Abstract
Bees are a key component of biodiversity as they ensure a crucial ecosystem service: pollination. This ecosystem service is nowadays threatened, because bees suffer from agricultural intensification. Yet, bees rarely benefit from the measures established to promote biodiversity in farmland, such as agri-environment schemes (AES). We experimentally tested if the spatio-temporal modification of mowing regimes within extensively managed hay meadows, a widespread AES, can promote bees. We applied a randomized block design, replicated 12 times across the Swiss lowlands, that consisted of three different mowing treatments: 1) first cut not before 15 June (conventional regime for meadows within Swiss AES); 2) first cut not before 15 June, as treatment 1 but with 15% of area left uncut serving as a refuge; 3) first cut not before 15 July. Bees were collected with pan traps, twice during the vegetation season (before and after mowing). Wild bee abundance and species richness significantly increased in meadows where uncut refuges were left, in comparison to meadows without refuges: there was both an immediate (within year) and cumulative (from one year to the following) positive effect of the uncut refuge treatment. An immediate positive effect of delayed mowing was also evidenced in both wild bees and honey bees. Conventional AES could easily accommodate such a simple management prescription that promotes farmland biodiversity and is likely to enhance pollination services.
Introduction
Animal pollination is an essential ecosystem service, without which more than 80% of flowering plants could not properly set seeds [1] and many important food products would become difficult to grow [2]. Despite its key role, pollination is nowadays threatened by numerous factors [3]. For example, managed honey bees Apis mellifera are impacted by a global colony loss, which has recently decimated up to 53% of European colonies [3], [4]. Alternative pollinators that are not directly managed by humans, like wild bees, are also threatened by habitat loss, landscape fragmentation, use of agro-chemicals, and general degradation of ecological resources [5], [6]. This phenomenon is referred to as the “pollination crisis” [7] (but see [8]). The pollination role of wild bees in food production has long been debated, but recent studies indicate it might be much more important than previously thought [9], [10]. The situation for pollinators is likely to worsen in the future due to the rising demand for food production that will inevitably lead to further agricultural intensification, which will in turn translate into even more demand for, and pressure on pollination [10], [11].
The intensification of farming practices has been the main process eroding biodiversity in low-input farmland, which was the typical cultivated landscape across Europe until World War II [12]–[14]. Intensification is achieved via the application of fertilizers and pesticides, and through a growing reliance on heavy machinery that necessitates radical landscape simplification [15]. In order to counter this negative impact of agricultural intensification on biodiversity, agri-environment schemes (AES) were established in the early 1990’s throughout Europe in order to encourage farmers to adopt more environmental friendly farming practices [16]. AES primarily aim at protecting and restoring farmland biodiversity, thus securing or even enhancing several ecosystem services, including pollination. Some AES like the sown wildflower strips and areas were specifically designed to promote pollinating insects, but their temporary based management misses the restoration of semi-natural habitats [17]. In comparison, extensively managed (low-input) grasslands established under AES regulations are widespread [16], usually harbouring more wild bees than high-intensity grasslands [18], [19]. However, several studies have established that these AES have only a moderate positive impact on overall biodiversity and bees [20]. This calls for the development of more appropriate management practices that can favour biodiversity, including pollinating insects [21].
The main aim of this study was to test whether and how slight modifications of mowing regimes may improve wild bee biodiversity in extensively managed hay meadows, a widespread AES scheme [22]. These altered management regimes had to be easily implementable by farmers to ensure their potential future uptake; they consisted of 1) not mowing a fraction of the meadow so as to leave an uncut area as refuge that is expected to boost wild bee biodiversity by continuously providing them with food resources, essentially nectar and pollen, during the entire season; 2) delaying by one month the first cut in order to provide undisturbed meadows with flowers and other crucial resources during the whole peak of natural wild bee activity. These two experimental treatments were compared with the Swiss AES serving as ‘control’; the ecological compensation areas (ECA). Standard management for hay meadows complying with ECA regulations are: first cut not before 15 June and; with no uncut refuge left behind. These treatments were applied at the field scale, two years in a row (2010 and 2011), in order to test for both immediate (within season) and cumulative (from one year to the following) effects. For this purpose, wild bees were collected twice in 2011: firstly in June, before the onset of any mowing intervention in all meadows; secondly beginning of July, when meadows with uncut refuges and control meadows were mown, but not meadows with delayed mowing regimes. To our knowledge, this study is the first attempt to manipulate mowing regimes at the field scale to test whether such simple measures can promote bee diversity. Although drawn from the Swiss context, the resulting recommendations have far-reaching implications for the establishment of AES across Europe if not beyond. They will contribute to the development and implementation of pollinator-friendly management practices and could potentially complement wild-flower strips [23]. They are also timely given the current intention of the European Union to frame a more biodiversity-friendly common agricultural policy [23]. In order to avoid jeopardizing essential components of biodiversity such as the guilds of natural pollinators, innovative farming practices have to be developed. We also take the opportunity to investigate and provide new data about the effectiveness our sampling method.
Materials and Methods
Ethic Statement
Farmers that participated to this project were informed about, and approved, the studies before they started. No endangered or protected species were sampled in this study.
