Abstract
The metabolic peptide hormone nesfatin-1 has been linked to the reproductive axis in fishes. The purpose of this study was to determine how energy availability after spawning affects plasma levels of nesfatin-1, the metabolic peptide hormone ghrelin, and sex steroid hormones in rematuring female rainbow trout (Oncorhynchus mykiss). To limit reproductive maturation, a group of female trout was food-restricted after spawning and compared with a control group that was fed a standard broodstock ration. The experiment was conducted twice, once using two-year-old trout (second-time spawners) and once using three-year-old trout (third-time spawners). During monthly sampling, blood was collected from all fish, and a subset of fish from each treatment was sacrificed for pituitaries. Pituitary follicle-stimulating hormone-beta (fsh-β) mRNA expression was analyzed with q-RT-PCR; plasma hormone levels were quantified by radioimmunoassay (17β-estradiol and ghrelin) and enzyme-linked immunosorbent assay (11-keto-testosterone and nesfatin-1). Although plasma nesfatin-1 levels increased significantly in the months immediately after spawning within both feeding treatments, plasma nesfatin-1 did not differ significantly between the two treatments at any point. Similarly, plasma ghrelin levels did not differ significantly between the two treatments at any point. Food restriction arrested ovarian development by 15–20 weeks after spawning, shown by significantly lower plasma E2 levels among restricted-ration fish. Pituitary fsh-β mRNA levels were higher among control-ration fish than restricted-ration fish starting at 20 weeks, but did not differ significantly between treatment groups until 30 weeks after spawning. Within both treatment groups, plasma 11-KT was elevated immediately after spawning and rapidly decreased to and persisted at low levels; starting between 20 and 25 weeks after spawning, plasma 11-KT was higher among control-ration fish than restricted-ration fish. The results from these experiments do not provide support for plasma nesfatin-1 as a signal for the initiation of reproductive development in rematuring female rainbow trout.
Introduction
The reproductive endocrine axis–comprising the brain, pituitary, and gonads (BPG)–has been characterized for some fishes [1], [2], [3] including salmonids [4], and the basic mechanism by which the BPG axis secretes hormones to regulate initial maturation (puberty) in salmonid species are well understood [5]. However, there is a dearth of information concerning endocrine regulation and coordination of gonadal recrudescence (rematuration). While it is reasonable to suspect that puberty and rematuration are regulated similarly, this hypothesis has mostly gone untested. Furthermore, although the role of energetics in puberty is well described in many animals [6], [7], [8] including fishes [9], [10], [11], how metabolic and nutritional status regulate successive reproductive efforts has largely been overlooked.
In mammals, nesfatin-1 is an 82-amino acid hormone cleaved from the nucleobindin-2 (nucb2) gene product that is secreted primarily from the hypothalamus and adipose tissue, and that has both anorexigenic and growth inhibiting effects [12]. In rats, short-term (24-hour) fasting leads to a decrease in circulating levels of nesfatin-1, which return to normal levels upon refeeding [13]. In addition to behavioral and metabolic effects, nesfatin-1 appears to inform the reproductive endocrine axis regarding nutritional status [14]: in pigs, nesfatin-1 injections elevated plasma luteinizing hormone (LH) levels [15]. In goldfish (Carassius auratus), nucb2 mRNA is widely expressed in the brain and peripheral tissues, with the highest expression levels found in the liver and pituitary [16], nesfatin-1 injections reduce feeding [16], and nesfatin-1 suppresses the BPG axis at all three tiers [17]. Taken together, these results suggest that circulating nesfatin-1 may act to integrate metabolic or feeding status with reproductive development.
The metabolic peptide hormone ghrelin is synthesized and secreted from the stomach of vertebrates during an energy deficit [18]. In concert with other circulating endocrine factors, ghrelin coordinates the metabolic response to fasting and has behavioral effects such as stimulating appetite in salmonid fishes [19], [20]. Ghrelin and nesfatin-1 colocalize in stomach and hypothalamus of goldfish [21]. In unfed goldfish, i.c.v. nesfatin-1 injection suppressed preproghrelin and ghrelin receptor mRNA expression in forebrain, suppressed ghrelin and NUCB2 mRNA expression in hypothalamus, and suppressed NUCB2 mRNA expression in forebrain [21]. Along with leptin and other regulatory metabolic hormones, ghrelin acts at multiple levels of the BPG axis to inhibit reproduction, e.g., by suppressing pituitary LH and testicular androgen secretion [22]. Ghrelin acts directly on zebrafish follicles to inhibit both basal and MIH-induced germinal vesicle breakdown [23]. Ghrelin abnormalities have been implicated in a variety of human reproductive disorders [24], [25]. Thus, ghrelin may act as a peripheral metabolic signal that negatively regulates reproductive development under conditions of nutritional deficit among iteroparous fishes such as rainbow trout [7], [26], [27].
Among salmonid fishes, the onset of puberty occurs up to a year in advance of spawning [28], [29]. The current working hypothesis states that puberty in salmonids is ultimately regulated by energy availability [7], [26]. When energy is deficient, some peripheral metabolic hormone inhibits or arrests reproductive development [23], [27]. When energy is sufficient, some peripheral metabolic hormone stimulates or permits the reproductive axis to proceed with reproductive development [17], [30]. Upon receiving the stimulatory or permissive signal from such a metabolic cue, maturation is initiated in the brain by gonadotropin releasing hormones (GnRHs). GnRHs originate primarily in the hypothalamus [31] and act hierarchically to stimulate pituitary release of the gonadotropin hormones (GtHs), follicle stimulating hormone (FSH) and luteinizing hormone (LH) [32]. In female fishes, the GtHs stimulate growth and development of the ovaries [33], and also activate steroidogenic pathways culminating in secretion of both the estrogenic sex steroid 17β-estradiol (E2) and the androgenic sex steroid 11-keto-testosterone (11-KT) [34], [35]. Increasing levels of circulating E2 both initiate and signal ovarian maturation [36], [37]. Recent work suggests a role for 11-KT in early oocyte growth among various species of teleost fishes [38], [39], [40]; in vitro evidence suggests such a role for 11-KT in salmonids [41].
The purpose of this study was to determine how energy availability after spawning affects plasma levels of nesfatin-1, ghrelin, and sex steroid hormones in rematuring female rainbow trout (Oncorhynchus mykiss). We hypothesized that by restricting food availability in a group of female trout, these fish would become energy deficient and thus arrest reproductive development [42] as individuals adopted a non-consecutive year or “skip-spawner” life history that has been described in fishes [43], [44]. We also hypothesized that this energy deficit would be initially reflected by increased plasma ghrelin and decreased plasma nesfatin-1 [43], [44]. We further hypothesized that this skip-spawner life history decision would be detectible first by reduced pituitary levels of fsh-β mRNA, then by reduced circulating levels of 11-KT, and finally by reduced circulating levels of E2.
