Abstract
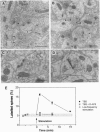
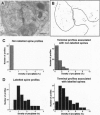
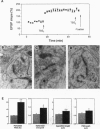
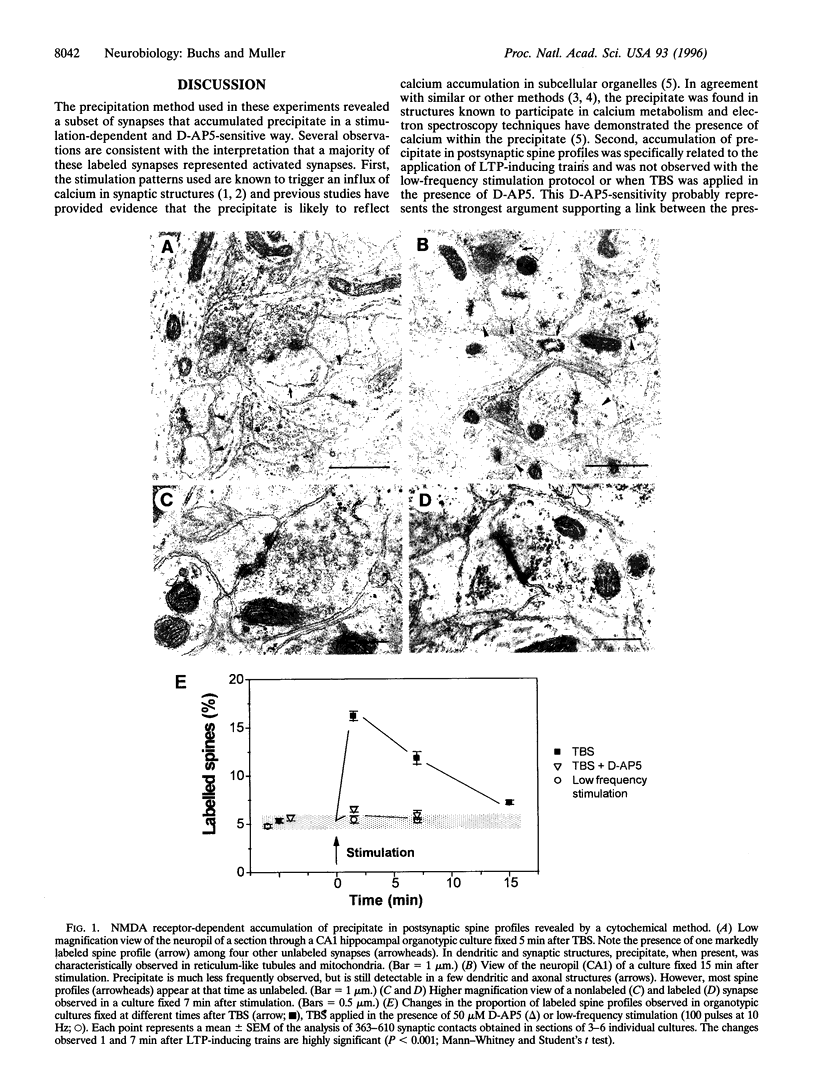
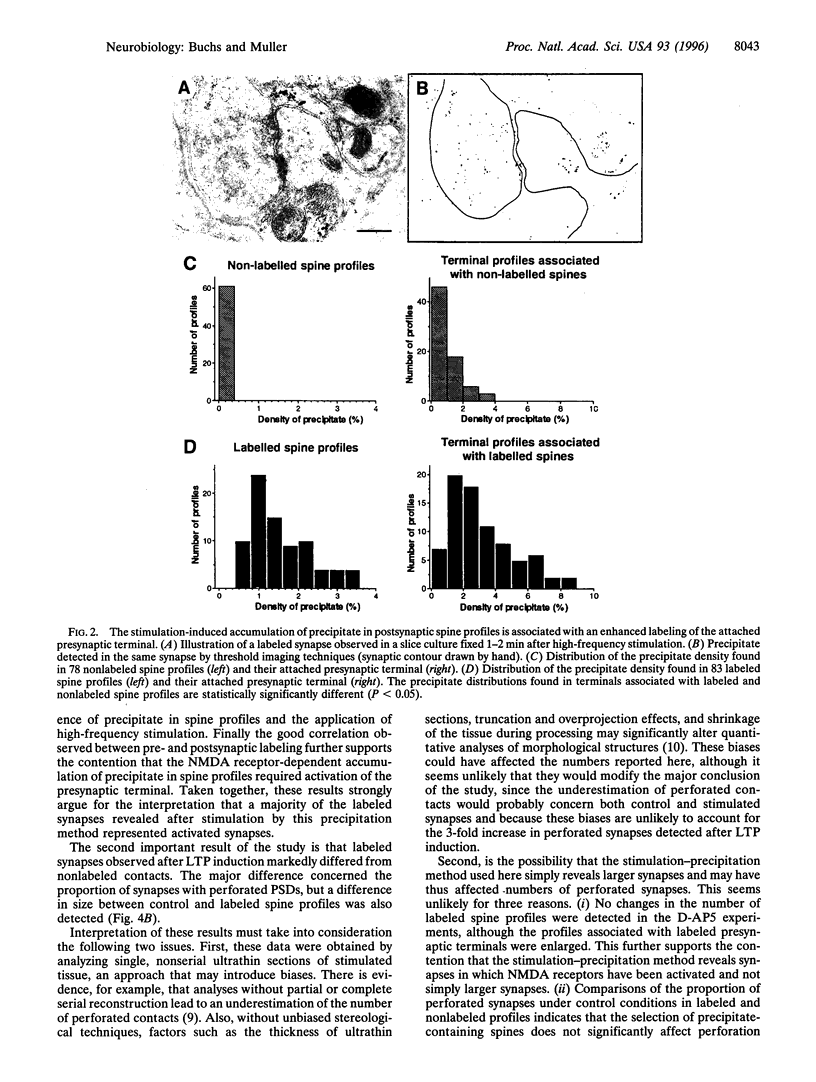
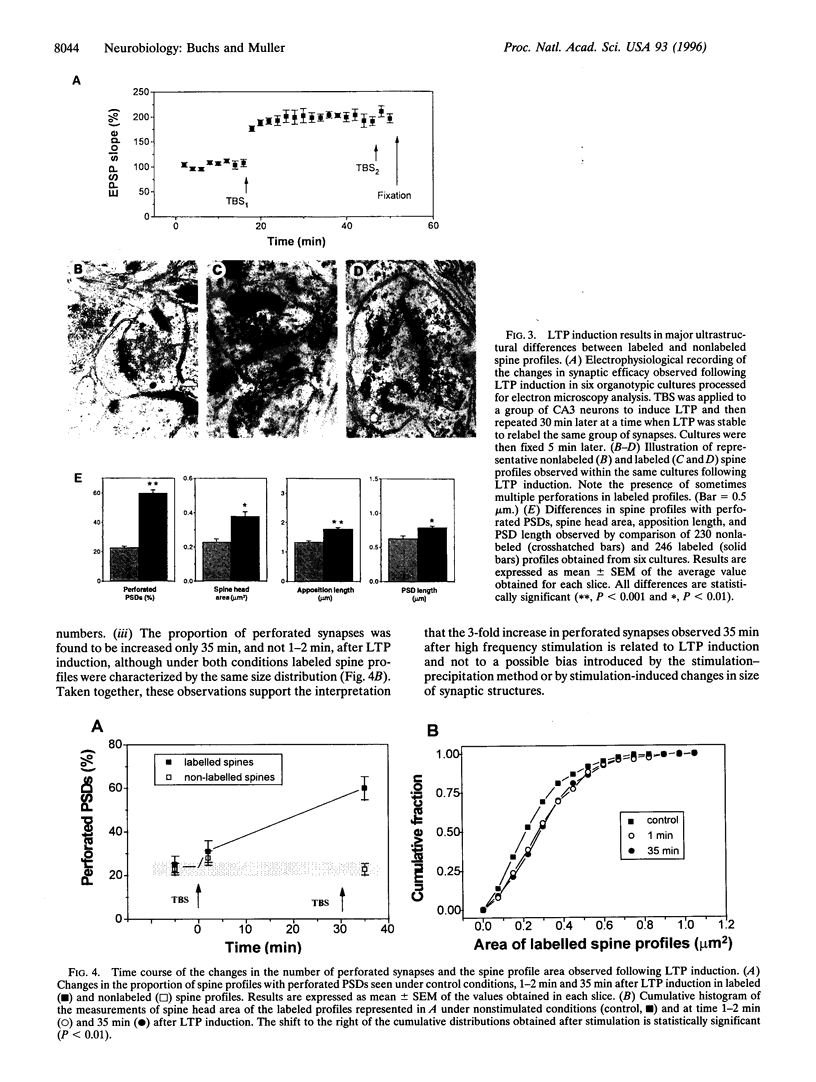
Long-term potentiation (LTP), an increase in synaptic efficacy believed to underlie learning and memory mechanisms, has been proposed to involve structural modifications of synapses. Precise identification of the morphological changes associated with LTP has however been hindered by the difficulty in distinguishing potentiated or activated from nonstimulated synapses. Here we used a cytochemical method that allowed detection in CA1 hippocampus at the electron microscopy level of a stimulation-specific, D-AP5-sensitive accumulation of calcium in postsynaptic spines and presynaptic terminals following application of high-frequency trains. Morphometric analyses carried out 30-40 min after LTP induction revealed dramatic ultrastructural differences between labeled and nonlabeled synapses. The majority of labeled synapses (60%) exhibited perforated postsynaptic densities, whereas this proportion was only 20% in nonlabeled synaptic contacts. Labeled synaptic profiles were also characterized by a larger apposition zone between pre- and postsynaptic structures, longer postsynaptic densities, and enlarged spine profiles. These results add strong support to the idea that ultrastructural modifications and specifically an increase in perforated synapses are associated