Background: LIM homeobox 8 (Lhx8) functions in the differentiation of cholinergic neurons.
Results: Lhx8 regulates functions of cholinergic neurons by modulating tropomyosin receptor kinase A (TrkA) expression. Lhx8 expression and function are regulated by NGF.
Conclusion: Lhx8 is a mediator of NGF signaling for the regulation of cholinergic functions.
Significance: Positive feedback regulation between Lhx8, TrkA, and NGF is an important regulatory mechanism for cholinergic functions.
Keywords: Cell Signaling, Neurons, Neurotrophic Factor, Receptor Tyrosine Kinase, Transcription Factors, Cholinergic Neuron, LIM Homeobox, Nerve Growth Factor, TrkA
Abstract
Basal forebrain cholinergic neurons play an important role in cognitive functions such as learning and memory, and they are affected in several neurodegenerative diseases, including Alzheimer disease and Down syndrome. Despite their functional importance, the molecular mechanisms of functional maturation and maintenance of these cholinergic neurons after the differentiation stage have not been fully elucidated. This study demonstrates that the LIM homeobox 8 (Lhx8) transcription factor regulates cholinergic function in rat septal cholinergic neurons in primary cultures from E18.5 embryos and in the adult brain. Lhx8 expression modulated tropomyosin receptor kinase A (TrkA) expression in septal cholinergic neurons in vitro and in vivo, resulting in regulated acetylcholine release as an index of cholinergic function. In addition, Lhx8 expression and function were regulated by nerve growth factor (NGF), and the effect of NGF was potentiated by Lhx8-induced TrkA expression. Together, our findings suggest that positive feedback regulation between Lhx8, TrkA, and NGF is an important regulatory mechanism for cholinergic functions of the septum.
Introduction
Neurons in the medial septum and diagonal band of Broca project to the hippocampus, modulating its activity and providing important input for cognitive functions (1). Within this system, basal forebrain cholinergic neurons (BFCNs)3 have been extensively studied in several species of mammals, and accumulating evidence indicates that they play important roles in arousal, attention, learning, and memory (2–5). By contrast, the molecular mechanisms of their functional maintenance and degeneration in certain neurological disorders have not been fully elucidated.
The genetic and developmental mechanisms that control the formation of BFCNs are just beginning to be clarified. Many transcription factors are expressed in the developing basal forebrain, and mice in which genes encoding those factors are disrupted exhibit forebrain malformation (6–11). Among these transcription factors, LIM homeobox 8 (Lhx8), a member of the LIM homeodomain family of transcription factors, has a pivotal role in BFCN development (12, 13). Several studies in mice revealed that Lhx8 is selectively expressed in the medial ganglionic eminence, and mice with disrupted expression of the Lhx8 gene lost a significant number of BFCNs (14–16). In addition, progenitors of striatal cholinergic interneurons switch their fate to striatal GABAergic interneurons in the absence of Lhx8 (17). Although these studies have suggested that Lhx8 functions in the differentiation and specification of BFCNs during development, the role of this transcription factor in the functional maturation and maintenance of BFCNs after the differentiation stage remains unclear.
Nerve growth factor (NGF), which is a member of the neurotrophin family, has been shown to play an essential role in promoting the survival and regulating the activity of cholinergic neurons of the medial septum and diagonal band of Broca (18–24). NGF acts via a high affinity receptor, tropomyosin receptor kinase A (TrkA), which is expressed by cholinergic neurons in the basal forebrain and striatum in the CNS (25). In TrkA-null mice, BFCNs do not mature fully and begin to die by the time of target innervation, suggesting that TrkA expression is required for normal BFCN maturation and survival (26). However, the regulation of TrkA expression in the BFCN after the differentiation stage is not well understood.
In this study, we sought to investigate the role of Lhx8 in differentiated cholinergic neurons in the CNS. Using rat E18.5 primary septum neurons and medial septum of adult brain, we demonstrated for the first time that Lhx8 directly regulates TrkA expression in the CNS and functions in the activity of differentiated cholinergic neurons. Moreover, we identified a new pathway in which NGF, TrkA, and Lhx8 work together to form a positive feedback loop.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague-Dawley rats (8 weeks old) were purchased from Charles River Laboratories (Japan). The animals were maintained on a 12-h light/dark cycle with unlimited access to food and water. All animal procedures conformed to Japanese regulations for animal care and use according to the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science and were approved by the Animal Care and Use Committee of Eisai Co., Ltd.
Cloning and Construction
Full-length Lhx8 cDNA was generated from mouse brain by PCR amplification using the following primer set: sense 5′-ATGTATTGGAAGAGCGATCA-3′ and antisense 5′-TCATTGGATGGGGTAACAAGGCT-3′. The following LacZ and Lhx8 miRNA sequences were designed using BLOCK-iT RNAi Designer (Invitrogen): Lhx8 miRNA sense 5′-AAGTCATTCACCTTGAGGAGGGTTTTGGCCACTGACTGACCCTCCTCAGTGAATGACTT-3′ and antisense 5′-AAGTCATTCACTGAGGAGGGTCAGTCAGTGGCCAAAACCCTCCTCAAGGTGAATGACTT-3′, and LacZ miRNA sense 5′-AAATCGCTGATTTGTGTAGTCGTTTTGGCCACTGACTGACGACTACACACATCAGCGATTT-3′ and antisense 5′-AAATCGCTGATGTGTGTAGTCGTCAGTCAGTGGCCAAAACGACTACACAAATCAGCGATTT-3′. These genes were inserted into the adeno-associated virus (AAV) vector pAAV-CAG-WPRE (woodchuck hepatitis virus post-transcriptional regulatory element) (28, 29). The TrkA promoter region was amplified using PCR from mouse genome using the following primers: sense 5′-CTAGCTAGCAAGAACTCAGGATGCTTAGT-3′ and antisense 5′-CCCAAGCTTCAGCCGTCCGTGCGCTCCTAG-3′. The PCR product was inserted into the pTK-placental alkaline phosphatase (PLAP) vector (27), creating the reporter vector TrkA promoter-PLAP. Site-directed mutagenesis of the Lhx-binding sequence in the TrkA promoter was carried out using the QuikChange site-directed mutagenesis kit (Stratagene) with the TrkA promoter-PLAP vector as a template and the sense primer 5′-TAAGAGATCTATCGCGTTCTCCGCACAGA-3′. The resulting vector was named TrkA mutant promoter-PLAP.
Primary Septal Neuron Culture
Septal areas (containing cholinergic neurons from the septum, diagonal band of Broca, and substantia innominata) of embryonic day (E)18.5 rat embryos were dissected in L-15 medium (Sigma) containing 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen). These tissues were dissociated at 37 °C with 0.25% trypsin (Invitrogen) and 1% DNase I (150 units/ml, Sigma) for 30 min (terminated with 10% heat-inactivated horse serum; Invitrogen). The dissociation was completed mechanically with a pipette. Cultures were plated at 1.4 × 105 cells per well in 100 μl of growth medium in poly-d-lysine-coated 96-well culture plates (BD Biosciences), and cultures were maintained at 37 °C and 5% CO2. The medium consisted of Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing N2 supplement (Invitrogen), 1 mm sodium pyruvate (Invitrogen), 10% horse serum, and 10% heat-inactivated FBS (Invitrogen). Twenty four hours after plating, the medium was replaced with serum-free medium containing N2 supplement and 1 mm sodium pyruvate.
