Background: The coxsackievirus and adenovirus receptor (CAR) acts as a docking factor during infection.
Results: Adenovirus fiber knob induces CAR internalization via a pathway involving lipid microdomain integrity, actin dynamics, and dynamin.
Conclusion: In neurons, CAR is linked to endocytic pathways that could modulate its function or regulate viral infection.
Significance: Learning how viruses use receptors to enter and spread in the nervous system.
Keywords: Adenovirus, Endocytosis, Lipid Raft, Lysosomes, Neurons, CAR, Axonal Transport
Abstract
The coxsackievirus and adenovirus receptor (CAR) serves as a docking factor for some adenovirus (AdV) types and group B coxsackieviruses. Its role in AdV internalization is unclear as studies suggest that its intracellular domain is dispensable for some AdV infection. We previously showed that in motor neurons, AdV induced CAR internalization and co-transport in axons, suggesting that CAR was linked to endocytic and long-range transport machineries. Here, we characterized the mechanisms of CAR endocytosis in neurons and neuronal cells. We found that CAR internalization was lipid microdomain-, actin-, and dynamin-dependent, and subsequently followed by CAR degradation in lysosomes. Moreover, ligands that disrupted the homodimeric CAR interactions in its D1 domains triggered an internalization cascade involving sequences in its intracellular tail.
Introduction
Cell surface molecules are often responsible for the initial recognition of pathogens by the host, and thus much effort has been devoted to characterize how cells capture pathogens. In the nervous system, several cell adhesion molecules (CAMs)7 located at nerve terminals bind viruses, including members of Herpesviridae and Picornaviridae (1). Virus propagation and/or latency depend on their ability to be internalized and to recruit the axonal transport machinery to be transported to the cell body of neurons. Axonal transport is a key mechanism in neuronal homeostasis that maintains efficient communication between the somatodendritic compartment and nerve termini. The integrity of this process is crucial for neuronal survival as impairment of factors regulating this pathway can be linked to neurodegenerative disease (2, 3). In this light, viruses are ideal tools to understand cellular processes: for instance, α-herpesvirus and rabies virus have been used as neuronal tracers and to understand signals regulating intracellular trafficking in neurons (1, 4).
The coxsackievirus and adenovirus receptor (CAR, encoded by CXADR) is a member of the immunoglobulin (Ig) superfamily (5). It has a single membrane-spanning domain, an extracellular domain of 216 residues composed of two Ig-like domains (D1 and D2), and an intracellular tail composed of 107 residues. The D1 domain can be recognized at the cell surface by many adenovirus (AdV) types and the group B coxsackieviruses (CVB). Our current understanding of its role in AdV entry is that it serves as “docking platform,” but does not significantly participate in internalization. This is based primary on the finding that when the entire intracellular tail of CAR is deleted AdV infection still progressed efficiently (6). In addition, during CVB entry at tight junctions in epithelial cells, CAR remained at the plasma membrane (7). However, during canine adenovirus type 2 (CAdV-2, commonly referred as CAV-2) entry in motor neurons, CAR and CAV-2 were co-internalized within axons and transported bidirectionally in pH-neutral endosomes (8). Incubation of motor neurons with recombinant CAV-2 fiber knob (FKCAV), which binds in theory three CAR molecules (9), also led to CAR internalization and transport (8). In addition, others have showed that CAR may regulate intracellular signaling pathways through engagement with co-receptors such as junctional adhesion molecule type L (10), or after human AdV type 5 (HAdV5) binding (11). Interestingly, HAdV5 FK was efficiently internalized in lacrimal acinar cells and may stimulate endocytic pathways such as macropinocytosis to regulate HAdV5 entry (12). Whether this effect was CAR-dependent was, however, not addressed.
CAMs and other receptors respond to external cues during neuronal development and in mature neurons by eliciting intracellular signaling through their endocytosis (13). This highlights the link between endocytosis and signaling as endosomes play a crucial role in signal transduction (14, 15). Furthermore, CAM depletion from the plasma membrane and subsequent degradation through the lysosomal pathway can change cell adhesion, a process important in the control of cell migration (16, 17). Understanding how CAR is linked to the intracellular machinery is also of particular importance for a better understanding of its endogenous function in the CNS as a role in neurite outgrowth was proposed (18), as well as in virus infection and gene transfer.
In this study, we characterized the mechanisms regulating CAR endocytosis and transport upon ligand binding in neurons. We found that CAR D1-D1 interaction disengagement, coupled to sequences in its cytoplasmic tail were responsible for ligand-induced CAR endocytosis. Moreover, we found that lipid microdomains, dynamin, and actin played crucial roles in CAR internalization. We also demonstrated that CAR was targeted to lysosomes for degradation in primary rodent neuron cultures and in the rodent brain.
EXPERIMENTAL PROCEDURES
Material, Constructs and Cellular Cultures
Labeled recombinant CTB (cholera toxin binding fragment), dextran and transferrin (Tf), as well as Alexa Fluor-conjugated secondary antibodies were purchased from Invitrogen. RmcB, a mouse monoclonal anti-CAR antibody was purchased from Millipore, the goat polyclonal anti-CAR antibody was from R&D Systems, and the rabbit polyclonal anti-CAR was generously provided by Joseph Zabner (University of Iowa). Polyclonal anti-flotillin-1 and monoclonal anti-β-tubulin were purchased from Sigma. Rat monoclonal anti-LAMP1 was purchased from Abcam. β3-Tubulin (Tuj-1) antibody was purchased from Covance. Anti-Rab5 antibody was from Synaptic Systems. OptiPrepTM, filipin, Latrunculin A (LatA), methyl-β-cyclodextrin, E-64D, and pepstatin A were from Sigma.
Plasmids from the pcDNA-RFP-C backbone and encoding CAR-RFP and mutants 348, 315, and 261 were previously described (19). The pcDNA-CAR-274 construct was generated using site-directed mutagenesis. The AP2μ2T156A construct was a gift from E. Smythe and the dynaminK44A construct was a gift from H. McMahon (Laboratory of Molecular Biology, Cambridge, UK).
Mouse hippocampal neurons were obtained from OF1 embryonic day 18 (E18) embryos using standard procedures. Briefly, hippocampi were isolated, dissociated with 0.025% trypsin, and plated in Neurobasal medium (Invitrogen) containing B27 (Invitrogen), l-glutamine (Sigma), Glutamax (Invitrogen), 10% fetal bovine serum (FBS, Sigma), and antibiotics. Hippocampal neurons were then incubated at 37 °C and 5% CO2 under a humidified environment. At day in vitro (DIV) 4, ⅔ of the medium was replaced with medium without l-glutamine and FBS. Neurons were used at DIV 10–14. Neu2A cells were cultured in DMEM (Invitrogen) containing 10% FBS and antibiotics.
siRNA Treatments
siRNAs against the murine clathrin heavy chain (CHC) were previously described (chc-1, 5′-AACAUUGGCUUCAGUACCUUGTT-3′; chc-2, 5′-AAUGGAUCUCUUUGAAUACGGTT-3) (20). Control siRNA was designed using the siRNA CHC-1 sequence and replacing 2 central nucleotides (control, 5′-AACAUUGGCAACAGUACCUUGTT-3′). siRNA duplexes were purchased from IDT Technologies and transfected in primary neurons according to a published method (21). Briefly, neurons at DIV 11 were co-transfected with a plasmid encoding GFP (pEGFP) and siRNA duplexes using Lipofectamine 2000. Three days later, ligands (FKCAV or Tf) were applied and internalization was monitored. Knock-down of CHC was monitored using anti-clathrin antibody (clone X22) from Abcam.
Adenoviral Vectors and Recombinant Fiber Knob (FKCAV)
CAV-GFP, CAV-CRE, and HAdV5-βGal were produced and purified using established methods (22, 23). To produce FKCAV (residues 358–542), the cDNA was cloned into pPROEX HTb (Invitrogen), expressed with a cleavable His6 tag, and purified as previously described (9). Labeled FKCAV was obtained as previously described (8).
Flow Cytometry
Neu2A cells transfected with a plasmid encoding for CAR-RFP were collected in PBS (Invitrogen) and incubated for 1 h at 4 °C with anti-CAR RmcB. Cells were washed with PBS and incubated for 30 min with Alexa Fluor 488-labeled anti-mouse IgG antibody. As control, nontransfected cells incubated with anti-CAR and cells transfected and incubated with only the secondary antibody were used. Cells were washed with PBS, fixed with 2% paraformaldehyde, and analyzed with FACSCalibur (BD Biosciences). Compensation to avoid overlapping fluorescence between RFP and Alexa Fluor 488 was performed. Data analysis was performed using the FlowJo software.
Lipid Microdomain Isolation
Lipid microdomains were isolated with a modified protocol previously described (24). Briefly, cells were lysed using 250 mm sucrose, 1 mm MgCl2, 1 mm CaCl2, and 20 mm Tris-HCl, pH 7.5. The cellular homogenate was passed through a 25-gauge needle several times and centrifuged at 16,000 × g for 10 min at 4 °C. The supernatant was put aside, the procedure repeated on the pellet, and the two supernatants pooled. Lipid microdomain fractions were obtained by ultracentrifugation in OptiPrepTM step density gradients. Two milliliters (ml) of the supernatant was mixed with an equal volume of 50% OptiPrep diluted in lysis buffer and transferred to a SW40 ultracentrifuge tube. 1.7 ml of 20, 15, 10, and 5% OptiPrep and lysis buffer (v/v) were successively overlaid. The tube was centrifuged at 75,000 × g for 90 min at 4 °C. Fractions of 1 ml were removed from the top of the tube and precipitated in 10% trichloroacetic acid overnight at 4 °C. Precipitates were then washed with cold acetone and solubilized in SDS-PAGE loading buffer.
Immunofluorescence and Confocal Microscopy
Hippocampal neurons plated on poly-d-ornithine coverslips or in microfluidic chambers (see below) were imaged using Leica LSM780 and Zeiss SP5 confocal microscopes, with ×63 1.4 NA Plan Apochromat oil-immersion objectives.
For indirect immunofluorescence, neurons were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 5 min at room temperature (RT), followed by a blocking step with 2% bovine serum albumin (BSA) and 10% horse serum for 30 min to 1 h at RT. Primary and secondary antibodies were diluted in blocking solution and incubated sequentially for 1 h at RT. Samples were then mounted with fluorescent mounting medium (Dako) with DAPI (Sigma) and imaged by confocal microscopy.
Live cell imaging was performed as previously described (8). Briefly, 100–150 frames were acquired at 0.5 frames/s. Kymographs were generated using the Image J software.
