Abstract
We address a critical aspect of antiretroviral therapy (ART) scale-up: poor clinic organization leading to long waiting times and reduced patient retention. Using a before and after study design, time and motion studies and qualitative methods we evaluated the impact of triage and longer clinic appointment intervals (triage) on clinic efficiency in a community-based program in Uganda. We compared time waiting to see and time spent with providers for various patient categories and examined patient and provider satisfaction with the triage. Overall, median time spent at the clinic reduced from 206 to 83 min. Total median time waiting to see providers for stable-ART patients reduced from 102 to 20 min while that for patients undergoing ART preparation reduced 88–37 min. Improved patient flow, patient and provider satisfaction and reduced waiting times allowed for service delivery to more patients using the same staff following the implementation of triage.
Keywords: Clinic efficiency, Waiting time, Time and motion, Triage, Antiretroviral therapy
Introduction
Antiretroviral therapy (ART) scale-up is challenged by an increasing caseload of patients, shortage of health workers and the labor-intensive nature of current delivery models. In 2009, 1.2 million people received ART for the first time representing a 30 % increase in a single year [1, 2]. In 2010, WHO issued revised treatment guidelines [3] recommending earlier initiation of ART at a CD4 count of < 350 cells/mm3 leading to an increase in the total number of people eligible for ART. To achieve universal access, ART scale-up must not only be sustained, but greatly accelerated [4]. However, clinics designed for acute care are challenged with the need to provide care for an enormous number of patients requiring life-long follow-up. The infrastructure, the health system and the human resources required to properly monitor treatment are becoming increasingly inadequate to support scaled-up ART programs [5–9]. Large ART clinics see 100–500 patients per day and patients generally spend 3–6 h at each clinic visit [10]. Rapid scale-up is only possible if clinic operations are optimally efficient.
ART delivery models affect the number of patients who can be treated and are thus critical in optimizing efficiency [11, 12] Models are multiple and diverse reflecting the difficult choices that have to be made which may include, home-based versus facility-based care, doctor versus nurse led, or decentralized versus vertical programs in the provision of care [13, 14]. The delivery model is critical for fully optimizing efficiency.
This study is part of an evaluation of the operational efficiency Reach Out Mbuya parish HIV/AIDS Initiative (ROM), a community-based ART program in Kampala, Uganda. Operational efficiency is defined as how fast a patient moves through the clinic from entry to exit during a given clinic visit including the waiting times to see and spent with providers. This is determined by how many patients are seen per day per provider (workload) and how many providers are seen per clinic visit per patient which is in turn affected by the stage of patient illness. Other factors include provider skills, motivation and the organization of the clinic including records retrieval, appointment scheduling and efficiency of the triage system.
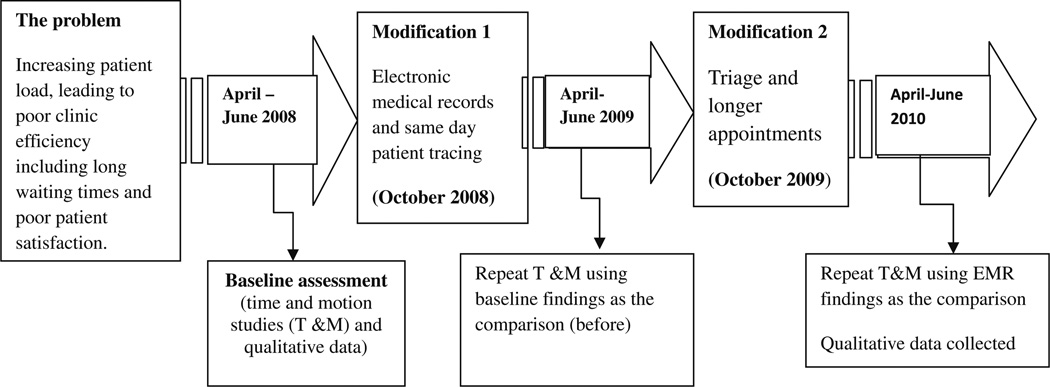
A baseline time and motion study performed between April–June 2008 [10] revealed three major efficiency barriers for patient care: (1) a manual record keeping system, which became unmanageable with the increasing caseload; (2) routine monthly clinic follow-up and a 30-day supply of medications for all patients leading to unnecessary visits for some patients and clinic congestion; and (3) also leading to long patient waiting times [15]. Reach Out’s health care workers proposed to increase the staff but did not mention the need to improve patient flow as an option for reducing the waiting times. Likewise, the patients advocated for more staff citing few staff as the reason for the long waiting times. These baseline findings were used to determine the modifications which were implemented to improve efficiency in two phases as illustrated in Fig. 1. In the first phase of the efficiency enhancement modifications, we implemented an Electronic Medical record (EMR) and patient tracing system and repeated the time and motion studies to evaluate the impact of the modifications on waiting times. We reported previously [16] that EMR significantly improved the efficiency of scheduling and tracing missed clinic appointments leading to a reduction in waiting times and the number of patients lost to follow-up. In the same paper we reported that a major reason the patients cited for missed appointments was being too busy with other commitments including work. We highlighted the need for strategies that take into consideration the improved quality of life of PLWHA including longer intervals between clinic appointments.
Fig. 1.
