Abstract
This article reviews the current state of knowledge regarding the potential of Müller glia to become neuronal progenitor cells in the avian retina. We compare and contrast the remarkable proliferative and neurogenic capacity of Müller glia in the fish retina to the limited capacity of Müller glia in avian and rodent retinas. We summarize recent findings regarding the secreted factors, signaling pathways and cell intrinsic factors that have been implicated in the formation of Müller glia-derived progenitors. We discuss several key similarities and differences between the fish, rodent and chick model systems, highlighting several of the key transcription factors and signaling pathways that regulate the formation of Müller glia-derived progenitors.
Keywords: retina, microglia, Müller glia, regeneration, progenitor
Introduction
Retinal regeneration in cold-blooded vertebrates has been known for more than one hundred years (reviewed by Stone 1950). There are reports of neuronal regeneration in the retinas of many different vertebrate classes, including frogs (Reh 1987; Reh et al. 1987), fish (Lombardo 1968; Lombardo 1972), embryonic rodents (Zhao et al. 1997; Zhao et al. 1995), and embryonic birds (Coulombre and Coulombre 1965; Coulombre and Coulombre 1970; Orts-Llorca and Genis-Galvez 1960). In recent years, evidence has been provided for neuronal regeneration in mature retinas of birds (Fischer and Reh 2001a) and rodents (Karl et al. 2008; Ooto et al. 2004). However, the retinal regeneration in birds and rodents is modest compared to the robust regeneration seen in cold-blooded vertebrates (Hitchcock et al. 1992; Hitchcock and Raymond 1992; Raymond 1991; Raymond et al. 1988). Across the different vertebrate classes retinal regeneration can arise from different cellular sources. These cellular sources include (1) the retinal pigmented epithelium (RPE), (2) retinal stem cells in the circumferential marginal zone (CMZ), and (3) Müller glia.
Retinal regeneration from RPE cells is known to occur in the eyes of neotenic amphibians and larval anurans (reviewed by Fischer and Reh 2001b; Hitchcock et al. 1992). The RPE is a densely pigmented monolayer of cells that lies directly adjacent to the photoreceptors of the retina. Like the retina, the RPE is derived from ventral diencephalon during early stages of development, but neurogenesis is suppressed when the pigment phenotype is established. In the mature eye, RPE cells are highly differentiated and post-mitotic. In salamanders and tadpole frogs, acute injury to the retina stimulates the RPE cells to de-differentiate, proliferate and become neurogenic retinal progenitors (reviewed by Barbosa-Sabanero et al. 2012). In chicks and rodents, during early stages of embryonic development, immature RPE cells have the potential to generate retina. This regeneration includes all types of neurons segregated into appropriate laminae in a reversed polarity with photoreceptors near the vitread surface and ganglion cells near the sclerad surface. However, as the phenotype of RPE cells becomes well-established, the ability to form proliferating retinal progenitors is lost (Park and Hollenberg 1989; Park and Hollenberg 1991; Park and Hollenberg 1993).
Distinct from the RPE, are CMZ stem cells which are located at the far peripheral edge of the retina, at the transition between neural retina and non-pigmented epithelium of the ciliary body. CMZ stem cells have been identified in the eyes of frogs, fish and birds. CMZ stem cells may act as a source of neuronal regeneration, but this form of regeneration may be restricted to the fish and frog retina (Reh 1987; Reh and Tully 1986; Stenkamp et al. 2001); the chick CMZ does not contribute to retinal regeneration (Fischer 2005; Fischer and Reh 2000). However, during early stages of chick development, progenitors in far-peripheral retina or a primitive CMZ-like region are capable of regenerating retina (Haynes et al. 2007; Spence et al. 2004). The regenerative potential of CMZ stem cells is discussed in-depth by a review in this issue (Fischer, Bosse and El-Hodiri, 2013).
Within the past dozen years, Müller glia have been identified as a source of retinal regeneration in fish, chicks and rodents. Until recently, it was believed that rod progenitors or hard-to-find quiescent stem cells that are seeded within the inner nuclear layer across the mature retina were responsible for the regeneration of neurons in injured fish retina (Hitchcock et al. 2004; Otteson et al. 2002; Otteson and Hitchcock 2003; Raymond 1991; Raymond and Hitchcock 1997; Raymond and Hitchcock 2000; Raymond et al. 1988; Wu et al. 2001). However, it was recently shown that Müller glia give rise to rod progenitors (Bernardos et al. 2007), and that Müller glia de-differentiate and become proliferating neurogenic progenitors in the injured retina (Fausett and Goldman 2006; Fausett et al. 2008). The neurogenic potential of Müller glia was first identified in the chick retina (Fischer and Reh 2001a), and thereafter described in the rodent retina (Ooto et al. 2004). There is also some evidence that Müller glia from the primate retina can become progenitor-like cells in vitro (Limb and Daniels 2008; Limb et al. 2006), but the potential of Müller glia to regenerate neurons in the intact primate retina remains unexplored. The remainder of this review will focus upon Müller glia in the avian retina, their potential to become progenitor-like cells and compare the factors influencing Müller glia de-differentiation, proliferation and neurogenesis across species.
Definitions: Müller glia, stem cells and retinal progenitors
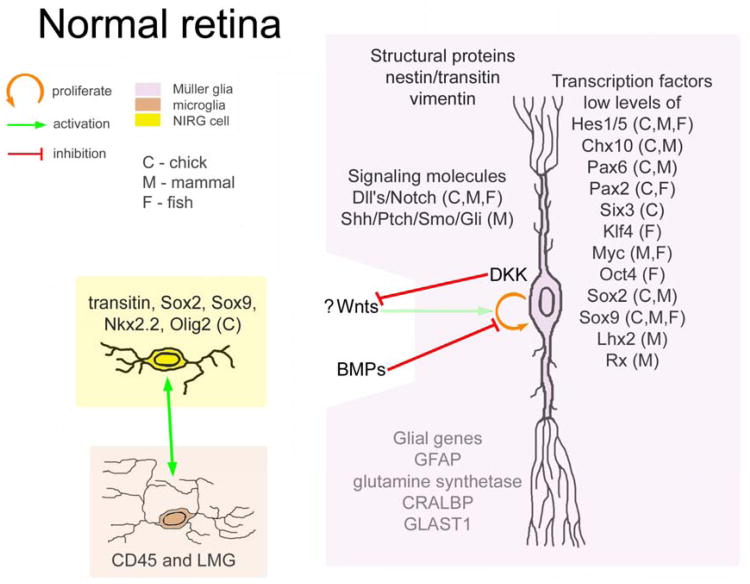
In normal healthy retina, Müller cells are the predominant type of retinal glia; these cells are abundant, have peripheral processes that densely infiltrate all retinal layers, and contribute significantly to retinal function. The nature of the Müller glia is clearly defined by structure, function and gene expression patterns (reviewed by Bringmann et al. 2009; Reichenbach and Bringmann 2013). The Müller glia function to provide structural support, metabolic support, ion homeostasis, and synaptic support (reviewed by Bringmann et al. 2009; Bringmann et al. 2006; Reichenbach and Bringmann 2013). Thus, Müller cells are bona fide glia by all measures of structure and function. However, it should be noted that normal Müller glia in rodent retina have significant transcriptome-overlap with that of retinal progenitors (Blackshaw et al. 2004; Roesch et al. 2008), and there appears to be a gradual transition in phenotype from neural progenitor to mature Müller glia during early postnatal retinal development (Nelson et al. 2011). Some of the signaling pathways and transcription factors that are maintained in normal Müller glia are listed in Figure 1. Nevertheless, Müller glia should not be referred to as stem cells, given that these glia do not function as stem cells in normal, healthy retina. Further, Müller glia have also been characterized as the “radial glia” of the retina based, at least in part, on their morphology and radially oriented processes that span the retina from outer to inner limiting membranes. Radial glia in the developing brain have been shown to function as progenitors, and provide “structural guides” for migrating and differentiating neurons (Noctor et al. 2001), functions that Müller glia do not provide during development, but can provide in a regenerating retina. Based on functional similarities such as structural, metabolic and synaptic support, Müller glia could be considered to be astrocyte-like cells (reviewed by Bringmann et al. 2009; Bringmann et al. 2006; Reichenbach and Bringmann 2013). The identity of a cell should be based on a combination of parameters, including function, morphology and gene expression profile, not on the potential functions of the cell. Müller glia may have the potential to become a stem or a progenitor cell with appropriate stimulation, but this potential does not define the Müller glia in normal retina. Furthermore, Müller glia have a limited potential to regenerate neurons in the avian and mammalian retina. Therefore, given their limited capacity for neurogenesis, it seems inappropriate to consider Müller glia as stem cells in warm-blooded vertebrates even when these cells are stimulated to de-differentiate and proliferate.