Materials and Methods
1. Ethics Statement
Experiments were approved by and conducted under approved protocols in accordance with the principles and procedures of the Institutional Animal Care and Use Committee, University of Idaho (Permit Number 2012-130). Fish were anesthetized with tricaine methanesulfonate (Finquel MS-222, Argent Laboratories, Redmond, WA) prior to handling.
2. Animals
Post-spawned female rainbow trout (Oncorhynchus mykiss) were purchased from Troutlodge (Sumner, WA) and transported to the University of Idaho (Moscow, ID). Fish had been manually strip-spawned 2 d prior to transport, and were fasted for one month (in the case of two-year-old trout) or two months (in the case of three-year-old trout) prior to spawning. Fish were held in 1,130 L tanks, in a recirculating system (flow rate 14 L min−1 per tank, temperature 12 to 15°C following a seasonal profile).
3. Experiments
Experiments were conducted as previously described [42]. Briefly, 150 two-year-old or three-year-old fish were started at week zero on a control ration (0.5% fish mass per day), or a restricted ration (0.1% fish mass per day). Fish were fed a commercial trout broodstock diet (6.4 mm pellets, Rangen, Inc., Buhl, ID), and were sampled every 5 weeks (two-year-old fish) or every 4 weeks (three-year-old fish). During sampling, blood was drawn from all fish, and a subsample of fish from each treatment group was lethally sampled for tissue collection (causing n to decrease as weeks progressed; see figure legends for relevant n for each analysis). PIT tags individually identified fish.
4. RNA Extractions & cDNA Synthesis
Pituitary samples were homogenized in 1.0 mL ice cold TRIzol (Invitrogen, Life Technologies, Carlsbad, CA), and RNA was isolated following the TRIzol protocol, using three chloroform:isoamyl alcohol extractions and three 70% ethanol washes. Resuspended nucleic acid fractions were treated with DNase (TURBO DNA-free, Ambion, Life Technologies, Carlsbad, CA). RNA purity was assessed by spectrophotometric absorbance (NanoDrop ND-1000, Thermo Fisher), and RNA concentration was measured using the RiboGreen RNA assay kit (Invitrogen) with a fluorometer. 1 µg total RNA was reversed transcribed with the SuperScript III First-Strand Synthesis Kit (Invitrogen) using random hexamer primers. cDNA was diluted 1∶5 in 1× Tris-EDTA.
5. q-RT-PCR
A quantitative real-time reverse-transcriptase PCR (q-RT-PCR) primer set (Table 1) was designed using ABI Primer Express 3.0 software (Life Technologies, Carlsbad, CA) to amplify a 60 bp fragment of the annotated O. mykiss fsh-β sequence (NM_001124586). Specificity was confirmed by bioinformatic analysis, agarose gel electrophoresis of PCR products, and melting curve analysis of PCR products. To carry out the q-RT-PCR, sample cDNA was amplified in 96-well optical reaction plates (Invitrogen) containing 20 µL PCR reactions made up of 2 µL cDNA, 10 µL Power SYBR Green PCR Master Mix (Life Technologies), 6 µL H2O, and 2 µL of a mix of forward and reverse primers at 2 pM each, in an Applied Biosystems ABI 7900 HT real-time PCR system (Life Technologies) (2 min @ 50°C; 10 min @ 95°C; 40 cycles of 15 sec @ 95°C and 1 min @ 60°C). Copy numbers in samples were quantified using standard curves of PCR amplicons. Positive and negative controls were included on each plate. Three technical replicate PCRs were completed for each sample. The mean of the mRNA copy number for the three replicate PCRs is the value reported. q-RT-PCR results were log2-transformed prior to statistical analysis.
Table 1. Primer sequence data for q-RT-PCRs.
| Gene | Accession Number | Direction | Sequence | Product Size |
| fsh-β | AB050835 | Fwd | AGAGCTGCGATTGCATCAAA | 61 bp |
| Rev | GCCATGCTTATGCGATCACA |
6. 17β-Estradiol Radioimmunoassay (RIA)
6.1. Solvent extraction
Plasma samples were extracted with methyl tert-butyl ether (MTBE) (Fisher Scientific, Hampton, NH) by combining 100 µL plasma with 4.0 mL MTBE and vortexing for 1 min [45]. Samples were incubated at room temperature for 7 min to allow phase separation to occur and then the aqueous phase was frozen. The solvent fraction was decanted, equilibrated 10 min at room temperature and incubated at 55°C until all solvent had volatilized (approximately 2 hour). A second extraction of the remaining aqueous fraction from each plasma sample was performed, using 3.0 mL MTBE, and pooled with the first extract. Dried extracts were resuspended in 250 µL E2 zero calibrator solution from the E2 RIA kit (Coat-A-Count, Siemens, Munich, Germany). Average extraction efficiency was 83%, as determined by RIA values for extracted versus unextracted assay standards included with the RIA kit.
6.2. RIA
Resuspended plasma extracts were analyzed in duplicate for E2 concentration using an antibody-coated tube E2 radioimmunoassay (RIA) kit (Coat-A-Count, Siemens, Munich, Germany), per the manufacturer’s instructions. Sensitivity for the assay is reported to be 8 pg mL−1. Positive and negative controls were included in all assays.
7. 11-keto Testosterone Enzyme-linked Immunosorbent Assay (EIA)
7.1. Solvent extraction
Plasma samples were extracted with anhydrous diethyl ether (JT Baker, Avantor Performance Materials, Inc.; Center Valley, PA, USA) by combining 100 µL plasma with 2.0 mL diethyl ether and vortexing for 1 min [46]. Samples were incubated at room temperature for 7 min to allow phase separation to occur, and then the aqueous phase was frozen. The solvent fraction was decanted. A second extraction of the remaining aqueous fraction from each plasma sample was then performed, using 2.0 mL diethyl ether, and pooled with the first extract. Diethyl ether extracts were dried down in a 49°C water bath under a gentle stream of N2 directed via a nitrogen evaporator manifold (N-EVAP 112; Organomation Associates, Inc; Berlin, MA). Dried extracts were resuspended in 1000 µL EIA buffer from the 11-KT EIA kit (described below). Extraction efficiency ranged from 90–102%, as determined by RIA values for extracted versus unextracted assay standards included with the RIA kit.
7.2. EIA
Resuspended plasma extracts were analyzed in duplicate for 11-KT concentration using an antibody-coated 96-well plate based enzyme-linked immunosorbent assay (EIA) kit (Cayman Chemical Company; Ann Arbor, MI). Sensitivity for the assay is reported to be 1 pg mL−1. Positive and negative controls were included in all assays.
8. Ghrelin RIA
Plasma ghrelin concentrations were measured in duplicate using a ghrelin radioimmunoassay established and validated for use in fish by Riley et al. [47], with the following minor variations. One hundred µL of rat ghrelin standards and plasma samples were incubated with 200 µL anti-rat ghrelin (from H. Hosoda) at a dilution of 1∶750,000. The anti-rat ghrelin (a.a. 1–11) recognizes the octanoylated epitope (biologically active region) of ghrelin [48] and detects only the biologically active forms of ghrelin (ghrelin-C8 and ghrelin-C10). After incubation at 4°C overnight, 100 µL of 125I-human ghrelin (Millipore, St. Charles, MO) was added before an additional overnight incubation at 4°C. Finally, 100 µL anti-rabbit IgG goat serum at 1∶35 (with 10% polyethylene glycol) was added, incubated for 2 hour at room temperature, and then centrifuged at 3200×g for 60 min to separate free and bound tracers. Radioactivity of aspirated pellet was then quantified using a gamma counter (Packard, Palo Alto, CA). Intra- and inter-assay CV’s were 6.2 and 9.8%, respectively. Positive and negative controls were included in all assays.