Study Sites
We selected 36 extensively managed hay meadows registered as ECA across the Swiss Plateau (lowlands between the Jura and the Alps) in 2010 (see Appendix S1 and S2). The Swiss Plateau can be characterized mainly as a simple landscape where non-crop habitats are still present, but constitute usually only 1–20% of the matrix [14]. The ECA retained for our experiments had to be registered since latest 2004 (range: 1993–2004) and had to have a minimal area of 0.3 ha (range: 0.3–1.7 ha). The meadows were situated between 390 and 833 m altitude. They were clustered in 12 study areas (our geographic replicates) distant from each other by ≥5 km, each area containing three meadows that were more than 400 m distant (range: 440–6170 m) but that were enclosed within a radius of 3.5 km.
We had first to assess the different land covers as these could be important covariates that should be accounted for in our analysis. Land covers were extracted from the Vector 25 data base of the Swiss Federal Office of Topography [24], using QGIS [25] and SpatiaLite [26] software. Land covers [proportion of forest, settlements, water bodies (including rivers), special crops (vineyards and orchards), and gravel pits] were quantified around each meadow within different nested concentric radii ranging from 250 to 3000 m, with steps of 250 m. A principal component analysis (PCA) was then conducted on land covers to draw synthetic information about the various landscape contexts at the different geographic replicates [27], [28]. The PCA was performed with land cover values averaged across these radii, this to avoid auto-correlation [28]–[30]. We retained only PCA axes that had a proportion of variance superior to a broken-stick model with heuristic for principal component selection [31], with the function PCAsignificance of the package BiodiversityR [32]. Then the coefficients of the Pearson product-moment correlation (eigenvectors) of the retained axes were used to select important land covers with 0.5 as cutting of value.
Study Design
A randomized block design was adopted [33], where the three mowing regimes (our two experimental treatments and a control) were applied once each within each study area. Hence, each area represented a geographic replicate (n = 12), i.e. an experimental block in the design, which allowed achieving data independency. The following treatments and control were randomly assigned to the three meadows within each area. We start with the control, because it represents the standard management that today prevails among extensively managed hay meadows within the ECA (ecological compensation areas) measures of the Swiss AES: 1) control meadow (abbreviated C, C-meadow): managed according to the Swiss regulations for ECA extensive hay meadows, i.e. first cut not before 15 June; 2) refuge treatment (R, R-meadow): same as C, but at each cut 10 to 20% of the meadow area were left uncut; 3) delayed mowing (D, D-meadow): same as C, but first possible cut not before 15 July (one month later than C). All other management aspects (such as non-application of fertilizers and pesticides or minimal duration of 6 years) abided by the present regulation [34]. Each farmer was interviewed about mowing dates and related management issues using a standardized questionnaire.
Wild Bees Sampling
In 2011, plastic bowl traps (13 cm in diameter and 12.5 cm deep) were used to sample wild bees (Hymenoptera: Apoidea) applying the following protocol: three bowl traps (blue, white and yellow) were fixed on a wooden pole just above the grass vegetation layer [35]. They were operated at daylight (8∶00–19∶00) during one day in order to avoid local population depletion [36]. Since this study aimed at comparing relative differences among mowing regimes and not at providing a full inventory per se, such standardized operating time was considered as being sufficient [37]–[41]. Three such poles equipped with three bowl traps were placed at the apexes of a virtual isosceles triangle (base: 14 m; sides: 10 m) randomly placed inside the meadow, within at least 10 m from meadow edges so as to reduce margin effects [18]. Meadows were sampled twice, a first time between 23 May and 14 June, i.e. before the onset of mowing in any treatment and control meadows (hereafter referred to as the ‘June’ samples) and a second time between 2 July and 12 July, i.e. before the first mowing of D-meadows but when C-meadows and R-meadows were regrowing (hereafter ‘July’ samples). Samplings took place on sunny, non-windy days with ambient temperature ≥15°C [35]. All the meadows within a given area were sampled simultaneously (Appendix S2 for exact dates). Samples were stored individually in plastic bags and frozen at −20°C. Before sorting them, defrozen samples were washed; bees pinned and dried [37]. Bees were identified according to identification keys for Central Europe [42]–[48].
Data Analysis
Data were analysed with generalized linear mixed models (GLMMs) using the lmer function from the lme4 package for R [49]. Wild bees consisted of the so-called “solitary bees” and of bumblebees pooled together. Fixed effects were the mowing treatments and the land covers selected in the previous part. The latter were added in the models, progressively increasing model complexity, following a bidirectional stepwise procedure [50], [51]. Areas (our geographic replicates) were designated as a random effect. Response variables were pooled for each meadow and resulted in: wild bee abundance; species richness and; diversity (Shannon-Wiener index), the former two variables were analysed fitting a Poisson error distribution and the latter one fitted a normal error distribution. Data of the two sampling periods (June and July) were first analysed pooled together, then separately, this in order to better appraise underlying patterns. Planned orthogonal comparisons were done to identify significant differences between the treatments. In addition, we also investigated in a similar way the effects of these mowing regimes on the abundance of managed honey bees, given that feral honey bee colonies apparently do not occur in Switzerland [52] and the effectiveness of the different colours of our traps. All the analyses were performed with statistical software R version 2.15.0 [53].