Figure 1.
Schematic diagrams summarizing the signaling, transcription factors and interactions between glial cells when the Müller glia have been stimulated to become progenitor-like. Legend: orange arrow – proliferation or transdifferentiate, green line – activate, red line – inhibit or suppress, magenta text – increased, blue text – decreased, C- chick, M – mammal, F - fish. Abbreviations: FGF2 – fibroblast growth factor 2, EGF - epidermal growth factor IGF1 – insulin-like growth factor 1, CNTF – ciliary neurotrophic factor, ascl1a – acheate schute-like 1a, MAPK – mitogen activation protein kinase, Dll – Delta-like ligand, LMG – lysosomal membrane glycoprotein, GFAP – glila fibrilliary acid protein, CRALBP – cellular retinaldehyde binding protein, GLAST1 – glutamate aspartate transporter 1.
Defining retinal regeneration
Retinal regeneration from Müller glia-derived progenitor cells (MGPCs) requires several cellular actions. These actions include (1) de-differentiation, (2) proliferation, (3) migration (4) neural differentiation, and (5) functional integration into retinal circuitry.
De-differentiation involves reversion to an immature state. In the case of Müller glia, these cells stop functioning as glia and acquire progenitor phenotype. No benchmark has been established to unambiguously indicate when or if Müller cells abandon glial functions and cell-distinguishing glial phenotypes in favor of becoming progenitor-like. Alternatively, the Müller glial may relinquish only some glial traits while maintaining others during the transition to a progenitor-like state. The suppression of different glial phenotypes during the transition to a progenitor-like state likely varies between different vertebrate classes. The glia-to-progenitor de-differentiation is most likely to be a continuum given that there is a gradual transition in phenotype from retinal progenitor to mature glial phenotype during postnatal rodent development (Nelson et al. 2011).
Proliferation is required during retinal regeneration to produce additional numbers of cells, including progeny to differentiate as new neurons and replacement of Müller glia to continue supporting neuronal function. Proliferation is a function of neural progenitors and is a symptom of glial de-differentiation; presumably glia must abandon some glial functions to re-enter the cell cycle. It is not evident whether proliferation occurs before, after or during glial de-differentiation. However, glial proliferation can also contribute to scar formation, and it is likely that much of the proliferation of Müller glia in damaged avian and mammalian retinas is part of scar formation.
Migration of dividing progenitors and post-mitotic neural precursors is a necessary step for retinal regeneration. Clearly, functional retinal regeneration requires that different types of neurons that are situated in different retinal layers are replaced; this requires migration of MGPCs and differentiating progeny to the appropriate retinal location. In the regenerating zebrafish retina, for example, the selective ablation of photoreceptors recruits progenitors to the ONL (Vihtelic and Hyde 2000), whereas ablation of inner retinal neurons retains MGPCs within the INL to replace amacrine cells and GCL to replace ganglion cells (Fimbel et al. 2007). These correlative findings are supported by functional studies during development which have demonstrated that migration of retinal progenitors is required for normal proliferation and neurogenesis (Del Bene et al. 2008; Leung et al. 2011; Norden et al. 2009). In the chick retina, the nuclei of Mülller glia and proliferating MGPCs are commonly observed delaminating away from the center of the INL to spread across the INL and into the ONL and the GCL when stimulated by acute damage or growth factors (Fischer et al. 2002; Fischer and Reh 2001a; Fischer and Reh 2002; Fischer et al. 2009a; Ghai et al. 2010). Similarly, nuclear migration occurs in the damaged rodent retina wherein MGPCs delaminate away from the center of INL, and migrate across the INL and into the ONL (Karl et al. 2008; Ooto et al. 2004; Osakada et al. 2007). However, it seems likely that the vast majority of this migration of MGPCs in chick and rodent retinas entails interkinetic nuclear migration, similar to that which occurs in developing neuroepithelia, and not migration of post-mitotic neuronal cells.
Neural differentiation requires that Müller glia-derived cells fully differentiate into neurons. Expression of a few neuronal proteins by Müller glia-derived cells is insufficient; there are examples of Müller glia and glia-derived cells expressing “neuronal” proteins while maintaining glial phenotype (Fischer et al. 2004a; Fischer et al. 2004b). The differentiation of Müller glia-derived progeny must be sufficient to permit neuronal function and abandon of glial phenotype. Integration into retinal circuitry requires that newly-generated neurons establish pre- and post-synaptic connections that participate in retinal function. Regenerated neurons in the fish retina have been shown to form synapses (Hitchcock and Cirenza 1994; Stuermer and Easter 1984). The establishment of functional synapses must occur for the restoration of visual function (Fimbel et al. 2007; Lindsey and Powers 2007; Mensinger and Powers 1999; Mensinger and Powers 2007; Sherpa et al. 2008). Currently, evidence demonstrating functional integration of Müller glia-mediated regeneration of neurons in birds and mammals is lacking.
Signaling pathways and the formation of Müller glia-derived progenitors
The formation of MGPCs in damaged retinas is driven by signals that originate from damaged or dying neurons. The identities of the signals that originate from damaged neurons to initiate the de-differentiation of Müller glia remain uncertain. However, a number of secreted factor and signaling-pathways have been identified that influence the formation of MGPCs and the regeneration of neurons. One of these factors is Epidermal Growth Factor (EGF). EGF-signaling is important during retinal histogenesis. During postnatal development, EGF-signaling promotes Müller glial fate and stimulates the proliferation of Müller glia (Lillien 1995). After this early developmental window, the proliferation-inducing efficacy of EGF decreases in parallel with a decline in EGF receptor expression in Müller glia (Close et al. 2006). However, following light-induced damage Müller glia re-express the EGF receptor, and exogenous EGF regains the ability to stimulate Müller glia to proliferate (Close et al. 2006). Similarly, in N-Methyl-D-Aspartate (NMDA) -damaged rodent retina, EGF can stimulate Müller glia to de-differentiate, proliferate, and differentiate into inner retinal neurons (Karl et al. 2008). EGF in the uninjured retina is unable to stimulate Müller glia to become progenitor-like cells in zebrafish, chicken and mouse (Fischer and Reh 2000; Fischer and Reh 2003; Karl et al. 2008; Wan et al. 2012). In the chick retina, EGF alone or in combination with IGF1/insulin does not influence the proliferation of MGPCs (Fischer and Reh 2003). Instead, EGF alone stimulates the proliferation of CMZ progenitors (Fischer and Reh 2000), and when combined with insulin acts synergistically to stimulate the proliferation of progenitor-like cells in non-pigmented epithelium of the pars plana, immediately anterior to the CMZ (Fischer and Reh 2003). By comparison Heparin-Binding EGF-like growth factor (HB-EGF) has recently been described to stimulate Müller glia to become multi-potent progenitors in the uninjured zebrafish retina (Wan et al. 2012). HB-EGF stimulates the Müller glia via the Mitogen-Activated Protein Kinase/extracellular-signal-regulated kinase (MAPK/ERK) pathway, as well as activating Notch- and Wnt-signaling pathways (Wan et al. 2012). In damaged retina, the HB-EGF appears to be produced by Müller glia to act in a paracrine/autocrine manner (Wan et al. 2012); the signals produced by the damaged neurons that are up-stream of HB-EGF expression in Müller glia remain unknown. The effects of HB-EGF on Müller glia in the chick and rodent retina remain unknown.