9. Nesfatin-1 EIA
9.1. Parallelism of trout plasma dilution
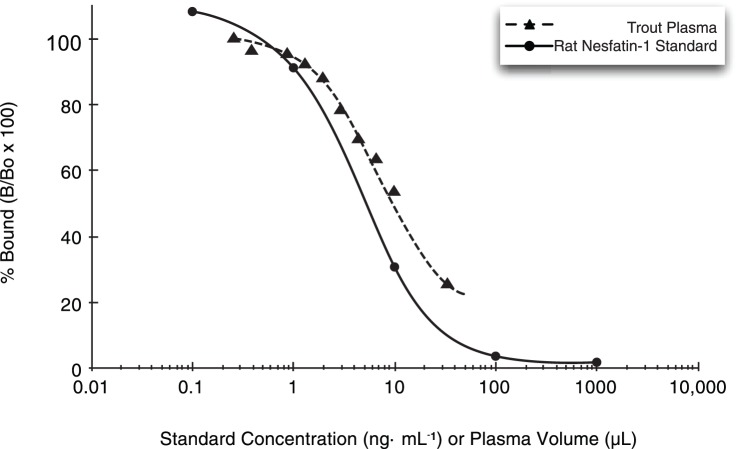
A rat nesfatin-1 EIA (EK 003–22; Phoenix Pharmaceuticals) was used for measuring nesfatin-1 immunoreactivity (nesfatin-1) in rainbow trout plasma. Although this kit has been previously validated for use in fish (goldfish [16]), the following procedure was conducted to establish its use in rainbow trout. A representative sample of female trout plasma was serially diluted 1.5 fold in 1× nesfatin-1 EIA buffer and measured using the nesfatin-1 EIA; displacement of label by standard and by trout plasma were then compared (Fig. 1). A four-parameter logarithmic regression line was fit to the relationship between displacement and plasma volume. Displacement of rat nesfatin-1 by trout plasma was parallel to the standard curve, indicating the presence of an immunologically similar protein in trout plasma. Displacement was linear over the range 1.3 µL– 9.9 µL trout plasma (R2 = 0.99).
Figure 1. Parallel displacement of rat nesfatin-1 by a serial dilution of trout plasma.
Displacement curve showing parallelism of the rat nesfatin-1 standard curve (circle points, solid line) and a dilution series of female rainbow trout plasma displacing rat-nesfatin-1 (triangle points, dashed line) in the nesfatin-1 EIA. Horizontal axis depicts volume of trout plasma (µL) used in the assay or concentration of rat-nesfatin-1 standard (ng⋅mL−1).
9.2. Measuring nesfatin-1 levels in trout plasma
Rainbow trout plasma samples were assayed for nesfatin-1 by diluting them 1∶10 in 1× EIA buffer to bring values within the linear range of the kit, as described above. Samples were run in duplicate, and had intra-sample CV values <10%. Positive control samples were all within the range specified in literature accompanying the kit, and negative controls were included for all assays.
10. Data Analysis
Only data from fish that survived until being terminally sampled were included in statistical analyses. Systematic tank differences were not detected within treatment for any variable (ANOVA, p>0.05). Therefore, tank replicates were pooled and analyzed together. For normality and homoscedasticity requirements of ANOVA and post-hoc tests, plasma hormone data were log-transformed prior to analysis. Two-way ANOVA was used to detect main and interaction effects (time, treatment, time*treatment). When ANOVA indicated a significant time effect, Tukey-Kramer Honestly Significant Difference (Tukey-Kramer HSD) tests were used to compare values at all time points within a given treatment. Within each time point, two-tailed t-tests were used to detect treatment differences. Statistical analyses were performed with JMP (Version 9, SAS Institute Inc., Cary, NC). Differences are reported as significant when p<0.05.
Results
As described previously [42], the feeding regime used here affected metrics of growth, metabolism, and reproduction in both two- and three-year-old rematuring female rainbow trout. However, in both experiments there was no significant difference in plasma nesfatin-1 immunoreactivity between the treatment groups at any time point (Fig. 2). Within each experiment, a two-way ANOVA determined that “week” was a significant effect, but neither “treatment” nor the “treatment*week” interaction were significant effects. Plasma nesfatin-1 immunoreactivity covaried significantly in the two treatment groups (ANCOVA, p<0.0001), suggesting that plasma nesfatin-1 was not affected by treatment. While the feeding regimes used here did not yield differences in trout plasma nesfatin-1 immunoreactivity between treatments, plasma nesfatin-1 immunoreactivity did change over time both within each treatment individually and when the feeding treatments were pooled and considered together. In both two-year-old and three-year-old trout, there was a marked increase over time in plasma nesfatin-1 immunoreactivity, before levels declined toward the end of the experiment. Similarly, no difference in plasma levels of acylated-ghrelin (ghrelin) was detected either among time points within treatment groups for a given age class, or between treatment groups at any time-point for a given age class (Fig. 3).
Figure 2. Plasma nesfatin-1 concentrations in female rainbow trout recovering from spawning.
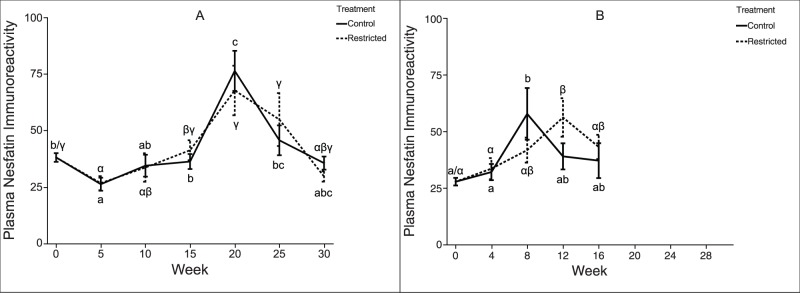
Mean (±SEM) plasma nesfatin-1 concentration over time in female rainbow trout (A: two-year-old; B: three-year-old) fed a control-ration or restricted-ration. When comparing treatment groups, mean values differ significantly (two-tailed t-test, p<0.05) at time-points marked “*” and marginally (two-tailed t-test, p<0.1) at time points marked “‡”. Within each figure, time-points sharing the same letter are not significantly different (Tukey’s HSD, p≥0.05). Latin letters (a, b, c, …) refer to control treatment fish; Greek letters (α, β, γ, …) refer to restricted ration fish. Figure A: Week 0, n = 42 per treatment; Week 5, n = 36 per treatment; Week 10, n = 30 per treatment; Week 15, n = 24 per treatment; Week 20, n = 18 per treatment; Week 25, n = 12 per treatment; Week 30, n = 6 per treatment. Figure B: Week 0, n = 25 per treatment; Week 4, n = 20 per treatment; Week 8, n = 15 per treatment; Week 12, n = 10 per treatment; Week 16, n = 5 per treatment.