Cell Culture and Transfection
PC12 cells were obtained from RIKEN. PC12 cells were grown in DMEM supplemented with 10% FBS and 10% horse serum and then seeded on collagen I-coated cell culture plates (Iwaki) and left to adhere for 1 day for the following plasmid transfection. Plasmid DNA was transfected with Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions.
AAV Preparation and Infection
pAAV-CAG-WPRE vectors in which the expression of the gene of interest, such as mouse Lhx8, Lhx8 miRNA, lacZ miRNA, or GFP, is driven by the CAG promoter (30) were constructed and co-transfected with plasmid pHelper (containing required E2A, E4, and VA RNA adenoviral genes) and pAAV-RC (encoding the rep and cap genes) into HEK293 cells. pAAV-TrkA promoter-PLAP-WPRE was also constructed. Resulting AAV particles were purified by iodixanol step gradients and heparin-agarose column chromatography as described previously (31). The viral titers were determined by AAV2 titration ELISA kit (Progen Biotechnik GmbH).
Anesthetized rats were placed in a stereotaxic frame (Narishige), and injections were made unilaterally into the left medial septum at 0.3 mm lateral to and 0.5 mm anterior to bregma and a depth of 8.0 mm from the skull. Four microliters of viral solution were injected with a 25-gauge Hamilton syringe at a speed of 250 nl/min using a microsyringe pump (World Precision Instruments). In the case of AAV-GFP and AAV-Lhx8 vectors, 2.8 × 1010 particles were injected, and in the case of AAV-LacZ miRNA and AAV-Lhx8 miRNA vectors, 5 × 1010 particles were injected. After injection, the needle was kept in place for an additional 4 min before withdrawal. In primary septal neurons, 2 × 1010 AAV particles were transmitted per well of a 96-well culture plate for the acetylcholine (ACh) release assay, and 1 × 1011 particles were transmitted per well of a 12-well culture plate for immunoblotting.
Intracerebroventricular Infusion
Murine NGF (0.1 μg/μl, 2.5S mNGF, Promega) dissolved in phosphate-buffered saline (PBS; Invitrogen) or vehicle alone was infused into the cerebroventricular space (0.8 mm posterior, 1.4 mm lateral to bregma) at 0.5 μl/h for 2 weeks using a brain infusion kit II (Alzet) and an osmotic pump (Alzet, 2001).
ACh Release and Quantification
Cells were rinsed with Krebs-like buffer (125 mm NaCl, 4.8 mm KCl, 1.2 mm KH2PO4, 25 mm HEPES, 1.2 mm MgSO4·7H2O, 2.2 mm CaCl2·2H2O, 10 mm glucose, pH adjusted to 7.4). This buffer was discarded and replaced for a 30-min period with fresh buffer containing 6 mm K+, 10 μm choline, and 100 nm physostigmine. During 30-min constitutive release periods, the culture plates were kept at 37 °C and 5% CO2, and ACh release was measured. ACh concentration was measured by high performance liquid chromatography (HPLC) with electrochemical detection. Measurement was performed as described previously (32).
Immunoblotting, Immunohistochemistry, and Immunocytochemistry
Primary neuron cultures infected with AAV and rat septum regions were homogenized with a lysis buffer (Cell Signaling Technology, 9803) containing 1 mm phenylmethylsulfonyl fluoride (PMSM) (Sigma), and the lysates were denatured at 95 °C for 5 min in LDS sample buffer (Invitrogen) containing 1 mm dithiothreitol (DTT). The samples (10–30 μg of protein per lane) were separated by Novex 12% BisTris gels (Invitrogen) and then electroblotted onto PVDF membranes (Invitrogen). These membranes were incubated with primary antibodies. After incubation with horseradish peroxidase-conjugated secondary antibodies, signals were detected with a luminescence assay kit (GE Healthcare). Signal intensities were quantified with an image analyzer (LAS-3000, Fujifilm). The following primary antibodies were used in this study: rabbit polyclonal anti-TrkA (1:500; Millipore); rabbit polyclonal anti-choline acetyltransferase (ChAT) (1:1000; Millipore); rabbit polyclonal anti-Lhx8 (1:300; Abcam and LifeSpan BioScience); mouse monoclonal anti-actin (1:2000; Sigma); mouse monoclonal anti-GFP (1:1000; Invitrogen), and mouse monoclonal anti-HA (1:500; Covance).
Immunohistochemistry was performed as described previously (33). Rat brains were fixed with 4% paraformaldehyde in PBS (pH 7.4), and 30-μm frozen sections were analyzed. Immunolabeled sections using peroxidase-labeled avidin-biotin complex were visualized using a 3,3-diaminobenzidine solution (Vector Laboratories). For double immunofluorescence labeling, Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen) were used as secondary antibodies, and the sections were analyzed with a Biorevo BZ-9000 fluorescence microscope (Keyence).
Immunocytochemistry was performed as described previously (33), with some modification. Primary neuron cultures were fixed with 4% paraformaldehyde for 20 min at room temperature. The cells were rinsed with PBS twice and pretreated with PBS containing 0.3% Triton X-100 for 5 min at room temperature. After blocking in PBS containing 3% normal goat serum for 1 h at room temperature, the cells were incubated with primary antibody at 4 °C overnight. Alexa Fluor 555-conjugated anti-mouse or anti-rabbit IgG (Invitrogen; 1:1000) was added and incubated for 1 h at room temperature. After washing, cells were observed with a Biorevo BZ-9000 fluorescence microscope (Keyence) and a confocal microscopy (LSM 510 META, Zeiss). The following primary antibodies were used in this study: mouse monoclonal anti-MAP2 (1:1000; Sigma); mouse monoclonal anti-choline transporter (SLC5A7) (1:1000; Abcam); rabbit polyclonal anti-ChAT (1:1000; Millipore); mouse monoclonal anti-HA (1:500; Covance), and rabbit polyclonal anti-TrkA (1:500; Millipore).
TrkA Reporter Assay
PC12 cells were transfected with PLAP reporter plasmid using Lipofectamine LTX (Invitrogen) in DMEM with 5% FBS. The next day, cells were pretreated with 10 μm U0126 (Sigma), 10 μm LY294002 (Sigma), or 2 μm H89 (Sigma) for 1 h prior to addition of 25 ng/ml NGF. Four days post-transfection, the culture supernatant was collected from each sample, and PLAP activity was determined as described previously (34).
ACh Esterase (AChE) Activity Assay
Hippocampi were dissected from rats injected with NGF, AAV-GFP, or AAV-Lhx8 and homogenized in 0.1 m PB (0.1 m Na2HPO4, 0.1 m KH2PO4 (pH 8.0)) containing 0.5% Triton X-100. Aliquots of the homogenate were diluted in 0.1 m PB. Reaction buffer was prepared, containing 100 mm acetylthiocholine iodide/H2O, 0.15% NaHCO3, 0.1 m phosphate buffer (pH 7.0), and 10 mm 5,5′-dithiobis(2-nitrobenzoic acid), 0.1 m phosphate buffer (pH 7.0) containing 0.15% NaHCO3, and then mixed with the diluted aliquots in Microtest tissue culture plates (Falcon). Absorbance was then immediately measured at 412 nm using a Spectra MAX 190 spectrophotometer (Molecular Devices).