Schematic framework illustrating the modifications and study periods
In this paper we report on findings from modifications introduced in the second phase: a triage system and longer (two monthly) intervals between clinic visits. We maintained the same staff and used the evaluation of the EMR system as the new baseline.
Methods
Study Setting
Located in Kampala, Uganda, Reach Out Mbuya parish HIV/AIDS Initiative (ROM) is a community based ART program which targets the urban poor with free comprehensive HIV services. To improve geographical access, ROM operates three satellite clinics of Mbuya, Banda and Kinawataka. This evaluation was conducted at the Mbuya clinic which serves 40 % of the patient population. A full description of the ROM model of care has been described elsewhere [15, 17].
Study Design
This study constitutes the second phase of a larger, multimethod study to evaluate the efficiency, quality of care and cost of models of ART scale up in Uganda. A before and after study design was employed. We used time and motion studies [18–20].and qualitative methods.
The time and motion studies involved tracking of patients from the time of arrival to exit, on a single clinic visit. We documented all activities, including services provided types of providers seen, and the time waiting to see and time spent with each provider A semi-structured one-page tool was placed in the patient files for completion by providers at each point of care and withdrawn after the patient saw the final provider at that visit. The detailed time and motion procedures are published elsewhere [10, 16].
Assessment Periods
Our study assessed clinic efficiency over sequential 6-month periods to reveal changes in clinic efficiency following the modifications as illustrated in Fig. 1. Baseline time and motion and qualitative data were collected between April–June 2008 [10].
The first modification was the EMR which was implemented in October 2008 and time and motion studies were repeated in April–June 2009 [16].The second modification was the triage and longer visits interval modifications which were implemented in October 2009 and time and motion studies repeated in April–June 2010. Qualitative interviews were also conducted between April–June 2010 to understand how the medications affected patient and provider satisfaction.
All evaluations were done 6 months following implementation of the modifications to allow staff time to understand and correctly implement the modified patient flow procedures. The analysis for this paper focuses on data regarding the triage and longer appointment interval modification.
The study procedures and data collection methods were approved by the Ethics committee of Makerere University School of Public Health and the Ugandan National Council of Science and Technology.
Implementation of Triage and two Monthly Appointments Interval Schedules
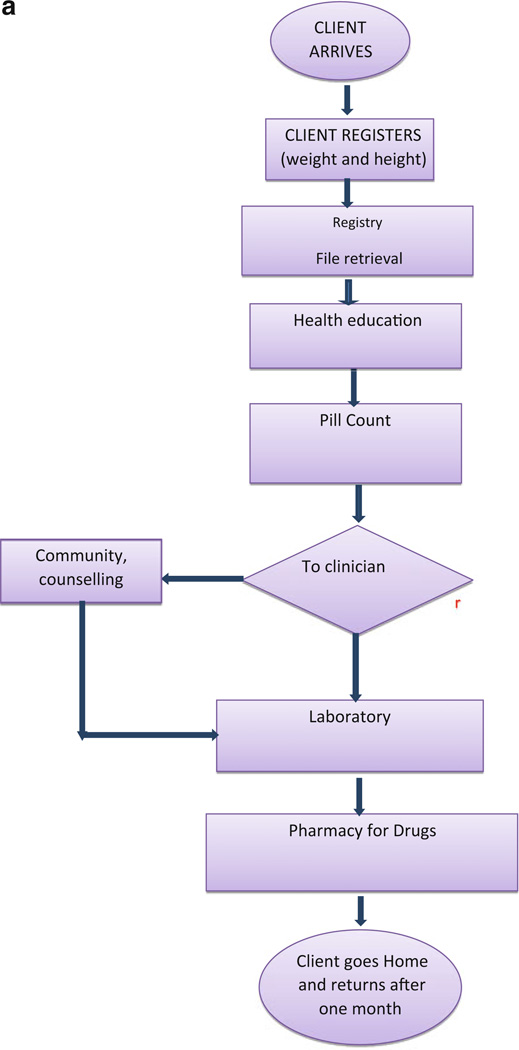
Before the implementation of triage, patients first interacted with the registry staff on arrival. The registry staff then transferred the patient files to the pill counters, the counselor and then to the nurse or doctor who requested laboratory tests and prescribed medications that the patient received at the pharmacy. Each patient interphased with 5–8 providers before exiting the clinic (Fig. 2a). All patients returned on a monthly basis for both clinical reviews and medication refills. However, new patients who had opportunistic infections returned on a bi-weekly basis until they stabilized. Stable patients with no adherence challenges or acute illness followed the same flow and visit schedule as all other patients. Patients undergoing preparation for ART had 3–5 counseling sessions prior to ART initiation. The median length of a clinic visit was reported to be 6 h at baseline [10] and only improved to 4 h following the implementation of EMR [16].
