Background: Adipocytes are known to metabolize but not synthesize de novo steroids or oxysterols.
Results: CYP11A1 in adipocytes is able to synthesize pregnenolone; 27-hydroxycholesterol (27HC) is a major cholesterol metabolite in adipocytes exhibiting anti-adipogenic activity.
Conclusion: Adipocytes have the ability to synthesize steroids and oxysterols de novo.
Significance: Formation of 27HC in adipocytes may prevent the enlargement of adipose tissue compartment.
Keywords: Adipocyte, Cholesterol, Differentiation, Mouse, Rat, Steroidogenesis, CYP11A1, CYP27A1, Human, Oxysterol
Abstract
Local production and action of cholesterol metabolites such as steroids or oxysterols within endocrine tissues are currently recognized as an important principle in the cell type- and tissue-specific regulation of hormone effects. In adipocytes, one of the most abundant endocrine cells in the human body, the de novo production of steroids or oxysterols from cholesterol has not been examined. Here, we demonstrate that essential components of cholesterol transport and metabolism machinery in the initial steps of steroid and/or oxysterol biosynthesis pathways are present and active in adipocytes. The ability of adipocyte CYP11A1 in producing pregnenolone is demonstrated for the first time, rendering adipocyte a steroidogenic cell. The oxysterol 27-hydroxycholesterol (27HC), synthesized by the mitochondrial enzyme CYP27A1, was identified as one of the major de novo adipocyte products from cholesterol and its precursor mevalonate. Inhibition of CYP27A1 activity or knockdown and deletion of the Cyp27a1 gene induced adipocyte differentiation, suggesting a paracrine or autocrine biological significance for the adipocyte-derived 27HC. These findings suggest that the presence of the 27HC biosynthesis pathway in adipocytes may represent a defense mechanism to prevent the formation of new fat cells upon overfeeding with dietary cholesterol.
Introduction
Adipocytes are not passive storage depots for fat; rather, they are active endocrine/paracrine/autocrine/intracrine cells that produce a wealth of factors that regulate lipid homeostasis, insulin sensitivity, glucose metabolism, and inflammation (1). In addition to secreting proteins such as leptin and adiponectin, adipocytes are major sites of steroid conversion. Indeed, adipocytes express several steroid-metabolizing enzymes (2) and can modulate local steroid concentrations. Thus, local production of steroids by adipocytes may contribute substantially to steroid action. To initiate the production of any steroid hormone, cholesterol must be delivered to the cytochrome P450 cholesterol side-chain cleavage enzyme (CYP11A1) located in the inner mitochondrial membrane. CYP11A1, aided by the electron transport partners ferredoxin (FDX)3 and ferredoxin reductase (FNR), converts cholesterol to pregnenolone, which is transformed into different steroid products through enzymatic reactions in a tissue-specific manner (Fig. 1A) (3).
FIGURE 1.
De novo synthesis of steroids, oxysterols, and bile acids from cholesterol. A, various specialized tissues can use cholesterol as the building block for the synthesis of steroid hormones, oxysterols, or bile acids. Cholesterol endogenously synthesized through the mevalonate pathway is transported by TSPO and STAR into the inner mitochondrial membrane, where it can be converted to the steroid pregnenolone or the oxysterol 27HC by CYP11A1 or CYP27A1, respectively. Pregnenolone is the precursor of all of the other steroids (e.g. aldosterone and cortisol in adrenal glands or sex steroids in gonads). The oxysterol 27HC serves as an intermediate for bile acid synthesis in hepatic cells. B, Oil Red O staining of lipid droplets in 3T3-L1 cells during differentiation (magnification, ×40).
In classic steroidogenic tissues, such as adrenal and testis, cholesterol availability to CYP11A1 limits steroidogenesis; the transport of cholesterol from the outer to the inner mitochondrial membrane is the rate-limiting step in steroidogenesis overall (4). The translocator protein (18 kDa) TSPO and the steroidogenic acute regulatory protein (STAR) are the two major components of the mitochondrial cholesterol transport machinery. Binding of the endogenous ligand diazepam-binding inhibitor/acyl-CoA binding domain 1 to TSPO accelerates the translocation of cholesterol into mitochondria and thus accelerates pregnenolone formation (Fig. 1A) (5). Because of the lack of evidence supporting the presence of mitochondrial cholesterol transport and metabolism in adipocytes, the ability to make steroids de novo has not been examined in these cells.
Recent findings in hepatic cells suggest that the mitochondrial cholesterol transport system may also be crucial for the activity of a second mitochondrial enzyme, the cytochrome P450 sterol 27-hydroxylase (CYP27A1). CYP27A1 metabolizes cholesterol into 27-hydroxycholesterol (27HC; Fig. 1A) (6), the most abundant oxysterol in human circulation (7). Based on isotope-labeling experiments that allow for monitoring the incorporation of labeled cholesterol into oxysterols in vivo, 80% of circulating 27HC is believed to be derived from a slowly exchangeable pool of cholesterol, representing production by extrahepatic tissues, including adipose, skeletal muscle, and skin (8). Thus, adipose tissue may be one of the sources for 27HC production.
There is evidence that Tspo gene transcription is induced during 3T3-L1 mouse preadipocyte differentiation (9) and that silencing of Acbd1 inhibits 3T3-L1 adipocyte differentiation (10). Based on these preliminary findings, we hypothesized that adipocytes are able to synthesize steroids or oxysterols, such as 27HC, de novo and that these endogenous products play a role in adipocyte differentiation and function. Here, we provide evidence that the expression and activity of the mitochondrial cholesterol delivery and metabolism machinery responsible for steroid hormone and 27HC biosynthesis are present in adipocytes. Moreover, inhibition of the CYP27A1 enzymatic pathway induces adipocyte differentiation, suggesting a local regulatory role of this pathway in adipocyte development and function.
EXPERIMENTAL PROCEDURES
Cell Culture, Differentiation, and Treatments
Mouse 3T3-L1 preadipocytes were cultured and differentiated as described previously (9, 11). In brief, 3T3-L1 preadipocytes (ATCC) were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) and antibiotics (100 IU/ml penicillin G and 100 μg/ml streptomycin) and maintained in a humidified chamber at 37 °C with 5% CO2. Two days post-confluency (designated day 0), cells were induced to differentiate by addition of DMEM supplemented with the same antibiotics and a standard induction mixture composed of 10% FBS, 0.5 mm 3-isobutyl-1-methylxanthine (IBMX), 1 μm dexamethasone, and 1 μg/ml insulin. After 48 h, the media were replaced with DMEM supplemented with the same antibiotics, 10% FBS, and 1 μg/ml insulin. The media were replaced every 48 h for an additional 8 days. For the treatment with 27HC or the specific CYP27A1 inhibitor GI268267X (a gift from GlaxoSmithKline), appropriate concentrations of compounds were added at the start of the differentiation, and the same concentrations of the compounds were added at a 2-day interval when the culture medium was replenished.
Simpson-Golabi-Behmel syndrome (SGBS) preadipocyte cell culture and differentiation was performed as described previously (12). SGBS preadipocytes were maintained in DMEM/Nutrient Mix F-12 (Invitrogen), supplemented with 8 μg/ml biotin, 4 μg/ml pantothenic acid, 10% FBS (not heat-inactivated, Wisent), and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin) at 37 °C with 5% CO2. Three days post-confluency, SGBS preadipocytes were induced to differentiate using the “quick” differentiation medium (DMEM/F-12, 8 μg/ml biotin, 4 μg/ml pantothenic acid, 0.01 mg/ml human transferrin, 100 nm cortisol, 200 pm triiodothyronine, 20 nm human insulin, 25 nm dexamethasone, 500 μm IBMX, 2 μm rosiglitazone, and antibiotics). On day 4 of differentiation, the medium was replaced by adipogenic medium lacking the dexamethasone, IBMX, and rosiglitazone of the quick differentiation medium. The adipogenic medium was replaced every 2–3 days. SGBS cells were fully differentiated on day 15.
Human mature adipocytes in culture (2-week post-differentiation) from subcutaneous or omental adipose tissues of nondiabetic male subjects were obtained from Zenbio Inc. Upon arrival, excess medium added to each well for shipping was immediately removed, and only sufficient volume of medium was left to cover the cell monolayer. After overnight incubation at 37 °C with 5% CO2, adipocytes were fed with fresh Omental Adipocyte Medium (OM-AM, Zenbio) for omental adipocytes and Adipocyte Maintenance Medium (AM-1, Zenbio) for subcutaneous adipocytes for another 24 h before using for de novo steroid or oxysterol studies.
MA-10 mouse Leydig cells were a gift from Dr. Mario Ascoli (University of Iowa) and cultured in DMEM/F-12 supplemented with 5% FBS and 2.5% heat-inactivated horse serum as described previously (13). HepG2 human liver hepatocellular carcinoma cell lines were obtained from ATCC and cultured in DMEM supplemented with 10% FBS.
Isolation, Culture, and Differentiation of Stromal Vascular Fraction (SVF)
10-Week-old male B6.129-Cyp27a1tm1Elt/J (Cyp27a1−/−) and their respective control male C57BL/6J (wild type) mice were purchased from The Jackson Laboratory. 55–58-Day-old male Sprague-Dawley rats were purchased from Charles River Laboratories. Following CO2 euthanasia, animals were decapitated, and adipose tissues were collected. Animals were handled according to protocols approved by the McGill University Animal Care and Use Committees. SVF isolation from adipose tissue and cell differentiation was performed as described previously (14). In brief, adipose tissue was rinsed immediately in phosphate-buffered saline (PBS), and connective tissues and blood vessels were carefully dissected and removed. The remaining tissues were minced into small pieces and digested in a collagenase solution containing 2% bovine serum albumin for 60 min at 37 °C under continuous shaking. The collagenase used was type II collagenase (Sigma, 1.2% v/v) for rat adipose tissue and type 1 collagenase (Worthington, 1 mg/ml) for mouse adipose tissue. The dispersed tissue was centrifuged for 5 min at 250 × g to give rise to the floating adipocytes and the sedimented SVF. The SVF pellets were resuspended in erythrocyte lysis buffer (8.26 g of NH4Cl, 1 g of KHCO3, and 0.037 g of EDTA in 1 liter of water (pH 7.3)) for 5 min at room temperature (RT) before adding complete growth medium (10% fetal bovine serum, 1% penicillin/streptomycin solution, and 1% amphotericin B in DMEM/Nutrient Mixture F-12) to stop the reaction. The resulting suspension was filtered through 100-μm nylon strainers and 40-μm nylon strainers. After centrifuging at 350 × g for 5 min at 4 °C, the pellets were resuspended in complete medium and cultured at 37 °C in a humid 5% CO2 environment. Three days post-seeding, SVF cells from rat adipose tissue were differentiated into adipocytes with complete growth medium supplemented with 0.5 mm IBMX, 1 μm dexamethasone, and 1.6 μm insulin for 2 days, followed by supplementation of 1.6 μm insulin alone for an additional 8 days. The medium was replaced every 2 days. The differentiation of SVF cells from mouse adipose tissue followed the same procedure as above, except that 5 μm rosiglitazone was added into the differentiation medium during the first 2 days.