Chromatin Immunoprecipitation Analysis
PC12 cells were transfected with GFP or Lhx8 plasmid using the NeonTM transfection device. Chromatin immunoprecipitation (ChIP) assays were performed 48 h after electroporation using the LowCell ChIP kit (Diagenode) according to the manufacturer's instructions. Using purified DNA and designed primers (forward 5′-AGGTTCACAAATACCGTGA-3′ and reverse 5′-TTCGAGGGCGGAGAAGAGCA-3′), quantitative real time PCR using qSTAR SYBR Master Mix (Invitrogen) was performed according to the manufacturer's instructions, consisting of 50 cycles at 94 °C for 15 s, 56 °C for 15 s, and 72 °C for 30 s. PCR products were detected by ethidium bromide.
RNA Preparation and Real Time PCR Analysis
Total RNA was isolated from the medial septum or primary septal neurons using TRIzol reagent (Invitrogen). The RNA was processed with the RNeasy mini kit (Qiagen) and then treated with DNase I. RNA was isolated as described previously (35). The RNA was reverse-transcribed to cDNA. cDNA was synthesized with the SuperScript VILO cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. The synthesized cDNA was subjected to real time PCR. All primers were obtained from Applied Biosystems (TrkA, Rn00572130_m1; Lhx8, Mm00802919_m1; Lhx6, Mm01333349_m1; and actin, Rn00667869_m1). Signal values of each gene were normalized by the expression of actin. Real time PCR was performed at a final volume of 20 μl using TaqMan Universal PCR Master Mix (Invitrogen) according to the manufacturer's instructions using the ABI PRISM 7900HT sequence detection system (Applied Biosystems). The PCR included 50 cycles with an annealing temperature of 60 °C.
RESULTS
Lhx8 Overexpression Induces Increased ACh Release from Rat Primary Medial Septal Neurons
Transcription factors that induce differentiation of certain cell types are often required for their specific functions. For instance, Nurr1 is known to play an essential role in a range of processes from generation to maintenance of dopaminergic neurons (36, 37). Although Lhx8 has been reported to play an important role in cholinergic neuron development, whether Lhx8 regulates cholinergic neuronal functions after the developmental stage has remained unclear. To examine the effect of Lhx8 on cholinergic neuronal function, we overexpressed Lhx8 in rat E18.5 medial septal neurons in vitro, including a certain number of cholinergic neurons, via an adeno-associated virus vector 2 (AAV2-Lhx8), and 5 days later these neurons were evaluated (Fig. 1A, panels a–i). The main function of cholinergic neurons is to release the neurotransmitter ACh (38). Overexpression of Lhx8 elevated ACh release in a titer-dependent manner compared with GFP-overexpressing cells used as control (Fig. 1B), but no difference in the number of choline transporter-positive and TrkA-positive cholinergic neurons was observed between control cells and Lhx8-overexpressing cells (Fig. 1, C and D). Note that virtually all neurons were transduced with the AAV vectors in these cultures even at the lower virus titer shown in Fig. 1B (Fig. 1A, panels a–c). Together, these data suggest that Lhx8 is involved in ACh release from post-differentiated cholinergic neurons.
FIGURE 1.
Effect of Lhx8 overexpression on cholinergic neurons. After 3 DIV, rat E18.5 primary septum neurons were infected with AAV-GFP or AAV-Lhx8, and those cells were fixed at 8 DIV (A, C, and D) or exposed to Krebs-like buffer containing 6 mm K+ (B). A, representative images of septum neurons infected with AAV-GFP and immunostained with anti-MAP2 (panels a–c) or anti-choline transporter (panels d–f and d′–f′) or anti-TrkA (panels g–i, and g′–i′) antibodies. Virtually all MAP2-positive cells expressed GFP. B, ACh release evaluated after 30 min of stimulation with 6 mm K+. Data are expressed as a percentage of the ACh concentration released from AAV-GFP-infected neurons. C and D, number of choline transporter (CHT)- and TrkA-positive cells counted. No significant difference in the number of cholinergic neurons was observed. Scale bar, 30 μm. Data are presented as the mean ± S.E. n = 4; *, p < 0.05; **, p < 0.01; analysis of variance with post hoc Dunnett's multiple comparison test.
Lhx8 Potentiates the Effect of NGF on ACh Release by Regulating TrkA Expression
To investigate further the role of Lhx8 in the regulation of cholinergic neuronal functions, we examined the expression of cholinergic functional markers related to ACh release in cultured rat E18.5 medial septal neurons. TrkA and ChAT expressions were up-regulated by overexpression of Lhx8 (Fig. 2, A–C), whereas partial knockdown of Lhx8 by the miRNA expressed via an AAV vector resulted in a significant reduction in TrkA expression (Fig. 2, E–G). TrkA plays an important role in cholinergic neuron maturation and maintenance. The activation of TrkA via binding of its ligand NGF has been shown to induce an increase of ChAT expression and facilitates ACh release (26, 39–43). Because these results suggest the possibility that Lhx8 regulates NGF signals through modulating TrkA expression, we next examined Lhx8 function on ACh release induced by NGF in the primary culture of rat E18.5 medial septal neurons. These neurons infected with AAV-GFP or AAV-Lhx8 were exposed to NGF for 24 h. The effect of NGF on ACh release was potentiated by overexpression of Lhx8 (Fig. 2D). In addition, TrkA down-regulation caused by Lhx8 knockdown led to a significant decline in NGF-induced ACh release (Fig. 2H), whereas no significant reduction in base-line ACh release was observed in neurons in which Lhx8 was down-regulated in the absence of exogenous NGF. Therefore, these results indicate that Lhx8 modulates the efficacy of NGF on cholinergic neurons by regulating the expression level of TrkA.
FIGURE 2.