with LTP induction in field CA1 of hippocampus and they suggest that a majority of activated contacts may exhibit such changes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. Synaptic integration in hippocampal CA1 pyramids. Prog Brain Res. 1990;83:215–222. doi: 10.1016/s0079-6123(08)61251-0. [DOI] [PubMed] [Google Scholar]
- Andrews S. B., Leapman R. D., Landis D. M., Reese T. S. Distribution of calcium and potassium in presynaptic nerve terminals from cerebellar cortex. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1713–1717. doi: 10.1073/pnas.84.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Buchs P. A., Stoppini L., Párducz A., Siklós L., Muller D. A new cytochemical method for the ultrastructural localization of calcium in the central nervous system. J Neurosci Methods. 1994 Sep;54(1):83–93. doi: 10.1016/0165-0270(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Fifková E., Markham J. A., Delay R. J. Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res. 1983 Apr 25;266(1):163–168. doi: 10.1016/0006-8993(83)91322-7. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., deToledo-Morrell L., Morrell F., Heller R. E., Rossi M., Parshall R. F. Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993 Oct;3(4):435–445. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., deToledo-Morrell L., Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991 Dec 6;566(1-2):77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Glanzman D. L., Kandel E. R., Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990 Aug 17;249(4970):799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., West R. W., DeVoogd T. J. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978 Dec 8;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Harris K. M., Jensen F. E., Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992 Jul;12(7):2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Kater S. B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Larson J., Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986 May 23;232(4753):985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Lüthl A., Laurent J. P., Figurov A., Muller D., Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994 Dec 22;372(6508):777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Mayford M., Barzilai A., Keller F., Schacher S., Kandel E. R. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992 May 1;256(5057):638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- Schuster T., Krug M., Wenzel J. Spinules in axospinous synapses of the rat dentate gyrus: changes in density following long-term potentiation. Brain Res. 1990 Jul 16;523(1):171–174. doi: 10.1016/0006-8993(90)91654-y. [DOI] [PubMed] [Google Scholar]
- Sorra K. E., Harris K. M. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci. 1993 Sep;13(9):3736–3748. doi: 10.1523/JNEUROSCI.13-09-03736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L., Buchs P. A., Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991 Apr;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]