Results
We collected a total of 1′620 wild bees (Appendix S2) and 281 honey bees. Cryptic, sibling species of bumblebees that were difficult to identify were grouped within their respective taxonomic groups, mostly subgenera (Bombus sensu stricto, Megabombus and Thoracobombus [47]). Cryptic, sibling species of solitary bees were grouped within the following categories: Halictus simplex group (Halictus simplex; H. eurygnathus and H. langobardicus and Andrena ovatula group (Andrena ovatula and A. albofasciata). Altogether, we could identify 62 wild bee species (9 bumblebee and 56 solitary bee species; full species list in Appendix S3).
Bowl Trap Efficiency
Yellow bowl traps were generally more efficient (greater number of captures of wild bees) than white traps which were themselves more attractive than the blue ones. These differences were significant when the June and July samples were pooled, and when the June data was considered separately. In July, however, yellow and white traps did not differ in efficiency between each other though they were still more attractive than the blue traps (detailed analysis in Appendix S4).
Management and Land Cover
Our study meadows were mown, on average (±SD), 1.92 times (0.56) and 1.81 times (0.49) in 2010 and 2011, respectively, with a minimum number of cuts of one and a maximum of three. There was no significant difference in the yearly number of cuts between 2010 and 2011. In 2011, the first cut took place between 15 June and 26 June in C– and R–meadows, and between 15 July and 15 August in D–meadows (exact dates are provided in the appendix S5). In R-meadows, uncut grass refuges covered, on average, 15% of the meadow area.
Regarding the PCA on landscape covers, only the first component fulfilled the broken-stick criteria (73.41% of variance explained vs 45.66% expected). The following land covers were identified as significant based on their eigenvalues (Pearson product-moment correlation) and retained for subsequent analyses: forest (−0.511); special crops (0.566) and water bodies (0.515).
Effect of Mowing Treatments on Wild Bees
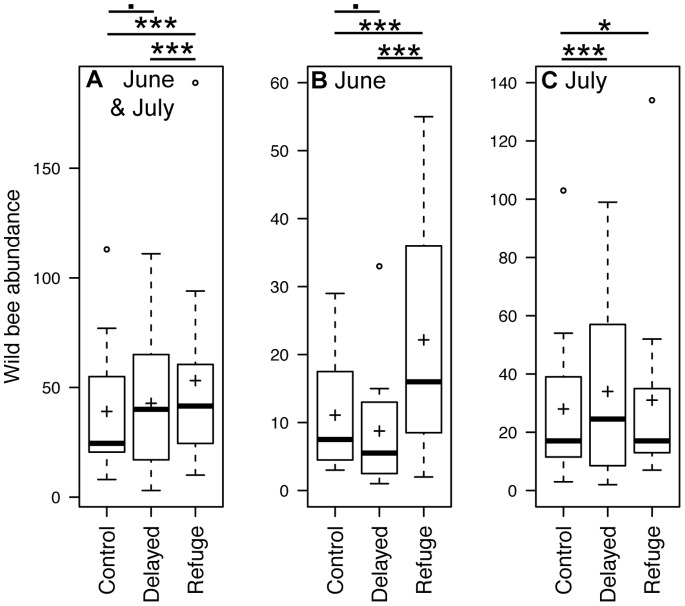
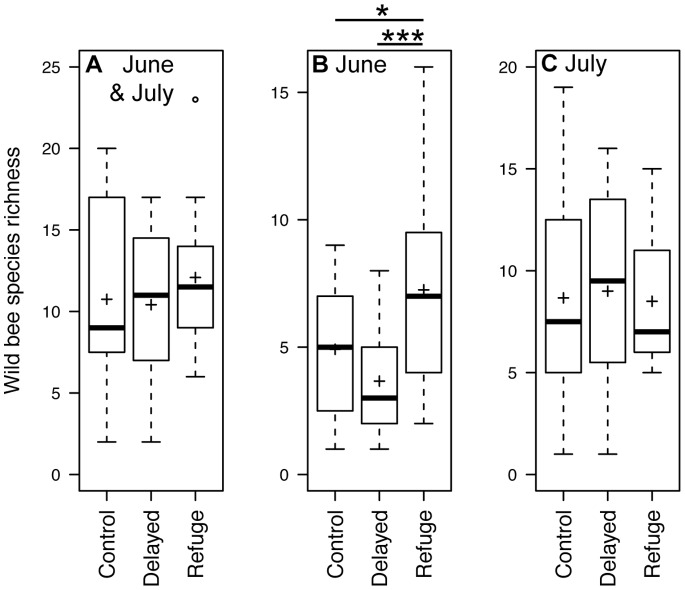
In the analyses performed with data from June and July pooled together, the mean abundance (±SE) of wild bees was 53.16 (±14.15) in R-meadows (refuge) and was significantly higher compared to C-meadows (control, 39.08±8.9; Fig. 1a and Table 1). Abundance in D-meadows (delayed mowing) was only marginally higher than in C-meadows. Finally, significantly fewer individuals were found in D-meadows compared to R-meadows (Z = 3.677, P<0.001). The land covers retained in this first model were forest and water bodies with both a negative effect on wild bee abundance; in contrast, special crops had a positive effect. Species richness did not show any significant difference among the mowing regimes with the June and July samples pooled (Fig. 2a and Table 2). Neither did we find any difference for the Shannon-Wiener index of diversity for pooled data.