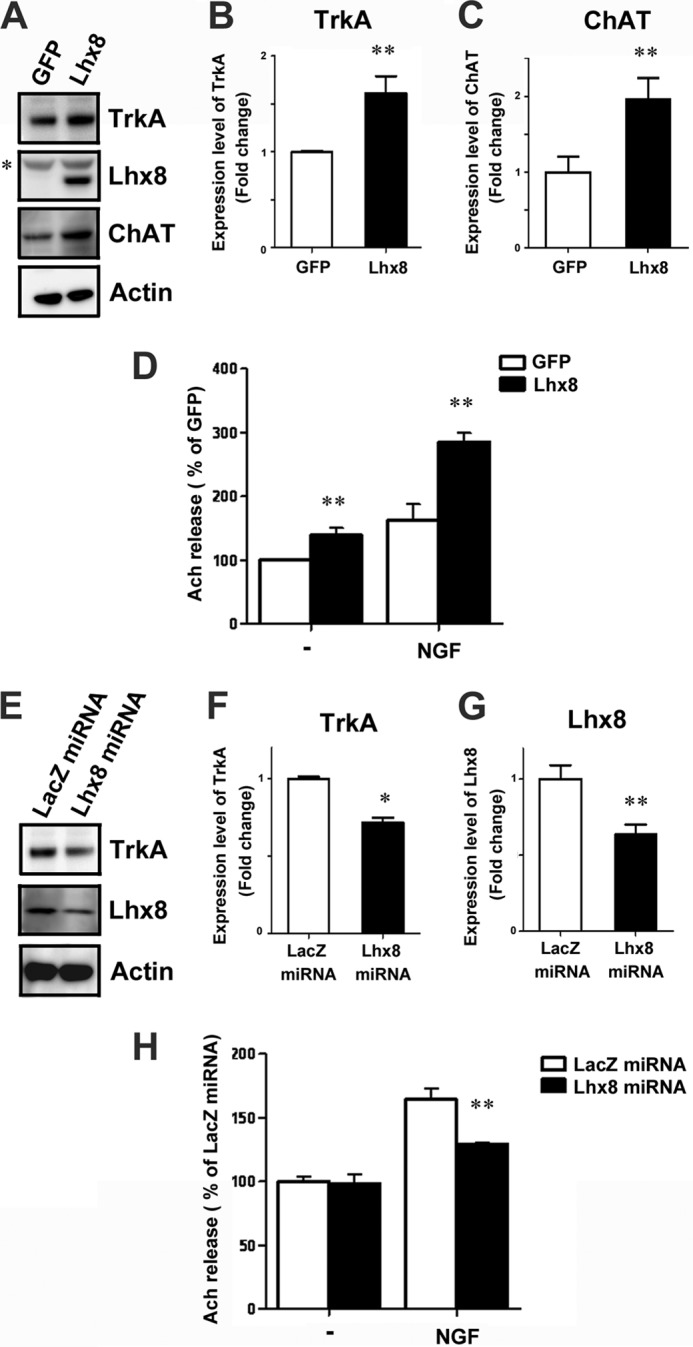
Lhx8 regulates TrkA expression and potentiates the effect of NGF on ACh release. Primary septum neurons at 4 DIV were infected with AAV-GFP, AAV-Lhx8, AAV-LacZ miRNA, or AAV-Lhx8 miRNA for 10 days and harvested for immunoblotting assay (A–C and E–G) or exposed to Krebs-like buffer containing 6 mm K+ for ACh release assay (D and H). Cell lysates were immunoblotted with an antibody directed against TrkA, Lhx8, ChAT, or actin (A and E). An asterisk indicates nonspecific labeling. Quantification of TrkA protein level between GFP- and Lhx8-treated neurons (B) or between LacZ miRNA- and Lhx8 miRNA-treated neurons (F) is shown. C, quantification of ChAT protein level between GFP- and Lhx8-treated neurons. The level of TrkA and ChAT protein was normalized to that of actin and expressed as the fold-change compared with GFP- or LacZ miRNA-treated neurons (n = 3). Specific reduction of Lhx8 expression by Lhx8 miRNA was confirmed (G). NGF (25 ng/ml) was added at 13 DIV, and quantification of ACh release assay was performed 24 h later (n = 4). Data are expressed as percentage of the GFP or LacZ miRNA control in the absence of NGF (D and H). Data are presented as the mean ± S.E. **, p < 0.01; *, p < 0.05; Student's t test.
Lhx8 Directly Regulates TrkA Expression
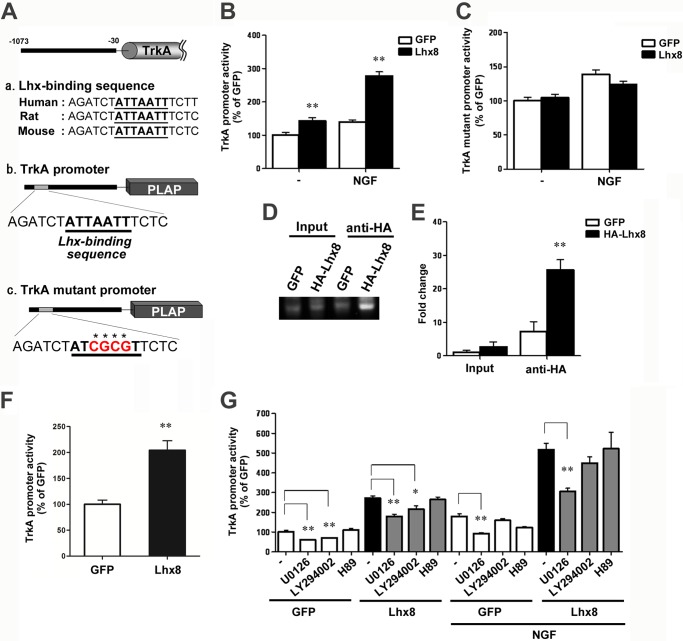
To determine whether the TrkA promoter responds to Lhx8, we performed a PLAP reporter assay of a 1073-bp upstream region of the mouse TrkA gene. This fragment contains a segment required for the appropriate spatial and temporal expression of TrkA in trigeminal, dorsal root, and sympathetic ganglia during development and is known to function as a TrkA enhancer in PC12 cells (44, 45). Based on previous reports, we identified one putative Lhx-binding sequence (position) conserved across several mammals in the TrkA promoter (Fig. 3A). This sequence (5′-ATTAATT-3′) was reported as an Lhx3 consensus binding site (46).
FIGURE 3.
Lhx8 directly regulates TrkA promoter activity. Placental alkaline phosphatase (PLAP) reporter constructs are schematically shown (A). The predicted Lhx-binding sequence in the TrkA promoter is shown. Its core region (ATTAATT) is shown in bold and underlined. In the TrkA mutant promoter, the Lhx core sequence was changed from ATTATTT to ATCGCGT, as shown in red. B, C, and G, PC12 cells were transiently co-transfected with reporter constructs and GFP or Lhx8 expression vector. Twenty four hours after transfection, the cells were treated with 25 ng/ml NGF, 10 μm U0126, 10 μm LY294002, 2 μm H89, or combinations as indicated. Placental alkaline phosphatase activity was assayed after 72 h of culture with or without NGF. Data are presented as the mean ± S.E. n = 5; **, p < 0.01; Student's t test. D and E, ChIP assay performed using PC12 cells transfected with GFP or Lhx8 tagged with human influenza hemagglutinin peptide (HA-Lhx8) expression vector. Soluble chromatin was immunoprecipitated with anti-HA antibody. Immunoprecipitates were subjected to PCR with a primer pair specific for the TrkA promoter (D). Quantitative PCR analysis was performed to measure the amount of DNA (E). Data are presented as the mean ± S.E. n = 3; **, p < 0.01; Student's t test in E. F, primary cultured septum neurons at 3 DIV were infected with AAV-GFP or AAV-Lhx8 with AAV-TrkA promoter placental alkaline phosphatase for 4 days, and placental alkaline phosphatase activity was assayed. Data are presented as the mean ± S.E. n = 5; **, p < 0.01; Student's t test.
The reporter vector and Lhx8 vector were co-transfected simultaneously into PC12 cells, and 24 h later those cells were treated with NGF. We measured the reporter activity 72 h after the addition of NGF. The level of reporter activity was significantly increased by Lhx8 overexpression, and NGF potentiated this increase in PC12 cells (Fig. 3B). In addition, when the Lhx-binding sequence was mutated (5′-ATCGCGT-3′), Lhx8 did not enhance the reporter activity. Furthermore, potentiation of the efficacy of NGF was not observed in PC12 cells transfected with reporter vector containing the mutated Lhx-binding sequence (Fig. 3C).