Knockdown of Cyp27a1 in 3T3-L1 Preadipocytes
At 80% confluence, 3T3-L1 preadipocytes were transfected with 50 nm siRNA for Cyp27a1 (Dharmacon, M-058118-01, siGENOME SMARTpool) using Lipofectamine 2000 reagent (Invitrogen) according to the protocol recommended by the manufacturer. The transfected cells were incubated for 48 h and subjected to differentiation.
Quantitative Real Time PCR (qPCR) Analysis
Total RNA was isolated from cells using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. The concentration and quality of the purified RNA samples were determined spectrophotometrically using NanoDrop ND-1000 (Thermo Scientific) at A260 and by the A260/A280 ratio, respectively. Total RNA (700 ng) was reverse-transcribed into cDNA using random hexamers (Roche Applied Science) according to the manufacturer's instructions. RNA expression levels were quantified using SYBR Green on a Light Cycler System 480 (Roche Applied Science). Briefly, samples were preincubated at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 63 °C for 10 s, and elongation at 72 °C for 10 s. For each primer pair, the identity of the qPCR amplification product was verified by electrophoresis on a 2% agarose gel and by melting curve analysis. Ribosomal protein S18 (Rps18) mRNA expression was constant under the experimental conditions, and all results were normalized to Rps18 mRNA levels.
The primers (upstream and downstream) used were as follows: Tspo, 5′-CCC GCT TGC TGT ACC CTT ACC-3′ and 5′-CAC CGC ATA CAT AGT AGT TGA GCA CGG TG-3′; Acbd1, 5′-GCT TGT TCC ACG AGT CCC ACT-3′ and 5′-CGG TAG ACA GTC ACT TCA AAC AAG CTA CCG-3′; Star, 5′-ATC TCC TTG ACA TTT GGG TTC CA-3′ and 5′-CGG TCT CTA TGA AGA ACT TGT GGA CCG-3′; Cyp11a1, 5′-GGT TCT CAG GCA TCA GGA TGA G-3′ and 5′-CGG AGC AGA ATT GAA GTT CAA AAT CTC CG-3′; Fdx, 5′-CGG ATC AAC AGA CTG TCG GAC ATC CG-3′ and 5′-CAA GGT TGG GCT GCC AAG TT-3′; Fnr, 5′-CGG CAG CAG CAG TTC TGT TAG CCG-3′ and 5′-TTG GGC CTC CAG GAC AGA ATT A-3′; Cyp17a1, 5′-ATG AGG AGG TGA GTC CGG TCA-3′ and 5′-CGA CTG TGA CCA GTA TGT AGG CTT CAG TCG-3′; Hsd3b1, 5′-TGT TGG TGC AGG AGA AAG AAC TG-3′ and 5′-CGA CCT CCT CCT TGG TTT CTG GTC G-3′; Cyp21, 5′-CGG CTT ATC CTT AGG GAT GTC ATA GCC G-3′ and 5′-ATT GCC GAG GTG CTG CGT TT-3′; Cyp11b1, 5′-AAG TCC CTT GCT ATC CCA TCC A-3′ and 5′-CGG CAA TGT TCT GTC ACC AAA AGC CG-3′; Cyp11b2, 5′-TCA GAC TCG GCA GCT CTC AGA C-3′ and 5′-CGG AAA AGA TCC CTG AGA TAT TAG TTC CG-3′; Hsd11b1, 5′-CAT GAC CAC GTA GCT GAG GAA G-3′ and 5′-CGC ACG ACG ACA TCC ACT CTG TGC G-3′; Cyp19a1, 5′-AGG GTC AAC ACA TCC ACG TAG C-3′ and 5′-CGG TCA TCA AGC AGC ATT TGG ACC G-3′; Hsd17b7, 5′-CGC AAT GCA AAG AAG GCT AAC TT-3′ and 5′-CGG AAT GGA AGA GCT GTA GGG TTC CG-3′; Cyp27a1, 5′-CGG CCA TTC CTG AGG ACA CTG CCG-3′ and 5′-TCC ATT TGG GAA GGA AAG TGA T-3′; Cyp7b1, 5′-GGT CTG CCT GGA AAG CAC TA-3′ and 5′-TTC TCG GAT GAT GCT GGA GT-3′; Cyp7a1, 5′-CGG GAC AAG TGA ATA GGG ACG CCC G-3′ and 5′-TGA CAG CTT CAA ACA ATT TGA CCA-3′; Nr5a1, 5′-GCT GGC ATA GGG CTC TGG AT-3′ and 5′-CGG TTC TCT ATC CTG CCT TCT CTA ACC G-3′; Pparg, 5′-CGG AAA TAA AGT CAC CAA AGG GCT TCC G-3′ and 5′-CTC ATC TCA GAG GGC CAA GGA-3′; Fabp4, 5′-TTT GGT CAC CAT CCG GTC AG-3′ and 5′-CGA GAT CCC AGT TTG AAG GAA ATC TCG-3′; Cebpa, 5′-TTG GCT TTA TCT CGG CTC TTG C-3′ and 5′-CGG TAA CAA GAA CAG CAA CGA GTA CCG 3′; Rps18, 5′-CAC GGG CTC CAC CTC ATC CTC CGT G-3′ and 5′-TGA GGA AAG CAG ACA TCG ACC T-3′.
Mitochondrial Preparation
Mitochondria were isolated as described previously (15), with minor modifications. All steps of the procedure were performed at 4 °C. Briefly, 3T3-L1 adipocytes (in 150-mm dishes) were rinsed twice in PBS, harvested in Buffer A (10 mm Hepes-KOH (pH 7.5), 200 mm mannitol, 70 mm sucrose, 1 mm EDTA, and 1× Complete Protease Inhibitor Mixture Tablet (Roche Diagnostics)) using a cell lifter and centrifuged at 500 × g for 10 min. The cell pellets were resuspended in 5 volumes of Buffer A, incubated for 10 min, and centrifuged at 500 × g for 10 min. The cell pellets were resuspended in Buffer B (40 mm Hepes-KOH (pH 7.5), 250 mm sucrose, 80 mm potassium acetate, 5 mm magnesium acetate, and 1× Complete Protease Inhibitor Mixture Tablet) and homogenized with an electric Teflon-glass homogenizer. The homogenate was centrifuged at 500 × g for 10 min; the supernatant was collected, and the pellet was homogenized and centrifuged again. The two supernatants were pooled and centrifuged at 10,000 × g for 10 min. The resulting mitochondrial pellet was resuspended in 1 ml Buffer B and centrifuged at 10,000 × g for another 10 min to enrich mitochondrial purity. The final mitochondrial pellets were either solubilized in Laemmli buffer for immunoblot analysis or resuspended in import buffer for pregnenolone or 27HC synthesis.
Protein Measurement
Protein concentrations were determined using a bicinchoninic acid assay (Thermo Fisher Scientific) according to the manufacturer's instructions.
Immunoblot Analysis
Whole cells or mitochondrial pellets were solubilized in Laemmli buffer, and proteins (30 μg) were separated by electrophoresis on a 4–20% SDS-polyacrylamide gradient gel and transferred to a polyvinylidene fluoride membrane. Nonspecific binding was blocked with 5% skim milk in Tris-buffered saline solution for 1 h at RT. Membranes were incubated with specific antibodies overnight at 4 °C. Antibodies used were as follows: anti-CYP11A1 (1:1000, Abcam, ab75497); anti-CYP27A1 (1:1000, Abcam, ab64889); anti-FDX (1:1000, Abcam, ab108257); anti-FNR (1:1000, Abcam, ab16874); anti-TSPO (1:500) (16); anti-ACBD1 (1:1000, Abcam, ab16871); anti-STAR (1:1000, provided by Dr. Buck Hales, Southern Illinois University); anti-adipocyte fatty acid-binding protein (FABP4; 1:1000, Cell Signaling, 3544); anti-β-actin (1:1000, Cell Signaling, 4970); and anti-cytochrome c oxidase (COX IV; 1:1000, Abcam, ab16056). Membranes were incubated with anti-rabbit IgG horseradish peroxidase-linked secondary antibodies for 1 h at RT. After developing membranes using an enhanced chemiluminescence kit (Amersham Biosciences), signals were visualized with a Fujifilm LAS-4000. The same membrane was probed with either anti-β-actin or anti-cytochrome c oxidase to detect the expression of the internal housekeeping control in cell or mitochondrial lysates.
CYP11A1 and CYP27A1 Activity Assays
In both isolated mitochondria and whole adipocytes, the activities of CYP11A1 or CYP27A1 were tested by using 22(R)-hydroxycholesterol (22RHC) or cholesterol, respectively, as substrate. When using isolated mitochondria for the activity assay, procedures from previous studies were followed (17). Freshly prepared adipocyte mitochondria pellets were suspended at a final concentration of 1 mg/ml in import buffer (250 mm sucrose, 10 mm potassium phosphate buffer (pH 7.5), 15 mm triethanolamine-HCl, 20 mm KCl, 5 mm MgCl2, 3% bovine serum albumin, and 1× Complete Protease Inhibitor Mixture tablet). Either 22RHC (substrate for CYP11A1, 20 μm) or cholesterol (substrate for CYP11A1 and CYP27A1, 1.5 μm [3H]cholesterol (specific activity of 43 Ci/mmol, Amersham Biosciences), and 20 μm unlabeled cholesterol) was added to the import buffer. For the CYP11A1 assay, the import buffer was also supplemented with 5 μm trilostane, a 3β-hydroxysteroid dehydrogenase (3β-HSD) inhibitor. These mitochondrial suspensions were incubated at 37 °C for 5 min in a shaking platform. The reaction was started by the addition of 15 mm sodium isocitrate and 0.5 mm NADP in a 250-μl final volume. After incubating for the indicated time periods at 37 °C, the reaction was stopped by adding 1 ml of ethyl acetate. After extraction, the organic phase was collected and evaporated to dryness. Pregnenolone formation was measured by RIA. Radiolabeled 27HC formation was detected by HPLC-radiometric assay.