Figure 1. Abundance of wild bees.
Number of individuals captured according to the different mowing treatments in: a) June and July (pooled data); b) June only; and c) July only. Bold transversal bars represent medians;+the means; box boundaries the first and last quartiles; whiskers the inter-quartile distance multiplied by 1.5; and open dots the outliers. Significance codes of statistical tests: · marginally significant results (0.1<P<0.05); *significant results, P<0.05; ***highly significant results, P<0.001.
Table 1. Abundance of wild bees.
| Total | June | July | ||||
| Parameters | Z-value | P-value | Z-value | P-value | Z-value | P-value |
| Delayed | 1.713 | 0.086 | −1.927 | 0.054 | 3.594 | <0.001 |
| Refuge | 4.036 | <0.001 | 5.487 | <0.001 | 2.472 | 0. 0.013 |
| Forest | −2.843 | 0.005 | – | – | −2.319 | 0. 021 |
| Water bodies | −2.683 | 0.00730 | – | – | −4.021 | <0.001 |
| Special crops | 2.669 | 0.008 | 2.044 | 0.041 | – | – |
GLMM outputs on the abundance of the wild bees recorded according to the different managements and the most important land covers. Analyses presented are the pooled data (June and July added); the June sampling session and; the sampling of July. Significant contrasts are highlighted in bold.
Figure 2. Species richness of wild bees.
Number of species captured according to the different mowing treatments in: a) June and July (pooled data); b) June only; and c) July only. Symbols as in Fig. 1.
Table 2. Species richness of wild bees.
| Total | June | July | ||||
| Parameters | Z-value | P-value | Z-value | P-value | Z-value | P-value |
| Delayed | −0.251 | 0.802 | 1.490 | 0.1363 | 0.274 | 0.784 |
| Refuge | 0.965 | 0.335 | 2.044 | 0.036 | −0.139 | 0.889 |
| Forest | – | – | – | – | – | – |
| Water bodies | – | – | – | – | – | – |
| Special crops | – | – | 2.044 | 0.041 | – | – |
GLMM outputs on the species richness of the wild bees recorded according to the different managements and the most important land covers. Analyses are presented in the same way as Table 1.
In June, the abundance of wild bees in R-meadows was, on average, 22.17 (±5.05), i.e. significantly higher than in C-meadows (11.08±2.44) and D-meadows (8.75±2.60; Z = 2.101, P = 0.035; Fig. 1b). Abundance in D-meadows was also marginally lower than in C-meadows. Only special crops were retained as a significant land cover in this model, with a positive effect. Species richness in R-meadows was 7.25 (±1.15), significantly higher than in both C-meadows (4.92±0.80; Table 2) and D-meadows (3.67±0.58; Z = 3.664, P<0.001; Fig. 2b). Special crops coverage had again a positive effect on species richness. In contrast, we detected no significant effect on the Shannon-Wiener index of diversity.
In July, the mean abundance of wild bees collected in D-meadows was 34.00 (±8.78) individuals, i.e. significantly higher than in C-meadows (28.00±8.17; Fig. 1c). The abundance in R-meadows (31±10.07) was also higher than in C-meadows (Z = 2.472, P = 0. 0013). Forest (Z = 2.319, P = 0. 021) and water bodies (Z = −4.021, P<0.001) were the only land covers retained by the model; both had a significant negative effect. Neither species richness nor the Shannon-Wiener index of diversity showed any significant difference among mowing regimes.
When the data of June and July were pooled, honey bee abundance was, on average, 9.91±2.49 in D-meadows, i.e. significantly higher than the abundance recorded in C-meadows (6.5±1.08; GLMM with Poisson; Z = 2.894, P = 0.0038) and R-meadows (6.83±1.38, Z = 0.316, P = 0.75). In June, no significant difference was found, while in July honey bee abundance was significantly higher in D-meadows (6.83±2.05) compared with C-meadows (3.67±0.88; Z = 3.323, P<0.001) and R-meadows (3.08±0.91; Z = 3.221, P = 0.00128).
Discussion
This study shows that leaving 10–20% of the area of an extensive meadow uncut when mowing (R-meadows) is overall beneficial for wild bee populations, more so than delaying the date of mowing by one month (D-meadows). There were variations in the observed pattern according to whether we consider immediate (within the same season) or cumulative (from one year to the following) effects. Regarding cumulative effects [samples collected in June in the year following (i.e. yeart+1) the year of first application (yeart) of the different management treatments, but before any mowing event in yeart+1], positive effects were evidenced for both wild bee abundance and species richness. The average wild bee abundance was double so high in R-meadows compared to C-meadows, and even 2.4 times higher than in C-meadows that had the lowest values (Fig. 1b). Species richness was, on average, 1.75 and 1.4 higher in R-meadows compared to D-meadows and C-meadows, respectively (Fig. 2b). Immediate effects showed a reversed pattern, but only regarding wild bee abundance, with D-meadows harbouring, on average, 1.2 and 1.1 times more individuals than C- meadows and R-meadows, respectively; this is not very surprising given that D-meadows were not yet mown at the second sampling session. Concerning, honey bee population size estimates, we could not evidence a cumulative effect, while immediate effects showed that D-meadows supported ca. 1.8 and 2.3 times greater abundances than C-meadows and R-meadows, respectively.