FGF, IGF1, insulin and Mitogen-Activated Protein Kinase Signaling
Activation of the MAPK pathway may be a requirement common to MGPCs in fish, chick and rodent retinas. In acutely damaged chick retina, Müller glia transiently activate branches of the MAPK pathway, pERK1/2 and p38 MAPK, and downstream targets, Egr1, cFos and pCREB (Fischer et al. 2009a). Similarly, in the mammalian retina, NMDA-induced damage leads to Müller glia-specific accumulation of pERK1/2 and cFos (Nakazawa et al. 2008). In NMDA-damaged avian retina, interference with MAPK using small molecule inhibitors of MEK or FGF-receptors suppresses the proliferation of MGPCs (Fischer et al. 2009a). Inhibition of MEK or FGF-receptors also prevents the accumulation of Egr1 and pCREB in de-differentiating Müller glia (Fischer et al. 2009a). Interestingly, Egr1 and pCREB are normally expressed by retinal progenitors in the CMZ, whereas cFos and pERK1/2 are not (Fischer et al. 2009b). These findings suggest that activation of FGF-receptors, ERK1/2-pathway, Egr1 and pCREB promotes the de-differentiation of Müller glia and proliferation of MGPCs.
In the chick retina, the combination of FGF and insulin/IGF stimulates the formation of MGPCs (Fischer et al. 2002; Ritchey et al. 2012). Although 3 consecutive daily injections of insulin or FGF2 alone have no effect, the combination of insulin and FGF2 stimulates Müller glia to express transcripts common to retinal progenitors, and initiate a wave of proliferating MGPCs that begins at the retinal margin and spreads toward central retina (Fischer et al. 2002). Similar to the outcome in acutely damaged retinas, most of the newly formed cells remain as undifferentiated progenitor-like cells, whereas some differentiate into Müller glia, and a few (∼5%) differentiate into neurons. At least 3 consecutive daily injections of insulin and FGF2 are required to stimulate the formation of MGPCs, indicating that sustained activation is required (Fischer et al. 2002). The combination of IGF1 and FGF2, similar to insulin and FGF2, stimulates the formation of proliferating MGPCs, but with the added complication of transiently elevating intraocular pressure and damage to ganglion cells (Ritchey et al. 2012).
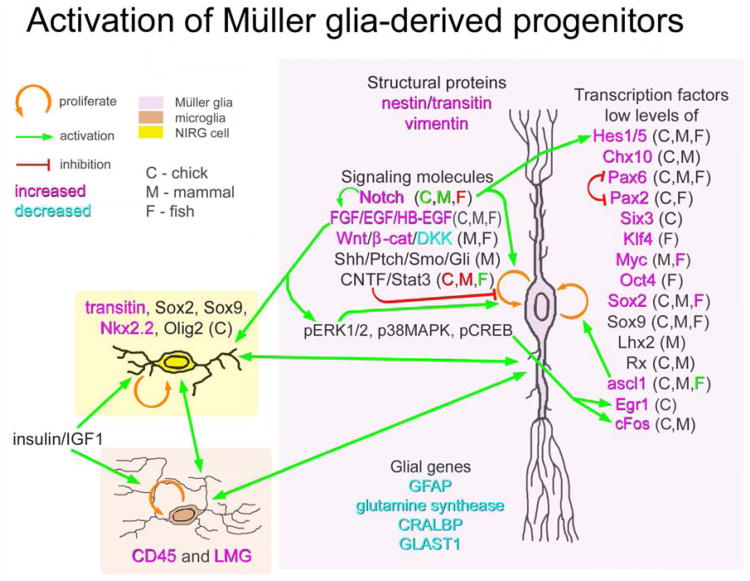
In the chick retina, FGF2 appears to primarily influence the Müller glia, whereas insulin and IGF1 appear to primarily influence the non-astrocytic inner retinal glial (NIRG) cells and microglia (Fig. 2; (Fischer et al. 2009a; Fischer et al. 2010a). FGF2 exerts its effects directly on Müller glia through the MAPK pathway (Fischer et al. 2009b). In support of this notion, intraocular injections of FGF2 stimulate the phosphorylation of ERK1/2, p38 MAPK, and CREB, and the expression of immediate early genes, cFos and Egr1, selectively in Müller glia, whereas neurons do not respond (Fischer et al. 2009a). By comparison, intraocular injections of insulin or IGF1 stimulate the reactivity of microglia and NIRG cells (Fischer et al. 2009a; Fischer et al. 2010a). FGF2 induces the formation of proliferating MGPCs in moderately damaged retinas, whereas insulin has no effect (Fischer et al. 2009a). Collectively, these findings indicate that MAPK-signaling through FGF receptors stimulates Müller glia to become more progenitor-like, whereas insulin acting at microglia and NIRG cells provides additional signals to stimulate the formation of MGPCs. Similar to the findings in the chick retina, the combination of insulin and FGF1 stimulates the formation of MGPCs in the damaged mammalian retina (Karl et al. 2008). In zebrafish, the progenitor-inducing effects of HB-EGF upon Müller glia are mediated through MAPK-signaling (Wan et al. 2012). Thus, activation of MAPK-signaling appears to be a requirement for the formation of MGPCs across vertebrate species.
Figure 2.
Schematic diagram summarizing the some of the genes (structural proteins, signaling pathways and transcription factors) that are expressed by normal Müller glia that may be related to maintaining progenitor-like properties. Wnts (from unidentified source) are blocked by Dkks (highly expressed by Müller glia) and BMPs from inner retinal neurons likely act to keep Müller glia post-mitotic. Legend: orange arrow – proliferation or transdifferentiate, green line – activate, red line – inhibit or suppress, C-chick, M – mammal, F - fish. Abbreviations: LMG – lysosomal membrane glycoprotein, GFAP – glila fibrilliary acid protein, CRALBP – cellular retinaldehyde binding protein, GLAST1 – glutamate aspartate transporter 1, Dll – delta-like ligand, Ptch – patched, Smo - smoothened.
Sonic Hedgehog (Shh) and Hedgehog (Hh) -signaling
Shh is a pleiotropic factor with numerous context-specific functions in the developing nervous system and in the developing eye. In the developing retina, Hh-signaling has many different roles including: (1) mitogen for retinal progenitors, (2) regulates the numbers of ganglion cells, (3) induces the formation of optic nerve glia, (4) promotes proper lamination, (5) promotes neuronal survival, (6) promotes the differentiation of the RPE, and (6) supports Müller glial differentiation (reviewed by Amato et al. 2004; Stadler et al. 2004; Wallace 2008; Yang 2004). Despite numerous important functioning during eye development, almost nothing is known about Hh-signaling in the mature retina and Müller glia. Addition of Shh in the uninjured mouse retina does not influence the Müller glia (Wan et al. 2007). However, in the N-methyl N-nitrosourea (MNU) -damaged mouse retina, intraocular injections of Shh stimulate Müller glia to de-differentiate and express progenitor markers, and inhibition of Hh-signaling prevents Müller glial dedifferentiation and acquisition of progenitor markers (Wan et al. 2007). There is evidence that Hh-signaling is maintained in mature Müller glia. Microarray data from single or sorted rodent Müller glia indicate expression of patched(ptch; integral membrane receptor for Hh and antagonist of smo), smoothened (smo; a co-receptor and activator of intracellular signaling), and Gli's (transcription adaptors of Hh-signaling) (Nelson et al. 2011; Roesch et al. 2008; Roesch et al. 2012). The influence of Hh-signaling on mature Müller glia and MGPCs in the avian and fish retina remains uncertain.