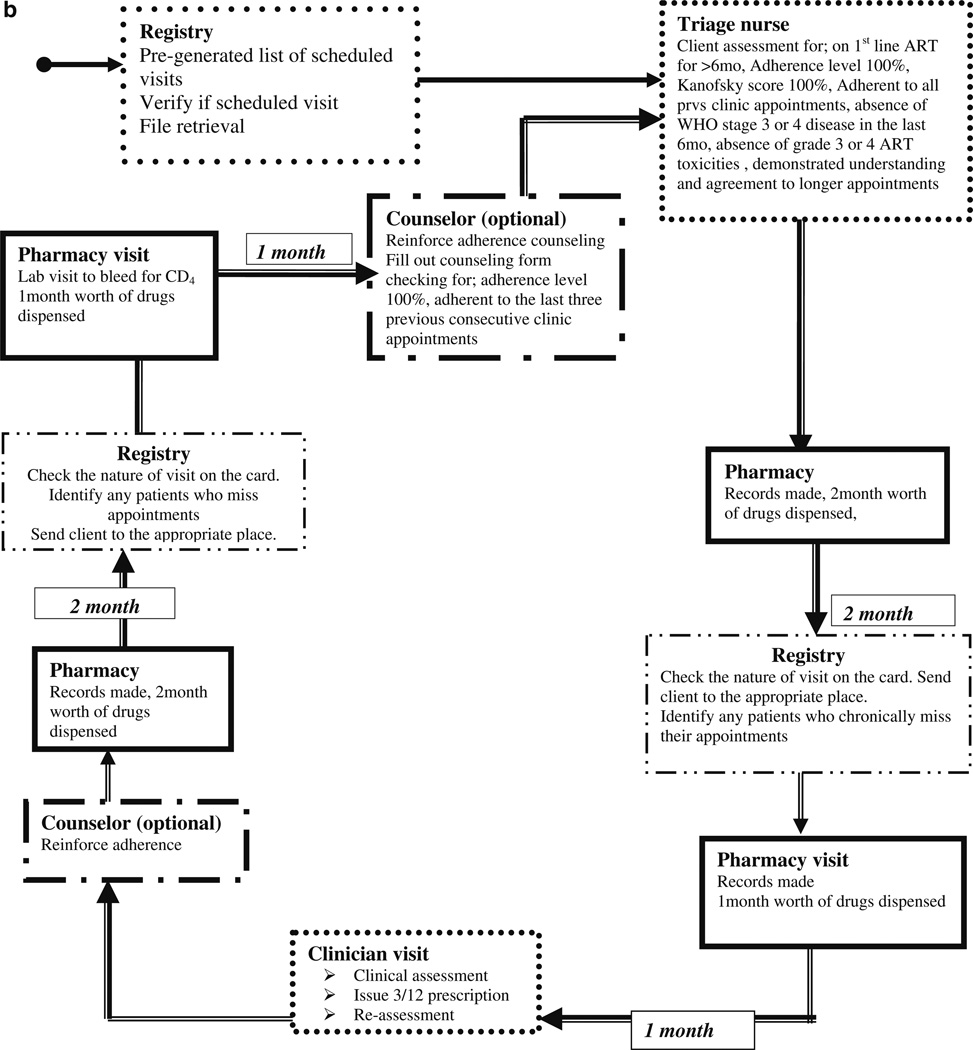
Fig. 2.
a Client flow before the implementation of triage and longer appointments. b Longer appointments and drug refill cycle
In October 2009, we implemented modifications to the patient flow (Fig. 2b) which included a triage system and two monthly intervals between clinic appointments instead of monthly visits. During the triage period, we electronically pre-generated the list of patients scheduled for a clinic visit and all files were retrieved and given to the triage nurse before the clinic starting time. Triage involved identification of very ill patients for urgent attention and stable patients who go directly to the pharmacy for medications refills and received two monthly appointment schedules. A triage nurse takes vital observations (temperature, blood pressure, weight, and height) and reviews the patients’ CD4 and medication adherence through pill counts. CD4 counts are done every 6 months and are indicated on the appointment card as a Lab visit (request filled out and filed in the lab) to ensure that lab visits are synchronized with pharmacy refills.
The stable patients receive a refill prescription, a 2 months appointment schedule and go straight to the pharmacy for their drug refills. The nurses and doctors received guidelines defining the eligibility criteria and flow plan for those patients who require longer appointment intervals and streamlined clinic visits (seeing only 3–4 providers as opposed to 5–8 during their visit). Patients who did not honor more than one of the appointments in the 6 month cycle, have a drug adherence below 95 %, or became pregnant were not eligible for longer appointment schedules or pharmacy only refills.
Data Collection
Waiting Times
We conducted a time and motion study on a random sample of 262 patients (every 12th patient) attending routine clinic services to estimate waiting times. Eligible patients included all adults receiving care and treatment from ROMs’ Mbuya clinic. To determine whether a patient’s stage of care influences waiting times and clinic efficiency, we categorized the patients as: new patients (in the clinic for the first time), new-ART (within 6 months of ART initiation), stable-ART (more than 6 months taking ART), stable pre-ART (not taking ART and stable medical condition) and patients undergoing preparation for ART.
Qualitative Interviews
All 262 patients who participated in the time and motion studies received an individual exit semi-structured interview which was conducted in English or the local language best known to the patient. Study patients also received a drink and a snack after the interview. No transport and time compensation was given since they came on their scheduled clinic day. In addition, 7 conveniently selected clinic staff (2 doctors, 3 nurses, the clinic supervisor and the medical director) received in-depth interviews. All interviews were conducted in person by a research assistant. Open–ended questions were used to explore new and unexpected leads and to identify areas that were most important to respondents. Open-ended questions were followed by standard probes and closed–ended questions that served to verify, compare, and contrast responses. The interviewers worked in pairs (one took notes while the other conducted the interview). The interviews, which ranged from 30 to 45 min, were tape-recorded.