Microfluidic Chambers
Microfluidic chambers (MCs) for neuronal compartmentalization were produced as previously described (25, 26). Briefly, polydimethylsiloxane inserts were covalently bound to glass bottom dishes (Wilco Dish) using a plasma cleaner (Deiner Electronic). MCs were then coated with 4% BSA for 2 h at 37 °C followed by poly-d-ornithine for 2 h at 37 °C. Devices were washed and further coated with laminin for 90 min at 37 °C. Dissociated neurons were plated in the proximal compartment and axonal growth through the microgrooves to the distal compartment was allowed for 5–8 days. To perform axonal transport experiments, distal compartments were fluidically isolated by using less volume than in proximal compartments.
Ethic Statement
The animals were treated in accordance with the European Community Council Directive 86/609, modified by the decrees 87/848 and 2001/464. The Animal Welfare Committee at the University of Montpellier 2 approved all protocols and all efforts were made to minimize the number of animals used and potential pain and distress. Pertinent license and permits (34-132) were obtained prior to institutional and regional animal welfare committee approval.
Intraparenchymal Injection of FKCAV
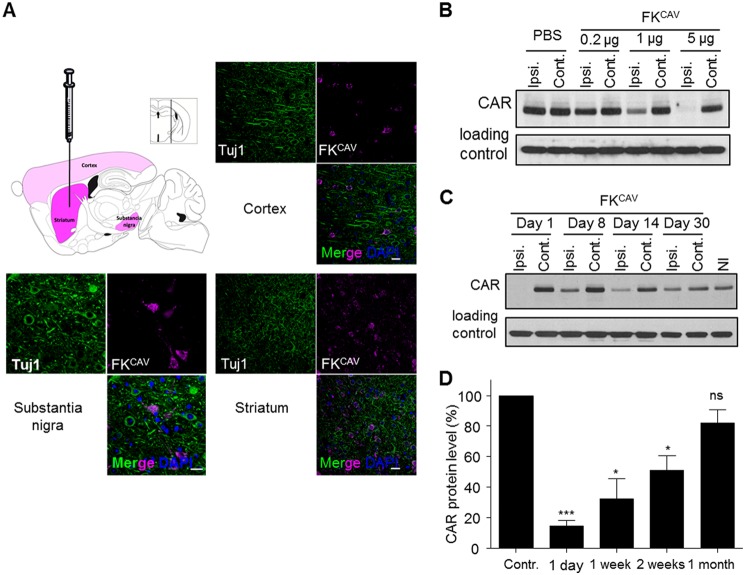
6–7-Week-old female OF1 mice (Charles River) weighing 26–30 g were housed in groups prior to injections, and allowed food and water ad libitum. They were maintained in a controlled environment (22 ± 1 °C, 55 ± 5% humidity) with a 12 h:12 h light/dark cycle. Intrastriatal injections were performed in anesthetized animals with an intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). To cover the entire striatum, we targeted four coordinates of injection according to a mouse brain atlas (80) (first and second sites of injection: anterior-posterior, +0.14 mm; lateral, +2 mm; dorsal-ventral, −3/−4 mm; third and fourth sites of injection: anterior-posterior, +1.10 mm; lateral, +1.5 mm; dorsal-ventral, −3/−3.75 mm). Animals were divided into several groups. One group received injections of PBS and a second group injections of FKCAV (0.2, 1, or 5 μg/striatum). Each mouse was injected in one hemisphere; the contralateral striatum was used as internal control. Mice were sacrificed by intracardiac fixation or decapitation 1, 7, 14, or 30 days post-injection. For the study of CAR expression by immunoblot, striata were dissected and proteins were extracted by sonication with 5% SDS. The location of the Cy5-tagged FKCAV was evaluated in the striatum and the substantia nigra 1 day post-injection.
Statistical Analyses
Data were analyzed using Student's t test for unpaired data and one sample t test for data compared with control set as 100% (*, p value <0.05; **, p value <0.01; ***, p value <0.001 versus control). Results are expressed as mean ± S.E.
RESULTS
Multiple Ligands Induced CAR Internalization in Primary Neurons and Neu2A Cells
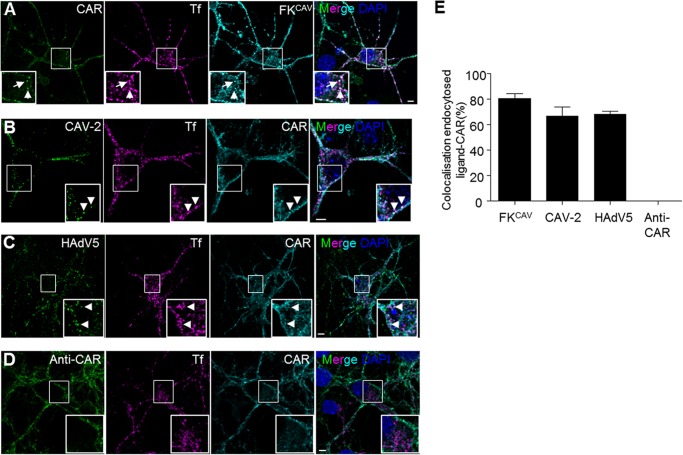
Receptor engagement by viruses or viral proteins is often linked with conformational changes that can ultimately lead to endocytosis of the receptor-pathogen complex. To better understand the mechanism leading to CAR internalization, the role of ligand valency and CAR-CAR interactions, we incubated hippocampal neurons for 20 min at 37 °C after a pre-binding step on ice with multiple CAR ligands: FKCAV, CAV-2, HAdV5, and anti-CAR antibodies (Fig. 1, A–E). Cultured hippocampal neurons from E18 express CAR at the membrane and possibly in intracellular compartments (data not shown), as we previously showed in primary motor neurons (8, 27). CAV-2 and FKCAV led to CAR internalization in puncta that were also positive for Tf, which label endocytic structures (as observed in motor neurons (8)) (Fig. 1, A, B, and E). We also detected internalized CAR after incubation with HAdV5, suggesting that other CAR-tropic AdVs also triggered CAR internalization (Fig. 1, C and E). One possible explanation for ligand-induced CAR internalization was that its clustering through multivalent interactors initiate subsequent endocytosis. To test whether clustering per se was responsible for CAR entry, we used anti-CAR antibodies recognizing epitopes on the extracellular domains of CAR (see “Experimental Procedures”). In contrast to trivalent (FKCAV) or multivalent (AdV) ligands, anti-CAR antibodies did not lead to CAR internalization in hippocampal neurons (Fig. 1, D and E), suggesting that clustering of CAR through multimeric ligands was not sufficient for its internalization. Notably, recombinant FKs and AdVs can disrupt CAR D1-D1 interactions (28).
FIGURE 1.
CAR was endocytosed after viral ligand binding in hippocampal neurons. A–D, viral ligands induced CAR internalization. Hippocampal neurons were incubated with FKCAV (0.6 μg/ml) (A), CAV-2 (2,000 pp/c) (B), HAdV5 (2,000 pp/c) (C), or anti-extracellular CAR (D) together with Alexa 555-Tf for 15 min on ice, and washed, and internalization was allowed for 20 min at 37 °C. Fixed cells were labeled for endogenous CAR. CAR can be found in endocytic vesicles positive for Tf (indicated by arrows) after FKCAV, CAV-2, and HAdV5 engagement, but not after anti-CAR binding. E, quantification of three independent experiments shows that internalized ligands (co-localizing with Tf) are found with CAR (n = 3, ≥107 vesicles counted for each condition; results are expressed as mean ± S.E.). Scale bars: A–D, 5 μm.
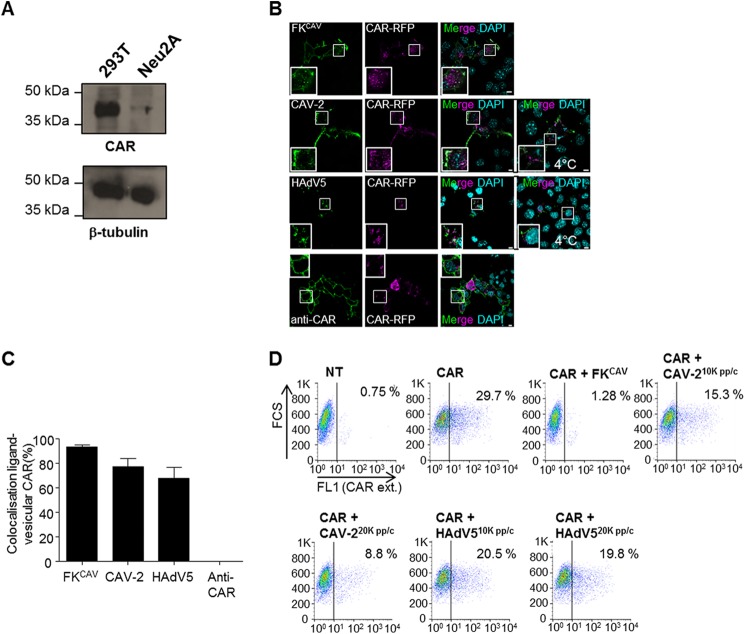
This differential ligand-induced internalization was also seen in CAR-transfected Neu2A cells, a mouse line derived from a murine neuroblastoma with undetectable CAR expression (Fig. 2A). In Neu2A cells transfected with a plasmid expressing CAR-RFP and then incubated with FKCAV, CAV-2, or HAdV5, the ligands were found in vesicular structures together with CAR, similarly to hippocampal neurons. Notably, cells incubated with anti-CAR antibodies did not contain intracellular puncta of CAR (Fig. 2, B and C). To test these observations using another method, we monitored CAR plasma membrane levels using flow cytometry. Neu2A cells were transfected with a plasmid encoding CAR-RFP, and then incubated overnight with FKCAV, CAV-2, or HAdV5. In these conditions, we found a decrease in plasma membrane CAR (Fig. 2D), and therefore conclude that viral ligands triggered CAR internalization. Of note, at similar physical particle per cell ratio (pp/c), CAV-2 induced CAR internalization more efficiently than HAdV5 (Fig. 2D). Together, these data showed that CAR can be internalized in neuronal cells, but different efficacies in ligand-dependent internalization existed.
FIGURE 2.