Notch Signaling
Notch-signaling is required for normal histogenesis throughout retinal development. During early stages of development in chick and rodent retina, Notch-signaling maintains progenitors in a proliferative, undifferentiated state (Furukawa et al. 2000; Jadhav et al. 2006a; Jadhav et al. 2006b; Nelson et al. 2011). During later stages of retinal development, Notch-signaling promotes the formation of Müller glia (Furukawa et al. 2000; Nelson et al. 2011). In developing zebrafish retina, elevated Notch-signaling inhibits neuronal differentiation, leading to progenitors taking on a glial fate or remaining undifferentiated (Scheer et al. 2001). In maturing retinas, where the cells are post-mitotic, Notch expression is highest in the peripheral retina and expression decreases as the retina matures (Bao and Cepko 1997; Ghai et al. 2010; Nelson et al. 2011). Notch-signaling is maintained at relatively low levels in mature Müller glia in uninjured chick retinas (Ghai et al. 2010; Hayes et al. 2007). Similarly, Notch-signaling is maintained in mature Müller glia in the zebrafish retina (Bernardos et al. 2005). Mature Müller glia in the rodent retina express the Notch-related genes Dll3, Dll4, Hes1 and Hes5, indicating that post-mitotic Müller glia maintain Notch-signaling (Furukawa et al. 2000; Nelson et al. 2011). The function of sustained Notch-signaling in the mature Müller glia in the rodent retina remains uncertain, but during the first 2 weeks of postnatal development Notch-signaling is required to establish glial phenotype (Nelson et al. 2011). Inhibition of Notch-signaling in mature chick retina does not overtly influence the phenotype of the Müller glia, but does diminish the ability of these glia to protect retinal neurons from acute damage (Ghai et al. 2010).
After retinal injury, elements of the Notch pathway are up-regulated in the zebrafish, avian and mammalian retina. In zebrafish, retinal injury results in up-regulation of deltaC, notch1 and notch3 (Raymond et al. 2006; Yurco and Cameron 2005). NMDA-induced damage to the chicken retina leads to up-regulation of Notch1 and its target Hes5 in Müller glia (Ghai et al. 2010; Hayes et al. 2007). Notch-signaling is up-regulated in MGPCs and inhibition of Notch prevents the proliferation of the MGPCs (Hayes et al. 2007). Inhibition of Notch after MGPCs have progressed through the cell cycle, increases numbers of differentiated neurons (Hayes et al. 2007). In the chick retina, the combination of insulin and FGF2 stimulates MGPCs to proliferate without incurring retinal damage, and these factors act, at least in part, through the Notch-pathway. Intraocular injections of insulin and FGF2 increase Notch-signaling, and inhibition of Notch prevents the formation of MGPCs in response to insulin and FGF2 (Ghai et al. 2010). Notch is required for MAPK-signaling, as Notch-inhibition prevents FGF2-induced accumulation of p38 MAPK and pCREB, and the formation of MGPCs in mildly damaged retinas (Ghai et al. 2010). In mice, EGF treatment after NMDA damage leads to an increase in MGPC proliferation and elevated expression of Notch1 and Delta1 (Karl et al. 2008). Using in vitro neurosphere assays, Notch-signaling has been shown to promote the formation of Müller glia-derived spheres from rodent retina (Das et al. 2006). Taken together, these results suggest Notch is an important component in the formation of MGPCs. These findings indicate that low levels of Notch-signaling are maintained in mature Müller glia, elevated levels of Notch-signaling are required for the de-differentiation Müller glia and proliferation of MGPCs, and inhibition of Notch can increase neuronal differentiation of the glia-derived progeny.
Interestingly, the roles of Notch-signaling in MGPCs appear to be starkly different in the fish retina compared to the roles seen in the MGPCs of birds and mammals. Similar to birds and rodents, Notch-signaling is activated in zebrafish MGPCs after retinal injury (Wan et al. 2012). In contrast to birds, inhibition of Notch expands the zone of proliferating MGPCs, whereas increases in Notch-signaling suppress the formation of MGPCs in the injured retina of zebrafish (Wan et al. 2012). Further, activation of Notch in damaged zebrafish retina biases MGPCs to take on a photoreceptor fate at the expense of neurons and Müller glia (Wan et al. 2012). Thus, Notch-signaling plays roles in MGPCs of zebrafish that are distinct from the roles of Notch in MGPCs and embryonic retinal progenitors in birds and mammals. It remains to be determined whether differences in the expression of Hes-related genes that are down-stream of Notch-signaling underlie differences in the roles of Notch in fish, birds and mammals.
Wnt-signaling
Similar to the MAPK-pathway, activation of Wnt-signaling may be common across species and important to the formation of MGPCs. In normal retina, Müller glia are known to express Dkk's which are secreted to sequester Wnt-ligands (Fig.1; Roesch et al. 2008; Nelson et al., 2011; Ramachandran et al. 2011). In damaged zebrafish retinas, canonical Wnt-signaling has been shown to enhance the formation of MGPCs (Meyers et al. 2012; Ramachandran et al. 2011). In addition, activation of Wnt-signaling with a GSK3-inhibitor leads to the formation of MGPCs in the uninjured zebrafish retina (Ramachandran et al. 2011). The induction of Ascl1a in Müller glia leads to activation of the Wnt pathway by inducing expression of Wnt ligands and suppressing the Wnt inhibitor Dkk, thereby enabling MGPCs to proliferate (Ramachandran et al. 2011). In addition, HB-EGF partially exerts its mitogenic effects by acting upstream of the Wnt-pathway (Wan et al. 2012). Consistent with findings in the fish retina, Wnt/β-catenin signaling promotes the formation of MGPCs in vitro and in vivo in the mammalian retina. Components of the Wnt-signaling pathway are expressed in rodent Müller glia (Jadhav et al. 2009; Roesch et al. 2008). In retinal explants, Wnt3a and inhibition of GSK3β increases the proliferation of MGPCs (Osakada et al. 2007). Neurospheres derived from enriched Müller glia showed increased proliferation when cultured in the presence of the Wnt agonist Wnt2b (Das et al. 2006). However, in vivo injections of Wnt2b in the kainate-damaged mouse retina only led to a modest albeit significant increase in proliferating MGPCs (Das et al. 2006). In Axin2-/- mice, where Wnt-signaling is enhanced because of the loss a negative regulator, the retina contains a subpopulation of Müller glia that are Wnt-responsive and proliferate at elevated levels (Liu et al. 2012). It remains uncertain whether Wnt-signaling influences the formation of MGPCs in the chick retina. In summary, Wnt-signaling acts to enhance the progenitor-like properties of the Müller glia and enhance retinal regeneration.