Figure 3. Plasma ghrelin concentrations in female rainbow trout recovering from spawning.
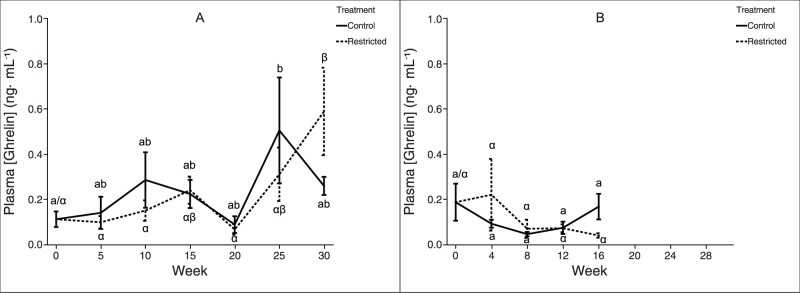
Mean (±SEM) plasma acylated ghrelin levels over time in female rainbow trout (A: two-year-old; B: three-year-old) fed a control-ration or restricted-ration. See Figure 2 for an explanation of what each symbol signifies. Figure A: n = 6 per treatment. Figure B: Week n = 5 per treatment.
The feeding treatments produced a significant difference in reproductive development during this period of initial ovarian recrudescence, as indicated by plasma E2 levels (Fig. 4): among two-year-old trout fed the control-ration, plasma E2 concentration was elevated compared to restricted-ration fish by week ten and continued to increase dramatically over the course of the experiment. Plasma E2 levels showed a similar but non-significant elevation in control versus restricted-ration fish among three-year-old trout starting at week eight. Interestingly, at week zero, three-year-old trout exhibited plasma levels of E2 that were an order of magnitude greater than those observed among two-year-old trout, before decreasing significantly as the experiment progressed.
Figure 4. Plasma estrogen concentrations in female rainbow trout recovering from spawning.
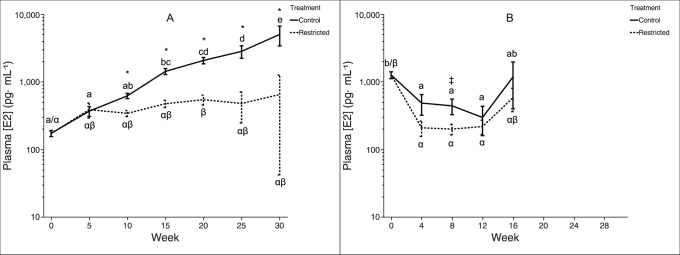
Mean (±SEM) plasma 17β-estradiol concentration over time in female rainbow trout (A: two-year-old; B: three-year-old) fed a control-ration or restricted-ration. See figure 2 for an explanation of what each symbol signifies. Figure A: Week 0, n = 42 per treatment; Week 5, n = 36 per treatment; Week 10, n = 30 per treatment; Week 15, n = 24 per treatment; Week 20, n = 18 per treatment; Week 25, n = 12 per treatment; Week 30, n = 6 per treatment. Figure B: Week 0, n = 25 per treatment; Week 4, n = 20 per treatment; Week 8, n = 15 per treatment; Week 12, n = 10 per treatment; Week 16, n = 5 per treatment.
Among two-year-old trout, pituitary fsh-β mRNA expression decreased from weeks 0–25 within restricted-ration fish, while fsh-β expression increased from weeks 10–30 within control-ration fish (Fig. 5). This trend led to a significant difference between the treatment groups: restricted-ration fish exhibited higher pituitary fsh-β mRNA levels shortly after spawning, at week 10, and control-ration fish exhibited higher pituitary fsh-β mRNA levels later, at weeks 25–30. Among three-year-old trout, pituitary fsh-β mRNA expression did not significantly change over time within either treatment group, and pituitary fsh-β mRNA expression levels did not significantly differ between the two groups at any time point.
Figure 5. Pituitary fsh-β mRNA expression in female rainbow trout recovering from spawning.
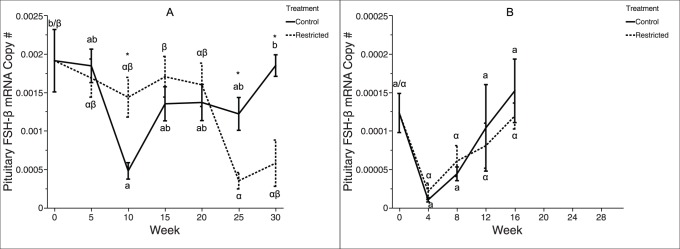
Mean (±SEM) normalized ratio of pituitary fsh-β to β-actin mRNA levels over time in female rainbow trout (A: two-year-old; B: three-year-old) fed a control-ration or restricted-ration. See Figure 2 for an explanation of what each symbol signifies. Figure A: n = 6 per treatment at all time points. Figure B: n = 5 per treatment at all time points.
Plasma concentration of 11-KT (Fig. 6) showed a similar trend within both year classes: plasma 11-KT levels were elevated at week zero before precipitously declining and remaining low through the remainder of the experiment. Among two-year-old trout, plasma 11-KT diverged at week 25, with control-ration fish exhibiting significantly elevated plasma 11-KT levels compared to restricted-ration fish; the experiment with three-year-old trout was presumably not long enough to capture this effect, as no difference was detected.
Figure 6. Plasma androgen concentrations in female rainbow trout recovering from spawning.
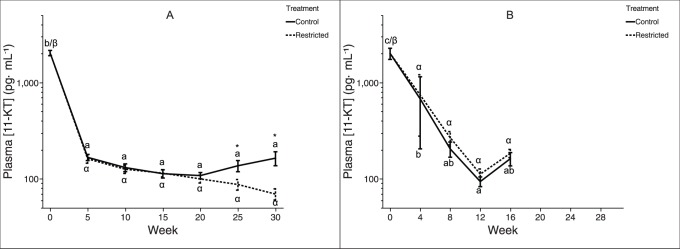
Mean (±SEM) plasma 11-keto testosterone concentration over time in female rainbow trout (A: two-year-old; B: three-year-old) fed a control-ration or restricted-ration. See Figure 2 for an explanation of what each symbol signifies. Figure A: Week 0, n = 42 per treatment; Week 5, n = 36 per treatment; Week 10, n = 30 per treatment; Week 15, n = 24 per treatment; Week 20, n = 18 per treatment; Week 25, n = 12 per treatment; Week 30, n = 6 per treatment. Figure B: Week 0, n = 25 per treatment; Week 4, n = 20 per treatment; Week 8, n = 15 per treatment; Week 12, n = 10 per treatment; Week 16, n = 5 per treatment.