Next, to examine whether Lhx8 directly binds to the TrkA promoter, we performed ChIP followed by quantitative real time PCR analysis with chromatin obtained from PC12 cells overexpressing GFP or Lhx8. As shown in Fig. 3, D and E, significant enrichment of the TrkA promoter region was observed in Lhx8-overexpressing cells compared with the GFP-overexpressing cells used as control. Moreover, we confirmed Lhx8-induced TrkA promoter activity in neurons. Primary septal neurons at 3 DIV were co-infected with AAV-GFP or AAV-Lhx8 and AAV-TrkA reporter, and 4 days later these neurons were evaluated by reporter activity. As a result, Lhx8 overexpression increased TrkA promoter activity in cultured septal neurons (Fig. 3F). Taken together, these results provide evidence that Lhx8 can activate the transcription of TrkA through direct binding to the Lhx-binding sequence present in the TrkA promoter.
Interestingly, the increase of TrkA reporter activity by Lhx8 was enhanced by NGF, suggesting that Lhx8 function is modulated downstream of NGF signaling. Therefore, we examined the effects of pharmacological inhibitors H89, U0126, and LY294002, which inactivate PKA, ERK, and PI3K as known to be downstream genes of NGF signaling, respectively, on reporter activity in PC12 cells. These inhibitors were added 1 h prior to NGF treatment. We found that the increase of Lhx8-induced TrkA reporter activity was significantly suppressed by U0126 in the absence or presence of NGF (Fig. 3G). However, U0126 did not change the relative increase of the reporter activity by the ectopic expression of Lhx8 both in the presence and absence of NGF, suggesting that Lhx8 function itself is not regulated by ERK. Because U0126 suppressed TrkA promoter activity in any experimental condition, albeit to a different extent, the enhancement of Lhx8 function on the TrkA promoter by NGF signaling may occur as a consequence of synergistic action of the ERK pathway and Lhx8 downstream in the NGF signaling pathway. Indeed, the increase of Lhx8-induced TrkA reporter activity by NGF was almost completely cancelled by U0126, although changes in the absolute activity of the reporter by U0126 and NGF in the absence of Lhx8 were not as much as in the presence of Lhx8.
Lhx8 Regulates TrkA Expression and Cholinergic Neuronal Function in Vivo
To evaluate the role of Lhx8 in mature cholinergic neurons in vivo, AAV-Lhx8 tagged with HA was injected into the adult rat medial septum. Four weeks after injection, a significant increase of TrkA immunoreactivity, but not the number of cholinergic neurons recognized by TrkA or ChAT immunoreactivity in the medial septum, was observed in HA-Lhx8-infected animals compared with control animals expressing GFP (Fig. 4, A–C). The increase of TrkA expression by overexpression of Lhx8 was confirmed by immunoblotting (Fig. 4, D and E). Also, the increase of ChAT expression was directly or indirectly induced by overexpression of Lhx8 (Fig. 4, D and F). Thus, Lhx8 can regulate TrkA expression in mature cholinergic neurons in vivo. Furthermore, either intracerebroventricular infusion of NGF for 2 weeks or overexpression of Lhx8 led to significant enhancement of hippocampal AChE activity that generally has been monitored as another index of cholinergic neuronal functions because cholinergic neurons project from the medial septum to the hippocampus (Fig. 5, A and B) (47, 48). Together, these results suggest that increased TrkA expression induced by Lhx8 may enhance the functions of mature cholinergic neurons in adult brain, as demonstrated by AChE activity.
FIGURE 4.
Lhx8 regulates TrkA expression and cholinergic function in vivo. AAV-GFP or AAV-HA-Lhx8 was injected into the medial septum of 8-week-old rats. One month after AAV injection, the rats were sacrificed. Frozen sections of brain were prepared for immunohistochemical detection of TrkA (A and B) and ChAT (A and C), and tissue extracts of medial septum were used for immunoblotting (D–F). A, bright field microscopy revealed more intense brown 3,3′-diaminobenzidine staining for TrkA and ChAT in the medial septum of rats injected with AAV-HA-Lhx8 than AAV-GFP-infected ones. Scale bar, 300 μm. B and C, no significant difference in the number of TrkA- and ChAT-positive cholinergic neurons in a section with the same coordinate was observed. D, protein extracts from AAV-GFP- or AAV-Lhx8-injected medial septums were immunoblotted with an antibody directed against TrkA, ChAT, Lhx8, GFP, HA, or actin. E and F, level of TrkA and ChAT protein was normalized to that of actin and expressed as a percentage of the value of AAV-GFP-injected medial septum. Data are presented as the mean ± S.E. n = 5; **, p < 0.01; Student's t test in E. n = 5; *, p < 0.05; Student's t test in F.
FIGURE 5.
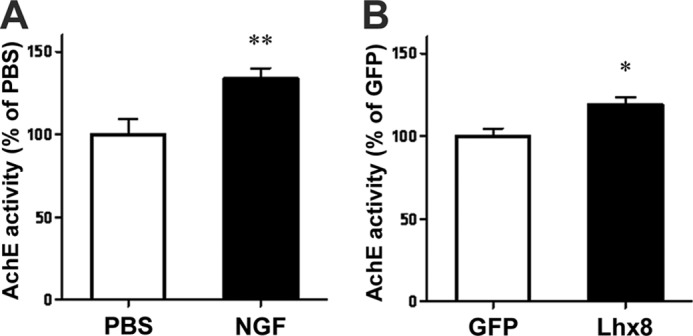
Lhx8 elevates cholinergic function in vivo. Measurement of AChE activity in medial septum was prepared from rats treated with PBS or NGF by osmotic minipump for 2 weeks (A). ACh activity was measured in rat medial septum injected with AAV-GFP or AAV-Lhx8 1 month later (B). Data are presented as the mean ± S.E. n = 4; **, p < 0.01; Student's t test in A. n = 7; *, p < 0.05; Student's t test in B.