The patient interviews focused on their perceptions of how the newer triage system and longer appointment schedules influenced patient flow, satisfaction, and the efficiency of the information and records management. The staff interviews further explored themes that emerged from the patient interviews in order to gain additional insights into the impact of the modifications on patient and provider satisfaction.
Quantitative data analysis: Data was entered and analyzed using Stata version 11.0 (Stata Corp Texas). Descriptive analysis was employed for the basic clinic characteristics and the waiting times to see and spent with providers. We compared waiting times before and after implementation of the triage using the Wilcoxon signedrank test (one sample median test). p values < 0.05 (twosided) were considered statistically significant.
Qualitative data analysis was performed by using theme and content qualitative methods. All transcripts were read several times to identify themes and categories. All, the transcripts were read by author 1 and a subsample was read by author 4.
After discussion a coding frame was developed and the transcripts coded by author 1. If new codes emerged the coding frame was changed and the transcripts were re-read according to the new structure. This process was used to develop categories, which were then conceptualized into broad themes after further discussion. Representative quotes were selected to illustrate identified themes.
Results
Quantitative Results
The clinic characteristics and distribution of the patient categories studied during the two periods are shown in Table 1a. Overall, the clinic was similar in most of the evaluated characteristics. In the period after triage the clinic cared for more patients (3625 vs. 3400) using fewer staff (less by one nurse). Sixty-three percent of the patients were on ART. In addition 836 patients received two monthly appointment intervals after the triage compared to the monthly appointments before the triage. There was also an increase in the percentage of children after the triage (10 vs. 7 %) and the percentage of pregnant women (3 vs. 2 %).
Table 1.
(a) Clinic characteristics before and after the triage and longer appointments intervals and (b) Patients sampled for time and motion study before and after the triage
| (a) | ||
|---|---|---|
| Clinic characteristics |
aBefore (April-June 2009) |
bAfter (April-June 2010) |
| Total patients | 3,400 | 3,625 |
| Total patients on ART | 1,951 | 2,293 |
| No of new patients/week (Mean: SD) | 14 (5) | 20 (7) |
| No of new ART patients/week (Mean: SD) | 2 (1) | 7 (2) |
| Patients seen/day (Mean: SD) | 71 (11) | 80 (16) |
| % Second line ART | 2% | 3% |
| % Pregnant women | 2% | 3% |
| % Children | 7% | 10 % |
| Baseline CD4 (n, %) | ||
| <100 | 1,020 (34) | 1,124 (31) |
| 100–250 | 1,700 (46) | 1,523 (42) |
| >250 | 680 (20) | 27 (978) |
| Staffing | ||
| Nurses | 6 | 5 |
| Support staff | 6 | 6 |
| Doctors | 3 | 3 |
| Pharmacy technician | 1 | 1 |
| (b) | ||
|---|---|---|
| Category | (n = 232) | (n = 262) |
| New patients | 38 (16.3 | 50 (19) |
| Stable Pre-ART | 48 (20.7) | 56 (21.3) |
| ART Preparation | 32 (13.8) | 27 (10) |
| Early ART | 38 (16.4) | 45 (17) |
| Stable ART | 75 (32) | 84 (34) |
monthly follow-up
two monthly follow-up
Most patients arrived at the clinic between 7 am and 9 am. An attempt to streamline appointments into either morning or afternoon was not successful as almost 90 % of the interviewed patients preferred to come to the clinic between 7 am and 10 am.
Of the patients sampled for the time and motion studies, the proportions in the five patient categories were similar before and after the triage with each patient category representing about 1/5 of the sampled patients (Table 1b).
Waiting Time
Overall time spent at the clinic significantly reduced from a median time of 206 min (IQR, 159–250) to 85.5 min (IQR, 59–116) (Z = −1.996; p = 0.046). The reduction was mainly attributed to significant reductions in times waiting to see providers with reductions as high as 20-fold for time waiting to see registry staff. The overall time spent with the providers also reduced with significant reductions in time spent with registry staff (19.0 min; IQR, 10–30) versus (3.0 min; IQR, 2–6) (Z = −13.072; p = 0.000), laboratory (23.0 min; IQR, 8–35) versus (14.0 min; IQR, 10–18) (Z = −9.063; p = 0.000) and pharmacy (21.0 min; IQR, 14–32) versus (11.0 min; IQR, 7–15) (Z = 15.526; p = 0.000) (Table 2).
Table 2.