When using the whole cells for the activity assay, fully differentiated adipocytes grown on 100-mm culture plates were washed twice with PBS before the incubation with 22RHC (1.5 μCi of 22(R)-[22-3H] hydroxycholesterol (specific activity of 20 Ci/mmol, American Radiolabeled Chemicals) and 20 μm unlabeled 22RHC) or cholesterol (1.5 μCi of [3H]cholesterol and 20 μm unlabeled cholesterol) in Aim-V serum-free medium. After different time intervals, cells and media were collected, extracted with ethyl acetate, and dried. The residues were reconstituted in methanol, and the radiolabeled products were analyzed by HPLC-radiometric assay.
De Novo Steroid or Oxysterol Synthesis
Mature adipocytes differentiated from mouse 3T3-L1 preadipocytes, rat SVFs, human SGBS preadipocytes, and human primary preadipocytes were used for de novo synthesis of steroids or oxysterols. As described previously (18), cells were washed twice with warm PBS and incubated at 37 °C with either cholesterol (1.5 μCi of [3H]cholesterol and 20 μm unlabeled cholesterol) or its direct precursor mevalonate (1.5 μCi of [3H]mevalonolactone (specific activity, 37.1 Ci/mmol, PerkinElmer Life Sciences) and 20 μm unlabeled mevalonolactone) in the media, which are as follows: for 3T3-L1 adipocytes, Aim-V serum-free medium; for human SGBS adipocytes, DMEM/F-12 supplemented with 8 μg/ml biotin, 4 μg/ml pantothenic acid; for differentiated adipocytes from rat primary SVF, DMEM/F-12 supplemented with 5% charcoal-stripped FBS; for human omental adipocytes, Omental Adipocyte Medium; and for human subcutaneous adipocytes, Adipocyte Maintenance Medium. After terminating the reaction at various time points, media were collected, and cells were harvested in PBS. Aliquots of cell suspensions were frozen at −20 °C for protein concentration measurement later. The remaining cell suspensions were combined with the media and stored at −20 °C before extraction and analysis.
HPLC Radiometric Assay and TLC
Cell and media homogenates collected at various intervals of the substrate treatment were extracted three times with 4 volumes of ethyl acetate and evaporated. Extracts were reconstituted in 200 μl of methanol. Steroid or oxysterol products were separated by a Beckman Gold HPLC system using a Beckman Ultrasphere ODS column (250 × 4.6 mm, 5 μm) at RT and monitored by ultraviolet detection (190–300 nm). HPLC separation was achieved with a gradient of solution A (acetonitrile/methanol/water, 40:40:20) and solution B (100% methanol) at a flow rate of 1.5 ml/min, as described previously (19). Runs were started with 0% solution B (100% solution A) increasing linearly to 100% solution B during the initial 15 min, after which the flow was kept at 100% solution B for another 15 min. Fractions were collected every 30 s and counted by liquid scintillation spectrometry. Products were identified by respective retention time compared with standards under the same chromatographic conditions. Results were presented as counts/min minus background and normalized to the protein concentrations of the sample. In some experiments, steroid or oxysterol products were also separated by TLC on silica gel plates (Fluka) and developed in hexane/ethyl acetate/acetic acid (50:50:1, by volume). The bands were detected with iodine vapor.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Pooled extracts from de novo 27HC assays were air-dried, derivatized with bis-(trimethylsilyl)trifluoroacetamide/trimethylchlorosilane (99:1), and subjected to GC-MS using a Shimadzu 17A/QP5050A single quadrupole mass spectrometer operated in the electron impact ionization mode. The gas chromatograph was equipped with a 3-m × 0.25-mm 0.25-μm film thickness analytical column (Restek Canada, Chromatographic Specialties Inc., catalog no. 12223; serial no. 559805). A 0.5-μl sample was injected in splitless mode (inlet was set at 280 °C with the helium flow at 59 μl/min) at the initial 120 °C. The oven was first kept at 120 °C for 3 min, ramped at 40 °C/min to 310 °C, and held for 7 min isothermally. Based on the full scan (total ion current, m/z = 50–650) of the trimethylsilyl-derived reference standard 27HC, the ions detected at m/z 75 (C4H11O+), m/z 129 (C6H13OSi+), and m/z 457 (C30H53O2Si+) were used to confirm the production of 27HC by selected ion monitoring (SIM).
Quantification of the Rate of Bile Acid Synthesis
Rates of bile acid synthesis were determined via time point conversion of [3H]cholesterol to 3H-labeled bile acids, which were methanol/water-extractable products. As described previously (6), cells grown on 150-mm tissue culture dishes were washed twice with PBS and incubated in Aim-V serum-free medium containing 2.5 μCi of [3H]cholesterol. Aliquots (100 μl) of the medium were collected in duplicate in microcentrifuge tubes at various time points and kept frozen until analysis. A mini-Folch extraction buffer (50 μl of water, 250 μl of methanol, 537 μl of chloroform, and 3 μl of 1 m Na2CO3) was added to each 100-μl culture medium sample. After vortexing vigorously, the tubes were centrifuged at 16,000 × g for 6 min. The phases were collected separately and counted.
Oil Red O Staining
Cells were washed twice with PBS and fixed with 10% formaldehyde solution in PBS for 1 h. After removing the formaldehyde, cells were washed with 60% isopropyl alcohol and dried completely. Diluted Oil Red O solution (6 parts 0.35% Oil Red O in isopropyl alcohol and 4 parts water) was added for 10 min. After repeatedly washing with water, cells were visualized microscopically.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4.02 software. The significance of the results was determined by using Student's t test or one-way analysis of variance followed by Bonferroni's post hoc test for multiple comparisons.
RESULTS
Characterization of the Steroidogenic Pathway and Ability of 3T3-L1 Cells to Form Steroids during Differentiation
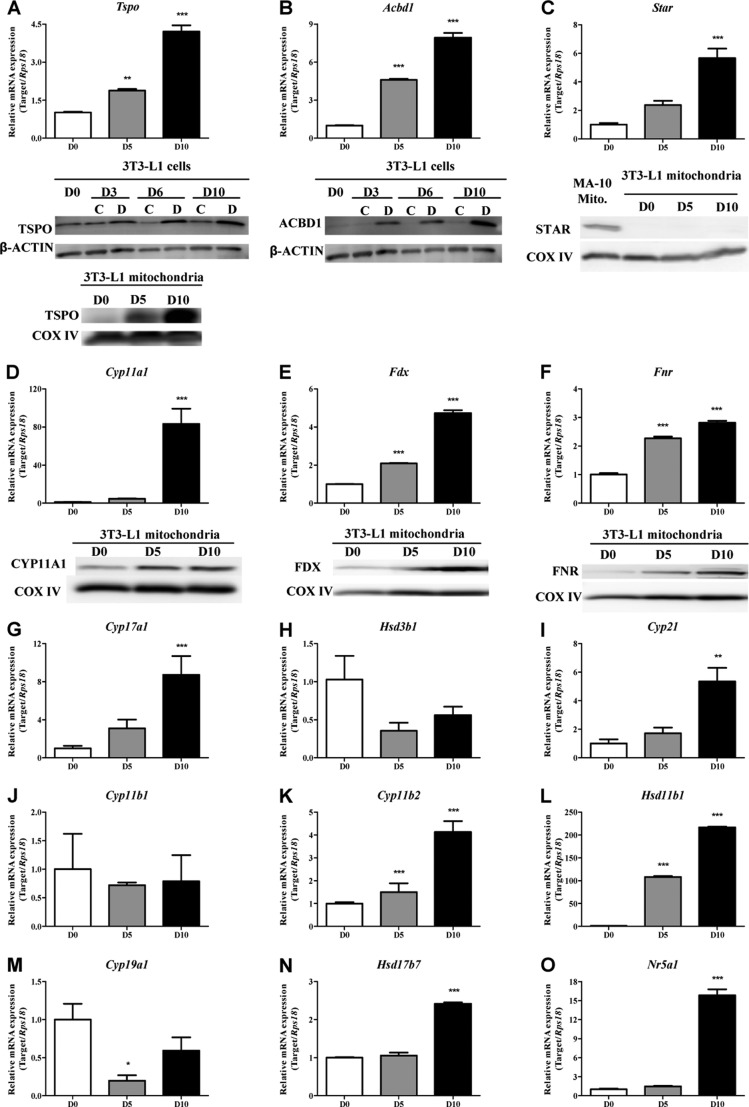
The 3T3-L1 preadipocyte cell line is a well established model used to study adipocyte differentiation. The differentiation stages of this cell line were defined by Oil Red O staining of lipid droplets (Fig. 1B). Upon induction of 3T3-L1 differentiation into adipocytes, Tspo gene expression was significantly elevated, rising ∼4-fold at D10 post-differentiation (Fig. 2A). In cell extracts, TSPO protein was present in preadipocytes, and TSPO levels increased during differentiation. In these studies, undifferentiated (control) cells were maintained in growth medium, whereas other cells were incubated in differentiation medium. TSPO levels in these cells were not changed compared with D0 preadipocytes. TSPO was observed in mitochondria isolated from D5 differentiating adipocytes, and TSPO levels were greatly increased in D10 mature adipocyte mitochondria (Fig. 2A). Similarly, Acbd1 mRNA and protein levels were induced during 3T3-L1 differentiation (Fig. 2B). Although Star (Stard1) mRNA levels at D10 were 4.7-fold higher than those at D0, STAR protein was not detected in isolated mitochondria from either preadipocytes or mature adipocytes (Fig. 2C).
FIGURE 2.
Steroidogenic pathway is present in 3T3-L1 adipocytes. A–F, qPCR and immunoblot analysis of TSPO (A), ACBD1 (B), STAR (C), CYP11A1 (D), FDX (E), and FNR (F). C, control cells maintained in growth medium; D, cells exposed to the differentiation medium. G–O, qPCR analysis of Cyp17a1 (G), Hsd3b1 (H), Cyp21 (I), Cyp11b1 (J), Cyp11b2 (K), Hsd11b1 (L), Cyp19a1 (M), Hsd17b7 (N), and Nr5a1 (O). qPCR results are expressed as means ± S.E. (S.E.; n = 3) and presented as fold increase compared with the value on day 0 (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Immunoblot results shown are representative of three independent experiments.
CYP11A1 is the first enzyme in the steroidogenic pathway, acting by converting cholesterol into pregnenolone. The enzymatic activity of mitochondrial P450s is regulated by the rate of electron transfer from the P450 redox partners FDX and FNR. qPCR analysis revealed that Cyp11a1 was elevated ∼80-fold in D10 mature adipocytes compared with D0 preadipocytes (Fig. 2D). Fdx and Fnr mRNA levels were both induced significantly at D5 and D10 during differentiation (Fig. 2, E and F). Each of these three proteins was present at low levels in preadipocyte mitochondria. However, these mitochondrial proteins were up-regulated in differentiated adipocytes (Fig. 2, D–F).