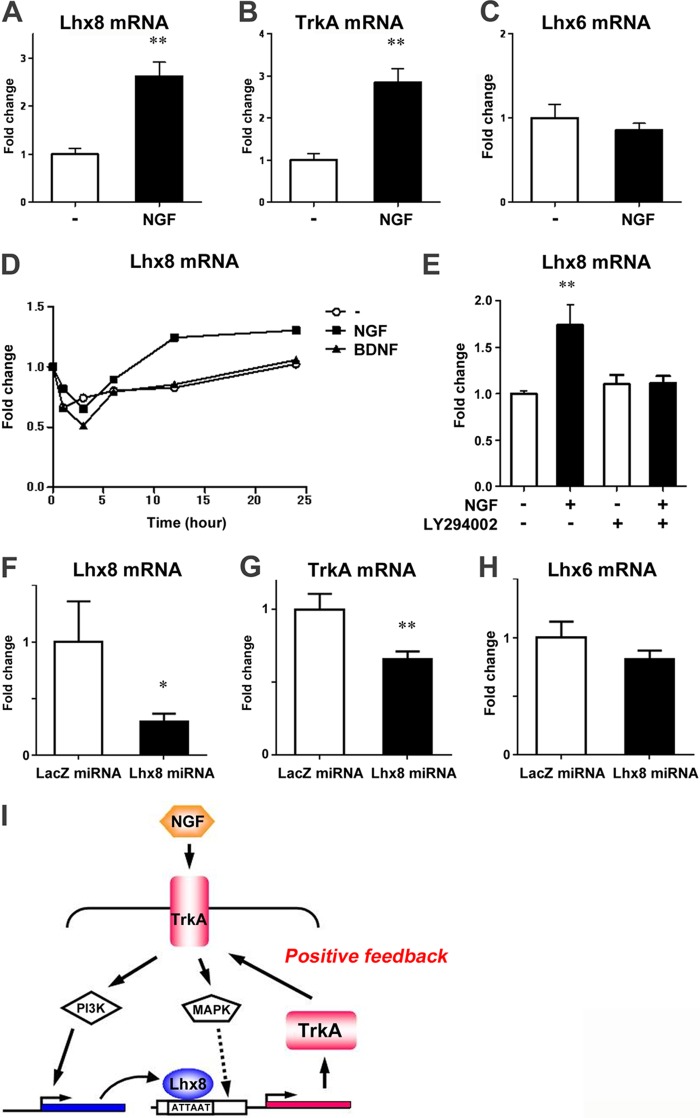
Lhx8 Expression Is Regulated by NGF as a Positive Feedback System
To better understand the overall mechanisms of the enhancement of cholinergic neuron function by NGF signaling, we exposed adult rat brain to NGF continuously for 2 weeks via intracerebroventricular administration and assessed the expression of downstream genes Lhx8 and TrkA in the medial septum. Interestingly, Lhx8 as well as TrkA expression was up-regulated in NGF-treated rats, whereas Lhx6 expression was unchanged (Fig. 6, A–C). Likewise, NGF increased Lhx8 expression also in primary medial septal neurons in a time-dependent manner, whereas brain-derived neurotrophic factor, which is a member of the neurotrophin family of growth factors (49), did not (Fig. 6D). We further examined whether PI3K is involved in the regulation of Lhx8 expression by NGF signal, because PI3K has been reported to regulate cholinergic gene expression induced by NGF (50). The primary septum neurons were cultured for 7 days prior to 1 h of pretreatment with LY294002 followed by 24 h of exposure to NGF in the presence or absence of the inhibitor. As shown in Fig. 6E, up-regulation of Lhx8 expression by NGF was suppressed by LY294002, indicating that Lhx8 expression is regulated by the NGF-TrkA-PI3K pathway. Furthermore, knockdown of Lhx8 by the miRNA expressed via an AAV vector resulted in a significant reduction in TrkA expression in the adult brain, although Lhx6 expression was not changed (Fig. 6, F–H). Taken together, these results suggest that Lhx8 modulates TrkA expression, and NGF signaling regulates TrkA expression by inducing Lhx8 expression in the adult brain, thereby generating a positive feedback loop (Fig. 6I).
FIGURE 6.
Lhx8 expression is regulated by NGF. A–C, total RNA was extracted from the medial septum of rat brains infused with PBS or NGF for 2 weeks by osmotic minipumps. Expression of Lhx8, TrkA, and Lhx6 was analyzed by quantitative RT-PCR and normalized to actin. D and E, rat E18.5 primary septum neurons at 6 DIV were treated with NGF, brain-derived neurotrophic factor, or LY294002. Total RNA was prepared from these neurons at the indicated times (D) or 24 h later (E), and Lhx8 expression was analyzed by quantitative RT-PCR and normalized to actin. F–H, total RNA was extracted from the medial septum of rat brains 1 month after injection of AAV-LacZ miRNA or AAV-Lhx8 miRNA for 1 month. Expression of Lhx8, TrkA, and Lhx6 mRNA was analyzed by quantitative RT-PCR and normalized to β-actin mRNA. I, schematic diagram of the pathway showing a positive feedback loop of NGF-TrkA-PI3K-Lhx8-TrkA, which is positively regulated by Lhx8. Data are presented as the mean ± S.E. n = 4; **, p < 0.01; Student's t test in A and B. n = 3; **, p < 0.01; Student's t test in E. n = 12; **, p < 0.01; *, p < 0.05; Student's t test in F–H.
DISCUSSION
The focus of this study was to analyze the role of Lhx8 in the maturation and maintenance of cholinergic neurons using E18.5 primary septum neuron cultures and the medial septum of the adult brain. With this approach, we identified Lhx8 as a novel regulator of cholinergic neuron function.
Many reports to date have indicated that Lhx8 has prominent functions in regulating the development of cholinergic neurons generated in the medial ganglionic eminence (12–16). In addition, Lhx8 is a pivotal factor for cholinergic differentiation of murine embryonic stem cells (51). However, the precise role of Lhx8 in the maturation and maintenance of cholinergic neurons had not been elucidated. Here we demonstrated, for the first time, that Lhx8 induces an increase of ACh release and regulates TrkA expression in differentiated cholinergic neurons, suggesting that Lhx8 has an important role in cholinergic neuron maturation and maintenance. TrkA has been shown to regulate maturation and survival of cholinergic neurons, with binding of NGF to TrkA demonstrated to produce an increase of ACh release (25). NGF is also known to have a potent survival-promoting effect on cholinergic neurons and to increase the expression of acetylcholine-related enzymes, including ChAT and vesicular acetylcholine transporter and functional release of ACh from these neurons (18–22, 42). Because elevation of TrkA level should increase their sensitivity to NGF, the increase of ChAT level by overexpression of Lhx8 could be mediated by NGF signaling. Moreover, Lhx8 knockdown in the primary septum cultures inhibited only NGF-dependent increase of ACh release (Fig. 2H). Thus, the regulation of ACh release by Lhx8 should include indirect modulation of ChAT expression at the downstream of TrkA (Figs. 2, A and C, and 4, D and F). Taken together, Lhx8 may be a key regulator of cholinergic neuron function, as in the case of Nurr1 for dopaminergic neurons (36, 37).
To determine how Lhx8 influences TrkA expression, we performed a reporter assay using the TrkA enhancer region. Previously, Lhx3 was reported to bind to an AT-rich consensus sequence that is present in the TrkA enhancer region (44–46). We found that Lhx8 directly bound to the AT-rich consensus sequence in the TrkA enhancer region and activated TrkA transcription in PC12 cells. Moreover, Lhx8 overexpression increased TrkA promoter activity in cultured septal neurons (Fig. 3F). Therefore, this enhancer may also be required for maturation and maintenance of forebrain cholinergic neurons.
Our further experiments demonstrated that NGF potentiated the Lhx8-induced TrkA transcription, and ERK pathway was required for this potentiation in PC12 cells. Because the ERK pathway has been shown to modulate various transcription factors (52, 53), some of these factors may possibly regulate TrkA expression cooperatively with Lhx8 downstream in the NGF signaling pathway. Future studies may include identification of such factors to investigate this mechanism in more detail.
We have shown that the Lhx8 expression level was elevated in response to NGF but not to another neurotrophin, brain-derived neurotrophic factor, in vitro and in vivo. This increase was mediated via activation of the PI3K pathway. Because Lhx8 is able to activate the TrkA gene enhancer, Lhx8 may be required for NGF-induced TrkA expression downstream of NGF-TrkA signaling to amplify the effect of NGF. Taken together, these results suggest that NGF-TrkA signaling may regulate TrkA expression by increasing Lhx8 expression and modulating Lhx8 function, leading to promotion of the function and maintenance of forebrain cholinergic neurons. We propose a positive feedback regulatory loop formed by NGF, TrkA, and Lhx8 for that regulation (Fig. 6I).