Ciliary Neuronotrophic Factor (CNTF) and Jak/Stat signaling
CNTF may have different effects upon Müller glia in fish compared to those seen in birds and mammals. CNTF is known to predominantly act through the Jak/Stat pathway and is known to influence the Müller glia in different vertebrate species. In uninjured fish retina, CNTF has been shown to stimulate the proliferation of a subset of MGPCs via the Stat3-pathway (Nelson et al. 2012), whereas CNTF-mediated activation of the MAPK-pathway protects photoreceptors from light-induced damage (Kassen et al. 2009). This is in contrast to the effects of CNTF in the avian and rodent retina. In the rodent and chick retinas, CNTF/Jak/Stat-signaling appears to stimulate glial reactivity (up-regulation of GFAP) and enhance neuroprotection (Fischer et al. 2004a); (Kahn et al. 1997; Peterson et al. 2000; Wang et al. 2002), instead of stimulating the formation of proliferating MGPCs. In the chick retina, CNTF applied before NMDA-induced damage is potently neuroprotective, with no indication of activation of the MAPK-pathway (Fischer et al. 2004a). Furthermore, CNTF applied after NMDA-induced damage suppresses the formation of proliferating MGPCs (Fischer et al. 2004a). Taken together, these findings indicate that the effects of CNTF on Müller glia differ between cold- and warm-blooded vertebrates. However, zebrafish do not have a well-defined orthologue to CNTF. This suggests that signaling through receptors for other class-I cytokines such as Leukemia inhibitory factor or interleukin 6 could mediate the effects of CNTF on zebrafish Müller glia.
Stem cell transcription factors and Müller glia
Transcription factors are coordinated with the secreted factors and signaling pathways to regulate the stem cell-like properties of Müller glia. Transcription factors play a pivotal role in enabling the formation of MGPCs. Compelling examples of the important role of transcription factors in promoting “stemness” have been provided by studies regarding embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). It has been well established that a small set of key transcription factors, including Sox2 (Avilion et al. 2003), Oct3/4 (Nichols et al. 1998; Niwa et al. 2000), Nanog (Chambers et al. 2003; Mitsui et al. 2003), Klf4 (Takahashi and Yamanaka 2006), and c-myc (Cartwright et al. 2005) are essential for maintaining self-renewal and pluripotency of undifferentiated stem cells. Sox2 and Nanog positively regulate transcription of all pluripotency factors in the LIF (Leukemia inhibitory factor) signaling pathway, which maintains the “stemness” of mouse embryonic stem cells (Niwa et al. 2009). Furthermore, Sox2 combined with Oct4, c-myc and Klf4 are sufficient for producing iPSCs from fibroblasts (Takahashi and Yamanaka 2006). Interestingly, Müller glia express some of these key stem cell transcription factors. In normal conditions, the Müller glial of avian and mammalian retinas express Sox2, and continue to express Sox2 when stimulated to become MGPCs (Fischer et al. 2009a; Fischer et al. 2010b). A microarray analyses has demonstrated that Müller glia express n-myc in early postnatal mice, and Klf's, including Klf4, Klf6, Klf9 and Klf10, are up-regulated during Müller glial maturation (Nelson et al. 2011). Interestingly, c-myc, sox2, nanog and oct4 may be expressed by MGPCs in regenerating zebrafish retina. Evidence suggests that let-7 is expressed by Müller glia to repress expression of retinal levels of c-myc and oct4, and this repression is alleviated in damaged retina where MGPCs are formed (Ramachandran et al. 2010). In zebrafish, RT-PCR analyses have shown that the pluripotency factors klf4, oct4 and c-mycA are expressed in uninjured retina, and are transiently increased around 15 hours post damage (Ramachandran et al. 2010). In contrast, in the uninjured retina, pluripotency factors sox2, nanog and c-mycB are undetectable, but are induced between 15 and 48 hours after injury (Ramachandran et al. 2010). Unlike Müller glia in the fish retina, Sox2 is normally expressed by mature Müller glia in avian and mammalian species, including primates (Fischer et al. 2010b). It remains uncertain whether Nanog, Klf4 or Oct4 are expressed by Müller glia or MGPCs in the avian or rodent retina.
Transcription factors common to retinal progenitors, Müller glia and MGPCs
It has been well established that retinal stem cells are multipotent, giving rise to all cell types during retinal histogenesis (Holt et al. 1988; Jensen and Wallace 1997; Turner and Cepko 1987; Turner et al. 1990; Wetts and Fraser 1988; Wong and Rapaport 2009). Transcription factors play a major role in endowing retinal progenitors with the capabilities of proliferation and multipotency. It has been shown that the eye-field transcription factors Rx1, Pax6, Six6, Six3, Tlx, Lhx2, and ET are involved in the establishing retinogenic competence among neuroepithelial cells in Xenopus (Zuber et al. 2003), although subtle differences may be present in different vertebrates. Common to vertebrates, Pax6, Rx1, Six3/6 and Lhx2 and Otx2 are expressed in the retinal anlage preceding the formation of the optic sulci (Crossley et al. 2001; Furukawa et al. 1997; Jean et al. 1999; Kenyon et al. 2001; Li et al. 1994; Mathers et al. 1997; Oliver et al. 1995; Walther and Gruss 1991), and Pax6, Rx1, Six3/6 and Lhx2 continue to be present during subsequent steps of retinal neurogenesis (Chow et al. 1999; Jean et al. 1999; Loosli et al. 1999; Mathers et al. 1997; Oliver et al. 1995; Walther and Gruss 1991; Zuber et al. 1999). Combinations of homeodomain and basic-Helix-Loop-Helix (bHLH) transcription factors are required for fate determination of different retinal cell types (reviewed by Marquardt 2003). For instance, Pax6 is necessary for the expression of bHLH transcription factors during the specification multiple retinal cell types (reviewed by Marquardt and Pfaff 2001).
Many of the eye-field transcription factors have been detected in Müller glia and MGPCs. The transcriptome of Müller glia, unlike the transcriptome of retinal neurons, overlaps significantly with that of retinal progenitors (Blackshaw et al. 2004; Nelson et al. 2011; Roesch et al. 2008). In avian and mammalian retinas, Müller glia normally express the transcription factors Sox2 and Sox9, which are also expressed by retinal stem cells (Fischer and Bongini 2010; Fischer et al. 2009a; Fischer et al. 2009b; Hayes et al. 2007; Poche et al. 2008). In addition, normal Müller glia in the chick retina express low levels of Pax6, Six3 (Fischer 2005), Hes1 and Hes5 (Ghai et al. 2010). In response to acute retinal damage Müller glia begin to express and up-regulate transcription factors found in embryonic retinal progenitors. These transcription factors include Ascl1a, Pax6, Chx10 (Fischer and Reh 2001a), Six3 (Fischer 2005), and the Notch-related factors Hes1 and Hes5 (Ghai et al. 2010; Hayes et al. 2007). Lhx2 is expressed by both retinal progenitors and mature Müller glia. Lhx2 may convey progenitor-like properties to Müller glia; the loss of Lhx2 in mature Müller glia results in non-proliferative reactive phenotype (de Melo et al. 2012). Rx, an eyefield-enriched homeobox gene is known to induce Hes1 and to play an instructive role in the differentiation of Müller glia (Furukawa et al. 2000). There is evidence that Rx is required for regeneration of larval Xenopus retina (Martinez-De Luna et al. 2011). However, the cellular source of retinal regeneration in Xenopus may not be Müller glia, and it remains uncertain whether Rx is expressed by MGPCs in birds and mammals.
