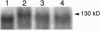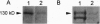Abstract
The Abeta peptide of Alzheimer disease is derived from the proteolytic processing of the amyloid precursor proteins (APP), which are considered type I transmembrane glycoproteins. Recently, however, soluble forms of full-length APP were also detected in several systems including chromaffin granules. In this report we used antisera specific for the cytoplasmic sequence of APP to show that primary bovine chromaffin cells secrete a soluble APP, termed solAPPcyt, of an apparent molecular mass of 130 kDa. This APP was oversecreted from Chinese hamster ovary cells transfected with a full-length APP cDNA indicating that solAPPcyt contained both the transmembrane and Abeta sequence. Deglycosylation of solAPPcyt showed that it contained both N- and O-linked sugars, suggesting that this APP was transported through the endoplasmic reticulum-Golgi pathway. Secretion of solAPPcyt from primary chromatin cells was temperature-, time-, and energy-dependent and was stimulated by cell depolarization in a Ca2+-dependent manner. Cholinergic receptor agonists, including acetylcholine, nicotine, or carbachol, stimulated the rapid secretion of solAPPcyt, a process that was inhibited by cholinergic antagonists. Stimulation of solAPPcyt secretion was paralleled by a stimulation of secretion in catecholamines and chromogranin A, indicating that secretion of solAPPcyt was mediated by chromaffin granule vesicles. Taken together, our results show that release of the potentially amyloidogenic solAPPcyt is an active cellular process mediated by both the constitutive and regulated pathways. solAPPcyt was also detected in human cerebrospinal fluid. Combined with the neuronal physiology of chromaffin cells, our data suggest that cholinergic agonists may stimulate the release of this APP in neuronal synapses where it may exert its biological functions. Moreover, vesicular or secreted solAPPcyt may serve as a soluble precursor of Abeta.
Full text
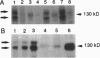
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Chen Y., Kim K. S., Robakis N. K. An alternative secretase cleavage produces soluble Alzheimer amyloid precursor protein containing a potentially amyloidogenic sequence. J Neurochem. 1992 Dec;59(6):2328–2331. doi: 10.1111/j.1471-4159.1992.tb10128.x. [DOI] [PubMed] [Google Scholar]
- Anderson J. P., Refolo L. M., Wallace W., Mehta P., Krishnamurthi M., Gotlib J., Bierer L., Haroutunian V., Perl D., Robakis N. K. Differential brain expression of the Alzheimer's amyloid precursor protein. EMBO J. 1989 Dec 1;8(12):3627–3632. doi: 10.1002/j.1460-2075.1989.tb08536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin F., Rosenberg R. N., Greenberg B. D. Increased release of an amyloidogenic C-terminal Alzheimer amyloid precursor protein fragment from stressed PC-12 cells. J Neurosci Res. 1991 May;29(1):127–132. doi: 10.1002/jnr.490290115. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Weidemann A., Multhaup G., Salbaum J. M., Lemaire H. G., Kang J., Müller-Hill B., Masters C. L., Beyreuther K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J. 1988 Apr;7(4):949–957. doi: 10.1002/j.1460-2075.1988.tb02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthimiopoulos S., Felsenstein K. M., Sambamurti K., Robakis N. K., Refolo L. M. Study of the phorbol ester effect on Alzheimer amyloid precursor processing: sequence requirements and involvement of a cholera toxin sensitive protein. J Neurosci Res. 1994 May 1;38(1):81–90. doi: 10.1002/jnr.490380111. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Barger S. W., Blalock E. M., Mattson M. P. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996 Jan 4;379(6560):74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- Haas C., Hung A. Y., Citron M., Teplow D. B., Selkoe D. J. beta-Amyloid, protein processing and Alzheimer's disease. Arzneimittelforschung. 1995 Mar;45(3A):398–402. [PubMed] [Google Scholar]
- Haass C., Lemere C. A., Capell A., Citron M., Seubert P., Schenk D., Lannfelt L., Selkoe D. J. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995 Dec;1(12):1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Eiden L. E. (Met)enkephalin and carboxypeptidase processing enzyme are co-released from chromaffin cells by cholinergic stimulation. Biochem Biophys Res Commun. 1985 Apr 30;128(2):563–570. doi: 10.1016/0006-291x(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krog L., Olsen M., Dalseg A. M., Roth J., Bock E. Characterization of soluble neural cell adhesion molecule in rat brain, CSF, and plasma. J Neurochem. 1992 Sep;59(3):838–847. doi: 10.1111/j.1471-4159.1992.