CAR was endocytosed after viral ligand binding in Neu2A cells. A, Neu2A cells did not express CAR. Western blot analyses showed that in contrast to 293T cells, Neu2A did not express detectable levels of CAR. B, viral ligands triggered CAR-RFP internalization in Neu2A cells. Cells were transfected with a plasmid encoding for CAR-RFP and 24 h later, incubated with FKCAV, CAV-2 (2,000 pp/c), HAdV5 (2,000 pp/c), or anti-extracellular CAR on ice for 20 min and placed at 37 °C for 30 min. Only viral ligands triggered CAR relocalization in vesicular structures (insets show colocalization of CAR and viral ligands compared with CAV-2 and HAdV5 incubation only on ice or anti-CAR applied live). C, quantification of three independent experiments showed that ligands were found with vesicular CAR (n = 3, ≥93 vesicles counted for each condition; results are expressed as mean ± S.E.). D, flow cytometry analyses showed a decrease of plasma membrane CAR after viral ligand engagement. Neu2A cells were transfected or not with a plasmid encoding for CAR for 24 h, incubated on ice for 20 min with either 1 μg of FKCAV, 10,000 or 20,000 pp/c of CAV-2, or 10,000 or 20,000 pp/c of HAdV5, and shifted to 37 °C overnight. Cells were scrapped in PBS and labeled for external CAR (CAR ext.) according to “Experimental Procedures.” Scale bars: A, 10 μm; C, 5 μm.
CAR Is Located within Lipid Microdomains at the Plasma Membrane and during Internalization in Neurons
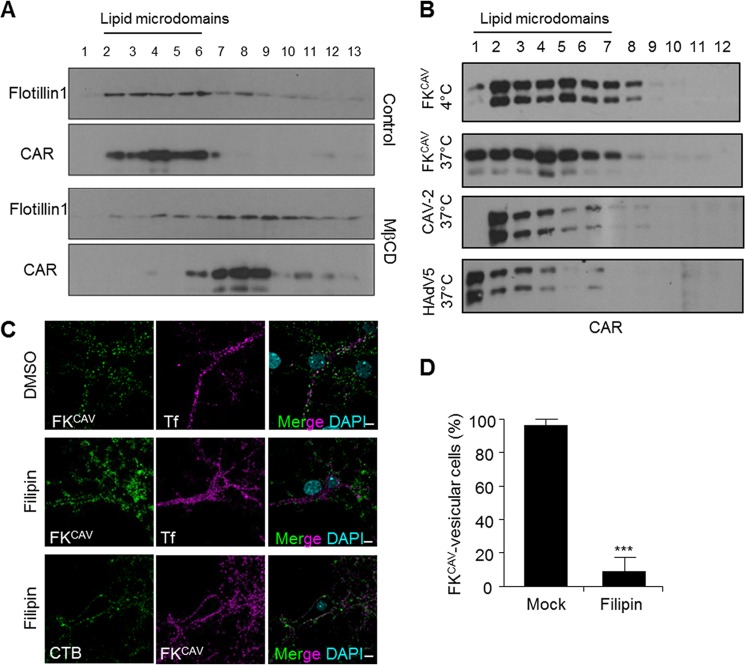
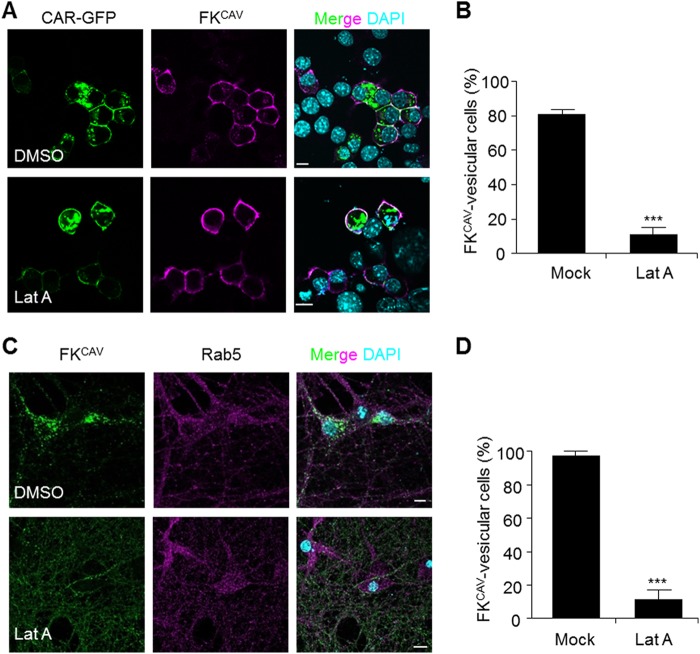
The plasma membrane is organized in subdomains where proteins and lipids can elicit specific signals, regulate cell adhesion, and other processes. Among these domains, lipid microdomains have unique properties that diversify membrane trafficking and signaling (29). Because numerous viral and toxin receptors, including CAR (30, 31), are located within lipid microdomains, we assayed the membrane sublocalization of CAR in primary neuron cultures and in the mouse brain. When using a detergent-free method (24) to isolate lipid microdomains from primary cortical neurons, flotillin-1, a marker for lipid microdomains, was found in the top fractions of the density gradient (Fig. 3A). CAR was mainly found in flotillin-1+ fractions, suggesting that CAR was a resident of lipid microdomains in neurons (Fig. 3A). To monitor the dynamic association of CAR with lipid microdomains, we used methyl-β-cyclodextrin to remove cholesterol from membranes and shift lipid microdomain-associated proteins toward the bottom of the density gradient. Under these conditions, CAR and flotillin-1 were found in the bottom fractions of gradient of cortical neurons (Fig. 3A).
FIGURE 3.
CAR location in lipid microdomains at the plasma membrane and during internalization. A, CAR was found in flotillin-1+ fractions, a lipid microdomain protein in mouse cortical neuron extract subjected to biochemical isolation through OptiPrep gradient. Cortical neurons were incubated with 10 mm methyl-β-cyclodextrin (MβCD), a known lipid microdomain destabilizator, and subjected to lipid microdomain isolation. Methyl-β-cyclodextrin triggered flotillin-1 and CAR relocalization in lower fractions. Western blots are representatives of 3 independent experiments. B, CAR did not exit lipid microdomains after ligand binding and internalization. Lipid microdomain isolation was performed on cortical neurons incubated either with FKCAV on ice, or shifted to 37 °C for 20 min after incubation on ice with FKCAV, 10,000 pp/c CAV-2, or 10,000 pp/c of HAdV5. Western blots are representatives of three independent experiments. C, filipin blocked CAR internalization. Hippocampal neurons were treated with 5 μg/ml of filipin for 30 min prior to FKCAV/Tf or FKCAV/CTB incubation on ice for 20 min. Cells were washed and then shifted to 37 °C for 1 h to trigger internalization. In contrast to Tf, CTB and FKCAV did not enter after filipin treatment. D, quantification of three independent experiments showed that filipin efficiently blocked FKCAV internalization (n = 3, ≥45 cells counted for each condition; results are expressed as mean ± S.E. ***, p value <0.001 versus control). Scale bar, 5 μm.
AdVs use multiple receptors to engage and enter cells. The paradigm is that CAR-bound AdVs are internalized via interaction with integrins through an RGD motif in the penton base (32). To circumvent the role of co-receptors, which could bias the interpretation of CAR membrane dynamics, we used FKCAV as a CAR-specific ligand. We previously showed that when motor neurons were incubated with a mutant form of FKCAV, FKCAVΔCAR, where the CAR-binding site was ablated, we could not detect CAR interaction via surface plasma resonance, cell binding, or CAR endocytosis (8, 9). These data were consistent with the specificity of FKCAV to detect CAR.
Some proteins, such as the epidermal growth factor receptor (EGFR), can diffuse laterally out of lipid microdomains during clathrin-dependent internalization (33). To test whether CAR would also leave these microdomains during binding or the first steps of entry, we assayed its lipid microdomain location after ligand engagement. FKCAV was incubated with cells at 4 °C to allow attachment, and then ±20 min at 37 °C to allow internalization. Using this assay, we found that CAR did not exit lipid microdomains after FKCAV binding or internalization (Fig. 3B). Similarly, neither CAV-2 nor HAdV5 displaced CAR from these microdomains (Fig. 3B). We then asked whether lipid microdomain integrity was needed for CAR internalization. We incubated hippocampal neurons with filipin, a lipid microdomain-disruptor, prior to FKCAV binding. In this condition, the internalization of FKCAV/CAR and the binding domain of CTB, which recognizes the ganglioside GM1, a well characterized lipid microdomain component, was prevented. By contrast, Tf internalization, which uses a non-lipid microdomain entry pathway, was not (Fig. 3, C and D). This suggested that the main route of entry of CAR in neurons required the integrity of lipid microdomains.
Together, these data demonstrated that CAR was a permanent resident of lipid microdomains in neurons in native conditions, and during ligand-induced internalization. Furthermore, the association of CAR with lipid microdomains was crucial in its ability to undergo endocytosis as sequestration of cholesterol blocked its internalization.
Clathrin-independent, Dynamin- and Actin-dependent CAR Endocytosis in Neurons
Lipid microdomain-dependent and clathrin-dependent endocytosis were, until recently, thought to be largely independent. This is illustrated by the data concerning EGF/EGFR endocytosis, which can happen in lipid microdomains, or after lateral diffusion outside them, and then engaging the clathrin machinery (33). However, this view has been tempered by reports suggesting that clathrin-dependent endocytosis could take place into lipid microdomains (34). In epithelial cells, numerous studies showed that AdV entry involves the clathrin machinery (35). Notably, the intracellular domain of CAR has a functional tyrosine-based AP (adaptor protein) binding site YXXφ (YNQV; Fig. 4A) that is present in numerous receptors that use clathrin machinery for endocytosis. This motif was reported to be involved in the basolateral sorting of CAR through AP1 in epithelial cells (36). However, the role of YNQV as an internalization sequence has not been characterized for CAR. We therefore used transfected Neu2A cells with plasmids encoding CAR-RFP deletion mutants lacking the PDZ (PSD-95/Discs-large/ZO-1) binding domain and the potential AP binding site (Fig. 4, A and B). When we incubated FKCAV with cells expressing the full-length (FL) CAR-RFP at 4 °C, we found FKCAV bound only on transfected cells, showing the specificity for plasma membrane CAR (data not shown). We then shifted cells to 37 °C to monitor CAR endocytosis. Under these conditions, FKCAV and CAR were co-internalized in endocytic vesicles (Fig. 4, C and D). Neu2A cells expressing CAR-RFP with a deletion in the AP binding site (CAR-315) also bound FKCAV at 4 °C. After inducing internalization at 37 °C, endocytosis was similar to full-length CAR (Fig. 4, C and D), suggesting that clathrin-dependent endocytosis was unlikely to be the major route of internalization of CAR.
FIGURE 4.