To further map the presence of components of the steroidogenic pathway in adipocytes and identify the final steroid products, we evaluated the expression levels of transcripts encoding steroidogenic enzymes during 3T3-L1 adipocyte differentiation. The enzymes investigated included the following: pregnenolone-metabolizing enzymes 17α-hydroxylase/17,20-lyase (CYP17A1) and 3β-HSD; 21-hydroxylase (CYP21), 11β-hydroxylase (CYP11B1), aldosterone synthase (CYP11B2), and 11β-hydroxysteroid dehydrogenase (11β-HSD), which are involved in glucocorticoid and mineralocorticoid biosynthesis; and 17β-hydroxysteroid dehydrogenase (17β-HSD) and aromatase (CYP19A1), which are involved in sex steroid synthesis. Regarding pregnenolone-metabolizing enzymes, Cyp17a1 mRNA expression (Fig. 2G) was induced at D10, whereas Hsd3b1 mRNA levels (Fig. 2H) in D5 and D10 cells were lower than those in D0 preadipocytes. However, no statistical difference was detected in Hsd3b1 gene expression throughout differentiation. The mRNA levels of Cyp21, a key enzyme in the synthesis of both glucocorticoids and mineralocorticoids, increased in D10 adipocytes (Fig. 2I). Gene expression of Cyp11b2 (Fig. 2K) also increased during differentiation. The mRNA levels of Cyp11b1, the enzyme converting 11-deoxycortisol to cortisol, were not significantly different between preadipocytes and adipocytes (Fig. 2J). Not surprisingly, Hsd11b1, which predominantly catalyzes the reduction of metabolically inactive cortisone to active cortisol, was induced over 200-fold during 3T3-L1 adipogenesis (Fig. 2L). In the sex steroid synthesis pathway, Cyp19a1 (Fig. 2M) gene expression was higher in preadipocytes. Although Hsd17b3, encoding the androgenic form of 17β-HSD, was not detected in 3T3-L1 cells (data not shown), the Hsd17b7 gene encoding the estrogenic 17β-HSD (Fig. 2N) was slightly induced in differentiated cells.
To further confirm the steroidogenic potential of adipocytes, we evaluated the expression of steroidogenic factor 1 (SF-1; NR5A1), a nuclear receptor positively regulating steroidogenic protein and enzyme expression (20). As shown in Fig. 2O, Nr5a1 mRNA levels were significantly increased during 3T3-L1 adipogenesis. Gene expression of dosage-sensitive sex reversal gene 1 (Dax1), another nuclear receptor that acts as a global negative regulator of steroid hormone production (21), was not detected during adipocyte differentiation (data not shown). Taken together, these data suggest that differentiated adipocytes express the enzymes and proteins needed to produce steroids.
CYP11A1 Enzymatic Activity in 3T3-L1 Adipocytes
The ability of CYP11A1 to convert cholesterol to pregnenolone defines a “steroidogenic” cell. Based on the elevated expression pattern of the CYP11A1 enzyme system during 3T3-L1 adipogenesis, we used D10 mature adipocytes to investigate the enzymatic activity of CYP11A1. Mitochondria isolated from D10 3T3-L1 adipocytes were incubated in an NADPH-containing reaction buffer together with the substrate 22RHC. This substrate is a soluble cholesterol analog that bypasses the mitochondrial cholesterol transport system and reaches CYP11A1 in the inner mitochondrial membrane to be metabolized to pregnenolone. Fig. 3A shows that adding 22RHC to the cells resulted in the time-dependent production of pregnenolone, albeit at low levels. Statistical differences were only seen after 3 h of 22RHC incubation in adipocyte mitochondrial suspensions. To test the pregnenolone synthesis rate at the cell level, D10 3T3-L1 adipocytes were incubated with 20 μm 22RHC and [3H]22RHC as a tracer before extraction followed by HPLC-radiometric analysis (Fig. 3B). Fig. 3C shows that after 48 h only 6% of [3H]22RHC was converted to pregnenolone (retention time ≈5.5 min), indicating low CYP11A1 enzyme activity in 3T3-L1 adipocytes. However, HPLC-radiometric analysis of radioactive products after 48 h of incubation with [3H]22RHC showed the presence of peaks at a retention time distinct to that of pregnenolone (Fig. 3B), suggesting that the low pregnenolone production in adipocytes could be the result of pregnenolone metabolism to the downstream steroid hormones.
FIGURE 3.

CYP11A1 is active in adipocytes. A, RIA of pregnenolone levels formed from 22RHC in isolated mitochondria of mature 3T3-L1 adipocytes. Results shown are means ± S.E. (n = 3); *, p < 0.05. B, HPLC-radiometric assay of [3H]pregnenolone formation from [3H]22RHC in mature 3T3-L1 adipocyte cells after 48 h. The results shown are representative of three separate experiments. C, conversion rate of [3H]22RHC into [3H]pregnenolone in 3T3-L1 adipocytes, measured by HPLC-radiometric assay. Results shown are means ± S.E. (n = 3); *, p < 0.05.
CYP27A1 Enzymatic Activity and de Novo Synthesis of 27HC in 3T3-L1 Adipocytes
Because CYP11A1 is present and active in mature 3T3-L1 adipocytes, we incubated 3T3-L1 adipocytes with cholesterol to test whether it could be converted to form de novo steroids in adipocytes. After a 48-h incubation with [3H]cholesterol, not much radiolabeled pregnenolone was detected (Fig. 4A). However, significant amounts of some unknown radiolabeled cholesterol products were eluted at different retention times. Thus, we extended our hypothesis to other pathways that may be involved in cholesterol metabolism. The pathway for biosynthesis of oxysterols, the oxidized derivatives of cholesterol, is independent of steroidogenesis. HPLC and TLC separation of the samples incubated with [3H]cholesterol for 48 h indicated the separation of a radiolabeled product eluted at a retention time identical to a 27HC standard examined under the same HPLC separation conditions or on the same TLC plate (Fig. 4A). The inner mitochondrial membrane CYP27A1 generates 27HC from cholesterol. The presence of CYP27A1 enzymatic activity in D10 3T3-L1 adipocytes was confirmed by incubating isolated adipocyte mitochondria in an NADPH-containing reaction buffer supplemented with cholesterol. Under these conditions, formation of radiolabeled 27HC from [3H]cholesterol was observed after 3 h (Fig. 4B). CYP27A1 protein was also detected in both 3T3-L1 cells and mitochondrial lysates (Fig. 4C). Both Cyp27a1 mRNA and protein levels of CYP27A1 were induced during 3T3-L1 adipocyte differentiation. When treated with [3H]mevalonolactone (MVA, a cell-permeable form of mevalonate, the direct precursor of cholesterol), de novo formation of radiolabeled cholesterol was observed at a significant rate after 24 h (Fig. 4D). Thus, cells treated with MVA for 24 h or longer were used in subsequent experiments to generate a readily detectable amount of cholesterol metabolites. Incubation of mature 3T3-L1 adipocytes for 48 h with [3H]MVA generated the metabolite 27HC, detected both by HPLC-radiometric assay and TLC (Fig. 4E). These data suggest that oxysterol synthesis might be dominant over the steroid biosynthesis pathway in cholesterol metabolism in adipocytes, with 27HC being the major mitochondrial cholesterol product. The other peaks seen in the HPLC-radiometric assays of cells incubated with [3H]cholesterol or [3H]MVA (Fig. 4, A and E) suggest the existence of other oxysterols or cholesterol metabolites in adipocytes to be identified.
FIGURE 4.
3T3-L1 adipocytes can synthesize 27HC de novo. A, HPLC-radiometric assay and TLC analysis of [3H]27HC formation from [3H]cholesterol in 3T3-L1 adipocytes after 48 h. B, HPLC-radiometric assay of [3H]27HC formation from [3H]cholesterol in mitochondria isolated from 3T3-L1 adipocytes after 3 h. C, RT-PCR and immunoblot analysis of CYP27A1 during 3T3-L1 differentiation. Results shown are means ± S.E. (n = 3); **, p < 0.01. D, de novo cholesterol synthesis rate from MVA in 3T3-L1 adipocytes, measured by HPLC-radiometric assay. Results shown are means ± S.E. (n = 3); ***, p < 0.001. E, HPLC-radiometric assay and TLC analysis of [3H]27HC formation from [3H]MVA in 3T3-L1 adipocytes after 48 h. All results from HPLC-radiometric and TLC assays are representative of at least three independent experiments. F, SIM chromatogram of the extracts from the serum-free medium (AIM-V medium) used to incubate 3T3-L1 adipocytes during the de novo 27HC synthesis assay; TIC, total ion current. G, SIM chromatogram of the reference standard 27HC (50 ng/ml) dissolved and extracted from the AIM-V medium. RT, retention time. H, SIM chromatogram of the extracts from 3T3-L1 adipocytes incubated with unlabeled MVA in AIM-V medium for 72 h. I, SIM chromatogram of the extracts from the zero time control of 3T3-L1 adipocytes incubated in AIM-V medium.
The 27HC formed de novo by 3T3-L1 adipocytes was further identified by GC-MS analysis. The serum-free medium (AIM-V medium) used to incubate 3T3-L1 adipocyte cells in 27HC synthesis assays was used as a control and shown to contain no 27HC (Fig. 4F). A 27HC standard (50 ng/ml) was used as a positive control (Fig. 4G). Extracts of cells incubated with unlabeled MVA for 72 h contained material with a retention time identical to the reference 27HC and had the expected selected ions at m/z of 75 and 129 (Fig. 4H). This material was not detected in zero time reaction mixtures (Fig. 4I). These measurements corroborate HPLC-radiometric assay and TLC data on the formation of 27HC from MVA in mature adipocytes.
De Novo Synthesis of 27HC in Rat or Human Primary Adipocytes
To determine whether the biosynthesis of 27HC in 3T3-L1 adipocytes is a cell-specific event, we examined mature adipocytes that were differentiated in culture from isolated SVF from Sprague-Dawley rat retroperitoneal adipose (RETRO) or epididymal adipose (EPI) tissues. The differentiation stages of the SVF cells were defined by Oil Red O staining of lipid droplets (Fig. 5A). TSPO and CYP27A1 proteins were detected in SVF cells (D0) and differentiated adipocytes (D10) from both sites (Fig. 5B). During SVF cell differentiation, TSPO expression remained constant. CYP27A1 was seen mainly as a dimer in differentiated rat adipocytes, as reported previously in retina (22). Radiolabeled 27HC was formed from [3H]MVA in adipocytes differentiated from SVF cells derived from both RETRO and EPI depots (Fig. 5, C and D).
FIGURE 5.