Accumulating evidence has implicated dysfunction of NGF signaling in the etiology of Alzheimer disease (AD). Individuals with early to late stage AD demonstrate reduced TrkA expression in the brain, and this reduction is correlated with their memory impairment (54, 55). In addition, NGF replacement therapy has emerged as a potential treatment for AD (56–58). In a recent phase 1 clinical trial, implantation of NGF-producing fibroblasts into the basal forebrain of AD patients significantly slowed the rate of cognitive decline and increased cortical glucose uptake (59). Increasing Lhx8 expression levels in the brains of AD patients may enhance NGF signaling and survival of cholinergic neurons, thus improving cognitive functions in patients. Therefore, identification of modulators of Lhx8 function and pharmacological approaches to control their action via modulation of the PI3K and ERK pathways should be significant steps toward the treatment of AD.
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Funding Program for World-leading Innovative R&D on Science and Technology (to H. O.), and Eisai Co., Ltd.
- BFCN
- basal forebrain cholinergic neuron
- Lhx8
- LIM homeobox protein 8
- ACh
- acetylcholine
- AChE
- ACh esterase
- DIV
- days in vitro
- PLAP
- placental alkaline phosphatase
- AAV
- adeno-associated virus
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- AD
- Alzheimer disease.
REFERENCES
- 1. Winson J. (1978) Loss of hippocampal θ rhythm results in spatial memory deficit in the rat. Science 201, 160–163 [DOI] [PubMed] [Google Scholar]
- 2. Everitt B. J., Robbins T. W. (1997) Central cholinergic systems and cognition. Annu. Rev. Psychol. 48, 649–684 [DOI] [PubMed] [Google Scholar]
- 3. Fischer W., Chen K. S., Gage F. H., Björklund A. (1992) Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol. Aging 13, 9–23 [DOI] [PubMed] [Google Scholar]
- 4. Leanza G., Nilsson O. G., Wiley R. G., Björklund A. (1995) Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioural, biochemical and stereological studies in the rat. Eur. J. Neurosci. 7, 329–343 [DOI] [PubMed] [Google Scholar]
- 5. Leanza G., Muir J., Nilsson O. G., Wiley R. G., Dunnett S. B., Bjorklund A. (1996) Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur. J. Neurosci. 8, 1535–1544 [DOI] [PubMed] [Google Scholar]
- 6. Kimura S., Hara Y., Pineau T., Fernandez-Salguero P., Fox C. H., Ward J. M., Gonzalez F. J. (1996) The T/ebp null mouse: thyroid-specific enhancer binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10, 60–69 [DOI] [PubMed] [Google Scholar]
- 7. Casarosa S., Fode C., Guillemot F. (1999) Mash1 regulates neurogenesis in the ventral telencephalon. Development 126, 525–534 [DOI] [PubMed] [Google Scholar]
- 8. Hallonet M., Hollemann T., Pieler T., Gruss P. (1999) Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 13, 3106–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sussel L., Marin O., Kimura S., Rubenstein J. L. (1999) Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370 [DOI] [PubMed] [Google Scholar]
- 10. Toresson H., Potter S. S., Campbell K. (2000) Genetic control of dorsal ventral identify in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127, 4361–4371 [DOI] [PubMed] [Google Scholar]
- 11. Yun K., Potter S., Rubenstein J. L. (2001) Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128, 193–205 [DOI] [PubMed] [Google Scholar]
- 12. Grigoriou M., Tucker A. S., Sharpe P. T., Pachnis V. (1998) Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development 125, 2063–2074 [DOI] [PubMed] [Google Scholar]
- 13. Asbreuk C. H., van Schaick H. S., Cox J. J., Kromkamp M., Smidt M. P., Burbach J. P. (2002) The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience 109, 287–298 [DOI] [PubMed] [Google Scholar]
- 14. Zhao Y., Marín O., Hermesz E., Powell A., Flames N., Palkovits M., Rubenstein J. L., Westphal H. (2003) The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc. Natl. Acad. Sci. U.S.A. 100, 9005–9010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mori T., Yuxing Z., Takaki H., Takeuchi M., Iseki K., Hagino S., Kitanaka J., Takemura M., Misawa H., Ikawa M., Okabe M., Wanaka A. (2004) The LIM homeobox gene, L3/.Lhx8, is necessary for proper development of basal forebrain cholinergic neurons. Eur. J. Neurosci. 19, 3129–3141 [DOI] [PubMed] [Google Scholar]
- 16. Fragkouli A., Hearn C., Errington M., Cooke S., Grigoriou M., Bliss T., Stylianopoulou F., Pachnis V. (2005) Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur. J. Neurosci. 21, 2923–2938 [DOI] [PubMed] [Google Scholar]
- 17. Lopes R., van Wijk N. V., Neves G., Pachnis V. (2012) Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc. Natl. Acad. Sci. U.S.A. 109, 3119–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hefti F. (1986) Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 6, 2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mobley W. C., Rutkowski J. L., Tennekoon G. I., Gemski J., Buchanan K., Johnston M. V. (1986) Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 387, 53–62 [DOI] [PubMed] [Google Scholar]
- 20. Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. (1986) Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc. Natl. Acad. Sci. U.S.A. 83, 9231–9235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilcox B. J., Applegate M. D., Portera-Cailliau C., Koliatsos V. E. (1995) Nerve growth factor prevents apoptotic cell death in injured central cholinergic neurons. J. Comp. Neurol. 359, 573–585 [DOI] [PubMed] [Google Scholar]
- 22. Bäckman C., Rose G. M., Hoffer B. J., Henry M. A., Bartus R. T., Friden P., Granholm A. C. (1996) Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J. Neurosci. 16, 5437–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gustilo M. C., Markowska A. L., Breckler S. J., Fleischman C. A., Price D. L., Koliatsos V. E. (1999) Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons. J. Comp. Neurol. 405, 491–507 [DOI] [PubMed] [Google Scholar]
- 24. Ha D. H., Robertson R. T., Roshanaei M., Weiss J. H. (1999) Enhanced survival and morphological features of basal forebrain cholinergic neurons in vitro: role of neurotrophins and other potential cortically derived cholinergic trophic factors. J. Comp. Neurol. 406, 156–170 [PubMed] [Google Scholar]
- 25. Huang E. J., Reichardt L. F. (2003) Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 26. Fagan A. M., Garber M., Barbacid M., Silos-Santiago I., Holtzman D. M. (1997) A role for TrkA during maturation of striatal and basal forebrain cholinergic neurons in vivo. J. Neurosci. 17, 7644–7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi E., Murata Y., Oki T., Miyamoto N., Mori Y., Takada N., Wanifuchi H., Wanifuchi N., Yagami K., Niidome T., Tanaka I., Katayama K. (1999) Isolation and functional characterization of the 5′-upstream region of mouse P/Q-type Ca2+ channel α1A subunit gene. Biochem. Biophys. Res. Commun. 260, 54–59 [DOI] [PubMed] [Google Scholar]
- 28. Donello J. E., Beeche A. A., Smith G. J., 3rd, Lucero G. R., Hope T. J. (1996) The hepatitis B virus posttranscriptional regulatory element is composed of two subelements. J. Virol. 70, 4345–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fitzsimons H. L., Bland R. J., During M. J. (2002) Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods 28, 227–236 [DOI] [PubMed] [Google Scholar]