Cytoplasmic sequence necessary for CAR internalization. A, CAR intracellular domain sequence. AP binding site is highlighted in blue, PDZ-binding domain in magenta, and minimum sequence needed for internalization in green. B, expression of CAR constructs in Neu2A cells. Cells were transfected, collected 24 h later and total extracts subjected to SDS-PAGE analyses using an anti-CAR raised against the extracellular domain. C, the AP binding site is not necessary for CAR internalization. Neu2A cells were transfected with CAR-RFP or its mutants. 24 h later, FKCAV was added for 20 min on ice, the medium was replaced and internalization was allowed for 1 h at 37 °C. Cells were fixed and stained using an antibody anti-FK. Only the construct CAR-261 did not allow FKCAV internalization. D, quantification of FKCAV internalized cells (n = three independent experiments, ≥50 cells counted for each condition; results are expressed as mean ± S.E. ***, p value <0.001 versus control). E, hippocampal neurons were transfected with CAR-RFP or CAR-261 and similar procedures were applied. As above, CAR-261 did not internalize FKCAV. Insets show CAR-FL and FKCAV in vesicular structures (asterisks), whereas in CAR-261-transfected cells, FKCAV was mainly at the plasma membrane. F, quantification of FKCAV-internalized cells (n = 3 independent experiments, ≥21 cells counted for each condition; results are expressed as mean ± S.E. **, p value <0.01 versus control). Scale bars, 10 μm.
To identify the sequence involved in CAR internalization, we carried out a more extensive deletion analyses. We found that the sequence necessary for FKCAV-mediated endocytosis of CAR was between amino acids 261 and 274, as CAR-274 was internalized but CAR-261 was not (Fig. 4, C and D). To test if this conclusion held true in primary neurons, CAR-RFP plasmids were transfected in hippocampal neurons that were subsequently incubated with FKCAV. Similarly to that found in Neu2A, CAR-261 failed to internalize FKCAV (Fig. 4, E and F).
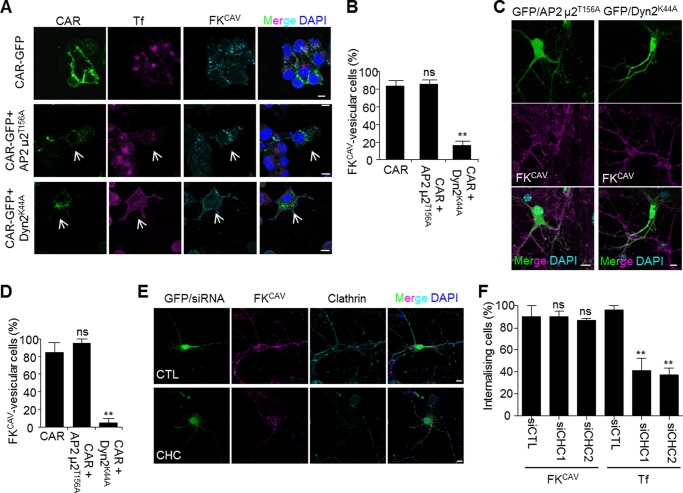
To address the role of clathrin-mediated endocytosis using another approach, we transfected Neu2A cells and hippocampal neurons with a plasmid expressing AP2μ2T156A, a dominant-negative construct that blocks clathrin-dependent endocytosis (37). AP2μ2T156A inhibited Tf uptake, which is dependent on the clathrin machinery (Fig. 5A). However, in Neu2A (Fig. 5, A and B) and hippocampal neurons (Fig. 5, C and D) expressing AP2μ2T156A and CAR-GFP, FKCAV was efficiently internalized. Similarly, hippocampal neurons co-transfected with a plasmid harboring a GFP expression cassette to identify transfected cells and two different siRNAs against the CHC was not blocking FKCAV internalization, which supports our conclusion that CAR did not use the clathrin machinery for its internalization upon FKCAV engagement (Fig. 5, E and F). Similar results were obtained in Neu2A cells transfected with CAR-GFP (data not shown). We then assayed the role of dynamin, a GTPase involved in the fission of clathrin-coated vesicles, and also in lipid microdomain-mediated pathways (38). We transfected plasmids expressing the dominant-negative construct dynamin2K44A (Dyn2K44A) that cannot hydrolyze GTP and therefore inhibits dynamin-mediated membrane fission (39). Expression of Dyn2K44A strongly decreased Tf and CAR internalization in Neu2A cells co-transfected with CAR-GFP, and in hippocampal neurons (Fig. 5, A–D), suggesting that dynamin was involved in CAR endocytosis after FKCAV engagement.
FIGURE 5.
Clathrin-independent and dynamin-dependent CAR internalization. A and B, dynamin mutant blocked CAR internalization in Neu2A cells, whereas inhibition of the clathrin-mediated entry did not have an effect. Neu2A cells were transfected with CAR-GFP alone, or in combination with plasmids encoding AP2μ2T156A or dynamin2K44A and 24 h later incubated with FKCAV on ice for 20 min, rinsed, and internalization was allowed for 1 h at 37 °C. Cells were fixed and the FKCAV/CAR location was analyzed by confocal microscopy. Arrows show cells expressing dominant-negative mutants where Tf internalization was inhibited. FKCAV was not internalized in cells expressing Dynamin2K44A. B, quantification showed that inhibition of clathrin entry had no effect on FKCAV/CAR internalization, whereas the dynamin mutant significantly decreased FKCAV/CAR vesicular pattern (n = three independent experiments, ≥58 cells counted for each condition; results are expressed as mean ± S.E. **, p value <0.01 versus control). C, hippocampal neurons were co-transfected with pEGFP and plasmids encoding AP2μ2T156A or dynamin2K44A, and FKCAV/CAR internalization was observed using similar methods than with Neu2A cells. Dynamin inhibition showed similar blockade in the uptake of FKCAV/CAR, whereas inhibition of clathrin-mediated endocytosis did not show a detectable effect. D, quantification of three independent experiments (≥19 cells counted for each condition; results are expressed as mean ± S.E. **, p value <0.01 versus control). E, hippocampal neurons were co-transfected with pEGFP and siRNAs against CHC or control siRNA. Three days post-transfection FKCAV or Tf were applied and allowed to internalize for 90 min. CHC was visualized by indirect immunofluorescence to monitor knock-down. F, quantification of FKCAV and Tf-internalized cells (n = three independent experiments, ≥20 cells counted for each condition; results are expressed as mean ± S.E. **, p value <0.01 versus control). Scale bars, 10 μm.
As actin filaments are involved in the endocytosis of numerous receptors (40), we then assayed the role of actin in CAR internalization. Huang et al. (41) previously showed that CAR interacts with actin, suggesting that the actin cytoskeleton could play a role in its trafficking. To monitor the role of actin, we used LatA, which induces the disassembly of actin polymers. We transfected Neu2A cells with CAR-GFP, incubated them at 4 °C with FKCAV and then shifted them at 37 °C for 1 h. In these conditions, cells pre-treated with LatA failed to internalize FKCAV, whereas in DMSO-treated cells FKCAV was found in endosomes (Fig. 6, A and B). Similarly, in mock-treated cells FKCAV was present in endosomes 1 h post-internalization, whereas LatA treatment maintained a membrane-bound pattern of FKCAV in hippocampal neurons, consistent with an inhibition of internalization (Fig. 6, C and D).
FIGURE 6.
Actin dynamic regulation of CAR internalization. A–D, inhibition of actin disassembly blocked CAR internalization. A, Neu2A cells transfected with a plasmid encoding for CAR-GFP and pre-treated 24 h later with LatA (10 μm) for 2 h at 37 °C and further incubated for 1 h at 37 °C with FKCAV. B, quantification show that perturbation of actin dynamics blocked FK-induced internalization of CAR (n = three independent experiments, ≥105 cells counted for each conditions; results are expressed as mean ± S.E. ***, p value <0.001 versus control). C, hippocampal neurons were pre-treated with LatA (10 μm) for 2 h at 37 °C and further incubated for 1 h at 37 °C with FKCAV. D, quantification to show efficient impairment of FKCAV internalization by LatA (n = three independent experiments, ≥26 cells counted for each condition; results are expressed as mean ± S.E. ***, p value <0.001 versus control). Scale bars, 10 μm.
Taken together, these data suggested that in neurons, CAR was linked to a lipid microdomain-, actin-, and dynamin-dependent route of internalization. In addition, the AP binding site in the intracellular tail of CAR was not necessary for its endocytosis, consistent with its uptake via a clathrin-independent route.
FKCAV-induced CAR Internalization in Neurons Is Coupled to the Endolysosomal Pathway
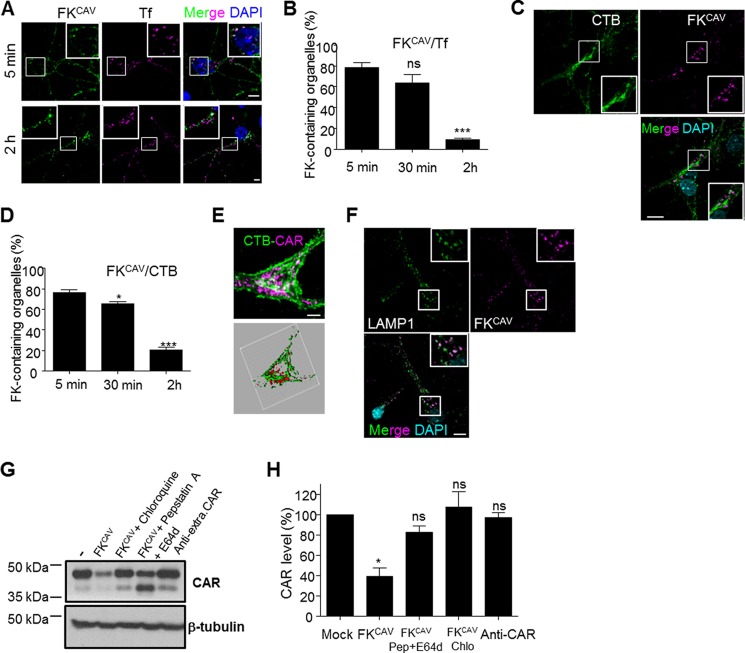
Receptor function can be modulated by the preferential targeting to subcellular compartments upon ligand-induced internalization. After endocytosis, ligand-receptor complexes can be recycled back to the plasma membrane, targeted to the trans-Golgi network and endoplasmic reticulum, or degraded by lysosomes. During their endocytosis, AdVs trigger endosomal lysis to reach the cytosol. This phenomenon precludes the identification of the trafficking of CAR after endocytosis. We therefore used FKCAV to induce CAR internalization and characterized its trafficking post-entry. When hippocampal neurons were incubated with FKCAV, we detected a relocalization of endogenous CAR that led to its disappearance from neurites and its accumulation in the cell body (data not shown). This could be interpreted in at least two ways: active transport (as previously shown) or distal degradation. To determine whether CAR entered the recycling pathway, we co-incubated hippocampal neurons with FKCAV and Tf on ice, then shifted the neurons to 37 °C for various times. Tf and its receptor TfR are markers of the recycling pathway and reach early endosomes prior to targeting recycling endosomes and the plasma membrane. FKCAV and Tf colocalized at the early time point but then separated, suggesting that CAR was not targeted to recycling endosomes (Fig. 7, A and B).