Adipocytes differentiated from rat SVF, human SGBS preadipocytes, and human primary preadipocytes can synthesize 27HC de novo. A, Oil Red O staining of lipid droplets in rat RETRO and EPI SVF during differentiation (magnification, ×20). B, immunoblot analysis of TSPO and CYP27A1 during differentiation of rat primary SVF. C, HPLC-radiometric assay of [3H]27HC formation from [3H]MVA in adipocytes differentiated from rat RETRO SVF after 48 h. D, HPLC-radiometric assay of [3H]27HC formation from [3H]MVA in adipocytes differentiated from rat EPI SVF after 48 h. E, Oil Red O staining of lipid droplets in human SGBS cells during differentiation. (Magnification, ×20.) F, immunoblot analysis of CYP27A1, CYP11A1, FDX, and FNR during differentiation of human SGBS preadipocytes. G, TLC analysis of [3H]27HC formation from [3H]MVA in human SGBS adipocytes after 5 h. H, HPLC-radiometric assay of [3H]27HC formation from [3H]MVA in human SGBS adipocytes after 4 days. The data presented are representative of three independent experiments. I, immunoblot analysis of CYP27A1 in primary preadipocytes (Preadip), differentiated adipocytes (Adip), and mature adipocytes (Mature Adip) from human subcutaneous adipose tissue of healthy subjects. J, immunoblot analysis of CYP27A1 in primary preadipocytes and differentiated adipocytes from human omental adipose tissue of healthy subjects or differentiated omental adipocytes from diabetic donors. K and L, HPLC-radiometric analysis of [3H]27HC formation from [3H]MVA in human differentiated subcutaneous adipocytes (K) and omental adipocytes (L) after 48 h.
The mitochondrial P450 system (CYP27A1, CYP11A1, FDX, and FDR) was also present and elevated during the differentiation of SGBS human preadipocytes (Fig. 5F). The differentiation stages of this cell line were defined by Oil Red O staining of lipid droplets (Fig. 5E). After incubating D10 adipocytes with [3H]MVA for 5 h (Fig. 5G) or 4 days (Fig. 5H), 27HC was detected.
In normal human subjects with body mass index between 24 and 27, expression levels of CYP27A1 protein were enriched in both subcutaneous and omental newly differentiated adipocytes, compared with their corresponding primary preadipocytes (Fig. 5, I and J). In contrast to the newly differentiated adipocytes, CYP27A1 expression was further induced in primary mature adipocytes isolated directly from human subcutaneous adipose tissue (Fig. 5I), suggesting that our discovery of CYP27A1 metabolic pathway in newly differentiated adipocytes could be extrapolated to mature adipocytes already present in the adipose tissue. Moreover, differentiated omental adipocytes from a diabetic donor (body mass index = 42.4) exhibited a higher CYP27A1 expression than those from normal subjects (Fig. 5J), indicating a potential elevation of 27HC production in adipose tissues of obese patients. In terms of the enzyme activity, a significant amount of 27HC was synthesized after incubating the newly differentiated human omental adipocytes with [3H]MVA for 48 h (Fig. 5L). However, despite the similar expression levels of CYP27A1 between the two depots, the activity of CYP27A1 in synthesizing 27HC was tremendously reduced in subcutaneous adipocytes (Fig. 5K), implying that omental fat, often associated with central obesity, might have a more pronounced capacity in producing 27HC.
Accumulation of 27HC and Absence of Bile Acid Synthesis in Adipocytes
The cellular level of 27HC depends on the expression of both generating and metabolizing enzymes. CYP27A1 is the sole enzyme metabolizing cholesterol to 27HC, whereas oxysterol and steroid 7α-hydroxylase (CYP7B1) is the most important enzyme to metabolize 27HC in extrahepatic cells (23). During 3T3-L1 differentiation, the increased CYP27A1 (Fig. 4C) and reduced CYP7B1 expression (Fig. 6A) suggest high production and low elimination rates for 27HC in mature adipocytes. This would increase local concentrations of 27HC in adipocytes.
FIGURE 6.
Adipocytes do not synthesize bile acids. A and B, qPCR analysis of Cyp7b1 (A) and Cyp7a1 (B) during 3T3-L1 differentiation. Results shown are means ± S.E. (n = 3); *, p < 0.05; **, p < 0.01. C and D, bile acid synthesis rates quantified as conversion of [3H]cholesterol to methanol/water-extractable products (C), and cholesterol uptake quantified as a function of change in [3H]cholesterol in the medium (D). Results shown are the mean values of a single experiment performed in duplicate. Experiments were repeated three times, yielding the same conclusion. HepG2 cells were used as the positive control.
Oxysterols, such as 27HC, are obligatory intermediates for bile acid synthesis (24). There are two bile acid formation pathways in mammalian cells. The classical pathway starts with the 7α-hydroxylation of cholesterol by cholesterol 7α-hydroxylase (CYP7A1), and the alternative pathway is initiated with the 27-hydroxylation of cholesterol by CYP27A1 (Fig. 1A) (25). During 3T3-L1 adipogenesis, the mRNA levels of Cyp7a1 reached the highest point in D5 differentiating cells and fell when the cells were fully differentiated (Fig. 6B), whereas Cyp27a1 mRNA levels gradually increased during differentiation (Fig. 4C). Therefore, if mature adipocytes are able to synthesize bile acid, the alternative pathway involving CYP27A1 might be favored over the classical pathway involving CYP7A1.
Upon production in extrahepatic cells, 27HC is transported to the liver, where it serves as an intermediate for bile acid synthesis (26). Thus, the presence of the bile acid biosynthesis pathway in adipocytes could also modulate local levels of 27HC. To test this hypothesis, the conversion of [3H]cholesterol to 3H-labeled methanol/water-extractable products over time was examined in D0, D5, and D10 3T3-L1 cells. HepG2 liver cells, known for their high capacity to form bile acids, were used as a positive control. Bile acid synthesis rates steadily increased in HepG2 cells up to 48 h, and no changes were observed in D0 preadipocytes or differentiating D5 or mature D10 adipocytes (Fig. 6C). To determine whether this difference between HepG2 and 3T3-L1 cells in bile acid synthesis is due to differences in cholesterol uptake, the rate of cholesterol uptake was determined by measuring [3H]cholesterol “disappearance” (chloroform phase) from the cell culture medium. Data shown in Fig. 6D indicate that the cellular cholesterol uptake rates were similar in all cell types used. These data exclude the possibility that adipocytes synthesize bile acids from cholesterol. This conclusion was further supported by the absence of Tgr5 and Fxr mRNA, two major targets of bile acid-modulated effects (25), in 3T3-L1 cells throughout differentiation (data not shown). Therefore, 27HC could be accumulated in adipocytes as an end product rather than an intermediate for bile acid synthesis.
Potential Role of CYP27A1 in Adipogenesis
To investigate the role of CYP27A1 in adipogenesis, we assessed the effects of GI268267X (a specific inhibitor of CYP27A1) (27) on 3T3-L1 differentiation. GI268267X-treated 3T3-L1 cells subjected to differentiation had higher mRNA and protein levels of the differentiation marker fatty acid-binding protein (FABP4) (Fig. 7, A and D) compared with untreated differentiated 3T3-L1 adipocytes. mRNA levels of other differentiation markers such as CCAAT/enhancer-binding protein-α (Cebpa) and peroxisome proliferator-activated receptor-γ (Pparg) were also induced in GI268267X-treated cells (Fig. 7, B and C). Oil Red O staining of the cells treated with GI268267X revealed a dose-dependent induction of lipid droplets (Fig. 7E).
FIGURE 7.
CYP27A1 is a negative regulator of adipocyte differentiation. A–C, qPCR analysis of the differentiation markers Fabp4 (A), Cebpa (B), and Pparg (C) in GI268267X-treated 3T3-L1 cells subjected to differentiation. Results shown are means ± S.E. (n = 3); ***, p < 0.001. D, immunoblot analysis of the differentiation marker FABP4 in GI268267X-treated 3T3-L1 cells subjected to differentiation. Immunoblot results shown are representative of three independent experiments. Protein quantification results are shown as means ± S.E. (n = 3); *, p < 0.05; ***, p < 0.001. E, Oil Red O staining of lipid droplets in GI268267X-treated 3T3-L1 cells subjected to differentiation. Images are representative of three independent experiments. (Magnification, ×10.) DMSO at the concentration of 0.1% v/v was used as the vehicle treatment in the above experiments. F, immunoblot analysis of CYP27A1 in 3T3-L1 preadipocytes after 48 h of siRNA transfection. G–I, qPCR analysis of the differentiation markers Fabp4 (G), Cebpa (H), and Pparg (I) in mock-transfected or siRNA-transfected 3T3-L1 cells subjected to differentiation. J and K, RT-PCR analysis of the differentiation markers Fabp4 (J) and Cebpa (K) in 27HC-treated 3T3-L1 cells subjected to differentiation. Results shown are means ± S.E. (n = 3); ***, p < 0.001. L, immunoblot analysis of the differentiation marker FABP4 in 27HC-treated 3T3-L1 cells subjected to differentiation. M, Oil Red O staining of lipid droplets in 27HC-treated 3T3-L1 cells subjected to differentiation. Images are representative of three independent experiments. (Magnification, ×20.) Methanol at the concentration of 0.05% v/v was used as the vehicle control in 27HC-treated experiments.
To ascertain that the effects of GI268267X on adipogenesis were due to CYP27A1 inhibition, 3T3-L1 preadipocytes at 80% confluence were transfected with Cyp27a1 siRNA, before the initiation of differentiation. Two days after transfection, CYP27A1 protein levels in preadipocytes (D0) were significantly knocked down by 50% (Fig. 7F). CYP27A1-deficient cells seemed to have a higher rate of differentiation compared with mock-transfected controls as assessed by the increased mRNA expression of adipogenesis markers Fabp4, Cebpa, and Pparg (Fig. 7, G–I) in differentiated adipocytes. Thus, CYP27A1 likely acts as a negative regulator of adipogenesis.
Considering the finding that 27HC is the major product synthesized by CYP27A1 in adipocytes, we hypothesized that 27HC might inhibit adipogenesis. Supplementation of the differentiation mixture with 27HC moderately decreased the differentiation of 3T3-L1 cells, as measured by mRNA and/or protein expression of the differentiation markers FABP4 (Fig. 7, J and L) and C/EBPα (Fig. 7K), as well as indicated by the amount of Oil Red O staining in lipid droplets (Fig. 7M) on day 10 after addition of the differentiation media. Taken together, these data suggest that the local 27HC biosynthesis pathway in adipocytes might act as a protective mechanism by limiting the potential of adipocyte differentiation.