The positive cumulative effect of the refuge treatment (R) on wild bee abundance indicates that populations could build up thanks to the grass refuge installed the year before. This demonstrates that uncut grass refuges have a positive impact on these pollinating insects beyond the season they are applied in. This cumulative effect is crucial for the maintenance of pollination services because pollination efficiency is based on the redundancy principle, which emphasizes the importance of pollinator abundance above species richness [7], [54], [55]. Our results further confirm that wild bees can react extremely rapidly to changes in management practices: this first analysis stems from just one year of field experimentation (June 2010– July 2011). Such a rapid positive reaction is consistent with the responses of bumblebees to modifications in grazing management [56] and manipulation of the cutting management of flower patches [27]. Similar responses were also observed in other taxa, such as orthopterans [57], spiders [58] and the only other pollinator taxon studied, butterflies [59]. Finally, the absence of a similar effect in honey bees in the present study can be due to the fact that these Hymenoptera depend neither on the structures nor on the food resources offered by the refuges for building their colonies, while they furthermore profit from artificial feeding at the hives (feral honey bees are extremely rare in Switzerland [52]). The continuation of our experiments during the coming years will allow assessing whether cumulative effects may further grow with additional years of implementation of the treatments.
Regarding immediate effects, delayed mowing (D-meadows) appeared to be more efficient than the creation of an uncut refuge (R-meadows) for increasing abundance of both wild and honey bees. Yet, the magnitude of these positive effects was strikingly lower than the cumulative effects obtained with the refuge treatment. Furthermore, this effect reflects the fact that D-meadows were not yet mown at the time of the second sampling session, contrary to R-meadows and C-meadows that were already regrowing after the first cut. These D-meadows were thus the main sources of nectar and pollen left in the agricultural matrix at that time of the year, which corresponds to the peak of hymenopteran pollinator activity [27], thus typically generating some short-term spatio-temporal concentration [60]. This hypothesis of a temporary concentration effect is further supported by the lower wild bee abundance and species richness in D-meadows compared to C- and R-meadows in June: for many species that firstly depend on vegetation with a late phenology, mowing around mid-July could still be too early. Notwithstanding the fact that bowl trapping is not the most efficient method to capture honey bees [35], [61], their concentration in D-meadows in July highlights the need for valuable flowering patches at this time of the year. Maintaining uncut meadows in the middle of the summer could indeed provide them with precious floral resources between the massive spring blossoming of both natural flowers or some crops (mainly oilseed rape Brassica napus, L.) and other crops with a later phenology, e.g. sunflowers Helianthus annuus Linnaeus, 1753 [62].
Improvement of species richness was only detected as a cumulative effect (June samples) and occurred furthermore only in R-meadows, but not in D-meadows. This result is in accordance with the outcome of the main study on Hymenoptera retrieved in the meta-analysis on delaying mowing done by Humbert et al. [63]. To the contrary of the main trend for arthropods, no effect of postponing mowing could be evidenced for bumblebee species richness [64].
Surprisingly, the effect of our mowing treatments did not affect species diversity (Shannon-Wiener index). An explanation could be that the relative population sizes of different sympatric wild bee species do not vary in relation to the number of co-occurring species [65], which would lead to little spatial variation in the index. Moreover, although R-meadows harboured, on average, more species than C-meadows and D-meadows in June, there was no new species specifically profiting from the refuge that appeared in the samples. Actually, among the 62 different species captured, only twelve occurred in more than seven of the 12 study areas (Appendix S4). This high level of spatial differentiation in bee communities, i.e. apparent high level of functional redundancy, was particularly striking within bee genera having similar ecological requirements, such as Lasioglossum and Halictus [6].
Land covers have an important influence on bees that are relatively mobile organisms [28]–[30]. The major land covers selected through the PCA were forest, water bodies and special land managements. The two first ones had the most part of time a negative influence, because they represent less suitable habitats for bees. Thus a high proportion of such features in the surroundings have a negative influence. Special land management had a positive influence in spring. This could be due to the kind of crops present in this land cover, especially orchards that are reputed to be major nectar sources.
Concerning the difference observed between the colours of the traps, the conclusion of the effectiveness of the yellow is in accordance with the literature [35]. Interestingly, other colours, especially white, can be as effective and more representative of the plant flowering community and thus illustrates the complementarity of the different colours for this traps.
Management Recommendations and Conclusions
This study constitutes to our knowledge the first attempt to experimentally test, moreover at real field-scale, the effects of different grassland management regimes in hay meadowland on wild bee communities and population sizes. It demonstrates that creating uncut refuges on a relatively small fraction of a hay meadow can quickly and efficiently promote pollinating insects such as wild bees during the following year, which is likely to enhance an essential ecosystem service. Although it remains to be established whether the inter-annual positive effects we observed further cumulate beyond one year, this measure represents a promising agri-environmental option, especially given that its simplicity of implementation might ensure a quick up-take by farmers, of course providing that financial incentives exist. In contrast, delaying mowing seems to have comparatively much smaller positive effects on bees as it simply leads to a temporary concentration of bees on the few patches with flowering plants that remain in farmland matrices that otherwise become hostile for pollinators after late spring mowing operations. Uncut refuges could enter the toolkit for promoting pollinators within farmland, similar to, for instance, wildflower sown margins [27].