Discussion
In our previous report [42], we showed that the control-ration described in the current study was sufficient to maintain positive growth rates, while the restricted-ration was sufficient to maintain near-zero or negative growth rates. Our current results provide evidence that plasma nesfatin-1 levels are not affected by this long-term feeding restriction in post-spawned female rainbow trout; no difference in plasma nesfatin-1 levels was detected between the control-ration and restricted-ration fish at any time point in two-year-old or three-year-old trout. Additionally, circulating nesfatin-1 levels observed during the months after spawning do not predict rematuration in rainbow trout: restricted-ration fish failed to undergo initial sexual maturation, but nonetheless exhibited plasma nesfatin-1 levels that were similar to control-ration fish. While i.p. injections of nesfatin-1 have been shown to inhibit the reproductive endocrine axis in fish [17], the observed inhibitory effect may be due to relatively high (i.e., pharmacological) dosage, and thus not accurately reflect the native (i.e., physiological) role of circulating nesfatin-1. Alternatively, nesfatin-1 may act differently in fish with group-synchronous ovary development (rainbow trout) compared to fish with asynchronous ovary development (goldfish). Plasma nesfatin-1 levels did exhibit a pattern suggesting nesfatin-1 may be regulated seasonally in rainbow trout, with a peak occurring near the summer solstice (Fig. 2A). Further studies are required to determine the significance of seasonal changes in trout plasma nesfatin-1 levels.
Similarly, within both age classes, plasma ghrelin levels did not differ significantly between treatment groups at any time point. Among two-year-old trout fed the restricted-ration, plasma ghrelin did significantly increase between week 20 and week 30. However, plasma ghrelin levels also exhibited a transient spike among two-year-old trout fed the control-ration around week 25. Among three-year-old trout, the later increase in plasma ghrelin (between week 20 and week 30) was not observed; this is likely the result of the experiment using three-year-old trout not running long enough. Taken together, these findings do not support a role for ghrelin as an indicator of long-term nutritional status in rainbow trout. Although these data do not support our original hypothesis, this contradiction with previous results in other systems may be partially explained by the observation that ghrelin physiology differs substantially between fishes and mammals [49], [50]. For instance, in rainbow trout, ghrelin administration does not increase feed intake, and plasma ghrelin levels are reduced during fasting [20], [51]. Also in rainbow trout, in vitro ghrelin treatment of gastrointestinal tissue does not affect GI contractility [52]. In channel catfish, neither plasma ghrelin nor stomach ghrl mRNA expression changes with feeding status [53]. In two species of sturgeon [54], and in grass carp [55], ghrelin appears to play a principally developmental role. It appears that more work needs to be done to clarify the disparate roles of ghrelin among non-mammalian vertebrates. However, our results do not support regulation of plasma ghrelin levels by either recovery from spawning or nutritional status in post-spawned rainbow trout.
The experimental design used in this study affected nutrition sufficiently to arrest reproductive development among two-year-old trout, as evidenced by a divergence in plasma E2, GSI [42], plasma 11-KT and pituitary fsh-β mRNA expression. This suggests that the nutritional limitation imposed upon the restricted-ration fish sufficed to induce a physiological trade-off between investment in continued survival and growth versus investment in reproductive development. While the experiment using three-year-old trout did not continue long enough to capture this arrest, it is clear from the experiment using two-year-old trout that between 15 and 20 weeks after spawning, the control-ration fish began to increasingly partition energy to the ovary for developing oocytes.
At intake, three-year-old trout exhibited plasma E2 levels that were approximately an order of magnitude greater than those observed in two-year-old trout (Fig. 4), while ovarian masses were similar between the two age classes [42]. This difference in circulating E2 levels may be due to differences in the duration of pre-spawn fasting used by the facility from which trout were obtained (as described in Methods section), or to altered clearance of steroids by the liver [56], [57]. Previous work suggests that animals may experience an aging-associated decrease in the rate of steroid clearance via liver conjugation and hydroxylation reactions [58], [59], or an increasing sequestration of steroids by sex hormone-binding globulin [60], or some diffuse age-related effects associated with a diminished basal metabolic rate similar to that described in humans [61], [62] and other animals [63]. Regardless, this difference between age classes was transient: two-year-old and three-year-old fish exhibited similar plasma E2 levels by the second sampling date.
Our previous study [42] described how this feeding regime induced remodeling of organs and a general redistribution of energy stores, with control-ration fish accumulating lipid in muscle tissue and increasing liver mass. Interestingly, differences in plasma E2 between the two treatment groups were already significant at week ten and presumably were present sometime between weeks five and ten of the experiment. This would indicate that a trajectory of reproductive rematuration for the following year’s spawning effort had been at least partially determined approximately six weeks after spawning (as fish were obtained approximately one week after spawning), similar to the current understanding of the progression of puberty in salmonid fishes [29]. This implies that the energy deficit incurred during spawning and feeding conditions during the period immediately after spawning interact to determine whether rematuration is initiated within an approximately six week window. Restricted ration fish appear to have arrested rematuration before significant energy was invested in ovarian development.
Classic reproductive endocrine axis theory [2], [4] predicts that an increase in secretion of pituitary (i.e., FSH) factors should precede an increase in plasma E2. However, among control ration fish, we detected no elevation in pituitary fsh-β mRNA (Fig. 5) expression prior to elevation of plasma E2 levels (Fig. 4). In vitro work suggests that FSH is regulated at the level of both transcription and secretion by the interaction of GnRH hierarchical stimulation and E2 feedback [64], and thus, pituitary expression of fsh-β may not be directly correlated with circulating levels of FSH peptide [65]. In addition, our ability to detect treatment differences in pituitary fsh-β mRNA was less than for plasma E2 due to the number of lethal and non-lethal samples at each time point (e.g., for two-year-old trout at week 10, n = 60 for plasma E2, while n = 12 for pituitary fsh-β mRNA). Interestingly, within two-year-old trout, pituitary fsh-β mRNA expression was significantly higher among restricted-ration fish than control-ration fish between weeks 10 and 15; plasma E2 levels show the opposite trend and were elevated among control-ration fish during this time. This combination of observations is explained by the well-documented inhibitory tone of sex steroids on pituitary gonadotropin synthesis [66], [67], [68]. Among control-ration fish, increasing levels of plasma E2 exert negative feedback inhibition of fsh-β expression, as has been described in rainbow trout [69] and other fishes [70], [71]. Conversely, the lower plasma E2 levels observed among restricted-ration fish around this time releases pituitary fsh-β mRNA transcription from inhibition, causing an apparent increase in pituitary fsh-β mRNA levels among restricted-ration fish.