Potential Local Effects of CYP27A1 Metabolic Pathway in Adipose Tissues
To further analyze the impact of the 27HC biosynthetic pathway in adipose tissue function, we isolated and characterized the properties of adipose tissues from 10-week-old Cyp27a1 knock-out mice. There was no significant difference in body weights (Fig. 8A), total white adipose tissue composition (Fig. 8B), or the weights of various fat depots (Fig. 8C) between male Cyp27a1−/− mice and the wild-type controls. However, as determined by the induced gene expression levels of the differentiation marker Fabp4 in differentiated adipocytes, there was a marked elevation in the adipocyte differentiation potential of SVF cells isolated from Cyp27a1−/− mouse adipose depots, including retroperitoneal (Fig. 8D) and epididymal adipose tissues (Fig. 8E) compared with those obtained from wild-type mice. These data are consistent with our previous findings in CYP27A1-deficient 3T3-L1 cell line studies. Cyp27a1−/− mice at a relatively young age (10 weeks) might not have developed obesity, but the absence of the CYP27A1 pathway may be associated with the accelerated ability of adipose tissue to recruit newly committed preadipocytes and to promote their subsequent differentiation, which might make the mice prone to obesity when given a high fat diet.
FIGURE 8.
SVFs isolated from Cyp27a1−/− mice have higher adipogenic potential than wild-type controls. A, body weight of 10-week-old male Cyp27a1−/− versus wild-type mice. B, percentage of total white fat mass normalized to the body weight. C, weights of individual fat depots. MES, mesenteric adipose tissue; ING, inguinal adipose tissue; BAT, brown adipose tissue. Two equal depots from one animal were combined for EPI, RETRO, inguinal adipose tissue, and brown adipose tissue. All results shown above are means ± S.E. (n = 3). D and E, qPCR analysis of the differentiation marker Fabp4 in 10-day post-differentiated RETRO adipocytes (D) and EPI adipocytes (E) compared with undifferentiated SVFs.
DISCUSSION
Investigation of cholesterol transport and metabolism machinery responsible for steroid and oxysterol synthesis in adipocytes is an essential step in understanding how locally produced steroids or oxysterols mediate adipocyte function and other metabolic parameters.
A steroidogenic cell is defined by its ability to convert endogenous cholesterol to pregnenolone (3). “Classical” steroidogenic tissues include gonads and adrenal glands, in which the biosynthesis of steroids is regulated by the action of trophic hormones, and placenta, a hormone-independent steroidogenic tissue. Several other tissues, such as brain (28, 29), heart (30), and skin (31), also express CYP11A1 and therefore can be considered steroidogenic. Although the absolute amount of steroids synthesized in these “nonclassical” tissues is small, local steroid concentrations achieved within tissues could be high enough to exert significant biological influence in an intracrine, autocrine, or paracrine manner. For instance, locally produced progesterone and estradiol in the brain are neuroprotective (32, 33); disturbance of local glucocorticoid biosynthesis in the skin affects the functions of epidermis and adnexal structures, leading to inflammatory disorders (34). Adipose tissue is one of the largest steroid reservoirs and sites of steroid metabolism. Modulation of active steroid levels in adipose tissue through steroid-converting enzymes regulates adipocyte functions at the local level (2). It has been widely recognized that the increased local regeneration of cortisol from circulating inert cortisone, through the increased activity of 11β-HSD1 in adipose tissues of obese individuals, amplifies fat cell differentiation and accounts for the metabolic complications of obesity (35). Most recently, adipocyte-derived aldosterone is found to induce adipocyte differentiation in an autocrine manner and contributes to vascular dysfunctions in obesity (36). Despite the association between abnormal adipocyte functions and local steroid levels, adipocytes have not been regarded as steroidogenic cells. MacKenzie et al. (37) were the first to detect the transcription of STAR, CYP11A1, HSD3B2, CYP21B, and HSD17B7 in both human omental and subcutaneous adipose tissues by qRT-PCR. However, no protein expression, localization, or activity studies about STAR and CYP11A1 in adipocytes have ever been reported. In this study, we demonstrated for the first time that the mitochondrial cholesterol transport machinery and CYP11A1 enzyme system is present and active in adipocyte cells. This finding substantially broadens our knowledge of the steroidogenic capacities of adipocytes.
SF-1 (NR5A1) and DAX-1 are positive and negative transcriptional regulators of genes involved in steroidogenesis, including Star, Cyp11a1, and Cyp17a1 (20, 21), and are therefore important indicators of steroidogenic capacity. Our study confirmed the gene expression and induction of Sf1 (Nr5a1) during 3T3-L1 adipocyte differentiation. This may be responsible for the increased levels of Star, Cyp11a1, and Cyp17a1 mRNAs during adipogenesis. Although we did not detect gene expression of Dax1, it has been reported that Dax1 levels are significantly lower in differentiated 3T3-L1 cells compared with preadipocytes (38). These authors suggested that DAX-1 acted as a co-repressor of peroxisome proliferator-activated receptor-γ (Pparg) gene and was involved in regulating peroxisome proliferator-activated receptor γ-mediated adipogenesis. Although additional experiments are needed to determine whether SF-1 or DAX-1 can also regulate the transcription of genes involved in adipocyte steroidogenesis, the elevated levels of Sf1 and the reduced levels of Dax1 suggest that mature 3T3-L1 adipocytes possess enhanced steroidogenic capacity.
The rate-determining step of all steroid hormone biosynthesis is transport of cholesterol from the outer to the inner mitochondrial membrane, where CYP11A1 is located. This process is regulated mainly by TSPO and STAR (5). Although mitochondrial TSPO expression in adipocytes was confirmed in this study, STAR protein was not seen in the various stages of 3T3-L1 cells during differentiation by immunoblotting, probably due to an inability of the method to detect STAR protein when the overall level of STAR is relatively low. In human omental adipose tissues, the mRNA level of STAR is only 0.6% of that in the adrenal gland (37). There might also be a possibility that adipocyte steroidogenesis is STAR-independent. In tissues that lack STAR but express the CYP11A1 enzyme system, such as placenta and brain, conversion of cholesterol to pregnenolone occurs at ∼14% of the STAR-induced rate (3). The cholesterol content in adipocyte mitochondria may be at near-saturating concentrations, like that in placenta (39). The electron supply by the P450 redox partners, rather than cholesterol transport, may limit the rate of cholesterol metabolism in mitochondria. Oxysterols accumulated inside adipocytes could also serve as the direct substrate for the CYP11A1 enzyme system, bypassing the action of STAR.
We next focused on the presence and activity of the first enzyme in the pathway, CYP11A1, which metabolizes cholesterol to pregnenolone, the precursor of all of the other steroids, an event that defines a cell as steroidogenic. Our results clearly indicate the presence of CYP11A1 in adipocyte mitochondria and its ability to synthesize pregnenolone. However, the limited rate of formation of pregnenolone indicated that this steroid might be quickly used up for the synthesis of downstream steroids, because both pregnenolone-metabolizing enzymes 3β-HSD and CYP17A1 are present. The fact that blocking the pregnenolone metabolism by trilostane or SU10603, enzyme inhibitors for 3β-HSD and CYP17A1, respectively, failed to increase the rate of pregnenolone formation (data not shown) suggests that CYP11A1 does not play a major metabolic role in adipocyte cholesterol consumption. Besides cholesterol, vitamins D2 and D3 and their precursors can also serve as substrates for CYP11A1 either in a reconstituted system or isolated adrenal mitochondria (40–43). These CYP11A1-derived hydroxyvitamin D products have essential biological activities on skin cells, such as inhibition of proliferation and induction of differentiation in keratinocytes (44). Because vitamin D deficiency is often linked to abnormal metabolic status of adipose tissue (45), the possible involvement of adipocyte CYP11A1 in metabolizing vitamin D and its subsequent impact on adipocyte development and functions are worth studying in the future.
All mitochondrial P450s receive electrons from NADPH via the same redox chain consisting of FDX and FNR; a similar ferredoxin-binding site is conserved among all mitochondrial P450s (46). Identification of another mitochondrial P450 enzyme in adipocytes, CYP27A1, raised the possibility that CYP11A1 and CYP27A1 might compete for the common redox partner FDX to perform the hydroxylation reaction. CYP27A1 binds significantly tighter (∼30-fold) to FDX than CYP11A1, due to an additional electrostatic interaction between CYP27A1 and ferredoxin (47). Differences in binding affinity for FDX between CYP27A1 and CYP11A1 may explain why the major mitochondrial enzyme metabolism of cholesterol in adipocytes involves 27-hydroxylation rather than conversion to pregnenolone.
CYP27A1 is a ubiquitous enzyme involved in bile acid synthesis by catalyzing multiple oxidation reactions at the C27 atom of steroids in the classical (hepatic) pathway (48) and cholesterol in the alternative (peripheral) pathway (26). The classical pathway begins with CYP7A1 and is considered the quantitatively most important pathway in bile acid synthesis. However, there is evidence that human CYP7A1 deficiency causes a doubling of CYP27A1 activity and up-regulation of the alternative pathway (49). During 3T3-L1 differentiation, we observed that Cyp7a1 mRNA levels were lower in mature adipocytes, when Cyp27a1 levels peaked. Thus, there might be a compensatory increase in 27-hydroxylation of cholesterol by CYP27A1 in mature adipocytes that accounts for the predominance of the alternative pathway. To yield bile acids via the alternative pathway, 27HC must be hydroxylated by CYP7B1, and the resulting 7α-hydroxylated products are further metabolized to bile acids (Fig. 1A) (23). Human CYP7B1 deficiency impairs the hydroxylation of 27HC, thus leading to elevated levels of circulating 27HC (50). In our study, the decrease of Cyp7b1 gene expression during 3T3-L1 adipogenesis may explain the accumulation of 27HC and absence of bile acids in mature adipocytes.
The formation of 27HC by differentiated 3T3-L1 adipocytes was assessed in metabolic labeling studies using either cholesterol or its precursor, MVA. The steroid formed was identified by HPLC and TLC, and its identity was further confirmed by GC-MS. These results are not limited to 3T3-L1 cells. Adipocytes differentiated in culture from stromal vascular cells of rat retroperitoneal and epididymal adipose tissues, human SGBS cells, and human primary preadipocytes were all able to produce 27HC. Noteworthy, a depot-specific difference in 27HC generation was observed in our study, with human omental adipocytes producing significantly higher amounts of 27HC than subcutaneous adipocytes. This result is consistent with previous findings that gene expression of CYP27A1 is more pronounced in omental than subcutaneous fat in both lean and obese women (51). Compared with subcutaneous adipose tissue, omental fat, often referred as “central fat,” is more metabolically active and closely involved in the co-morbidities associated with obesity (52). Thus, disruption of 27HC production in omental fat may offer a causative link between central obesity and metabolic parameters.