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Culwell A. R., Esch F. S., Lieberburg I., Rydel R. E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993 Feb;10(2):243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Mroczkowski B., Reich M., Chen K., Bell G. I., Cohen S. Recombinant human epidermal growth factor precursor is a glycosylated membrane protein with biological activity. Mol Cell Biol. 1989 Jul;9(7):2771–2778. doi: 10.1128/mcb.9.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992 Oct 9;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Olsen M., Krog L., Edvardsen K., Skovgaard L. T., Bock E. Intact transmembrane isoforms of the neural cell adhesion molecule are released from the plasma membrane. Biochem J. 1993 Nov 1;295(Pt 3):833–840. doi: 10.1042/bj2950833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overly C. C., Fritz L. C., Lieberburg I., McConlogue L. The beta-amyloid precursor protein is not processed by the regulated secretory pathway. Biochem Biophys Res Commun. 1991 Dec 16;181(2):513–519. doi: 10.1016/0006-291x(91)91218-2. [DOI] [PubMed] [Google Scholar]
- Papa V., Russo P., Gliozzo B., Goldfine I. D., Vigneri R., Pezzino V. An intact and functional soluble form of the insulin receptor is secreted by cultured cells. Endocrinology. 1993 Sep;133(3):1369–1376. doi: 10.1210/endo.133.3.8396017. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Refolo L. M., Salton S. R., Anderson J. P., Mehta P., Robakis N. K. Nerve and epidermal growth factors induce the release of the Alzheimer amyloid precursor from PC 12 cell cultures. Biochem Biophys Res Commun. 1989 Oct 31;164(2):664–670. doi: 10.1016/0006-291x(89)91511-8. [DOI] [PubMed] [Google Scholar]
- Regland B., Gottfries C. G. The role of amyloid beta-protein in Alzheimer's disease. Lancet. 1992 Aug 22;340(8817):467–469. doi: 10.1016/0140-6736(92)91780-c. [DOI] [PubMed] [Google Scholar]
- Ripellino J. A., Vassilacopoulou D., Robakis N. K. Solubilization of full-length amyloid precursor proteins from PC12 cell membranes. J Neurosci Res. 1994 Oct 1;39(2):211–218. doi: 10.1002/jnr.490390211. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Pangalos M. N. Involvement of amyloid as a central step in the development of Alzheimer's disease. Neurobiol Aging. 1994;15 (Suppl 2):S127–S129. doi: 10.1016/0197-4580(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Brugge K. Is amyloid causally involved in pathophysiology of Alzheimer's disease? Neurobiol Aging. 1994 Jul-Aug;15(4):461–462. doi: 10.1016/0197-4580(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Sundsmo M., Roch J. M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D. B. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989 Aug 25;58(4):615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Sambamurti K., Shioi J., Anderson J. P., Pappolla M. A., Robakis N. K. Evidence for intracellular cleavage of the Alzheimer's amyloid precursor in PC12 cells. J Neurosci Res. 1992 Oct;33(2):319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Oltersdorf T., Lee M. G., Barbour R., Blomquist C., Davis D. L., Bryant K., Fritz L. C., Galasko D., Thal L. J. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993 Jan 21;361(6409):260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simons M., Ikonen E., Tienari P. J., Cid-Arregui A., Mönning U., Beyreuther K., Dotti C. G. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J Neurosci Res. 1995 May 1;41(1):121–128. doi: 10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Hoffman P. N., Perry G., Price D. L. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci. 1993 Jul;13(7):3136–3142. doi: 10.1523/JNEUROSCI.13-07-03136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slunt H. H., Thinakaran G., Von Koch C., Lo A. C., Tanzi R. E., Sisodia S. S. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP). J Biol Chem. 1994 Jan 28;269(4):2637–2644. [PubMed] [Google Scholar]
- Takeda M., Tanaka S., Kido H., Daikoku S., Oka M., Sakai K., Katunuma N. Chromaffin cells express Alzheimer amyloid precursor protein in the same manner as brain cells. Neurosci Lett. 1994 Feb 28;168(1-2):57–60. doi: 10.1016/0304-3940(94)90415-4. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Vassilacopoulou D., Ripellino J. A., Tezapsidis N., Hook V. Y., Robakis N. K. Full-length and truncated Alzheimer amyloid precursors in chromaffin granules: solubilization of membrane amyloid precursor is mediated by an enzymatic mechanism. J Neurochem. 1995 May;64(5):2140–2146. doi: 10.1046/j.1471-4159.1995.64052140.x. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yee N. S., Langen H., Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down-regulation of c-kit receptors in mast cells. J Biol Chem. 1993 Jul 5;268(19):14189–14201. [PubMed] [Google Scholar]