FIGURE 7.
CAR was not linked to recycling or retrograde pathways, but was targeted to lysosomes. A and B, FKCAV/CAR did not accumulate in recycling endosomes after internalization. A, hippocampal neurons were incubated with FKCAV and Alexa 555-Tf on ice, the ligands were washed out, and the cells were placed at 37 °C for various times. Representative images of early (5 min) and late (2 h) endocytosis of FKCAV and Alexa 555-Tf. Insets show colocalization early between FKCAV and Tf but not at later points. B, quantitative analyses of three independent experiments showed significant loss of colocalization of FKCAV and Alexa 555-Tf during progression of endocytosis (n = 3, ≥140 structures were counted for each time point; results are expressed as mean ± S.E. ***, p value <0.001 versus control). C–E, FKCAV/CAR did not accumulate in the trans-Golgi network after internalization. C, representative images of late (2 h) endocytosis of FKCAV and Alexa 488-CTB. D, quantitative analyses of three independent experiments showed loss of colocalization of FKCAV and Alexa 488-CTB during progression of endocytosis (n = 3, ≥190 structures were counted for each time point; results are expressed as mean ± S.E. *, p value <0.05; ***, p value <0.001 versus control). E, three-dimensional reconstruction shows a separation between FKCAV and CTB. F, CAR was targeted to lysosomes after internalization. Hippocampal neurons were incubated with FKCAV for 2 h and stained for lysosomes (LAMP1). Insets show strong colocalization (87.7 ± 3.5%; n = three independent experiments, 315 structures were counted. Results are expressed as mean ± S.E.). G, biochemical analyses of hippocampal neurons incubated overnight with FKCAV ± lysosomal chloroquine and pepstatin A+E-64d showed CAR degradation. Anti-CAR antibodies did not trigger CAR lysosomal hydrolysis. H, quantification of CAR lysosomal degradation (n = three independent experiments, results are expressed as mean ± S.E. *, p value <0.05 versus control set as 100%). Scale bars: A and D, 5 μm. C and F, 10 μm.
As CTB uses a lipid microdomain-dependent endocytic pathway (42), which transports the toxin from endosomes to the Golgi apparatus, we monitored the possible co-transport of CAR within this route. To this end, we co-incubated hippocampal neurons with FKCAV and CTB as above. Although FKCAV/CAR colocalized with CTB at early time points and was in perinuclear organelles at late time points, they did not colocalize with CTB at the trans-Golgi network (Fig. 7, C and D), as seen by three-dimensional rendering (Fig. 7E).
We repeated the above assay and stained FKCAV-incubated neurons with LAMP1, a lysosomal marker. At 2 h post-internalization, numerous organelles were FKCAV/LAMP1+ (Fig. 7F). To control for targeting of FKCAV to lysosomes, we assayed the colocalization of FKCAV and endogenous CAR 2 h post-internalization by immunofluorescence (data not shown). Similarly, when we incubated hippocampal neurons with FKCAV overnight, we detected an ∼60% decrease in CAR levels by immunoblot analyses, consistent with ligand-induced degradation (Fig. 7, G and H). CAR degradation was almost completely blocked by pre-treating cells with pepstatin A/E-64d or chloroquine, drugs that perturb lysosomal function (Fig. 7, G and H). Incubating cells with an anti-CAR antibody did not lead to its degradation, consistent with the immunofluorescence data regarding its internalization (Fig. 7, G and H). From these data, we concluded that the primary fate of CAR after ligand-induced internalization was toward lysosomal degradation, and not the retrograde or recycling pathways.
Lipid Microdomain- and Actin-dependent Axonal-specific CAR Endocytosis and Somatodendritic Lysosomal Targeting
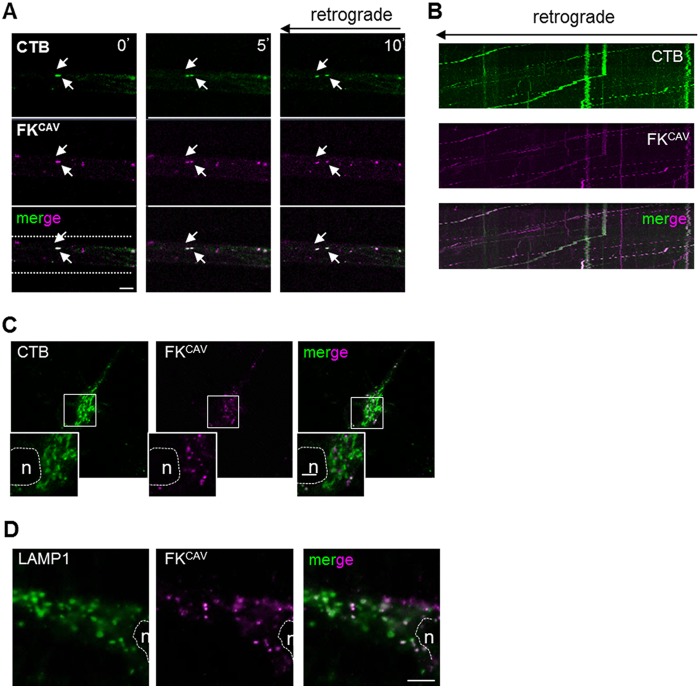
The composition of neuronal subcompartments such as dendrites and axons can differ greatly and possibly influence ligand-receptor endocytosis. To better understand ligand uptake at different neuronal sites, we used MCs (43). Hippocampal neurons were cultured for 5–8 days to allow axons to cross the microgrooves. Incubation in the axonal compartment with FKCAV led to efficient retrograde transport of FKCAV and accumulation of CAR in cell bodies (data not shown). As observed in batch cultures, ∼75% of the CAR+ retrograde carriers were also CTB+ when the axonal compartment was co-incubated with FKCAV and CTB, suggesting that lipid microdomain-rich structures mediated the axonal retrograde transport of CAR (Fig. 8, A and B). However, after axonal transport, FKCAV and CTB did not colocalize in the trans-Golgi network (Fig. 8C), whereas retrograde FKCAV was found in LAMP1+ somatodentritic structures (Fig. 8D). These data suggest a sorting mechanism once axonal cargoes reached the cell body and that CAR was targeted to lysosomes after retrograde transport.
FIGURE 8.
CAR and CTB co-transport and sorting after axonal retrograde transport. A–C, FKCAV/CAR were co-transported with CTB in axonal carriers. A, hippocampal neurons cultured in MCs were incubated with Cy5-FKCAV and Alexa 488-CTB in the axonal compartment for 60 min at 37 °C and then imaged in the microgrooves. Still frames of a movie show Cy5-FKCAV and Alexa 488-CTB in the same axonal carriers undergoing retrograde transport. Arrows show moving compartments positive for both ligands (co-transport of 75% ± 9%; n = 3, 94 structures were counted; results are expressed as mean ± S.E.). B, kymographs show similar profiles of transported Cy5-FKCAV and Alexa 488-CTB. C, CTB and CAR were sorted differently after axonal transport. Hippocampal neurons cultured in MCs were incubated with Cy5-FKCAV and Alexa 488-CTB in the axonal compartment for 3 h at 37 °C, and imaged in the cell body compartment. Dashed line and n identify the nucleus. Insets show minimal colocalization between FKCAV and CTB. D, CAR was targeted to lysosomes after axonal transport. Hippocampal neurons cultured in MCs were incubated with Cy5-FKCAV in the axonal compartment for 3 h at 37 °C, fixed, stained for the lysosomal marker LAMP1, and imaged at the cell body compartment. Numerous FKCAV-positive structures contain LAMP1 (88% ± 3%; n = three independent experiments, 140 structures were counted. Results are expressed as mean ± S.E.). Dashed line and n identify the nucleus. Scale bars, 5 μm.
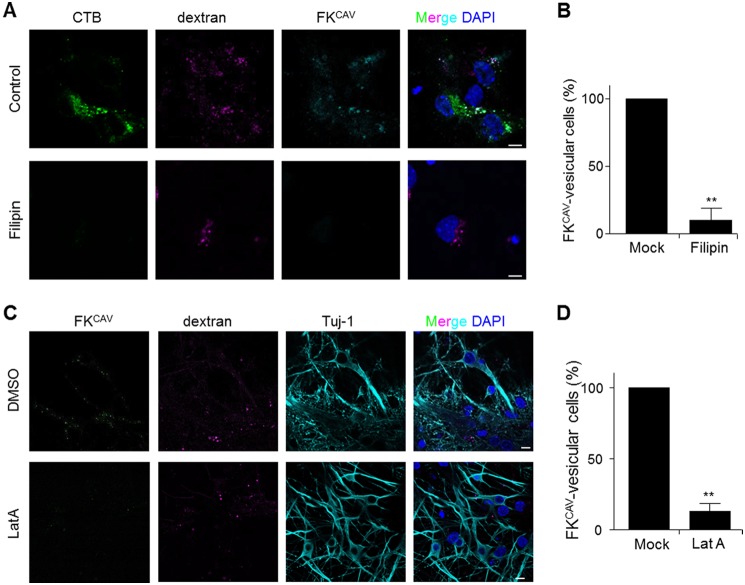
To monitor whether the endocytic pathway regulating axonal entry and transport was similar to that in batch cultures, we assayed the effect of lipid microdomain disruption on FKCAV/CAR axonal transport. When axon termini were preincubated with filipin, FKCAV and CTB failed to accumulate in the soma, whereas rhodamine/dextran, a fluid phase marker, did (Fig. 9, A and B). Similarly, when axons were treated with LatA prior to FKCAV incubation, we did not detect efficient FKCAV accumulation in cell bodies after axonal transport (Fig. 9, C and D).
FIGURE 9.