In a recent in vitro study in coho salmon, 11-KT stimulated growth of late perinucleolar stage follicles, suggesting a role for this androgen in early ovarian development [41]. However, it is not clear whether circulating or local levels of 11-KT are physiologically relevant in this scenario. In the present study, plasma 11-KT levels dropped rapidly between zero and five weeks after spawning and did not differ between treatment groups until week 25, which was 15 weeks after plasma E2 diverged. Therefore, our results do not support the hypothesis that plasma 11-KT stimulates ovarian rematuration during the period immediately following spawning. However, it cannot be ruled out that local 11-KT (i.e., 11-KT produced in the ovary) may be involved in stimulating early ovarian development immediately after spawning, in a paracrine fashion. Between 20 and 25 weeks after spawning, treatment groups within the two-year-old trout diverged, with control ration fish exhibiting 2.4-fold elevated plasma levels of 11-KT versus restricted-ration fish by 30 weeks. This elevation in plasma 11-KT coincided with a significant elevation in GSI among control ration fish [42], suggesting that plasma 11-KT among maturing female fish may be of ovarian origin, as has previously been proposed [72]. Both age classes of trout started the experiment at the first sampling point immediately after spawning with elevated plasma 11-KT (Fig. 6), supporting the proposed role for 11-KT in female salmonid spawning physiology and behavior [37], [73], [74].
In conclusion, by restricting food availability in a group of female rainbow trout, growth and energy partitioning were both affected, with restricted-ration fish generally existing in a catabolic state and control-ration fish existing in an anabolic state for the duration of the experiment. Furthermore, the treatments were sufficient to induce differences in gonadal recrudescence between the two treatment groups: while restricted-ration fish arrested ovarian growth, control ration fish accumulated ovarian tissue until the end of the experiment [42]. Differences in ovary size were preceded by differences in circulating levels of E2, which diverged between one and two months after the start of the experiment. Although pituitary secretion of FSH presumably drives the increase in ovarian steroidogenesis underlying elevated plasma E2, our results suggests that this purported increase in FSH is not regulated at the level of transcription during the months immediately after spawning. In addition, our study provides no evidence to support the notion that circulating 11-KT is involved in early maturation of recrudescing female rainbow trout. Finally, food-restriction and the subsequent difference in reproductive trajectory did not measurably affect plasma levels of nesfatin-1 or ghrelin, suggesting that circulating levels of neither peptide link metabolic status to reproduction in O. mykiss. Future work should focus on elucidating the different roles of endocrine and paracrine nesfatin-1, as well as clarifying differences in chronic and acute nesfatin-1 responses to nutrient availability.
Acknowledgments
The authors wish to acknowledge Dr. Tim Cavileer for assistance with primer design, Scott Williams for coordinating fish husbandry and housing, and David de Avila for assistance with steroid RIA.
Funding Statement
This research was funded by the Bonneville Power Administration (www.bpa.gov) (CRITFC (www.critfc.org) Project 2007-401-00, Steelhead Kelt Reconditioning and Reproductive Success Evaluation Research). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Carrillo M, Zanuy S, Felip A, Bayarri MJ, Molès G, et al. (2009) Hormonal and environmental control of puberty in perciform fish. Annals of the New York Academy of Sciences 1163: 49–59. [DOI] [PubMed] [Google Scholar]
- 2. Weltzien F-A, Andersson E, Andersen Ø, Shalchian-Tabrizi K, Norberg B (2004) The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 137: 447–477. [DOI] [PubMed] [Google Scholar]
- 3. Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O (2010) Neuroendocrinology of reproduction in teleost fish. General and Comparative Endocrinology 165: 438–455. [DOI] [PubMed] [Google Scholar]
- 4. Davies B, Bromage N, Swanson P (1999) The brain-pituitary-gonadal axis of female rainbow trout Oncorhynchus mykiss: effects of photoperiod manipulation. General and Comparative Endocrinology 115: 155–166. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto Y, Luckenbach JA, Goetz FW, Young G, Swanson P (2011) Disruption of the salmon reproductive endocrine axis through prolonged nutritional stress: Changes in circulating hormone levels and transcripts for ovarian genes involved in steroidogenesis and apoptosis. General and Comparative Endocrinology 172: 331–343. [DOI] [PubMed] [Google Scholar]
- 6. Castellano JM, Bentsen AH, Sánchez-Garrido MA, Ruiz-Pino F, Romero M, et al. (2011) Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology 152: 3396–3408. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, et al. (2006) Novel signals for the integration of energy balance and reproduction. Molecular and Cellular Endocrinology 254–255: 127–132. [DOI] [PubMed] [Google Scholar]
- 8. Mueller CA, Augustine S, Kooijman SALM, Kearney MR, Seymour RS (2012) The trade-off between maturation and growth during accelerated development in frogs. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 163: 95–102. [DOI] [PubMed] [Google Scholar]
- 9. Shearer K, Parkins P, Gadberry B, Beckman B, Swanson P (2006) Effects of growth rate/body size and a low lipid diet on the incidence of early sexual maturation in juvenile male spring Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 252: 545–556. [Google Scholar]
- 10. Augustine S, Gagnaire B, Floriani M, Adam-Guillermin C, Kooijman SALM (2011) Developmental energetics of zebrafish, Danio rerio . Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 159: 275–283. [DOI] [PubMed] [Google Scholar]
- 11. Baker DM, Davies B, Dickhoff WW, Swanson P (2000) Insulin-like growth factor I increases follicle-stimulating hormone (FSH) content and gonadotropin-releasing hormone-stimulated FSH release from coho salmon pituitary cells in vitro . Biology of Reproduction 63: 865–871. [DOI] [PubMed] [Google Scholar]
- 12. Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, et al. (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712. [DOI] [PubMed] [Google Scholar]
- 13. Goebel M, Stengel A, Tache Y, Sachs G, Lambrecht NW (2009) T1806: Fasting decreases nesfatin-1/NUCB2 serum levels in rats. Gastroenterology 136: A–584. [Google Scholar]
- 14. Tena-Sempere M (2012) Deciphering puberty: novel partners, novel mechanisms. European Journal of Endocrinology 167: 733–747. [DOI] [PubMed] [Google Scholar]