Another advantage of the uncut refuge option, over the delayed mowing option, is that it does not affect hay production to the same extent, given that only a fraction of the meadow remains unmown. The hay extracted from the non-refuge area would furthermore be of overall better quality because the timing of mowing operations can take place earlier than in D-meadows, i.e. closer to the period of forage quality peak. A systematic implementation of this measure within extensive hay meadows across the agricultural matrix might efficiently boost wild bee populations and communities. We may furthermore expect that the overall impact of a network of such refugial structures reaches beyond the sum of the local effects, due to opportunities for reconstituting functional meta-populations and integral communities, this especially given the short flight radius of numerous pollinators [29], [30]. This simple measure could also easily be integrated in extant AES which – given the extension of grassland AES [20], [22] – would theoretically lead to widespread improvement of pollination services in agriculture. Finally, the fact that this measure is already suggested as a voluntary enrolment for farmers in such schemes will enhance the probability of its uptake [66], [67]. Future research must investigate whether extra positive cumulative effects will, in the mid and long run, add to the short-term effects observed in this study. It must also establish whether other plant and animal taxa benefit from uncut refuges, and whether combining this measure with delayed mowing on, for instance, another small fraction of the same meadow might multiply the benefits for biodiversity, especially pollinating insects.
Supporting Information
Sampling sites. Map of Switzerland with the lowland and mountainous areas as defined in the Swiss agricultural cadastre. Sampling points are indicated with coloured dots.
(PDF)
Geographic coordinates of all sampling sites with indication of total number of individuals trapped. Numbers of individuals of solitary bees, bumblebees, honey bees and other insets caught are provided for each meadow.
(XLS)
List of the species of wild bees identified per area. The nomenclature follows the one proposed in the identification keys of Félix Amiet.
(XLS)
Study of the effects of different colours of bowl traps. Comprehensive analyses of the attractiveness of the different colours of the most numerous species.
(DOC)
List of the different mowing dates for the year 2011. Dates were communicated by the farmers.
(XLSX)
Acknowledgments
We thank Markus Fischer and Jérôme Pellet for their input in proposal preparation. We are grateful to Sabine Oertli for the identification of solitary bees, Christophe Praz for advice with sampling design, Nastassja Rieder and Franck Gandiaga for general assistance. We also thank Vincent Dietemann for fruitful discussions about honey bees. Special thanks go to all the farmers who participated in this project. This project was done with the technical support of the Federal Office of Environment, and the following cantonal offices of environment and/or agriculture: Bern, Vaud, Basel-Landschaft, Aargau, Neuchâtel, Fribourg, Valais and Graubünden. We also express our gratitude to all the members of the project accompanying group for their strong support and to the anonymous reviewers for their helpful comments.
Funding Statement
This project was supported by a grant of the Swiss National Science Foundation to R.A. (No. 31003A_125398/1), the Federal Office of Environment, and the following cantonal offices of environment and/or agriculture: Bern,Vaud, Basel-Landschaft, Aargau, Neuchâtel, Fribourg, Valais and Graubünden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bawa KS (1990) Plant-pollinator interactions in Tropical rain forests. Annual Review of Ecology and Systematics 21: 399–422. [Google Scholar]
- 2. Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, et al. (2007) Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, et al. (2010) Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution 25: 345–353. [DOI] [PubMed] [Google Scholar]
- 4. Carreck N, Neumann P (2010) Honey bee colony losses. Journal of Apicultural Research 49: 1–1. [Google Scholar]
- 5. Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, et al. (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313: 351–354. [DOI] [PubMed] [Google Scholar]
- 6. Winfree R, Aguilar R, Vàzquez DP, Lebuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
- 7. Steffan-Dewenter I, Potts SG, Packer L (2005) Pollinator diversity and crop pollination services are at risk. Trends in ecology & evolution 20: 651–652. [DOI] [PubMed] [Google Scholar]
- 8. Ghazoul J (2005) Buzziness as usual? Questioning the global pollination crisis. Trends in ecology & evolution 20: 367–373. [DOI] [PubMed] [Google Scholar]
- 9. Aizen MA, Garibaldi LA, Cunningham SA, Klein A-M (2009) How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Annals of Botany 103: 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breeze TD, Bailey AP, Balcombe KG, Potts SG (2011) Pollination services in the UK: How important are honeybees? Agriculture, Ecosystems & Environment 142: 137–143. [Google Scholar]
- 11. Diekötter T, Kadoya T, Peter F, Wolters V, Jauker F (2010) Oilseed rape crops distort plant-pollinator interactions. Journal of Applied Ecology 47: 209–214. [Google Scholar]
- 12. Bignal EM, McCrackent DI (1996) Low-intensity farming systems in the conservation of the countryside. Journal of Applied Ecology 33: 413–424. [Google Scholar]
- 13. Hendrickx F, Maelfait J-P, Van Wingerden W, Schweiger O, Speelmans M, et al. (2007) How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. Journal of Applied Ecology 44: 340–351. [Google Scholar]
- 14. Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity - ecosystem service management. Ecology Letters 8: 857–874. [Google Scholar]
- 15.Warren J, Lawson CS, Blecher K (2008) The Agri-Environment. Cambridge, United Kingdom. 1–232 p.