Several studies have been centered on understanding the roles of transcription factors that are expressed by MGPCs. In zebrafish, Pax6a and Pax6b are required at different times to facilitate the proliferation of MGPCs (Thummel et al. 2010). Pax6 is up-regulated in MGPCs in rodent and avian retinas (Fischer and Reh 2001a; Karl et al. 2008; Ooto et al. 2004) and is assumed to be necessary for the formation of MGPCs. In zebrafish, the bHLH factor Ascl1a is necessary for Müller glial de-differentiation and retina regeneration (Fausett et al. 2008). The function of Ascl1 likely differs between the MGPCs in avian and zebrafish retina. In zebrafish Müller glia, ascl1a is expressed within 4hrs of injury and is required in the initial steps of Müller glial de-differentiation (Fausett et al. 2008; Ramachandran et al. 2010). By comparison, chick MGPCs express Ascl1 about 48 hrs after injury (Fischer and Reh 2001a) and peaks at 4 days after injury (Hayes et al. 2007), long after the onset of injury-induced MAPK-signaling in MGPCs has been observed (Fischer et al. 2009a; Fischer et al. 2009b). Recent studies have shown that gene repression is important for retinal regeneration. In zebrafish, the transcription factor Insm1a acts as repressor that functions to keep pro-progenitor factors in check in the MGPCs (Ramachandran et al. 2012). In the embryonic mouse, Insm1 is expressed by retinal progenitors and nascent neurons (Duggan et al. 2008). However, it remains uncertain whether Insm1a or related genes are expressed by mature Müller glia or are involved in the formation of MGPCs in the retinas of birds and mammals.
In the chick retina, Pax2 is expressed by mature Müller glia in central regions; the expression of Pax2 is gradually decreased by Müller glia with increasing distance toward the periphery (Boije et al. 2010; Stanke et al. 2010). The expression levels of Pax2 are increased by acute retinal damage or treatment with insulin and FGF2 (Stanke et al. 2010). In addition, Pax2 is expressed by most Müller glia in central regions of the lizard retina (Romero-Aleman et al. 2012), and some of the Müller glia in central regions of the zebrafish retina (Boije et al. 2010). Pax2 is not expressed by Müller glia in the mammalian retina (Stanke et al. 2010). Given that Pax2 is known to negatively regulate Pax6, induce glial cell fate (Graw 1996; Schwarz et al. 2000), and that Müller glia in central regions of the chick have a diminished capacity to form MGPCs (Fischer et al. 2002; Fischer and Reh 2001a), it is possible that Pax2 negatively regulate the plasticity of Müller glia and inhibits the formation of MGPCs (Fig. 2). The roles of Pax2 in Müller glia and MGPCs remain to be functionally examined.
What retinal cells influence the ability of Müller glia to become progenitors?
The cellular signals that initiate the cascade of events that lead to glia-mediated retinal regeneration remain uncertain. It remains unknown whether stressed neurons produce factors that directly stimulate the formation of MGPCs and/or whether reactive microglia initiate the de-differentiation of Müller glia in damaged retinas. There is evidence to suggest that the activity of microglia, astrocytes, astrocyte-like cells and Müller glia is coordinated in normal and damaged retinas (Harada et al. 2002; Zelinka et al. 2012). It is possible that NIRG cells influence the ability of Müller glia to become proliferating progenitor-like cells. The NIRG cells have been described as being distinctly different from retinal astrocytes (Fischer et al. 2010a), NIRG-like cells may be present in the retinas of canines and primates but not rodents (Fischer et al. 2010b), and these glial cells originate from optic nerve progenitors during embryonic development (Rompani and Cepko 2010). The reactivity of NIRG cells is stimulated by neuronal damage and NIRG cell reactivity can exacerbate retinal damage, resulting in retinal folds wherein the Müller glia are absent (Fischer et al. 2010a). Furthermore, the survival and reactivity of the NIRG cells has been directly linked to microglia (Zelinka et al. 2012). In addition, in damaged retinas and in undamaged retinas where MGPCs have been generated, both the NIRG cells and microglia are coincidently activated (Fischer 2011; Fischer et al. 2002; Fischer et al. 2009b; Fischer et al. 2010a; Zelinka et al. 2012). In the developing zebrafish retina, the ablation of microglia results in microphthalmia, delayed cell cycle exit of retinal progenitors and diminished neuronal differentiation, indicating that microglia are required for normal retinal histogenesis (Huang et al. 2012). Collectively, these data suggest that coordinated activity of microglia, astrocytes and NIRG cells may influence the formation of MGPCs (Fig. 2), but definitive functional studies are currently lacking.
Conclusions
Is there a realistic potential for neuron replacement in mature, damaged human retina? On-going research to enhance the neurogenic potential of MGPCs provides some hope that the Müller glia in warm-blooded vertebrate could be stimulated to result in meaningful neuronal regeneration. It is possible that the replacement of photoreceptors from MGPCs is a viable goal. This notion is supported by a significant understanding of the mechanisms that guide photoreceptor differentiation (Hennig et al. 2008) and data from transplantation studies indicating that functional photoreceptors can be incorporated into degenerating retinas (Barber et al. 2012; MacLaren et al. 2006; Pearson et al. 2012; West et al. 2012). It seems less likely that Müller glia could replace functional ganglion cells, given the requirement for axonal growth and target acquisition. This notion is consistent with studies that have examined axonal regeneration following optic nerve damage. In these studies significant axonal growth can be induced by inactivating PTEN and SOCS3, but the axonal growth appears to be random and unguided, with no evidence of meaningful connections in higher visual centers (Park et al. 2008; Sun et al. 2011). There is some evidence that ganglion cells can be produced by MGPCs (Fischer and Reh 2002), but it seems unlikely that the regenerated ganglion cells produced axon and terminal field that establish functional connections in higher visual centers. By comparison, axonal regeneration is robust within the damaged optic nerve of fish (Becker and Becker 2008). Thus, neuronal regeneration in the fish is expected to include replacement of functional ganglion cells accompanied with appropriate axonal growth and target acquisition in higher visual centers of the brain (Fimbel et al. 2007; Sherpa et al. 2008).
Generating numerous proliferating MGPCs does not appear to be a significant obstacle. Many secreted factors, including Wnt's, FGF's, EGF and IGF1/insulin, can be applied to induce significant numbers of proliferating MGPCs. However, few of the progeny differentiate into retinal neurons in vivo in warm-blooded vertebrates (Fischer et al. 2002; Fischer and Reh 2001a; Karl et al. 2008). Thus, the major obstacle to functional restoration of vision from MGPCs in warm-blooded vertebrates appears to be enhancing the neuronal differentiation of the progeny of MGPCs. The environment provided by mature, damaged retina may not permit neuronal differentiation (Stanke and Fischer 2010). Thus, strategies to enhance the differentiation of MGPCs will need to be devised. Several studies have investigated whether the forced-expression of neurogenic bHLH transcription factors, such as NeuroD and Ngn2, can enhance the number of Müller glia-derived progeny that differentiate as neurons. These studies have demonstrated a tendency to enhance neuronal differentiation, but the majority of MGPC-progeny remain undifferentiated, or ectopically express neuronal proteins while retaining glial phenotype (Fischer et al. 2004b; Ooto et al. 2004). A recent study by Pollak and colleagues, has indicated that forced expression of Ascl1 in rodent significantly enhances the neurogenic ability of MGPCs to produce progeny that significantly differentiate toward a mature neuronal phenotype (Pollak et al. 2013). Another strategy has been to inhibit Notch-signaling after the formation of proliferating MGPCs. This study by Hayes and colleagues demonstrated increased neuronal differentiation, but the majority (∼90%) of MGPC-progeny remained undifferentiated (Hayes et al. 2007). It remains uncertain what fundamental cellular properties in fish Müller glia are lacking, or are inhibited, in chick/rodent Müller glia that enable widespread neuronal differentiation from MGPCs. Some studies have suggested that miRNA-regulated clusters of genes or epigenetic plasticity are important for the regenerative potential of Müller glia in fish (Ramachandran et al. 2010; Ramachandran et al. 2012). However, currently nothing is known about epigenetic and miRNA-imposed restrictions upon the plasticity of Müller glia in warm-blooded vertebrates.