Patient waiting times before and after implementation of triage system by provider categories
| Provider | Median time waiting to see providers |
Median time spent with providers |
||||
|---|---|---|---|---|---|---|
| (minutes; IQR) |
(minutes; IQR) |
|||||
| Before | After | Z-value | Before | After | Z-value | |
| Overall time | 115 (68–153) | 82 (55–109) | −1.996a | 94 (58–131) | 46 (13–127) | −14.032a |
| Registration | 20.0 (8–49) | 0.0 (0–7) | 13.196a | 19.0 (10–30) | 3.0 (2–6) | −13.702a |
| Pill counters | 21.0 (11–38) | 6.0 (3–8) | −7.174a | 2.00 (1–6) | 5.0 (3–7) | 11.847a |
| Nurses | 38.0 (18.0–62) | 5.0 (3–10) | −9.819a | 10.0 (4–20) | 14.0 (11–18) | 8.131a |
| Counselor | 13.0 (2–22) | 7.0 (5–10) | −7.610a | 43.0 (31–60) | 16.0 (11–20) | −10.481a |
| Laboratory | 42.0 (16–68.0) | 5.0 (3–9) | 11.195a | 23.0 (8–35) | 14.0 (10–18) | −9.063a |
| Pharmacy | 11.0 (3–36.0) | 6.0 (4–6) | −8.600a | 21.0 (14–32) | 11.0 (7–15) | 12.526a |
Z values derived from wilcoxon signed-rank test
IQR Interquartile range
p value<0.001
The median time spent waiting to see providers were longest for stable-ART patients prior to implementation of triage but significantly reduced from a median time of 102 to 20 min (Z = −13; p < 0.001). Patients undergoing preparation for ART waited longest to see providers during the triage period but still waited a median time of 51 min less compared to the same category of patients prior to the triage (Z = −15.3; p < 0.001). There was a significant reduction in time spent with providers for all patient categories but stable pre-ART patients and stable-ART patients spent the least time with providers during both study periods. Subsequently there was a reduction in total time spent at the clinic for all patient categories (Table 3).
Table 3.
Patient waiting times before and after implementation of triage by patient categories
| Patient category | Time waiting to see providers |
Time spent with providers |
Total time spent at the clinic |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Median time in minutes (IQR) |
Median time minutes (IQR) |
Median time minutes (IQR) |
|||||||
| Before | After | Z-value | Before | After | Z-value | Before | After | Z-value | |
| New patients | 95.5 (58–152) | 34.0 (11–14) | −13.2a | 12.5 (4–21) | 10.0 (6–16) | −4.5a | 174 (138–261) | 92.5 (75–110) | −6.1a |
| Stable pre-ART | 84.0(63–119) | 19.0 (9–38) | −10.8a | 12.0 (4–25) | 9.0 (5–13) | −6.6a | 183 (134–235) | 70 (47–89) | −6.4a |
| ART Preparation | 88.0 (32–150) | 37.0 (25–54) | −13.4a | 17.0 (7–30) | 12.0 (5–18) | −6.4a | 195 (160–273) | 115 (92–132) | −4.5a |
| Early ART(<6 months) | 95.0 (55–127) | 35.0 (19–19) | −15.3a | 14.0 (3–29) | 10.0 (6–15) | −7.8a | 223 (161–280) | 94 (81–120) | −5.8a |
| Stable ART (>6 months) | 102 (58–131) | 20.0 (7–32) | −13.7a | 11.0 (4–21) | 8.0 (5–13) | −6.3a | 185 (128–230) | 59 (45–85) | −7.9a |
Z values derived from wilcoxon signed-rank test
IQR Interquartile range
p value < 0.001
p value > 0.001
Qualitative Results
The themes were categorized into: Patient flow, waiting times, longer appointments and satisfaction.
Patient flow
The patients said there was less congestion around the registry because their files, which were previously piled at the registry, were retrieved on arrival and forwarded to the triage nurse immediately. They particularly liked the fact that they could now be seen soon after they arrived and the challenge of their files getting misplaced had reduced. “I used to come to the clinic by 5 am but some patients would already be here because getting the files would take long. I now come to the clinic at 8 am because I do not have to wait long to have my file retrieved” (male patient).
The provider interviews confirmed the improved patient flow. They mentioned that the triage decongested the nurse and doctor consultations as some of the patients went directly to the pharmacy which allowed them more time with the more sick patients. All providers also said the introduction of triage greatly reduced the congestion in the patient waiting area since stable patients had their pill counts, vital measurements and prescriptions at the triage point and saw fewer providers. “The patients used to crowd around the waiting area and many of them would keep asking how long more they had to wait to see a clinician. This has changed since the triage. The patients walk in and out very quickly and the look happier” (doctor).
Waiting Times and Longer Appointment Intervals
The patients mentioned that they waited much less to see the registry staff and spent much less time at the clinic which enabled them to get to their work earlier. In addition they liked the longer clinic appointment schedules which eased them from the burden of having to frequently ask for time off from their workplaces “sometimes I missed my clinic appointment because I had no more explanations to give at my work place about my late coming but now I walk into the clinic at 8 am and by 9 am I am getting to work” (Female patient).
Likewise the providers mentioned that the clinic which previously lasted 7–10 h now lasted 5–7 h which allowed them time to undertake other clinic based assignments including training and operations research However, the clinicians worried that patients were not revealing their acute illnesses because they wanted to be triaged to two monthly appointment schedules, which may compromise the quality of care in the longer term. “This push to improve waiting times is making the patients happy and motivates the staff but I am afraid that the patients may hide acute episodes from us. A couple of days ago I saw a female patient who was in her last trimester of pregnancy and she had an acute urinary tract infection yet she had requested to go straight for a drug refill for two months claiming no major illnesses.” (Medical Director). The clinic administrators also worried that the time the providers spent with the patients may compromise quality because of the rush. However, when asked about the adequacy of the time they spent with the providers, 95 % (249/ 262) of the patients said the time they spent with the nurses or doctors was adequate. Ninety-two percent (242/262) of the patients said the counseling time was adequate and the session was helpful.