Lipid raft- and actin-dependent CAR axonal endocytosis. A, cholesterol sequestration disrupted CAR axonal retrograde transport. Hippocampal neurons cultured in MCs for 8 DIV were treated for 30 min with 5 μg/ml of filipin in the axonal compartment and ligands (Alexa 488-CTB, Cy5-FKCAV, and rhodamine/dextran) were applied to the axonal compartment. Axonal transport was allowed for 3 h at 37 °C. Filipin-treated and control (dimethyl sulfoxide, DMSO) cells transported the fluid-phase marker dextran, but lipid microdomain receptors (GM1 and CAR) could not be transported back to cell bodies. B, quantification of filipin effect on FKCAV retrograde transport (n = three independent experiments, 5 fields were quantified for each experiment and each condition; results are expressed as mean ± S.E. compared with control cells set as 100%). C, actin disassembly inhibited CAR axonal retrograde transport. Hippocampal neurons cultured in MCs for 8 DIV were treated for 2 h with LatA (10 μm) in the axonal compartment and ligands (Cy5-FKCAV and rhodamine/dextran) were applied to the axonal compartment. Axonal transport was allowed for 3 h at 37 °C, cells were fixed and stained with β3-tubulin (Tuj-1). LatA-treated cells transported the fluid-phase marker dextran, but not FKCAV. D, quantification of the LatA effect on FKCAV retrograde transport (n = three independent experiments, 5 fields were quantified for each experiments and each condition; results are expressed as mean ± S.E. compared with control cells set as 100%. **, p value <0.01 versus control). Scale bars: A, 5 μm; B, 10 μm.
Together, these data showed that axonal endocytic trafficking of CAR was coupled to lipid microdomain- and actin-mediated endocytosis. In addition, retrograde co-transport with other lipid microdomain-dependent ligands was followed by a sorting to different somatodendritic destinations.
Ligand-induced CAR Axonal Transport and Degradation Occur in Vivo
Finally, we asked whether CAR trafficking and degradation occurred in the mammalian brain. To address this, we injected FKCAV in the striatum of 8-week-old mice. Using immunofluorescence, we found that FKCAV was efficiently retrogradely transported from the striatum to the cortex and subtantia nigra (SN) (Fig. 10A). This retrograde transport was also observed in the sciatic nerve after intramuscular injections (8). Eighteen hours post-injection, ipsilateral and controlateral striata were removed and proteins extracted. An ∼80% decrease in CAR levels in FKCAV-injected striata was detected compared with the contralateral structure, whereas mock (PBS) did not induce this phenomenon (Fig. 10B). Ligand-induced CAR degradation was dose-dependent (Fig. 10B) and CAR levels started to return to normal around 1 month post-injection (Fig. 10, C and D). Together, these data showed that after internalization, CAR was targeted to lysosomes for degradation in vivo.
FIGURE 10.
CAR is transported and degraded in vivo. A, intraparenchymal injections of FKCAV led to its axonal transport in connected regions. Scheme of brain regions showing Cy5-FKCAV localization after injection in the striatum. Slices were stained for β3-tubulin (Tuj-1). B and C, injection of 5 μg of FKCAV led to CAR degradation in the striatum. Representative immunoblots of at least three independent experiments show CAR expression in the mouse striatum. B, CAR levels 1 day post-injection of PBS or different doses of FKCAV. A dose effect of FKCAV injection on CAR expression was observed. C, time course effect of FKCAV injection on CAR expression. CAR levels were restored one month post-injection (Ipsi., ipsilateral; Cont., contralateral; NI., non-injected). D, quantification of three independent experiments; results are expressed as mean ± S.E. compared with control set as 100%. *, p value <0.05; ***, p value <0.001 versus control). Scale bars, 20 μm.
DISCUSSION
In this study, we defined the mechanisms regulating CAR endocytic trafficking upon ligand binding. We showed that CAR-tropic AdVs and adenoviral proteins induced CAR internalization in neurons and neuronal cells. Endocytosis was lipid microdomain-, actin-, and dynamin-dependent, and did not require clathrin and its adaptors. Somatodendritic and axonal endocytosis targeted CAR to lysosomes leading to its degradation. Although CAR and CTB/GM1 shared common a lipid-microdomain entry/transport pathway, they differed in terms of their final destination after axonal retrograde transport, suggesting a sorting mechanism at the soma-axon interface.
Numerous pathogens use plasma membrane receptors to access and spread in the CNS (1, 4). For instance, herpes simplex virus 1 (HSV-1) interacts with nectin-1, a CAM located at sensory terminals (44). Similarly, in motor neurons poliovirus binds CD155 to be endocytosed and retrogradely transported through direct interaction of the receptor with cytoplasmic dynein (45). As viral diseases can lead to severe pathologies such as paralysis or dementia, numerous efforts have been made to better characterize the host-pathogen interaction and the endogenous function of their cellular receptor(s).
Although AdVs are not quintessential human brain pathogens, they can be associated with encephalitis and brain tumors in humans and other species (46–50). Moreover, some AdV vectors are particularly promising for gene transfer to the CNS (51, 52). For instance, vectors derived from CAV-2 preferentially transduce neurons and their efficient axonal retrograde transport allows a widespread transduction in the CNS of rodents and dogs (27, 53). To better understand CAV-2 tropism and transport, we previously analyzed its axonal transport in motor neurons (8). We showed that CAV-2 transport from nerve termini to the cell body was in pH-neutral vesicles, which underwent a maturation process requiring the sequential recruitment of the small GTPases Rab5 and Rab7. These axonal carriers are also used by clostridial neurotoxins and neurotrophins (26, 54), as well as poliovirus (55), and may represent a common organelle providing a protective environment responsible for long-range transport in neurons (1). We also showed that CAR, the docking molecule of CAV-2 (56, 57), was found in endosomes together with CAV-2, suggesting that it used CAR to access the CNS (8).
As a virus receptor, the role of CAR was mainly considered as a docking platform, with no primordial function described in internalization. Typically, it is thought that some AdVs use CAR to dock, followed by integrin-mediated internalization. This is supported by data showing that deletion of the intracellular tail of CAR still allowed AdV-mediated transduction (6). Similarly, during CVB endocytosis in polarized epithelial cells CAR remained at the plasma membrane, whereas CVB was internalized (7). However, we previously showed that FKCAV triggered efficient CAR axonal transport, demonstrating that CAR could be directly linked to an endocytic pathway in motor neurons (8). We have now shown that CAV-2 and FKCAV triggered CAR internalization in different neuronal types and cell lines. Moreover, unlike that reported in other cell types, HAdV5 also triggered CAR internalization in neurons. As CAR engagement can regulate signaling pathways (10, 11), it is conceivable that CAR plays a yet undescribed role in endocytosis of AdVs and/or signaling cascades affecting AdV propagation in some cell types. Whether the neuronal signaling of CAR is pro- or antiviral is obviously unknown.
Notably, we show that not all CAR “ligands” behaved similarly. Antibodies directed against the extracellular D1 and D2 domains did not lead to CAR internalization, whereas FKCAV, CAV-2, and HAdV5 did. CAR can form homodimers in trans and in cis (18, 19, 28). The binding sites and affinity of viral and non-viral ligands have been measured and could explain why we observed differences in terms of ligand-induced internalization. Indeed, FKCAV, as a trimer, is theoretically able to engage three CAR molecules (9, 58), whereas CAV-2 and HAdV5 have 12 trimeric fibers and therefore are, in theory, able to engage 36 CAR monomers. Moreover, it is worth considering that the CAR-binding affinity of these ligands could also regulate its membrane trafficking: notably, FKCAV binds to CAR with a lower Kd than FKHAdV5 (1.1 versus 7.9 nm) (9, 59). Similarly, FK-CAR affinity is higher than the CAR D1-D1 homotypic interaction (Kd 16 μm) (60). Excess fiber production during HAdV5 propagation may perturb CAR-CAR homotypic interaction, as it binds similar residues in the D1 domain, and disrupts tight junctions during AdV epithelial infection (28).
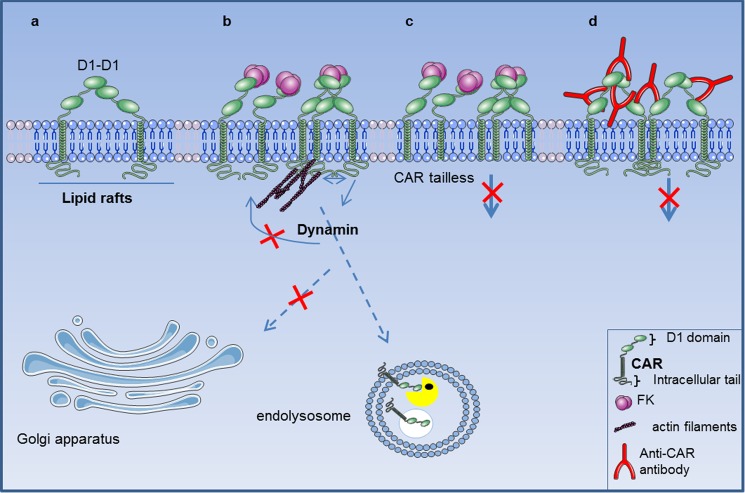
The hypothesis we favor for ligand-induced CAR internalization is that CAR-CAR interaction needs to be displaced, as during FKCAV or AdV engagement: it would not be clustering, but disengagement that triggers CAR internalization. In support of this view, polyclonal anti-CAR antibodies, in conditions that mimic multimeric ligand engagement, did not induce CAR endocytosis. We postulate that disruption of the CAR-CAR interaction then favors, or unmasks, intra- or intermolecular interactions that will induce endocytosis. This, coupled to amino acids between 261 and 274, could induce signals necessary for CAR entry (Fig. 11). Interestingly, CAR-binding CVB do not bind at the D1–D1 interface, but to residues situated on the side of the D1 domain (61). This observation is consistent with our hypothesis and why CAR would not be endocytosed during CVB entry in epithelial cells (7, 31). As viruses have been selected for their ability to structurally mimic endogenous host ligands (62), it is likely that a CAR ligand could trigger CAR-CAR disengagement and subsequent internalization. Interestingly, junctional adhesion molecule type L and AdVs bind similar domains on CAR with an affinity higher than CAR-CAR interaction (Kd 5 versus 16 μm) to elicit signaling (10). However, the junctional adhesion molecule type L-CAR interaction occurs in trans and was not reported to trigger CAR internalization.
FIGURE 11.
Mechanisms of ligand-induced CAR endocytosis in neuronal cells. a, CAR (in green) is located in lipid rafts (in blue) and homodimeric interaction occurs through the D1 domain. b, the trimeric fiber knob (FKCAV, in magenta), the CAR-interacting domain of some adenovirus types, binds with higher affinity to the D1-D1 interacting site and triggers D1-D1 disengagement. FKCAV can engage one, two, or three CAR molecules leading to either clustering, or D1 disengagement in FKCAV saturating conditions. Lipid raft integrity, dynamin, and actin (in purple) play crucial roles in subsequent internalization that will target CAR to endolysosomes for degradation (lysosomal enzymes represented by yellow Pacman). c, a region (amino acids 261–274) in the tail of CAR was essential for ligand-induced CAR endocytosis. d, clustering through antibody (in red) or coxsackievirus (7) binding, was insufficient to trigger CAR internalization, likely because they do not disrupt D1-D1 interactions and therefore do not favor or unmask, intra- or intermolecular interactions that regulate ligand-induced CAR endocytosis.