- 16. Kleijn D, Sutherland WJ (2003) How effective are European agri-environment schemes in conserving and promoting biodiversity ? Journal of Applied Ecology 40: 947–969. [Google Scholar]
- 17. Haaland C, Naisbit RE, Bersier L-F (2011) Sown wildflower strips for insect conservation: a review. Insect Conservation and Diversity 4: 60–80. [Google Scholar]
- 18. Knop E, Kleijn D, Herzog F, Schmid B (2006) Effectiveness of the Swiss agri-environment scheme in promoting biodiversity. Journal of Applied Ecology 43: 120–127. [Google Scholar]
- 19.Albrecht M, Duelli P, Müller C, Kleijn D, Schmid B (2007) The Swiss agri-environment scheme enhances pollinator diversity and plant reproductive success in nearby intensively managed farmland. Journal of Applied Ecology: 813–822.
- 20. Kleijn D, Baquero Ra, Clough Y, Díaz M, De Esteban J, et al. (2006) Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecology letters 9: 243–254 discussion 254–247. [DOI] [PubMed] [Google Scholar]
- 21. Littlewood NA, Stewart AJA, Woodcock BA (2012) Science into practice - how can fundamental science contribute to better management of grasslands for invertebrates? Insect Conservation and Diversity 5: 1–8. [Google Scholar]
- 22.FOAG (2012) Rapport Agricole 2011. Bern: Federal Office of Agriculture. 1–284 p.
- 23.Hart K, Baldock D (2011) Greening the CAP: Delivering environmental outcomes through pillar one. http://www.ieep.eu/assets/831/Greening_Pillar_1_IEEP_Thinkpiece_-_Final.pdf.
- 24.Swisstopo (2012) Vector 25. Federal Office of Topography, Wabern, Switzerland.
- 25.Quantum GIS Development Team (2012) QGIS. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project.
- 26.Furieri A (2008) SpatiaLite - A complete spatial DBMS in a nutshell. http://www.gaia447gis.it/gaia-sins/.
- 27. Pywell RF, Meek WR, Hulmes L, Hulmes S, James KL, et al. (2011) Management to enhance pollen and nectar resources for bumblebees and butterflies within intensively farmed landscapes. Journal of Insect Conservation 15: 853–864. [Google Scholar]
- 28. Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T, et al. (2002) Scale-dependant effects of landscape context on three pollinator guilds. Ecologgy 83: 1421–1432. [Google Scholar]
- 29. Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. Journal of Animal Ecology 71: 757–764. [Google Scholar]
- 30. Kohler F, Verhulst J, Van Klink R, Kleijn D (2007) At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? Journal of Applied Ecology 45: 753–762. [Google Scholar]
- 31. Peres-Neto PR, Jackson Da, Somers KM (2005) How many principal components? stopping rules for determining the number of non-trivial axes revisited. Computational Statistics & Data Analysis 49: 974–997. [Google Scholar]
- 32.Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. In: , editor. Nairobi.
- 33.Gotelli NJ, Ellison AM (2004) A Primer of Ecological Statistics. Sunderland, USA: Sinauer Associates Incorporated. 1–479 p.
- 34.Anonymous (1998) Verordnung über die Direktzahlungen an die Landwirtschaft. 93013: Bundesrat. pp. 229–265.
- 35. Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, et al. (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecological Monogrpahs 78: 653–671. [Google Scholar]
- 36. Russell KN, Ikerd H, Droege S (2005) The potential conservation value of unmowed powerline strips for native bees. Biological Conservation 124: 133–148. [Google Scholar]
- 37. Droege S, Tepedino VJ, Lebuhn G, Link W, Minckley RL, et al. (2010) Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conservation and Diversity 3: 15–23. [Google Scholar]
- 38. Heneberg P, Bogusch P, Řehounek J (2013) Sandpits provide critical refuge for bees and wasps (Hymenoptera: Apocrita). Journal of Insect Conservation 17: 473–490. [Google Scholar]
- 39. Kwaiser KS, Hendrix SD (2008) Diversity and abundance of bees (Hymenoptera: Apiformes) in native and ruderal grasslands of agriculturally dominated landscapes. Agriculture, Ecosystems & Environment 124: 200–204. [Google Scholar]
- 40. Le Féon V, Burel F, Chifflet R, Henry M, Ricroch A, et al. (2013) Solitary bee abundance and species richness in dynamic agricultural landscapes. Agriculture, Ecosystems & Environment 166: 94–101. [Google Scholar]
- 41. Murray TE, Fitzpatrick Ú, Byrne A, Fealy R, Brown MJF, et al. (2012) Local-scale factors structure wild bee communities in protected areas. Journal of Applied Ecology 49: 998–1008. [Google Scholar]
- 42.Amiet F (1996) Apidae 1. Teil. Allgemeiner Teil, Gattungsschlüssel, die Gattungen Apis, Bombus und Psithyrus. Neuchatêl, Switzerland: Centre Suisse de Cartogrpahie de la Faune. 1–98 p.
- 43.Amiet F, Herrmann M, Müller A, Neumeyer R (2001) Apidae 3. Halictus, Lasioglossum. Neuchatêl, Switzerland: Centre Suisse de Cartogrpahie de la Faune. 1–208 p.