Discussion
In this paper we address a critically important aspect of ART scale-up in resource limited settings: poor organization of clinics that wastes patients’ time, deters them from seeking care and reduces adherence including missed clinic visits [15, 16]. The task of providing long-term, quality care for the large volume of patients is overwhelming and effective management of clinic operations is therefore more critical now than ever, not only for clinic efficiency but also for job performance and satisfaction of program staff [5, 6] To achieve efficiency, clinics must consider the model of care including the composition of clinic staff, and the schedule of client visits and how this schedule varies by the client’s stage of care.
Optimizing Patient Flow
To significantly reduce the time that clients spend waiting to receive care, strategies are needed to make the flow of clients through the clinic smoother. The introduction of a triage in our clinic allowed for vital parameters and pill conting to be done using one service provider which reduced congestion at the clinicians table since stable patients with good adherence could go straight to the pharmacy. However, demographic, clinical events and medical prescriptions are still captured in patient files by providers and data entry clerks transfer the data into electronic data bases at the end of the clinic day. This makes the process of documentation and record keeping cumbersome. Movement of patient files from one provider to another (and sometimes back and forth) affects patient flow and could be improved by real time electronic data entry during consultations.
Optimizing Provider Efficiency
Efficiency will vary depending on the provider type and characteristics including training, experience, productivity, morale and attitude towards caring for persons living with HIV/AIDS.
A baseline efficiency evaluation of three HIV clinics between April–June 2008 revealed longest waiting times with providers at ROM, which utilizes a nurse-led model, compared to the other clinics that use a doctor-led model [10]. We speculated that the differences could be attributed to less skills, experience, and confidence in conducting consultations by nurses compared to doctors. Following the introduction of triage and longer intervals between appointments, training and introduction of clear guidelines we saw a dramatic reduction in the median nurse-time per patient of up to 30 % at ROM compared with the baseline findings. Other studies have demonstrated a reduction in the doctor-time needed per patient by a 14–33 %, after a reduction in the number of visits per patient [21, 22]. In this study however, in which compared waiting times with those after the implementation of EMR, we experienced an increase of 4 min in the median time spent with the nurses and an increase of 3 min in the median time spent with the pill counters.
(Although statistically significant, this difference may not be significant from a clinical care perspective). We observed significant reductions in the median times spent with the rest of the providers. These findings are contrary to what we expected. Since the clinic flow improved, the number of providers seen by each patient reduced, and the waiting times in between providers significantly reduced we expected that the providers would spend more time with those patients requiring more attention. We anticipate that this is possibly the result of reporting bias by the providers who were aware that they were being observed since they were involved in the efficiency improvement process. In addition, the efficiency improvement was being emphasized by the clinic managers. Furthermore, the distribution of patient types change with an increasing number of stable patients who require fewer laboratory investigations and counseling services.
Optimizing Efficiency Through Reducing the Intensity of Follow-up
The workload on any given clinic day is influenced by the number and type of patients on that day which also depends on the frequency and intensity of their clinic visits. On the other hand factors that influence the frequency of clinic visits include the maturity of the program, population targeted, the stage of the epidemic, severity of the patient’s illness, intensity of adherence management (including directly observed therapy) and occurrence of side effects) [23, 24]. Most people in sub-Saharan Africa start treatment late [25]. At the start up, many programs allow time to focus on high rates of patients with advanced AIDS and complications [26]. These individuals require frequent, longer consultation times and take longer to stabilize clinically leading to congestion in the clinics.
With improved access to HIV testing [27, 28], more asymptomatic individuals learn of their status early and are linked into care early. [29]. At ROM where we see ∼100 clients/day, more than half of the patients are in the pre-ART stage. Treating opportunistic infections and evaluating for ART eligibility requires generally 3–5 clinic visits and these patients therefore represent a greater proportion of the clinic burden yet newly enrolled patients tend to require more intensive clinical procedures and time with multiple providers. More clinician time is therefore required at the start and first months of ART in order to evaluate the patients’ health status, enforce adherence and manage side-effects. The number of clinic visits required for ART preparation could be reduced to 2 or incorporated into routine clinic consultations in order to reduce the intensity of visits for this category of patients. Over time, patients who are stable on ART and only need regular monitoring represent a disproportionate amount of the clinic burden. However, ROM required that all patients on ART be seen monthly, regardless of their health status. The total number of sampled stable patients [pre ART and ART] represented approximately one half of the patient population. Scheduling their appointments to longer intervals would be expected to reduce the work load devoted to them. We increased, as documented in this paper, the interval between clinic visits from one month to two months for stable, adherent patients (both ART and pre-ART) and the number of daily patient visits devoted to stable patients reduced by 50 %. Subsequently, there were fewer reminders to patients for clinic appointments per given month which also reduced the workload for the community health workers. Like-wise, the patients could save time and transport costs, and took less time off work to attend the clinic. It is clear, that the amount and type of clinic time needed for an individual patient will evolve over time. It is therefore important that clinic models continuously evolve alongside the different patient types so as to maintain efficiency, and provider and patient satisfaction.
How Can we Improve Efficiency and Maintain Quality of Care?