- 15. Lents CA, Barb CR, Hausman GJ, Nonneman D, Heidorn NL, et al. (2013) Effects of nesfatin-1 on food intake and LH secretion in prepubertal gilts and genomic association of the porcine NUCB2 gene with growth traits. Domestic Animal Endocrinology 45: 89–97. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez R, Kerbel B, Chun A, Unniappan S (2010) Molecular, cellular and physiological evidences for the anorexigenic actions of nesfatin-1 in goldfish. PLoS ONE 5: e15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez R, Shepperd E, Thiruppugazh V, Lohan S, Grey CL, et al. (2012) Nesfatin-1 regulates the hypothalamo-pituitary-ovarian axis of fish. Biology of Reproduction 87: 84. [DOI] [PubMed] [Google Scholar]
- 18. Picha M, Strom C, Riley L, Walker A, Won E, et al. (2009) Plasma ghrelin and growth hormone regulation in response to metabolic state in hybrid striped bass: Effects of feeding, ghrelin and insulin-like growth factor-I on in vivo and in vitro GH secretion. General and Comparative Endocrinology 161: 365–372. [DOI] [PubMed] [Google Scholar]
- 19. Hevrøy EM, Waagbø R, Torstensen BE, Takle H, Stubhaug I, et al. (2012) Ghrelin is involved in voluntary anorexia in Atlantic salmon raised at elevated sea temperatures. General and Comparative Endocrinology 175: 118–134. [DOI] [PubMed] [Google Scholar]
- 20. Jönsson E, Kaiya H, Bjornsson BT (2010) Ghrelin decreases food intake in juvenile rainbow trout (Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system. General and Comparative Endocrinology 166: 39–46. [DOI] [PubMed] [Google Scholar]
- 21. Kerbel B, Unniappan S (2012) Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. Journal of Neuroendocrinology 24: 366–377. [DOI] [PubMed] [Google Scholar]
- 22. Barreiro ML, Tena-Sempere M (2004) Ghrelin and reproduction: a novel signal linking energy status and fertility? Molecular and Cellular Endocrinology 226: 1–9. [DOI] [PubMed] [Google Scholar]
- 23. Shepperd E, Peng C, Unniappan S (2012) Ghrelinergic system in fish ovaries and ghrelin inhibition of germinal vesicle breakdown in zebrafish oocytes. General and Comparative Endocrinology 176: 426–431. [DOI] [PubMed] [Google Scholar]
- 24. Repaci A, Gambineri A, Pagotto U, Pasquali R (2011) Ghrelin and reproductive disorders. Molecular and Cellular Endocrinology 340: 70–79. [DOI] [PubMed] [Google Scholar]
- 25. Tena-Sempere M (2005) Exploring the role of ghrelin as novel regulator of gonadal function. Growth Hormone & IGF Research 15: 83–88. [DOI] [PubMed] [Google Scholar]
- 26. Roa J, García-Galiano D, Castellano JM, Gaytan F, Pinilla L, et al. (2010) Metabolic control of puberty onset: new players, new mechanisms. Molecular and Cellular Endocrinology 324: 87–94. [DOI] [PubMed] [Google Scholar]
- 27. Unniappan S (2010) Ghrelin: an emerging player in the regulation of reproduction in non-mammalian vertebrates. General and Comparative Endocrinology 167: 340–343. [DOI] [PubMed] [Google Scholar]
- 28. Campbell B, Dickey J, Beckman B, Young G, Pierce A, et al. (2003) Endocrine changes associated with the growth of pre-vitellogenic oocytes in coho salmon, Oncorhynchus kisutch . Fish Physiology and Biochemistry 28: 287–289. [Google Scholar]
- 29. Shearer KD, Swanson P (2000) The effect of whole body lipid on early sexual maturation of 1+ age male chinook salmon (Oncorhynchus tshawytscha). Aquaculture 190: 343–367. [Google Scholar]
- 30. Gonzalez R, Mohan H, Unniappan S (2012) Nucleobindins: Bioactive precursor proteins encoding putative endocrine factors? General and Comparative Endocrinology 176: 341–346. [DOI] [PubMed] [Google Scholar]
- 31. Ebling FJ, Cronin AS (2000) The neurobiology of reproductive development. Neuroreport 11: R23–33. [DOI] [PubMed] [Google Scholar]
- 32. Amano M, Urano A, Aida K (1997) Distribution and function of gonadotropin-releasing hormone (GnRH) in the teleost brain. Zoological Science 14: 1–11. [DOI] [PubMed] [Google Scholar]
- 33.Kah O (2009) Chapter 2: Endocrine targets of the hypothalamus and pituitary. In: Bernier NJ, Kraak GVD, Farrell AP, Brauner CJ, editors. Fish Physiology: Academic Press 75–112.
- 34. Nocillado JN, Elizur A (2008) Neuroendocrine regulation of puberty in fish: insights from the grey mullet (Mugil cephalus) model. Molecular Reproduction and Development 75: 355–361. [DOI] [PubMed] [Google Scholar]
- 35. Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, et al. (2010) Control of puberty in farmed fish. General and Comparative Endocrinology 165: 483–515. [DOI] [PubMed] [Google Scholar]
- 36. Chadwick EMP, Claytor RR, Léger CE, Saunders RL (1987) Inverse correlation between ovarian development of Atlantic salmon (Salmo salar) smolts and sea age. Canadian Journal of Fisheries and Aquatic Sciences 44: 1320–1325. [Google Scholar]
- 37. King HR, Pankhurst NW (2003) Ovarian growth and plasma sex steroid and vitellogenin profiles during vitellogenesis in Tasmanian female Atlantic salmon (Salmo salar). Aquaculture 219: 797–813. [Google Scholar]
- 38. Kortner TM, Rocha E, Arukwe A (2009) Previtellogenic oocyte growth and transcriptional changes of steroidogenic enzyme genes in immature female Atlantic cod (Gadus morhua L.) after exposure to the androgens 11-ketotestosterone and testosterone. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 152: 304–313. [DOI] [PubMed] [Google Scholar]
- 39. Lokman PM, Harris B, Kusakabe M, Kime DE, Schulz RW, et al. (2002) 11-Oxygenated androgens in female teleosts: prevalence, abundance, and life history implications. General and Comparative Endocrinology 129: 1–12. [DOI] [PubMed] [Google Scholar]
- 40. Setiawan AN, Ozaki Y, Shoae A, Kazeto Y, Lokman PM (2012) Androgen-specific regulation of FSH signalling in the previtellogenic ovary and pituitary of the New Zealand shortfinned eel, Anguilla australis . General and Comparative Endocrinology 176: 132–143. [DOI] [PubMed] [Google Scholar]
- 41. Forsgren KL, Young G (2012) Stage-specific effects of androgens and estradiol-17beta on the development of late primary and early secondary ovarian follicles of coho salmon (Oncorhynchus kisutch) in vitro. Biology of Reproduction 87: 64. [DOI] [PubMed] [Google Scholar]
- 42. Caldwell LK, Pierce AL, Nagler JJ (2013) Metabolic endocrine factors involved in spawning recovery and rematuration of iteroparous female rainbow trout (Oncorhynchus mykiss). General and Comparative Endocrinology 194: 124–132. [DOI] [PubMed] [Google Scholar]
- 43. Kennedy J, Witthames PR, Nash RDM, Fox CJ (2008) Is fecundity in plaice (Pleuronectes platessa L.) down-regulated in response to reduced food intake during autumn? Journal of Fish Biology 72: 78–92. [Google Scholar]
- 44. Rideout RM, Rose GA, Burton MPM (2005) Skipped spawning in female iteroparous fishes. Fish and Fisheries 6: 50–72. [Google Scholar]
- 45. Skillman AD, Nagler JJ, Hook SE, Small JA, Schultz IR (2006) Dynamics of 17α-Ethynylestradiol exposure in rainbow trout (Oncorhynchus mykiss): Absorption, tissue distribution, and hepatic gene expression pattern. Environmental Toxicology and Chemistry 25: 2997–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMaster ME, Munkittrick KR, van der Kraak GJ (1992) Protocol for measuring circulating levels of gonadal steroids in fish. Fisheries and Oceans Canada. 29 p.