- 30. Niwa H., Yamamura K., Miyazaki J. (1991) Efficient selection for high expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 31. Zolotukhin S., Potter M., Zolotukhin I., Sakai Y., Loiler S., Fraites T. J., Jr., Chiodo V. A., Phillipsberg T., Muzyczka N., Hauswirth W. W., Flotte T. R., Byrne B. J., Snyder R. O. (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167 [DOI] [PubMed] [Google Scholar]
- 32. Kosasa T., Kuriya Y., Matsui K., Yamanishi Y. (1999) Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. Eur. J. Pharmacol. 380, 101–107 [DOI] [PubMed] [Google Scholar]
- 33. Ohgoh M., Kimura M., Ogura H., Katayama K., Nishizawa Y. (1998) Apoptotic cell death of cultured cerebral cortical neurons induced by withdrawal of astroglial trophic support. Exp. Neurol. 149, 51–63 [DOI] [PubMed] [Google Scholar]
- 34. Goto M., Yamada K., Katayama K., Tanaka I. (1996) Inhibitory effect of E3330, a novel quinone derivative able to suppress tumor necrosis factor-α generation, on activation of nuclear factor-κB. Mol. Pharmacol. 49, 860–873 [PubMed] [Google Scholar]
- 35. Okada Y., Shimazaki T., Sobue G., Okano H. (2004) Retinoic acid- concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 275, 124–142 [DOI] [PubMed] [Google Scholar]
- 36. Zetterström R. H., Solomin L., Jansson L., Hoffer B. J., Olson L., Perlmann T. (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276, 248–250 [DOI] [PubMed] [Google Scholar]
- 37. Saucedo-Cardenas O., Quintana-Hau J. D., Le W. D., Smidt M. P., Cox J. J., De Mayo F., Burbach J. P., Conneely O. M. (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 4013–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Auld D. S., Day J. C., Mennicken F., Quirion R. (2000) Pharmacological characterization of endogenous acetylcholine release from primary septal cultures. J. Pharmacol. Exp. Ther. 292, 692–697 [PubMed] [Google Scholar]
- 39. Takei N., Tsukui H., Hatanaka H. (1989) Intracellular storage and evoked release of acetylcholine from postnatal rat basal forebrain cholinergic neurons in culture with nerve growth factor. J. Neurochem. 53, 1405–1410 [DOI] [PubMed] [Google Scholar]
- 40. Suzuki T., Kanagawa M., Takada Y., Fujimoto K., Kawashima K. (1994) Nerve growth factor treatment induces high potassium evoked calcium-dependent acetylcholine release in cultured embryonic rat septal cells. Brain Res. 665, 311–314 [DOI] [PubMed] [Google Scholar]
- 41. Pongrac J. L., Rylett R. J. (1996) Differential effects of nerve growth factor on expression of choline acetyltransferase and sodium-coupled choline transport in basal forebrain cholinergic neuron in culture. J. Neurochem. 66, 804–810 [DOI] [PubMed] [Google Scholar]
- 42. Oosawa H., Fujii T., Kawashima K. (1999) Nerve growth factor increases the synthesis and release of acetylcholine and the expression of vesicular acetylcholine transporter in primary cultured rat embryonic septal cells. J. Neurosci. Res. 57, 381–387 [PubMed] [Google Scholar]
- 43. Auld D. S., Mennicken F., Quirion R. (2001) Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: differentiation between neuromodulatory and neurotrophic influences. J. Neurosci. 21, 3375–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma L., Merenmies J., Parada L. F. (2000) Molecular characterization of the TrkA/NGF receptor minimal enhancer reveals regulation by multiple cis elements to drive embryonic neuron expression. Development 127, 3777–3788 [DOI] [PubMed] [Google Scholar]
- 45. Ma L., Lei L., Eng S. R., Turner E., Parada L. F. (2003) Brn3a regulation of TrkA/NGF receptor expression in developing sensory neurons. Development 130, 3525–3534 [DOI] [PubMed] [Google Scholar]
- 46. Bridwell J. A., Price J. R., Parker G. E., McCutchan Schiller A., Sloop K. W., Rhodes S. J. (2001) Role of the LIM domains in DNA recognition by the Lhx3 neuroendocrine transcription factor. Gene 277, 239–250 [DOI] [PubMed] [Google Scholar]
- 47. El Tamer A., Wülfert E., Hanin I. (1996) Age-dependent effect of AF64A on cholinergic activity in the septo-hippocampal pathway of the rat brain: decreased responsiveness in aged rats. Neurosci. Lett. 203, 123–126 [DOI] [PubMed] [Google Scholar]
- 48. Fischer W., Björklund A. (1991) Loss of AChE and NGFr labeling precedes neuronal death of axotomized septal-diagonal band neurons: reversal by intraventricular NGF infusion. Exp. Neurol. 113, 93–108 [DOI] [PubMed] [Google Scholar]
- 49. Xu B., Gottschalk W., Chow A., Wilson R. I., Schnell E., Zang K., Wang D., Nicoll R. A., Lu B., Reichardt L. F. (2000) The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 20, 6888–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madziar B., Lopez-Coviella I., Zemelko V., Berse B. (2005) Regulation of cholinergic gene expression by nerve growth factor depends on the phosphatidylinositol-3′-kinase pathway. J. Neurochem. 92, 767–779 [DOI] [PubMed] [Google Scholar]
- 51. Manabe T., Tatsumi K., Inoue M., Makinodan M., Yamauchi T., Makinodan E., Yokoyama S., Sakumura R., Wanaka A. (2007) L3/Lhx8 is a pivotal factor for cholinergic differentiation of murine embryonic stem cells. Cell Death Differ. 14, 1080–1085 [DOI] [PubMed] [Google Scholar]
- 52. Turjanski A. G., Vaqué J. P., Gutkind J. S. (2007) MAP kinases and the control of nuclear events. Oncogene 26, 3240–3253 [DOI] [PubMed] [Google Scholar]
- 53. Whitmarsh A. J. (2007) Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta 1773, 1285–1298 [DOI] [PubMed] [Google Scholar]
- 54. Salehi A., Delcroix J. D., Mobley W. C. (2003) Traffic at the intersection of neurotrophic factor signaling and neurodegeneration. Trends Neurosci. 26, 73–80 [DOI] [PubMed] [Google Scholar]
- 55. Saragovi H. U. (2005) Progression of age-associated cognitive impairment correlates with quantitative and qualitative loss of TrkA receptor protein in nucleus basalis and cortex. J. Neurochem. 95, 1472–1480 [DOI] [PubMed] [Google Scholar]
- 56. Fischer W., Sirevaag A., Wiegand S. J., Lindsay R. M., Björklund A. (1994) Reversal of spatial memory impairments in aged rats by nerve growth factor and neurotrophins 3 and 4/5 but not by brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 91, 8607–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martínez-Serrano A., Fischer W., Söderström S., Ebendal T., Björklund A. (1996) Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc. Natl. Acad. Sci. U.S.A. 93, 6355–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martínez-Serrano A., Fischer W., Björklund A. (1995) Reversal of age-dependent cognitive impairments and cholinergic neuron atrophy by NGF-secreting neural progenitors grafted to the basal forebrain. Neuron 15, 473–484 [DOI] [PubMed] [Google Scholar]
- 59. Tuszynski M. H., Thal L., Pay M., Salmon D. P., U H. S., Bakay R., Patel P., Blesch A., Vahlsing H. L., Ho G., Tong G., Potkin S. G., Fallon J., Hansen L., Mufson E. J., Kordower J. H., Gall C., Conner J. (2005) A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 11, 551–555 [DOI] [PubMed] [Google Scholar]