Efficiency interventions must balance the need to ensure that care provision is efficient with regard to time and staff allocation with the need to ensure services remain of a high quality that achieves treatment success. A danger of longer intervals between clinic visits is that patients may spend longer with co-morbidities, adverse events, and viraemia thus increasing the risk of resistance [30]. The importance of patient education to present for care as soon as they develop any new symptom should therefore be emphasized. In our study there was a reduction by a median of 27 min in the time spent with the counselors which could compromise opportunities for adherence counseling and HIV transmission risk reduction counseling. Efficiency strategies should focus on reducing waiting times in between seeing providers and allowing more patient time with the providers especially for the new patients, those undergoing preparation for ART, and those newly initiated on ART. Additionally, some populations will require more provider time such as pregnant women, children or those with tuberculosis and require close follow up making longer appointment intervals inappropriate in most cases. The optimal time required to see patients in different stages of HIV without compromising quality is not known and needs to be evaluated to inform efficiency improvement interventions.
Introduction of a patient tracking electronic medical records management system prior to implementation of triage and longer appointments facilitated tracking patients with longer appointment intervals, who are more likely to miss appointments due to forgetfulness. Giving patients even longer appointment intervals could improve efficiency further. However, the minimum number of visits required to achieve maximum adherence in the short and long term is not known.
A limitation of our study is the possible reporting bias by the providers who were part of the efficiency improvement team who in addition probably used their time more efficiently during the study period. However, our main aim was to reduce time waiting to see providers which in our view, had no reporting bias.
An important unintended consequence of this efficiency improvement intervention is the rush of nurses and counselors in carrying out consultations, emphasizing the need to include clinical outcomes and quality of care indicators in such studies.
Our results must be considered an attempt to systematically improve clinic efficiency within the context of a community-based program with strong patient follow up mechanisms. This may not be representative of other community based programs. Lastly, clinic efficiency may be embedded within specific patient and provider types which may evolve differently over time. Other factors will influence the number of staff required for efficient ART scale up such as adherence rates, non-clinical support needs, counseling needs, stigma, family, community support, and have not been accounted for in our evaluation.
Conclusion
We demonstrated that introduction of triage and longer intervals between clinic visits optimized efficiency at ROM. Substantial resources could be saved by less frequent monitoring of stable patients while allowing clinics to allocate more resources to patients at greatest risk of treatment failure and disease progression There was improved patient and provider satisfaction which supported the delivery of care and treatment to more patients with the same staff numbers and mix. However, efficiency improvement is a continuous process and programs intending to apply similar strategies need to train providers and integrate quality improvement techniques into their routine clinic operations.
Acknowledgments
We acknowledge clients and staff from ROM for providing all the information used in this study. We thank the Ugandan Ministry of Health for supporting care and treatment at ROM and the U.S. President’s Emergency Plan for AIDS Relief for the funding of HIV care. The work reported here was supported by the National Institutes of Health, Department of Health and Human Services, Public Health Services (grant number 1R24HD056651-D1).
Contributor Information
Stella T. Alamo, Email: stellaalamo@gmail.com, Medical Department, Reach Out Mbuya HIV/AIDS Initiative, P.O. Box 7303, Kampala, Uganda.
Glenn J. Wagner, Health Unit, RAND Corporation, Santa Monica, CA, USA
Joseph Ouma, Department of Strategic Planning, Management Sciences for Health, Kampala, Uganda.
Pamela Sunday, Monitoring and Evaluation Department, Reach Out Mbuya Parish HIV/AIDS Initiative, Kampala, Uganda.
Laga Marie, HIV Epidemiology and Control Unit, Institute of Tropical Medicine, Antwerp, Belgium.
Robert Colebunders, Department of Clinical Sciences, Institute of Tropical Medicine, Antwerp, Belgium; Epidemiology and Social Medicine, University of Antwerp, Antwerp, Belgium.
Fred Wabwire-Mangen, Department of Epidemiology and Biostatistics, Makerere University School of Public Health, Kampala, Uganda.
References
- 1.AIDS Epidemic Update 2009 Reports 2009 [web site]. UNAIDS, Geneva. [Accessed 11 October 2010]; http://www.unaids.org/en/dataanalysis/epidemiology/2009aidsepidemicupdate/ Country Progress All Countries.asp.