Given the irreversible nature of degenerative retinal diseases in humans, it is obvious that the spontaneous neuro-regenerative capacity of human Müller glia is minimal or non-existent with progressive retinal deterioration. Nevertheless, understanding the mechanisms that stimulate the formation of neurogenic MGPCs in experimental model systems could lead to treatments that stimulate Müller glia-mediated neuronal regeneration in degenerating retinas.
This manuscript reviews the current state of knowledge regarding the potential of Müller glia to become neuronal progenitor cells in the avian retina.
We compare and contrast the remarkable neurogenic capacity of Müller glia in the fish retina to the limited capacity of Müller glia in avian and rodent retinas.
We discuss and summarize findings regarding the secreted factors, signaling pathways and cell intrinsic transcription factors that have been implicated in the formation of Müller glia-derived progenitors.
We discuss similarities and differences between the fish, rodent and chick model systems, with emphasis on several of the key transcription factors and signaling pathways that regulate the formation of Müller glia-derived progenitors.
Acknowledgments
This work was supported by a grant (EY022030-01) from the National Institutes of Health. We thank Christopher Paul Zelinka for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato MA, Boy S, Perron M. Hedgehog signaling in vertebrate eye development: a growing puzzle. Cell Mol Life Sci. 2004;61(7-8):899–910. doi: 10.1007/s00018-003-3370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17(1):126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17(4):1425–34. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K, Luhmann UF, Lakowski J, Sowden JC, Ali RR. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2012;110(1):354–9. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: new perspectives from model organisms. Biochem J. 2012;447(3):321–34. doi: 10.1042/BJ20120813. [DOI] [PubMed] [Google Scholar]
- Becker CG, Becker T. Adult zebrafish as a model for successful central nervous system regeneration. Restor Neurol Neurosci. 2008;26(2-3):71–80. [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev Biol. 2005;278(2):381–95. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2(9):E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boije H, Ring H, Lopez-Gallardo M, Prada C, Hallbook F. Pax2 is expressed in a subpopulation of Muller cells in the central chick retina. Dev Dyn. 2010;239(6):1858–66. doi: 10.1002/dvdy.22309. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28(6):423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132(5):885–96. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126(19):4213–22. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54(2):94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12(1):79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature. 1970;228(271):559–60. doi: 10.1038/228559a0. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108(2):183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299(1):283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- de Melo J, Miki K, Rattner A, Smallwood P, Zibetti C, Hirokawa K, Monuki ES, Campochiaro PA, Blackshaw S. Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc Natl Acad Sci U S A. 2012;109(12):4657–62. doi: 10.1073/pnas.1107488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134(6):1055–65. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A, Madathany T, de Castro SC, Gerrelli D, Guddati K, Garcia-Anoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol. 2008;507(4):1497–520. doi: 10.1002/cne.21629. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26(23):6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28(5):1109–17. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27(7):1712–24. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24(2):161–82. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Muller glia, vision-guided ocular growth, retinal stem cells, and a little serendipity: the cogan lecture. Invest Ophthalmol Vis Sci. 2011;52(10):7705–10. doi: 10.1167/iovs.11-8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Bongini R. Turning Muller glia into neural progenitors in the retina. Mol Neurobiol. 2010;42(3):199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22(21):9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001a;4(3):247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Transdifferentiation of pigmented epithelial cells: a source of retinal stem cells? Dev Neurosci. 2001b;23(4-5):268–76. doi: 10.1159/000048710. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251(2):367–79. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259(2):225–40. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004a;27(4):531–42. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009a;57(14):1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009b;57(2):166–81. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Muller glia. Glia. 2010a;58(6):633–49. doi: 10.1002/glia.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Wang SZ, Reh TA. NeuroD induces the expression of visinin and calretinin by proliferating cells derived from toxin-damaged chicken retina. Dev Dyn. 2004b;229(3):555–63. doi: 10.1002/dvdy.10438. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS One. 2010b;5(6):e10774. doi: 10.1371/journal.pone.0010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91(4):531–41. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26(2):383–94. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci. 2010;30(8):3101–12. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J. Genetic aspects of embryonic eye development in vertebrates. Dev Genet. 1996;18(3):181–97. doi: 10.1002/(SICI)1520-6408(1996)18:3<181::AID-DVG1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22(21):9228–36. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312(1):300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes T, Gutierrez C, Aycinena JC, Tsonis PA, Del Rio-Tsonis K. BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proc Natl Acad Sci U S A. 2007;104(51):20380–5. doi: 10.1073/pnas.0707202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig AK, Peng GH, Chen S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 2008;1192:114–33. doi: 10.1016/j.brainres.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23(2):183–94. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Cirenza P. Synaptic organization of regenerated retina in the goldfish. J Comp Neurol. 1994;343(4):609–16. doi: 10.1002/cne.903430410. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Lindsey Myhr KJ, Easter SS, Jr, Mangione-Smith R, Jones DD. Local regeneration in the retina of the goldfish. J Neurobiol. 1992;23(2):187–203. doi: 10.1002/neu.480230209. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. Retinal regeneration. Trends Neurosci. 1992;15(3):103–8. doi: 10.1016/0166-2236(92)90020-9. [DOI] [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1(1):15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Huang T, Cui J, Li L, Hitchcock PF, Li Y. The role of microglia in the neurogenesis of zebrafish retina. Biochem Biophys Res Commun. 2012;421(2):214–20. doi: 10.1016/j.bbrc.2012.03.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006a;103(50):18998–9003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006b;133(5):913–23. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28(4):249–62. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech Dev. 1999;84(1-2):31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Wallace VA. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development. 1997;124(2):363–71. doi: 10.1242/dev.124.2.363. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Huang CJ, Caruso A, Barresi V, Nazarian R, Condorelli DF, de Vellis J. Ciliary neurotrophic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acidic protein in glial cells. J Neurochem. 1997;68(4):1413–23. doi: 10.1046/j.1471-4159.1997.68041413.x. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105(49):19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88(6):1051–64. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Zaghloul N, Moody SA. Transcription factors of the anterior neural plate alter cell movements of epidermal progenitors to specify a retinal fate. Dev Biol. 2001;240(1):77–91. doi: 10.1006/dbio.2001.0464. [DOI] [PubMed] [Google Scholar]
- Leung L, Klopper AV, Grill SW, Harris WA, Norden C. Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development. 2011;138(22):5003–13. doi: 10.1242/dev.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Yang JM, Jacobson RD, Pasko D, Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994;162(1):181–94. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377(6545):158–62. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- Limb GA, Daniels JT. Ocular regeneration by stem cells: present status and future prospects. Br Med Bull. 2008;85:47–61. doi: 10.1093/bmb/ldn008. [DOI] [PubMed] [Google Scholar]
- Limb GA, Daniels JT, Cambrey AD, Secker GA, Shortt AJ, Lawrence JM, Khaw PT. Current prospects for adult stem cell-based therapies in ocular repair and regeneration. Curr Eye Res. 2006;31(5):381–90. doi: 10.1080/02713680600681210. [DOI] [PubMed] [Google Scholar]
- Lindsey AE, Powers MK. Visual behavior of adult goldfish with regenerating retina. Vis Neurosci. 2007;24(3):247–55. doi: 10.1017/S0952523806230207. [DOI] [PubMed] [Google Scholar]
- Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA. Wnt signaling promotes muller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2012;54(1):444–53. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- Lombardo F. La rigenerazione della retina negli adulti di un Teleosteo. [Regeneration of the retina in an adult teleost] [in Italian] Accad Lincei-Rendiconti Scienze Fis Mat Nat Ser 8. 1968;45:631–635. [Google Scholar]
- Lombardo F. Andamento e localizzazione della mitosi durante la rigenerazione della retina di un Teleosteo adulto. [Time course and localization of mitoses during regeneration of the retina in an adult teleost.] [in Italian] Accad Lincei-Rendiconti Scienze Fis Mat Nat Ser 8. 1972;53:323–327. [Google Scholar]
- Loosli F, Winkler S, Wittbrodt J. Six3 overexpression initiates the formation of ectopic retina. Genes Dev. 1999;13(6):649–54. doi: 10.1101/gad.13.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22(5):567–77. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Pfaff SL. Cracking the transcriptional code for cell specification in the neural tube. Cell. 2001;106(6):651–4. doi: 10.1016/s0092-8674(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Martinez-De Luna RI, Kelly LE, El-Hodiri HM. The Retinal Homeobox (Rx) gene is necessary for retinal regeneration. Dev Biol. 2011;353(1):10–8. doi: 10.1016/j.ydbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387(6633):603–7. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis Neurosci. 1999;16(2):241–51. doi: 10.1017/s0952523899162059. [DOI] [PubMed] [Google Scholar]
- Mensinger AF, Powers MK. Visual function in regenerating teleost retina following surgical lesioning. Vis Neurosci. 2007;24(3):299–307. doi: 10.1017/S0952523807070265. [DOI] [PubMed] [Google Scholar]
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Ryu M, Nishida K, Pages G, Pouyssegur J, Endo S. ERK1 plays a critical protective role against N-methyl-D-aspartate-induced retinal injury. J Neurosci Res. 2008;86(1):136–44. doi: 10.1002/jnr.21472. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6(8):e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520(18):4294–311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460(7251):118–22. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, Harris WA. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138(6):1195–208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121(12):4045–55. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101(37):13654–9. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orts-Llorca F, Genis-Galvez JM. Experimental production of retinal septa in the chick embryo. Differentiation of pigment epithelium into neural retina. Acta Anat (Basel) 1960;42:31–70. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27(15):4210–9. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, Cirenza PF, Hitchcock PF. Persistent neurogenesis in the teleost retina: evidence for regulation by the growth-hormone/insulin-like growth factor-I axis. Mech Dev. 2002;117(1-2):137–49. doi: 10.1016/s0925-4773(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43(8):927–36. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134(1):201–5. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Induction of retinal regeneration in vivo by growth factors. Dev Biol. 1991;148(1):322–33. doi: 10.1016/0012-1606(91)90341-y. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Growth factor-induced retinal regeneration in vivo. Int Rev Cytol. 1993;146:49–74. doi: 10.1016/s0074-7696(08)60379-4. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20(11):4081–90. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J Comp Neurol. 2008;510(3):237–50. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA. Ascl1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013 doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12(11):1101–7. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108(38):15858–63. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Muller glia-derived progenitors in the injured retina. Nat Cell Biol. 2012 doi: 10.1038/ncb2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA. Retinal regeneration in teleost fish. Ciba Found Symp. 1991;160:171–86. doi: 10.1002/9780470514122.ch9. discussion 186-91. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. Retinal regeneration: common principles but a diversity of mechanisms. Adv Neurol. 1997;72:171–84. [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. How the neural retina regenerates. Results Probl Cell Differ. 2000;31:197–218. doi: 10.1007/978-3-540-46826-4_11. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19(5):431–63. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- Reh TA. Cell-specific regulation of neuronal production in the larval frog retina. J Neurosci. 1987;7(10):3317–24. doi: 10.1523/JNEUROSCI.07-10-03317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330(6143):68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- Reh TA, Tully T. Regulation of tyrosine hydroxylase-containing amacrine cell number in larval frog retina. Dev Biol. 1986;114(2):463–9. doi: 10.1016/0012-1606(86)90210-1. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61(5):651–78. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka CP, Tang J, Liu J, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509(2):225–38. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Stadler MB, Cepko CL. Gene expression changes within Muller glial cells in retinitis pigmentosa. Mol Vis. 2012;18:1197–214. [PMC free article] [PubMed] [Google Scholar]
- Romero-Aleman MM, Monzon-Mayor M, Santos E, Lang DM, Yanes C. Neuronal and glial differentiation during lizard (Gallotia galloti) visual system ontogeny. J Comp Neurol. 2012;520(10):2163–84. doi: 10.1002/cne.23034. [DOI] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J Neurosci. 2010;30(14):4970–80. doi: 10.1523/JNEUROSCI.3456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128(7):1099–107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127(20):4325–34. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Li L, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68(2):166–81. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131(18):4607–21. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- Stadler JA, Shkumatava A, Neumann CJ. The role of hedgehog signaling in the development of the zebrafish visual system. Dev Neurosci. 2004;26(5-6):346–51. doi: 10.1159/000082276. [DOI] [PubMed] [Google Scholar]
- Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010;518(12):2316–33. doi: 10.1002/cne.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke JJ, Fischer AJ. Embryonic retinal cells and support to mature retinal neurons. Invest Ophthalmol Vis Sci. 2010;51(4):2208–18. doi: 10.1167/iovs.09-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431(4):363–81. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stone L. Neural retina degeneration followed by regeneration from surviving pigment cells in grafted adult salamander eyes. Anatomical Records. 1950;106:89–110. doi: 10.1002/ar.1091060108. [DOI] [PubMed] [Google Scholar]
- Stuermer CA, Easter SS., Jr A comparison of the normal and regenerated retinotectal pathways of goldfish. J Comp Neurol. 1984;223(1):57–76. doi: 10.1002/cne.902230106. [DOI] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480(7377):372–5. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90(5):572–82. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328(6126):131–6. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4(6):833–45. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44(3):289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Proliferative and cell fate effects of Hedgehog signaling in the vertebrate retina. Brain Res. 2008;1192:61–75. doi: 10.1016/j.brainres.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113(4):1435–49. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22(2):334–47. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363(2):347–54. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- Wang Y, Smith SB, Ogilvie JM, McCool DJ, Sarthy V. Ciliary neurotrophic factor induces glial fibrillary acidic protein in retinal Muller cells through the JAK/STAT signal transduction pathway. Curr Eye Res. 2002;24(4):305–12. doi: 10.1076/ceyr.24.4.305.8408. [DOI] [PubMed] [Google Scholar]
- West EL, Gonzalez-Cordero A, Hippert C, Osakada F, Martinez-Barbera JP, Pearson RA, Sowden JC, Takahashi M, Ali RR. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30(7):1424–35. doi: 10.1002/stem.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239(4844):1142–5. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Wong LL, Rapaport DH. Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development. 2009;136(10):1707–15. doi: 10.1242/dev.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest Ophthalmol Vis Sci. 2001;42(9):2115–24. [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15(1):91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45(8):991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Zelinka CP, Scott MA, Volkov L, Fischer AJ. The Reactivity, Distribution and Abundance of Non-Astrocytic Inner Retinal Glial (NIRG) Cells Are Regulated by Microglia, Acute Damage, and IGF1. PLoS One. 2012;7(9):e44477. doi: 10.1371/journal.pone.0044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retinal pigment epithelium. Int Rev Cytol. 1997;171:225–66. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995;677(2):300–10. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130(21):5155–67. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Perron M, Philpott A, Bang A, Harris WA. Giant eyes in Xenopus laevis by overexpression of XOptx2. Cell. 1999;98(3):341–52. doi: 10.1016/s0092-8674(00)81963-7. [DOI] [PubMed] [Google Scholar]