We found that CAR is recruited to cholesterol-rich microdomains in neurons. In contrast to EGFR, which exits lipid microdomains prior to endocytosis, CAR location was maintained during ligand-induced entry. Furthermore, the integrity of lipid microdomains was necessary for CAR endocytosis. Lipid microdomain-mediated entry is a general term to describe cholesterol-dependent mechanisms. However, lipid microdomains are heterogenous by nature and can differ in terms of composition and size (63, 64). Caveolin-mediated entry is probably the best described lipid microdomain-mediated endocytic pathway. However, although neurons express caveolin (65), there is no evidence that caveolae can form in neurons (66), which would exclude this endocytic route in CAR internalization. Flotillin-dependent entry is another cholesterol-dependent pathway. In neurons, flotillin-1 can regulate the endocytosis of several proteins including the amyloid precursor protein (67) and semaphorin 3A (68). Our data does not exclude the possibility that CAR could enter via this pathway.
Because the intracellular domain of CAR has a potential AP2 binding site, we also tested the involvement of clathrin and dynamin in the internalization of CAV-2. This site has been well characterized in the basolateral sorting of CAR through AP1 in epithelial cells, but its role in endocytosis was recently questioned (36, 69). Using deletion mutants lacking this site and overexpression of a dominant-negative AP2 mutant, we showed that CAR was still efficiently endocytosed following FKCAV engagement, suggesting that clathrin had a nonessential role in this process. Perturbation of dynamin function and actin depolymerization, however, inhibited CAR internalization. Clathrin-independent, dynamin- and lipid microdomain-mediated endocytosis has been previously reported for cholera toxin (70), SV40 (71), and proteoglycan (72). Actin plays major roles in numerous internalization pathways (40), but its involvement in mammalian endocytosis remains controversial. Its role is best studied during clathrin-mediated endocytosis, but other routes also require actin dynamics, such as macropinocytosis and lipid-dependent entry (40). The analogy with SV40 and GM1-mediated internalization is pertinent, as SV40 also uses dynamin and lipid microdomains and also actin during entry (71). It was suggested that viral diffusion at the plasma membrane is blocked by actin assembly leading to its immobilization in lipid microdomains (73). Indeed, the formation of cholesterol-sensitive clusters may be regulated by cortical actin (74). CAR drifting at the plasma membrane has also been reported and was influenced by the actin cytoskeleton (75). Notably, actin interacts with the CAR cytoplasmic domain (41). Whether this interaction and its role in the regulation of CAR lateral diffusion at the plasma membrane are necessary for CAR endocytosis remain to be tested.
After FKCAV engagement ex vivo and in vivo, the majority of CAR was not recycled or transported to the Golgi network, but targeted to lysosomes for degradation. CTB and FKCAV shared a common axonal carrier, but differed in their final destination, suggesting that a general endocytic pathway was responsible for long-range transport and that somatodendritic sorting selectively targeted ligand-receptor complexes to distinct locations. As the transition from axon to soma after retrograde transport is still poorly understood, using these ligands may be of help to understand the nature of the endocytic compartments responsible for sorting.
Besides its role in AdV entry, what could CAR depletion from the plasma membrane, and targeting, for degradation, do to its neuronal function? As CAR is also coupled to signaling pathways (e.g. Ref. 11), one possibility is that its ligand-mediated degradation regulates mechanisms important for neuronal homeostasis such as migration or survival. Indeed, a potential role in neurite outgrowth has been postulated (18). In neurons, receptor-induced internalization following ligand binding is well described for nerve growth factor and TrkA where different signals are generated at the plasma membrane and in endosomes (76). Similarly, endocytosis at growth cones during development allows endosomes containing nerve growth factor, TrkA, and downstream signaling molecules to be transported to the cell body to trigger a transcriptional response necessary for survival (15). Moreover, CAMs can also be internalized to promote local and distal signaling, membrane remodeling, and to modulate the adhesive properties (17, 77). For example, fibronectin triggers integrin internalization and targeting to lysosomes to control cellular migration (16). Likewise, L1CAM is depleted from the plasma membrane to regulate axonal growth (17, 78) and can be degraded by the lysosomal system (79). This highlights the role of lysosomes in signal termination. It will also be particularly relevant to determine whether ligand-induced CAR endocytosis and degradation are associated with signal activation and the endogenous function of CAR in the CNS as was reported for other CAMs.
In conclusion, we describe the mechanisms behind ligand-induced CAR endocytosis in neurons. Characterizing how CAR interacts with molecules regulating internalization and the endocytic machinery may also help to better understand how pathogens access the CNS and how cell adhesion molecules convey signals necessary for neuronal homeostasis.
Acknowledgments
We thank the IGMM Animal Facility and Montpellier Rio Imaging for microscopy studies and Thierry Gostan for help with statistical analyses.
This work was supported in part by European Community 7th Framework Program FP7/2007–2013 Grant 222992—BrainCAV, the French Agence National de la Recherche, E-Rare, the Fondation de France, the Association pour la Recherche sur la Sclérose Amyotrophique Latérale (ARSLa), and the Association Française contre les Myopathies.
- CAM
- cell adhesion molecule
- CAR
- coxsackievirus and adenovirus receptor
- AdV
- adenovirus
- CVB
- group B coxsackieviruses
- HAdV5
- human AdV type 5
- CTB
- cholera toxin binding fragment
- Tf
- transferrin
- DIV
- day in vitro
- CHC
- clathrin heavy chain
- MC
- microfluidic chamber
- LatA
- latrunculin A
- RFP
- red fluorescent protein
- FK
- fiber knob
- pp/c
- physical particle per cell.
REFERENCES
- 1. Salinas S., Schiavo G., Kremer E. J. (2010) A hitchhiker's guide to the nervous system. The complex journey of viruses and toxins. Nat. Rev. Microbiol. 8, 645–655 [DOI] [PubMed] [Google Scholar]
- 2. Salinas S., Bilsland L. G., Schiavo G. (2008) Molecular landmarks along the axonal route. Axonal transport in health and disease. Curr. Opin. Cell Biol. 20, 445–453 [DOI] [PubMed] [Google Scholar]
- 3. Franker M. A., Hoogenraad C. C. (2013) Microtubule-based transport. Basic mechanisms, traffic rules and role in neurological pathogenesis. J. Cell Sci. 126, 2319–2329 [DOI] [PubMed] [Google Scholar]
- 4. Koyuncu O. O., Hogue I. B., Enquist L. W. (2013) Virus infections in the nervous system. Cell Host Microbe 13, 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freimuth P., Philipson L., Carson S. D. (2008) The coxsackievirus and adenovirus receptor. Curr. Top. Microbiol. Immunol. 323, 67–87 [DOI] [PubMed] [Google Scholar]
- 6. Wang X., Bergelson J. M. (1999) Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73, 2559–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coyne C. B., Bergelson J. M. (2006) Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124, 119–131 [DOI] [PubMed] [Google Scholar]
- 8. Salinas S., Bilsland L. G., Henaff D., Weston A. E., Keriel A., Schiavo G., Kremer E. J. (2009) CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 5, e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seiradake E., Lortat-Jacob H., Billet O., Kremer E. J., Cusack S. (2006) Structural and mutational analysis of human Ad37 and canine adenovirus 2 fiber heads in complex with the D1 domain of coxsackie and adenovirus receptor. J. Biol. Chem. 281, 33704–33716 [DOI] [PubMed] [Google Scholar]
- 10. Verdino P., Witherden D. A., Havran W. L., Wilson I. A. (2010) The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science 329, 1210–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamanini A., Nicolis E., Bonizzato A., Bezzerri V., Melotti P., Assael B. M., Cabrini G. (2006) Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J. Virol. 80, 11241–11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie J., Chiang L., Contreras J., Wu K., Garner J. A., Medina-Kauwe L., Hamm-Alvarez S. F. (2006) Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J. Virol. 80, 11833–11851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaefer A. W., Kamiguchi H., Wong E. V., Beach C. M., Landreth G., Lemmon V. (1999) Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J. Biol. Chem. 274, 37965–37973 [DOI] [PubMed] [Google Scholar]
- 14. Gonnord P., Blouin C. M., Lamaze C. (2012) Membrane trafficking and signaling. Two sides of the same coin. Semin. Cell Dev. Biol. 23, 154–164 [DOI] [PubMed] [Google Scholar]
- 15. Ibáñez C. F. (2007) Message in a bottle. Long-range retrograde signaling in the nervous system. Trends Cell Biol. 17, 519–528 [DOI] [PubMed] [Google Scholar]
- 16. Lobert V. H., Brech A., Pedersen N. M., Wesche J., Oppelt A., Malerød L., Stenmark H. (2010) Ubiquitination of α5β1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 19, 148–159 [DOI] [PubMed] [Google Scholar]
- 17. Castellani V., Falk J., Rougon G. (2004) Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol. Cell Neurosci. 26, 89–100 [DOI] [PubMed] [Google Scholar]
- 18. Patzke C., Max K. E., Behlke J., Schreiber J., Schmidt H., Dorner A. A., Kröger S., Henning M., Otto A., Heinemann U., Rathjen F. G. (2010) The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J. Neurosci. 30, 2897–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farmer C., Morton P. E., Snippe M., Santis G., Parsons M. (2009) Coxsackie adenovirus receptor (CAR) regulates integrin function through activation of p44/42 MAPK. Exp. Cell Res. 315, 2637–2647 [DOI] [PubMed] [Google Scholar]
- 20. Ezratty E. J., Bertaux C., Marcantonio E. E., Gundersen G. G. (2009) Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granseth B., Odermatt B., Royle S. J., Lagnado L. (2006) Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786 [DOI] [PubMed] [Google Scholar]
- 22. Kremer E. J., Boutin S., Chillon M., Danos O. (2000) Canine adenovirus vectors. An alternative for adenovirus-mediated gene transfer. J. Virol. 74, 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hnasko T. S., Perez F. A., Scouras A. D., Stoll E. A., Gale S. D., Luquet S., Phillips P. E., Kremer E. J., Palmiter R. D. (2006) Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc. Natl. Acad. Sci. U.S.A. 103, 8858–8863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Persaud-Sawin D. A., Lightcap S., Harry G. J. (2009) Isolation of rafts from mouse brain tissue by a detergent-free method. J. Lipid Res. 50, 759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor A. M., Rhee S. W., Jeon N. L. (2006) Microfluidic chambers for cell migration and neuroscience research. Methods Mol. Biol. 321, 167–177 [DOI] [PubMed] [Google Scholar]