The local concentration of 27HC achieved by de novo adipocyte synthesis may be notable and most likely play a local regulatory role in adipocyte development. In adult human white adipose tissues, new adipocytes constantly arise from a pre-existing population of undifferentiated pre-fat cells and replace the lost adipocytes, in a turnover rate of ∼10% annually (53). Thus, adipogenesis is probably responsible for the increase of fat cell number upon overfeeding and may have a key role in the pathology of obesity. Several studies have noted the possible role of oxysterols in anti-adipogenic differentiation. For example, the oxysterols 20(S)- or 22(S)-hydroxycholesterol regulate lineage-specific differentiation of mesenchymal stem cells in favor of osteoblastic differentiation and against adipogenic differentiation (54). Our data also demonstrate CYP27A1 as a negative regulator of adipogenesis. The presence of CYP27A1 able to synthesize 27HC in adipocytes might represent an adaptive mechanism for removal of excess cholesterol from adipose tissue, thus controlling the number of fat cells in the adipose compartment upon aging or overnutrition. It is evident that absence of the regulator oxysterol 27HC in patients with CYP27A1 deficiency (cerebrotendinous xanthomatosis) is associated with accumulation of lipids and accelerated tendency to develop atherosclerosis (55). Administration of 27HC to hyperlipidemic mice, followed by high fat diet, could reduce hepatic inflammation in nonalcoholic fatty liver disease (56). Both nonalcoholic fatty liver disease and atherosclerosis are major disorders of lipid metabolism, often related to obesity or abnormal distribution of body fat (57, 58). Thus, the existence of de novo 27HC synthesis in adipocytes might provide a new understanding of the connection between adipocyte function and lipid-related disorders.
The mechanism of 27HC action in adipocytes has not yet been established. Many effects of oxysterols in adipocytes are mediated by the nuclear liver X receptor (LXRα and -β) (59). Multiple in vitro and in vivo studies in adipose cells have demonstrated that endogenously expressed LXRs can be activated by oxysterols (60). However, the role of LXR in regulating adipogenesis is still open to debate. Different studies have reported induction (61), no effect (62), or even reduction of adipogenesis (63) by LXR ligands. The discrepancies between these studies could be due to the different degrees of differentiation of the cells used by the different groups. Nevertheless, LXR is not indispensable and might only act as a modulator for adipogenesis. This suggests that, in addition to LXR signaling, other adipogenic mediators may contribute to the inhibition of adipogenesis by 27HC observed in our study. Indeed, the previously reported anti-adipogenic activity of the oxysterol 20(S)-hydroxycholesterol is mediated predominantly through hedgehog signaling, despite the activation of LXRs at the same time, and is associated with inhibition of peroxisome proliferator-activated receptor γ levels (64). Another mechanism for 27HC action in adipocytes may involve interaction with ERs. As the first identified endogenous selective estrogen receptor modulator, 27HC has multiple functions in ER-positive tissues, through either activation or antagonism of ER (65). Human adipocytes express both functional ERα and ERβ (66), indicating that 27HC action may occur through either of these ERs in adipocytes.
In conclusion, this study provides the first evidence that adipocytes have the potential to synthesize steroids and/or oxysterols de novo from cholesterol and its precursors. The presence and activity of adipocyte mitochondrial cholesterol transport and metabolism machinery involved in the initial steps of steroid or oxysterol synthesis have been confirmed in our study. We propose that CYP11A1 activity in adipocytes is diminished due to competition from CYP27A1 for the same substrate and redox partners, which leads to the release of 27HC as the major mitochondrial cholesterol metabolite rather than pregnenolone. However, given the large inter-individual variations in adipocyte metabolic pathways, the final product of de novo synthesis could vary. The finding that local inhibition of CYP27A1 induced adipogenesis suggests that the presence of de novo adipocyte oxysterol synthesis might represent a defense mechanism for adipocytes to protect against intracellular cholesterol overloading and formation of new fat cells.
Acknowledgments
We thank M. Vindigni for expert technical assistance with the mass spectrometry studies and Drs. J. Fan, A. Midzak, and A. Batarseh for helpful discussions. We are grateful to GlaxoSmithKline for providing us the CYP27A1 inhibitor GI268267X. We also thank Drs. M. Ascoli (University of Iowa) for the MA-10 cells and D. B. Hales (Southern Illinois University) for the anti-STAR antiserum. The Research Institute of McGill University Health Centre is supported in part by a center grant from Fonds de Recherche du Quebec-Santé.
This work was supported in part by a grant from the Canadian Institutes of Health Research and a Canada Research Chair in Biochemical Pharmacology (to V. P.).
- FDX
- ferredoxin
- EPI
- epididymal adipose tissue
- ER
- estrogen receptor
- IBMX
- 3-isobutyl-1-methylxanthine
- FNR
- ferredoxin reductase
- HSD
- hydroxysteroid dehydrogenase
- 22RHC
- 22(R)-hydroxycholesterol
- 27HC
- 27-hydroxycholesterol
- qPCR
- quantitative real time PCR
- LXR
- liver X receptors
- MVA
- mevalonolactone
- RETRO
- retroperitoneal adipose tissue
- SF-1 or NR5A1
- steroidogenic factor 1
- SGBS
- Simpson-Golabi-Behmel syndrome
- SIM
- selected ion monitoring
- STAR
- steroidogenic acute regulatory protein
- SVF
- stromal vascular fraction
- TSPO
- translocator protein (18 kDa).
REFERENCES
- 1. Kershaw E. E., Flier J. S. (2004) Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 [DOI] [PubMed] [Google Scholar]
- 2. Bélanger C., Luu-The V., Dupont P., Tchernof A. (2002) Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm. Metab. Res. 34, 737–745 [DOI] [PubMed] [Google Scholar]
- 3. Miller W. L., Auchus R. J. (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lacapère J. J., Papadopoulos V. (2003) Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 68, 569–585 [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulos V., Liu J., Culty M. (2007) Is there a mitochondrial signaling complex facilitating cholesterol import? Mol. Cell. Endocrinol. 265, 59–64 [DOI] [PubMed] [Google Scholar]
- 6. Pandak W. M., Ren S., Marques D., Hall E., Redford K., Mallonee D., Bohdan P., Heuman D., Gil G., Hylemon P. (2002) Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J. Biol. Chem. 277, 48158–48164 [DOI] [PubMed] [Google Scholar]
- 7. Björkhem I., Meaney S., Diczfalusy U. (2002) Oxysterols in human circulation: which role do they have? Curr. Opin. Lipidol. 13, 247–253 [DOI] [PubMed] [Google Scholar]
- 8. Meaney S., Hassan M., Sakinis A., Lütjohann D., von Bergmann K., Wennmalm A., Diczfalusy U., Björkhem I. (2001) Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J. Lipid Res. 42, 70–78 [PubMed] [Google Scholar]
- 9. Wade F. M., Wakade C., Mahesh V. B., Brann D. W. (2005) Differential expression of the peripheral benzodiazepine receptor and gremlin during adipogenesis. Obes. Res. 13, 818–822 [DOI] [PubMed] [Google Scholar]
- 10. Mandrup S., Sorensen R. V., Helledie T., Nohr J., Baldursson T., Gram C., Knudsen J., Kristiansen K. (1998) Inhibition of 3T3-L1 adipocyte differentiation by expression of acyl-CoA-binding protein antisense RNA. J. Biol. Chem. 273, 23897–23903 [DOI] [PubMed] [Google Scholar]
- 11. Baumann C. A., Ribon V., Kanzaki M., Thurmond D. C., Mora S., Shigematsu S., Bickel P. E., Pessin J. E., Saltiel A. R. (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 [DOI] [PubMed] [Google Scholar]
- 12. Wabitsch M., Brenner R. E., Melzner I., Braun M., Möller P., Heinze E., Debatin K. M., Hauner H. (2001) Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 25, 8–15 [DOI] [PubMed] [Google Scholar]
- 13. Liu J., Rone M. B., Papadopoulos V. (2006) Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J. Biol. Chem. 281, 38879–38893 [DOI] [PubMed] [Google Scholar]
- 14. Grégoire F., Todoroff G., Hauser N., Remacle C. (1990) The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol. Cell 69, 215–222 [DOI] [PubMed] [Google Scholar]
- 15. Krueger K. E., Papadopoulos V. (1990) Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J. Biol. Chem. 265, 15015–15022 [PubMed] [Google Scholar]
- 16. Li H., Yao Z., Degenhardt B., Teper G., Papadopoulos V. (2001) Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. U.S.A. 98, 1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Culty M., Luo L., Yao Z. X., Chen H., Papadopoulos V., Zirkin B. R. (2002) Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J. Androl. 23, 439–447 [PubMed] [Google Scholar]
- 18. Guarneri P., Papadopoulos V., Pan B., Costa E. (1992) Regulation of pregnenolone synthesis in C6–2B glioma cells by 4′-chlorodiazepam. Proc. Natl. Acad. Sci. U.S.A. 89, 5118–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pikuleva I. A., Babiker A., Waterman M. R., Björkhem I. (1998) Activities of recombinant human cytochrome P450c27 (CYP27), which produce intermediates of alternative bile acid biosynthetic pathways. J. Biol. Chem. 273, 18153–18160 [DOI] [PubMed] [Google Scholar]
- 20. Jameson J. L. (2004) Of mice and men: The tale of steroidogenic factor-1. J. Clin. Endocrinol. Metab. 89, 5927–5929 [DOI] [PubMed] [Google Scholar]
- 21. Lalli E., Sassone-Corsi P. (2003) DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol. Endocrinol. 17, 1445–1453 [DOI] [PubMed] [Google Scholar]
- 22. Lee J. W., Fuda H., Javitt N. B., Strott C. A., Rodriguez I. R. (2006) Expression and localization of sterol 27-hydroxylase (CYP27A1) in monkey retina. Exp. Eye Res. 83, 465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin K. O., Reiss A. B., Lathe R., Javitt N. B. (1997) 7α-Hydroxylation of 27-hydroxycholesterol: biologic role in the regulation of cholesterol synthesis. J. Lipid Res. 38, 1053–1058 [PubMed] [Google Scholar]