- 2.WHO, UNICEF and UNAIDS. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. [Accessed 11 October 2010];World Health Organization, Geneva. 2010 http://www.who.int/hiv/pub/2008progressreport/en/
- 3.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 revision. World Health Organization, Geneva. [Accessed 17 October 2010]; http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 4.World Health Organization, UNAIDS, UNICEF: Towards Universal Access. Scaling up priority HIV/AIDS interventions in the health sector. Progress Report. 2007 Apr; [Google Scholar]
- 5.Kober K, Van Damme W. Scaling up access to antiretroviral treatment in southern Africa: who will do the job? Lancet. 2004;364(9428):103–107. doi: 10.1016/S0140-6736(04)16597-5. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme W, Kober K, Laga M. The real challenges for scaling up ART in sub-Saharan Africa. AIDS. 2006;20:653–656. doi: 10.1097/01.aids.0000216364.44409.b1. [DOI] [PubMed] [Google Scholar]
- 7.Mate KS, Bennett B, Mphatswe W, et al. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS ONE. 2009;4:e5483. doi: 10.1371/journal.pone.0005483. doi:10.1371/journal.pone.0005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffe HW. Universal access to HIV/AIDS treatment: promise and problems. JAMA. 2008;300:573–575. doi: 10.1001/jama.300.5.573. [DOI] [PubMed] [Google Scholar]
- 9.Miriam R, Waffa M, Kevin MD. The impact of HIV Scale-up on health systems: a priority research agenda. J Acquir Immune Defic Syndr. 2009;52:S6–S11. doi: 10.1097/QAI.0b013e3181bbcd69. [DOI] [PubMed] [Google Scholar]
- 10.Wanyenze RK, Wagner G, Alamo S, et al. Evaluation of the efficiency of patient flow at three Ugandan HIV clinics. AIDS Patient Care STDs. 2010;24:441–446. doi: 10.1089/apc.2009.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner G, Ryan G, Taylor S. Formative evaluation of antiretroviral therapy scale-up efficiency in sub-Saharan Africa. AIDS Patient Care STDs. 2007;21:871–887. doi: 10.1089/apc.2007.0008. [DOI] [PubMed] [Google Scholar]
- 12.Van Damme W, Kegels G. Health systems strengthening and scaling-up of antiretroviral therapy: the need for context specific delivery models: comment on Schneider. Reprod Health Matters. 2006;14(27):24–26. doi: 10.1016/S0968-8080(06)27243-4. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme W. World social health insurance: strengthening health systems in low-income countries. PLoS Med. 2007;4(3):e137. doi: 10.1371/journal.pmed.0040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang LW, Kagayi J, Nakigozi G, et al. Responding to the human resource crisis: peer health workers, mobile phones, and HIV care in Rakai, Uganda. AIDS Patient Care STDs. 2008;22(3):173–174. doi: 10.1089/apc.2007.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanyire G, Wanyenze R, Alamo S, et al. Client and provider perspectives of the efficiency and quality of care in the context of rapid scale-up of antiretroviral therapy. AIDS Patient Care STDs. 2010;24(11):719–27. doi: 10.1089/apc.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamo ST, Wagner GJ, Sunday P, et al. Electronic medical records and patient tracing improve adherence to clinic appointments and clinic efficiency in a resource limited setting. AIDS Behav. 2011 doi: 10.1007/s10461-011-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larry S, Alamo WC, Guma S, et al. Two year virological outcomes of an alternative AIDS care model: evaluation of a peer health worker and nurse-staffed community-based program in Uganda. J Acquir Immune Defic Syndr. 2009;50:276–282. doi: 10.1097/QAI.0b013e3181988375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xakellis GC, Jr, Bennett A. Improving clinic efficiency of a family medicine teaching clinic. Fam Med. 2001;33(7):533–538. [PubMed] [Google Scholar]
- 19.Hendrich A, Chow M, Skierczynski BA, Lu Z. A 36-hospital time and motion study: how do medical-surgical nurses spend their time? Permanente J. 2008;12:25–34. doi: 10.7812/tpp/08-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher J, Lotery H, Henderson C. Time in motion: testing efficiency in the dermatology procedure setting. Dermatol Surg. 2009;35:437–445. doi: 10.1111/j.1524-4725.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 21.Van Damme W, Kheang ST, Janssens B. How labour intensive is a doctor-based delivery model for antiretroviral treatment (ART)? Evidence from an observational study in Siem Reap, Cambodia. Hum Resour Health. 2007;5:12. doi: 10.1186/1478-4491-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reekiea J, Mocrofta A, Sambatakoub H, et al. Does less frequent routine monitoring of patients on a stable, fully suppressed cART regimen lead to an increased risk of treatment failure? AIDS. 2008;22:2381–2390. doi: 10.1097/QAD.0b013e328317a6eb. [DOI] [PubMed] [Google Scholar]
- 23.Colebunders R, Bukenya T, Pakker N, et al. Assessment of the patient flow at the infectious diseases institute out-patient clinic, Kampala, Uganda. AIDS Care. 2007;19(2):149–151. doi: 10.1080/09540120600762078. [DOI] [PubMed] [Google Scholar]
- 24.Wagner G, Ryan G, Taylor S. Formative evaluation of antiretroviral therapy scale-up efficiency in sub-Saharan Africa. AIDS Patient Care STDs. 2007;21:871–887. doi: 10.1089/apc.2007.0008. [DOI] [PubMed] [Google Scholar]
- 25.Antiretroviral Treatment in Lower Income Countries (ART-LINC) Collaboration [web site] Berne: ART-LINC; [Accessed 17 October 2010]. http://ije.oxfordjournals.org/content/34/5/979.full. [Google Scholar]
- 26.Bekker LG, Orrell C, Reader L, et al. Antiretroviral therapy in a community clinic-early lesson from a pilot project. S Afr Med J. 2003;93:458–462. [PubMed] [Google Scholar]
- 27.Uganda National Policy on HIV Counseling and Testing. Ministry of Health; Kampala: 2005. Sep, [Google Scholar]
- 28.WHO/UNAIDS. [Accessed 17 September 2009];Guidance on provider-initiated HIV testing and counseling in health facilities. 2007 May; http://www.who.int/mediacentre/news/releases/2007/pr24/en/index.html/
- 29.Warwick Z. The influence of antiretroviral therapy on the uptake of HIV testing in Tutume, Botswana. Int J STD AIDS. 2006;17:479–481. doi: 10.1258/095646206777689189. [DOI] [PubMed] [Google Scholar]
- 30.Deeks SG. Treatment of antiretroviral-drug-resistant HIV-1 infection. Lancet. 2003;362:2002–2011. doi: 10.1016/S0140-6736(03)15022-2. [DOI] [PubMed] [Google Scholar]