- 26. Restani L., Giribaldi F., Manich M., Bercsenyi K., Menendez G., Rossetto O., Caleo M., Schiavo G. (2012) Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 8, e1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soudais C., Laplace-Builhe C., Kissa K., Kremer E. J. (2001) Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15, 2283–2285 [DOI] [PubMed] [Google Scholar]
- 28. Walters R. W., Freimuth P., Moninger T. O., Ganske I., Zabner J., Welsh M. J. (2002) Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110, 789–799 [DOI] [PubMed] [Google Scholar]
- 29. Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 30. Ashbourne Excoffon K. J., Moninger T., Zabner J. (2003) The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain. J. Virol. 77, 2559–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi T., Suzuki T. (2011) Function of membrane rafts in viral lifecycles and host cellular response. Biochem. Res. Int. 2011, 245090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. (1993) Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319 [DOI] [PubMed] [Google Scholar]
- 33. Mineo C., Gill G. N., Anderson R. G. (1999) Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 274, 30636–30643 [DOI] [PubMed] [Google Scholar]
- 34. Sarnataro D., Caputo A., Casanova P., Puri C., Paladino S., Tivodar S. S., Campana V., Tacchetti C., Zurzolo C. (2009) Lipid rafts and clathrin cooperate in the internalization of PrP in epithelial FRT cells. PLoS ONE 4, e5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meier O., Greber U. F. (2003) Adenovirus endocytosis. J. Gene Med. 5, 451–462 [DOI] [PubMed] [Google Scholar]
- 36. Carvajal-Gonzalez J. M., Gravotta D., Mattera R., Diaz F., Perez Bay A., Roman A. C., Schreiner R. P., Thuenauer R., Bonifacino J. S., Rodriguez-Boulan E. (2012) Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXφ motif with the clathrin adaptors AP-1A and AP-1B. Proc. Natl. Acad. Sci. U.S.A. 109, 3820–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olusanya O., Andrews P. D., Swedlow J. R., Smythe E. (2001) Phosphorylation of threonine 156 of the mu2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11, 896–900 [DOI] [PubMed] [Google Scholar]
- 38. Ferguson S. M., De Camilli P. (2012) Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Damke H., Baba T., Warnock D. E., Schmid S. L. (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mooren O. L., Galletta B. J., Cooper J. A. (2012) Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81, 661–686 [DOI] [PubMed] [Google Scholar]
- 41. Huang K. C., Yasruel Z., Guérin C., Holland P. C., Nalbantoglu J. (2007) Interaction of the Coxsackie and adenovirus receptor (CAR) with the cytoskeleton. Binding to actin. FEBS Lett. 581, 2702–2708 [DOI] [PubMed] [Google Scholar]
- 42. Orlandi P. A., Fishman P. H. (1998) Filipin-dependent inhibition of cholera toxin. Evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richart S. M., Simpson S. A., Krummenacher C., Whitbeck J. C., Pizer L. I., Cohen G. H., Eisenberg R. J., Wilcox C. L. (2003) Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by Nectin-1/HveC. J. Virol. 77, 3307–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohka S., Matsuda N., Tohyama K., Oda T., Morikawa M., Kuge S., Nomoto A. (2004) Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J. Virol. 78, 7186–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang Y. C., Huang S. L., Chen S. P., Huang Y. L., Huang C. G., Tsao K. C., Lin T. Y. (2013) Adenovirus infection associated with central nervous system dysfunction in children. J. Clin. Virol. 57, 300–304 [DOI] [PubMed] [Google Scholar]
- 47. de Ory F., Avellón A., Echevarría J. E., Sánchez-Seco M. P., Trallero G., Cabrerizo M., Casas I., Pozo F., Fedele G., Vicente D., Pena M. J., Moreno A., Niubo J., Rabella N., Rubio G., Pérez-Ruiz M., Rodríguez-Iglesias M., Gimeno C., Eiros J. M., Melón S., Blasco M., López-Miragaya I., Varela E., Martinez-Sapiña A., Rodríguez G., Marcos M. Á., Gegúndez M. I., Cilla G., Gabilondo I., Navarro J. M., Torres J., Aznar C., Castellanos A., Guisasola M. E., Negredo A. I., Tenorio A., Vázquez-Morón S. (2013) Viral infections of the central nervous system in Spain. A prospective study. J. Med. Virol. 85, 554–562 [DOI] [PubMed] [Google Scholar]
- 48. Moore M. L., Brown C. C., Spindler K. R. (2003) T cells cause acute immunopathology and are required for long-term survival in mouse adenovirus type 1-induced encephalomyelitis. J. Virol. 77, 10060–10070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gralinski L. E., Ashley S. L., Dixon S. D., Spindler K. R. (2009) Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J. Virol. 83, 9398–9410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kosulin K., Haberler C., Hainfellner J. A., Amann G., Lang S., Lion T. (2007) Investigation of adenovirus occurrence in pediatric tumor entities. J. Virol. 81, 7629–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. (1993) An adenovirus vector for gene transfer into neurons and glia in the brain. Science 259, 988–990 [DOI] [PubMed] [Google Scholar]
- 52. Akli S., Caillaud C., Vigne E., Stratford-Perricaudet L. D., Poenaru L., Perricaudet M., Kahn A., Peschanski M. R. (1993) Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 3, 224–228 [DOI] [PubMed] [Google Scholar]
- 53. Bru T., Salinas S., Kremer E. (2010) An update on canine adenovirus type 2 and its vectors. Viruses 2, 2134–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. (2006) Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52, 293–305 [DOI] [PubMed] [Google Scholar]
- 55. Ohka S., Sakai M., Bohnert S., Igarashi H., Deinhardt K., Schiavo G., Nomoto A. (2009) Receptor-dependent and -independent axonal retrograde transport of poliovirus in motor neurons. J. Virol. 83, 4995–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soudais C., Boutin S., Hong S. S., Chillon M., Danos O., Bergelson J. M., Boulanger P., Kremer E. J. (2000) Canine adenovirus type 2 attachment and internalization. Coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 74, 10639–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chillon M., Kremer E. J. (2001) Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 12, 1815–1823 [DOI] [PubMed] [Google Scholar]
- 58. Lortat-Jacob H., Chouin E., Cusack S., van Raaij M. J. (2001) Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor-ligand interactions. J. Biol. Chem. 276, 9009–9015 [DOI] [PubMed] [Google Scholar]
- 59. Schoehn G., El Bakkouri M., Fabry C. M., Billet O., Estrozi L. F., Le L., Curiel D. T., Kajava A. V., Ruigrok R. W., Kremer E. J. (2008) Three-dimensional structure of canine adenovirus serotype 2 capsid. J. Virol. 82, 3192–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Raaij M. J., Chouin E., van der Zandt H., Bergelson J. M., Cusack S. (2000) Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7-Å resolution. Structure Fold Des. 8, 1147–1155 [DOI] [PubMed] [Google Scholar]
- 61. He Y., Chipman P. R., Howitt J., Bator C. M., Whitt M. A., Baker T. S., Kuhn R. J., Anderson C. W., Freimuth P., Rossmann M. G. (2001) Interaction of coxsackievirus B3 with the full-length coxsackievirus-adenovirus receptor. Nat. Struct. Biol. 8, 874–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drayman N., Glick Y., Ben-nun-shaul O., Zer H., Zlotnick A., Gerber D., Schueler-Furman O., Oppenheim A. (2013) Pathogens use structural mimicry of native host ligands as a mechanism for host receptor engagement. Cell Host Microbe 14, 63–73 [DOI] [PubMed] [Google Scholar]
- 63. Rajendran L., Simons K. (2005) Lipid rafts and membrane dynamics. J. Cell Sci. 118, 1099–1102 [DOI] [PubMed] [Google Scholar]
- 64. Simons K., Gerl M. J. (2010) Revitalizing membrane rafts. New tools and insights. Nat. Rev. Mol. Cell Biol. 11, 688–699 [DOI] [PubMed] [Google Scholar]
- 65. Boulware M. I., Kordasiewicz H., Mermelstein P. G. (2007) Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 27, 9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lang D. M., Lommel S., Jung M., Ankerhold R., Petrausch B., Laessing U., Wiechers M. F., Plattner H., Stuermer C. A. (1998) Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J. Neurobiol. 37, 502–523 [DOI] [PubMed] [Google Scholar]
- 67. Schneider A., Rajendran L., Honsho M., Gralle M., Donnert G., Wouters F., Hell S. W., Simons M. (2008) Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. Neurosci. 28, 2874–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carcea I., Ma'ayan A., Mesias R., Sepulveda B., Salton S. R., Benson D. L. (2010) Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J. Neurosci. 30, 15317–15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Diaz F., Gravotta D., Deora A., Schreiner R., Schoggins J., Falck-Pedersen E., Rodriguez-Boulan E. (2009) Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 106, 11143–11148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lajoie P., Kojic L. D., Nim S., Li L., Dennis J. W., Nabi I. R. (2009) Caveolin-1 regulation of dynamin-dependent, raft-mediated endocytosis of cholera toxin-B subunit occurs independently of caveolae. J. Cell. Mol. Med. 13, 3218–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pelkmans L., Püntener D., Helenius A. (2002) Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296, 535–539 [DOI] [PubMed] [Google Scholar]
- 72. Payne C. K., Jones S. A., Chen C., Zhuang X. (2007) Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic 8, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ewers H., Helenius A. (2011) Lipid-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 3, a004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goswami D., Gowrishankar K., Bilgrami S., Ghosh S., Raghupathy R., Chadda R., Vishwakarma R., Rao M., Mayor S. (2008) Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135, 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burckhardt C. J., Suomalainen M., Schoenenberger P., Boucke K., Hemmi S., Greber U. F. (2011) Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe 10, 105–117 [DOI] [PubMed] [Google Scholar]
- 76. Zhang Y., Moheban D. B., Conway B. R., Bhattacharyya A., Segal R. A. (2000) Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 20, 5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sakisaka T., Takai Y. (2005) Cell adhesion molecules in the CNS. J. Cell Sci. 118, 5407–5410 [DOI] [PubMed] [Google Scholar]
- 78. Kamiguchi H., Yoshihara F. (2001) The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J. Neurosci. 21, 9194–9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schäfer M. K., Schmitz B., Diestel S. (2010) L1CAM ubiquitination facilitates its lysosomal degradation. FEBS Lett. 584, 4475–4480 [DOI] [PubMed] [Google Scholar]
- 80. Paxinos G., Franklin K. B. J. (2003) The Mouse Brain in Stereotaxic Coordinates, 2nd Ed., pp. 22 and 30, Academic Press, San Diego, CA [Google Scholar]