- 47. Riley LG, Fox BK, Breves JP, Kaiya H, Dorough CP, et al. (2008) Absence of effects of short-term fasting on plasma ghrelin and brain expression of ghrelin receptors in the tilapia, Oreochromis mossambicus . Zoological Science 25: 821–827. [DOI] [PubMed] [Google Scholar]
- 48. Hosoda H, Kojima M, Matsuo H, Kangawa K (2000) Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochemical and Biophysical Research Communications 279: 909–913. [DOI] [PubMed] [Google Scholar]
- 49.Jönsson E (2013) The role of ghrelin in energy balance regulation in fish. General and Comparative Endocrinology IN PRESS. [DOI] [PubMed]
- 50.Riley L (2013) Different forms of ghrelin exhibit distinct biological roles in tilapia. Frontiers in Endocrinology 4. [DOI] [PMC free article] [PubMed]
- 51. Jönsson E, Forsman A, Einarsdottir IE, Kaiya H, Ruohonen K, et al. (2007) Plasma ghrelin levels in rainbow trout in response to fasting, feeding and food composition, and effects of ghrelin on voluntary food intake. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 147: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 52. Kitazawa T, Itoh K, Yaosaka N, Maruyama K, Matsuda K, et al. (2012) Ghrelin does not affect gastrointestinal contractility in rainbow trout and goldfish in vitro . General and Comparative Endocrinology 178: 539–545. [DOI] [PubMed] [Google Scholar]
- 53. Peterson BC, Waldbieser GC, Riley Jr LG, Upton KR, Kobayashi Y, et al. (2012) Pre- and postprandial changes in orexigenic and anorexigenic factors in channel catfish (Ictalurus punctatus). General and Comparative Endocrinology 176: 231–239. [DOI] [PubMed] [Google Scholar]
- 54. Miandare HK, Farahmand H, Akbarzadeh A, Ramezanpour S, Kaiya H, et al. (2013) Developmental transcription of genes putatively associated with growth in two sturgeon species of different growth rate. General and Comparative Endocrinology 182: 41–47. [DOI] [PubMed] [Google Scholar]
- 55.Feng KE, Zhang G-R, Wei K-J, Xiong B-X (2013) Molecular cloning, tissue distribution, and ontogenetic expression of ghrelin and regulation of expression by fasting and refeeding in the grass carp (Ctenopharyngodon idellus). Journal of Experimental Zoology, Part A: Ecological Genetics and Physiology: n/a-n/a. [DOI] [PubMed]
- 56. Baroiller JF, Fostier A, Zohar Y, Marcuzzi O (1987) The metabolic clearance rate of estradiol-17β in rainbow trout, Salmo gairdneri R., estimated by both single injection and constant infusion methods: Increase during oocyte maturation. General and Comparative Endocrinology 66: 85–94. [DOI] [PubMed] [Google Scholar]
- 57. James MO (2011) Steroid catabolism in marine and freshwater fish. The Journal of Steroid Biochemistry and Molecular Biology 127: 167–175. [DOI] [PubMed] [Google Scholar]
- 58. Bobe J, Guiguen Y, Fostier A (2010) Diversity and biological significance of sex hormone-binding globulin in fish, an evolutionary perspective. Molecular and Cellular Endocrinology 316: 66–78. [DOI] [PubMed] [Google Scholar]
- 59. You L (2004) Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chemico-Biological Interactions 147: 233–246. [DOI] [PubMed] [Google Scholar]
- 60. Hammond GL (2011) Diverse roles for sex hormone-binding globulin in reproduction. Biology of Reproduction 85: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fukagawa NK, Bandini LG, Young JB (1990) Effect of age on body composition and resting metabolic rate. The American Journal of Physiology 259: E233–238. [DOI] [PubMed] [Google Scholar]
- 62. Shimokata H, Kuzuya F (1993) Aging, basal metabolic rate, and nutrition. Nippon Ronen Igakkai Zasshi Japanese Journal of Geriatrics 30: 572–576. [DOI] [PubMed] [Google Scholar]
- 63. Broggi J, Hohtola E, Koivula K, Orell M, Thomson RL, et al. (2007) Sources of variation in winter basal metabolic rate in the great tit. Functional Ecology 21: 528–533. [Google Scholar]
- 64. Ando H, Swanson P, Kitani T, Koide N, Okada H, et al. (2004) Synergistic effects of salmon gonadotropin-releasing hormone and estradiol-17β on gonadotropin subunit gene expression and release in masu salmon pituitary cells in vitro . General and Comparative Endocrinology 137: 109–121. [DOI] [PubMed] [Google Scholar]
- 65. Hook SE, Nagler JJ, Cavileer T, Verducci J, Liu Y, et al. (2011) Relationships between the transcriptome and physiological indicators of reproduction in female rainbow trout over an annual cycle. Environmental Toxicology and Chemistry 30: 309–318. [DOI] [PubMed] [Google Scholar]
- 66.Yaron Z, Levavi-Sivan B (2011) Hormonal Control of Reproduction and Growth: Endocrine Regulation of Fish Reproduction. In: Farrell AP, editor. Encyclopedia of Fish Physiology - From Genome to Environment. San Diego: Academic Press. 1500–1508.
- 67.Yaron Z, Gur G, Melamed P, Rosenfeld H, Elizur A, et al.. (2003) Regulation of fish gonadotropins. In: Kwang WJ, editor. International Review of Cytology: Academic Press. 131–185. [DOI] [PubMed]
- 68. Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. General and Comparative Endocrinology 165: 412–437. [DOI] [PubMed] [Google Scholar]
- 69. Vacher C, Ferrière F, Marmignon M-H, Pellegrini E, Saligaut C (2002) Dopamine D2 receptors and secretion of FSH and LH: role of sexual steroids on the pituitary of the female rainbow trout. General and Comparative Endocrinology 127: 198–206. [DOI] [PubMed] [Google Scholar]
- 70. Mateos J, Mañanos E, Carrillo M, Zanuy S (2002) Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology 132: 75–86. [DOI] [PubMed] [Google Scholar]
- 71. Banerjee A, Khan IA (2008) Molecular cloning of FSH and LH β subunits and their regulation by estrogen in Atlantic croaker. General and Comparative Endocrinology 155: 827–837. [DOI] [PubMed] [Google Scholar]
- 72. Matsubara T, Nagae M, Ohkubo N, Andoh T, Sawaguchi S, et al. (2003) Multiple vitellogenins and their unique roles in marine teleosts. Fish Physiology and Biochemistry 28: 295–299. [Google Scholar]
- 73. Frantzen M, Damsgård B, Tveiten H, Moriyama S, Iwata M, et al. (2004) Effects of fasting on temporal changes in plasma concentrations of sex steroids, growth hormone and insulin-like growth factor I, and reproductive investment in Arctic charr. Journal of Fish Biology 65: 1526–1542. [Google Scholar]
- 74. Munakata A, Amano M, Ikuta K, Kitamura S, Aida K (2012) Involvement of sex steroids and thyroid hormones in upstream and downstream behaviors in masu salmon, Oncorhynchus masou . Aquaculture 362–363: 158–166. [Google Scholar]