCs.
Using shRNAs, we further demonstrated that CREB and Smad phosphorylation, induced by IGF-1 and BMP-2, respectively, were both required for induction of the adipogenic gene PPARγ2, confirming that the effects of PGE2 are transmited mainly via a CREB- and Smad-dependent mechanism.
In summary, the results of this study suggest that IGF-1 and BMP-2 together mediate PGE-2-induced adipogenic differentiation of TSCs. IGF-1 expression is up-regulated via the cAMP/PKA/CEBPδ pathway. IGF-1 and BMP2 phosphorylate and activate downstream CREB and Smad, respectively, which subsequently upregulate PPARγ2 expression, thus enhancing adipogenic differentiation. These findings provide the basis for further studies aimed at clarifying the pathogenesis of tendinopathies and identifying suitable therapeutic targets.
Funding Statement
This work was supported by the National Nature Science Foundation of China (grant nos. 81230040). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maffulli N, Khan KM, Puddu G (1998) Overuse tendon conditions: time to change a confusing terminology. Arthroscopy 14: 840–843. [DOI] [PubMed] [Google Scholar]
- 2. Rees JD, Wilson AM, Wolman RL (2006) Current concepts in the management of tendon disorders. Rheumatology (Oxford) 45: 508–521. [DOI] [PubMed] [Google Scholar]
- 3. Kannus P, Jozsa L (1991) Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 73: 1507–1525. [PubMed] [Google Scholar]
- 4. Chard MD, Cawston TE, Riley GP, Gresham GA, Hazleman BL (1994) Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis 53: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riley GP, Harrall RL, Constant CR, Cawston TE, Hazleman BL (1996) Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis 55: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenwick S, Harrall R, Hackney R, Bord S, Horner A, et al. (2002) Endochondral ossification in Achilles and patella tendinopathy. Rheumatology (Oxford) 41: 474–476. [DOI] [PubMed] [Google Scholar]
- 7. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, et al. (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Pan T, Liu Y, Wang JH (2010) Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res 28: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Wang JH (2010) Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rui YF, Lui PP, Li G, Fu SC, Lee YW, et al. (2010) Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A 16: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 11. Zhang J, Wang JH (2010) Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res 28: 198–203. [DOI] [PubMed] [Google Scholar]
- 12. de Mos M, Koevoet WJ, Jahr H, Verstegen MM, Heijboer MP, et al. (2007) Intrinsic differentiation potential of adolescent human tendon tissue: an in-vitro cell differentiation study. BMC Musculoskelet Disord 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, et al. (2003) Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res 287: 289–300. [DOI] [PubMed] [Google Scholar]
- 14. Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW (1984) The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol 2: 335–357. [DOI] [PubMed] [Google Scholar]
- 15. Almekinders LC, Banes AJ, Ballenger CA (1993) Effects of repetitive motion on human fibroblasts. Med Sci Sports Exerc 25: 603–607. [PubMed] [Google Scholar]
- 16. Almekinders LC, Baynes AJ, Bracey LW (1995) An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. Am J Sports Med 23: 119–123. [DOI] [PubMed] [Google Scholar]
- 17. Wang JH, Jia F, Yang G, Yang S, Campbell BH, et al. (2003) Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res 44: 128–133. [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Yang G, Khan M, Stone D, Woo SL, et al. (2004) Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med 32: 435–440. [DOI] [PubMed] [Google Scholar]
- 19. Devkota AC, Tsuzaki M, Almekinders LC, Banes AJ, Weinhold PS (2007) Distributing a fixed amount of cyclic loading to tendon explants over longer periods induces greater cellular and mechanical responses. J Orthop Res 25: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 20. Devkota AC, Weinhold PS (2010) Prostaglandin E(2), collagenase, and cell death responses depend on cyclical load magnitude in an explant model of tendinopathy. Connect Tissue Res 51: 306–313. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Chen L, Tao X, Tang K (2013) Phosphoinositide 3-kinase/Akt signaling is essential for prostaglandin E2-induced osteogenic differentiation of rat tendon stem cells. Biochem Biophys Res Commun 435: 514–519. [DOI] [PubMed] [Google Scholar]
- 22. Lui PP, Chan LS, Cheuk YC, Lee YW, Chan KM (2009) Expression of bone morphogenetic protein-2 in the chondrogenic and ossifying sites of calcific tendinopathy and traumatic tendon injury rat models. J Orthop Surg Res 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J, Wang JH (2012) BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res 30: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang H, Song TJ, Li X, Hu L, He Q, et al. (2009) BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 106: 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896. [DOI] [PubMed] [Google Scholar]
- 26. Scott A, Cook JL, Hart DA, Walker DC, Duronio V, et al. (2007) Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum 56: 871–881. [DOI] [PubMed] [Google Scholar]
- 27. Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, et al. (2003) Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 14: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ji C, Chang W (2003) Centrella M, McCarthy TL (2003) Activation domains of CCAAT enhancer binding protein delta: regions required for native activity and prostaglandin E2-dependent transactivation of insulin-like growth factor I gene expression in rat osteoblasts. Mol Endocrinol 17: 1834–1843. [DOI] [PubMed] [Google Scholar]
- 29. Archambault JM, Wiley JP, Bray RC (1995) Exercise loading of tendons and the development of overuse injuries. A review of current literature. Sports Med 20: 77–89. [DOI] [PubMed] [Google Scholar]
- 30. Kang Q, Song WX, Luo Q, Tang N, Luo J, et al. (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schulz TJ, Tseng YH (2009) Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev 20: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rui YF, Lui PP, Wong YM, Tan Q, Chan KM (2013) BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res 31: 746–753. [DOI] [PubMed] [Google Scholar]
- 33. Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, et al. (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gimble JM, Morgan C, Kelly K, Wu X, Dandapani V, et al. (1995) Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem 58: 393–402. [DOI] [PubMed] [Google Scholar]
- 35. Chen TL, Shen WJ, Kraemer FB (2001) Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem 82: 187–199. [DOI] [PubMed] [Google Scholar]
- 36. Tontonoz P, Hu E, Spiegelman BM (1995) Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev 5: 571–576. [DOI] [PubMed] [Google Scholar]