- 44.Amiet F, Herrmann M, Müller A, Neumeyer R (2004) Apidae 4. Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis: Centre Suisse de Cartogrpahie de la Faune. 1–273 p.
- 45.Amiet F, Herrmann M, Müller A, Neumeyer R (2007) Apidae 5. Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa: Centre Suisse de Cartogrpahie de la Faune. 1–356 p.
- 46.Amiet F, Neumeyer R (1999) Apidae 2. Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha. Neuchatêl, Switzerland: Centre Suisse de Cartogrpahie de la Faune. 1–219 p.
- 47.Rasmont P, Terzo M (2010) Catalogue et clé des sous-genres et espèces du genre Bombus de Belgique et du nord de la France (Hymenoptera, Apoidea) par Catalogue des sous-genre et espèces du genre Bombus (Apidae, Apinae, Bombini). Mons, Belgium. 1–28 p.
- 48.Schmid-Egger C, Scheuchl E (1997) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band III : Andrenidae. 1–180 p.
- 49.Bates D, Maechler M, Bolker B (2012) Package ‘lme4’.
- 50. Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends in ecology & evolution 19: 101–108. [DOI] [PubMed] [Google Scholar]
- 51. Pywell RF, Warman EA, Hulmes L, Hulmes S, Nuttall P, et al. (2006) Effectiveness of new agri-environment schemes in providing foraging resources for bumblebees in intensively farmed landscapes. Biological Conservation 129: 192–206. [Google Scholar]
- 52. Jaffé R, Dietemann V, Allsopp MH, Costa C, Crewe RM, et al. (2010) Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline censuses. Conservation biology 24: 583–593. [DOI] [PubMed] [Google Scholar]
- 53.R Development Core Team (2012) R: A Language and Environment for Statistical Computing. 2.15.0 ed. Vienna, Austria: R Foundation for Statistical Computing.
- 54. Fontaine C, Collin CL, Dajoz I (2008) Generalist foraging of pollinators: diet expansion at high density. Journal of Ecology 96: 1002–1010. [Google Scholar]
- 55. Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society B 271: 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carvell C, Meek WR, Pywell RF, Goulson D, Nowakowski M (2007) Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. Journal of Applied Ecology 44: 29–40. [Google Scholar]
- 57. Humbert J-Y, Ghazoul J, Richner N, Walter T (2012) Uncut grass refuges mitigate the impact of mechanical meadow harvesting on orthopterans. Biological Conservation 152: 96–101. [Google Scholar]
- 58. Schmidt MH, Rocker S, Hanafi J, Gigon A (2008) Rotational fallows as overwintering habitat for grassland arthropods: the case of spiders in fen meadows. Biodiversity and Conservation 17: 3003–3012. [Google Scholar]
- 59. Valtonen A, Saarinen K, Jantunen J (2006) Effect of different mowing regimes on butterflies and diurnal moths on road verges. Animal Biodiversity and Conservation 29: 133–148. [Google Scholar]
- 60. Veddeler D, Klein A-M, Tscharntke T (2006) Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos 112: 594–601. [Google Scholar]
- 61. Roulston TaH, Smith SA, Brewster AL (2007) A Comparison of Pan Trap and Intensive Net Sampling Techniques for Documenting a Bee (Hymenoptera : Apiformes) Fauna. Journal of the Kansas Entomological Society 80: 179–181. [Google Scholar]
- 62. Decourtye A, Mader E, Desneux N (2010) Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 41: 264–277. [Google Scholar]
- 63.Humbert J-Y, Pellet J, Buri P, Arlettaz R (2012) Does delaying the first mowing date benefit biodiversity in meadowland? A meta-analysis. Environmental Evidence: 1–9.
- 64. Potts SG, Woodcock BA, Roberts SPM, Tscheulin T, Pilgrim ES, et al. (2009) Enhancing pollinator biodiversity in intensive grasslands. Journal of Applied Ecology 46: 369–379. [Google Scholar]
- 65. Burel F, Baudry J, Butet A, Le Coeur D, Dubs F, et al. (1998) Comparative biodiversity along a gradient of agricultural landscapes. Acta Oecologica 19: 47–60. [Google Scholar]
- 66.Anonymous (2009) Bewirtschaftungsverträge Naturnahe Landwirtschaft Richtlinien. Aargau, Switzerland: Departement Bau Verkehr und Umwelt. 1–50 p.
- 67.OAN (2009) Projets de mise en réseau selon l’ordonnance sur la qualité écologique Directives cantonales l’ordonnance sur la préservation des bases naturelles de la vie et des paysages (OPBNP). Bern, Switzerland: Office de l’agriculture et de la nature du canton de Berne 1–17 p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampling sites. Map of Switzerland with the lowland and mountainous areas as defined in the Swiss agricultural cadastre. Sampling points are indicated with coloured dots.
(PDF)
Geographic coordinates of all sampling sites with indication of total number of individuals trapped. Numbers of individuals of solitary bees, bumblebees, honey bees and other insets caught are provided for each meadow.
(XLS)
List of the species of wild bees identified per area. The nomenclature follows the one proposed in the identification keys of Félix Amiet.
(XLS)
Study of the effects of different colours of bowl traps. Comprehensive analyses of the attractiveness of the different colours of the most numerous species.
(DOC)
List of the different mowing dates for the year 2011. Dates were communicated by the farmers.
(XLSX)