- 24. Crosignani A., Zuin M., Allocca M., Del Puppo M. (2011) Oxysterols in bile acid metabolism. Clin. Chim. Acta 412, 2037–2045 [DOI] [PubMed] [Google Scholar]
- 25. Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. (2008) Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 [DOI] [PubMed] [Google Scholar]
- 26. Javitt N. B. (2002) 25R,26-Hydroxycholesterol revisited: synthesis, metabolism, and biologic roles. J. Lipid Res. 43, 665–670 [PubMed] [Google Scholar]
- 27. Lyons M. A., Brown A. J. (2001) Metabolism of an oxysterol, 7-ketocholesterol, by sterol 27-hydroxylase in HepG2 cells. Lipids 36, 701–711 [DOI] [PubMed] [Google Scholar]
- 28. Mellon S. H., Griffin L. D. (2002) Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 13, 35–43 [DOI] [PubMed] [Google Scholar]
- 29. Baulieu E. E., Robel P., Schumacher M. (2001) Neurosteroids: beginning of the story. Int. Rev. Neurobiol. 46, 1–32 [DOI] [PubMed] [Google Scholar]
- 30. Kayes-Wandover K. M., White P. C. (2000) Steroidogenic enzyme gene expression in the human heart. J. Clin. Endocrinol. Metab. 85, 2519–2525 [DOI] [PubMed] [Google Scholar]
- 31. Thiboutot D., Jabara S., McAllister J. M., Sivarajah A., Gilliland K., Cong Z., Clawson G. (2003) Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J. Invest. Dermatol. 120, 905–914 [DOI] [PubMed] [Google Scholar]
- 32. Schumacher M., Guennoun R., Robert F., Carelli C., Gago N., Ghoumari A., Gonzalez Deniselle M. C., Gonzalez S. L., Ibanez C., Labombarda F., Coirini H., Baulieu E. E., De Nicola A. F. (2004) Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm. IGF Res. 14, S18–S33 [DOI] [PubMed] [Google Scholar]
- 33. Azcoitia I., Sierra A., Veiga S., Garcia-Segura L. M. (2005) Brain steroidogenesis: emerging therapeutic strategies to prevent neurodegeneration. J. Neural. Transm. 112, 171–176 [DOI] [PubMed] [Google Scholar]
- 34. Slominski A., Zbytek B., Nikolakis G., Manna P. R., Skobowiat C., Zmijewski M., Li W., Janjetovic Z., Postlethwaite A., Zouboulis C. C., Tuckey R. C. (2013) Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 137, 107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. (2001) A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 [DOI] [PubMed] [Google Scholar]
- 36. Briones A. M., Nguyen Dinh Cat A., Callera G. E., Yogi A., Burger D., He Y., Corrêa J. W., Gagnon A. M., Gomez-Sanchez C. E., Gomez-Sanchez E. P., Sorisky A., Ooi T. C., Ruzicka M., Burns K. D., Touyz R. M. (2012) Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 59, 1069–1078 [DOI] [PubMed] [Google Scholar]
- 37. MacKenzie S. M., Huda S. S., Sattar N., Fraser R., Connell J. M., Davies E. (2008) Depot-specific steroidogenic gene transcription in human adipose tissue. Clin. Endocrinol. 69, 848–854 [DOI] [PubMed] [Google Scholar]
- 38. Kim G. S., Lee G. Y., Nedumaran B., Park Y. Y., Kim K. T., Park S. C., Lee Y. C., Kim J. B., Choi H. S. (2008) The orphan nuclear receptor DAX-1 acts as a novel transcriptional corepressor of PPARγ. Biochem. Biophys. Res. Commun. 370, 264–268 [DOI] [PubMed] [Google Scholar]
- 39. Tuckey R. C. (1992) Cholesterol side-chain cleavage by mitochondria from the human placenta. Studies using hydroxycholesterols as substrates. J. Steroid Biochem. Mol. Biol. 42, 883–890 [DOI] [PubMed] [Google Scholar]
- 40. Nguyen M. N., Slominski A., Li W., Ng Y. R., Tuckey R. C. (2009) Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1). Drug Metab. Dispos. 37, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guryev O., Carvalho R. A., Usanov S., Gilep A., Estabrook R. W. (2003) A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc. Natl. Acad. Sci. U.S.A. 100, 14754–14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slominski A., Semak I., Zjawiony J., Wortsman J., Gandy M. N., Li J., Zbytek B., Li W., Tuckey R. C. (2005) Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17α,24-dihydroxyergosterol. Chem. Biol. 12, 931–939 [DOI] [PubMed] [Google Scholar]
- 43. Slominski A., Zjawiony J., Wortsman J., Semak I., Stewart J., Pisarchik A., Sweatman T., Marcos J., Dunbar C., C Tuckey R. (2004) A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur. J. Biochem. 271, 4178–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tuckey R. C., Li W., Shehabi H. Z., Janjetovic Z., Nguyen M. N., Kim T. K., Chen J., Howell D. E., Benson H. A., Sweatman T., Baldisseri D. M., Slominski A. (2011) Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 39, 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vimaleswaran K. S., Berry D. J., Lu C., Tikkanen E., Pilz S., Hiraki L. T., Cooper J. D., Dastani Z., Li R., Houston D. K., Wood A. R., Michaëlsson K., Vandenput L., Zgaga L., Yerges-Armstrong L. M., McCarthy M. I., Dupuis J., Kaakinen M., Kleber M. E., Jameson K., Arden N., Raitakari O., Viikari J., Lohman K. K., Ferrucci L., Melhus H., Ingelsson E., Byberg L., Lind L., Lorentzon M., Salomaa V., Campbell H., Dunlop M., Mitchell B. D., Herzig K. H., Pouta A., Hartikainen A. L., Streeten E. A., Theodoratou E., Jula A., Wareham N. J., Ohlsson C., Frayling T. M., Kritchevsky S. B., Spector T. D., Richards J. B., Lehtimäki T., Ouwehand W. H., Kraft P., Cooper C., Marz W., Power C., Loos R. J., Wang T. J., Jarvelin M. R., Whittaker J. C., Hingorani A. D., Hyppönen E. (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 10, e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wada A., Waterman M. R. (1992) Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J. Biol. Chem. 267, 22877–22882 [PubMed] [Google Scholar]
- 47. Pikuleva I. A., Cao C., Waterman M. R. (1999) An additional electrostatic interaction between adrenodoxin and P450c27 (CYP27A1) results in tighter binding than between adrenodoxin and p450scc (CYP11A1). J. Biol. Chem. 274, 2045–2052 [DOI] [PubMed] [Google Scholar]
- 48. Björkhem I. (1992) Mechanism of degradation of the steroid side chain in the formation of bile acids. J. Lipid Res. 33, 455–471 [PubMed] [Google Scholar]
- 49. Pullinger C. R., Eng C., Salen G., Shefer S., Batta A. K., Erickson S. K., Verhagen A., Rivera C. R., Mulvihill S. J., Malloy M. J., Kane J. P. (2002) Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 110, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schüle R., Siddique T., Deng H. X., Yang Y., Donkervoort S., Hansson M., Madrid R. E., Siddique N., Schöls L., Björkhem I. (2010) Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J. Lipid Res. 51, 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wamberg L., Christiansen T., Paulsen S. K., Fisker S., Rask P., Rejnmark L., Richelsen B., Pedersen S. B. (2013) Expression of vitamin D-metabolizing enzyme in human adipose tissue-the effect of obesity and diet-induced weight joss. Int. J. Obes. 37, 651–657 [DOI] [PubMed] [Google Scholar]
- 52. Wajchenberg B. L. (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 [DOI] [PubMed] [Google Scholar]
- 53. Spalding K. L., Arner E., Westermark P. O., Bernard S., Buchholz B. A., Bergmann O., Blomqvist L., Hoffstedt J., Näslund E., Britton T., Concha H., Hassan M., Rydén M., Frisén J., Arner P. (2008) Dynamics of fat cell turnover in humans. Nature 453, 783–787 [DOI] [PubMed] [Google Scholar]
- 54. Kha H. T., Basseri B., Shouhed D., Richardson J., Tetradis S., Hahn T. J., Parhami F. (2004) Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J. Bone Miner. Res. 19, 830–840 [DOI] [PubMed] [Google Scholar]
- 55. Dubrac S., Lear S. R., Ananthanarayanan M., Balasubramaniyan N., Bollineni J., Shefer S., Hyogo H., Cohen D. E., Blanche P. J., Krauss R. M., Batta A. K., Salen G., Suchy F. J., Maeda N., Erickson S. K. (2005) Role of CYP27A in cholesterol and bile acid metabolism. J. Lipid Res. 46, 76–85 [DOI] [PubMed] [Google Scholar]
- 56. Bieghs V., Hendrikx T., van Gorp P. J., Verheyen F., Guichot Y. D., Walenbergh S. M., Jeurissen M. L., Gijbels M., Rensen S. S., Bast A., Plat J., Kalhan S. C., Koek G. H., Leitersdorf E., Hofker M. H., Lutjohann D., Shiri-Sverdlov R. (2013) The cholesterol derivative 27-hydroxycholesterol reduces steatohepatitis in mice. Gastroenterology 144, 167–178 [DOI] [PubMed] [Google Scholar]
- 57. Pagadala M. R., McCullough A. J. (2012) Non-alcoholic fatty liver disease and obesity: not all about body mass index. Am. J. Gastroenterol. 107, 1859–1861 [DOI] [PubMed] [Google Scholar]
- 58. Alberti K. G., Eckel R. H., Grundy S. M., Zimmet P. Z., Cleeman J. I., Donato K. A., Fruchart J. C., James W. P., Loria C. M., Smith S. C., Jr. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 [DOI] [PubMed] [Google Scholar]
- 59. Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272, 3137–3140 [DOI] [PubMed] [Google Scholar]
- 60. Juvet L. K., Andresen S. M., Schuster G. U., Dalen K. T., Tobin K. A., Hollung K., Haugen F., Jacinto S., Ulven S. M., Bamberg K., Gustafsson J. A., Nebb H. I. (2003) On the role of liver X receptors in lipid accumulation in adipocytes. Mol. Endocrinol. 17, 172–182 [DOI] [PubMed] [Google Scholar]
- 61. Seo J. B., Moon H. M., Kim W. S., Lee Y. S., Jeong H. W., Yoo E. J., Ham J., Kang H., Park M. G., Steffensen K. R., Stulnig T. M., Gustafsson J. A., Park S. D., Kim J. B. (2004) Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor γ expression. Mol. Cell. Biol. 24, 3430–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hummasti S., Laffitte B. A., Watson M. A., Galardi C., Chao L. C., Ramamurthy L., Moore J. T., Tontonoz P. (2004) Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J. Lipid Res. 45, 616–625 [DOI] [PubMed] [Google Scholar]
- 63. Ross S. E., Erickson R. L., Gerin I., DeRose P. M., Bajnok L., Longo K. A., Misek D. E., Kuick R., Hanash S. M., Atkins K. B., Andresen S. M., Nebb H. I., Madsen L., Kristiansen K., MacDougald O. A. (2002) Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol. Cell. Biol. 22, 5989–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim W. K., Meliton V., Amantea C. M., Hahn T. J., Parhami F. (2007) 20(S)-hydroxycholesterol inhibits PPARγ expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. 22, 1711–1719 [DOI] [PubMed] [Google Scholar]
- 65. Umetani M., Shaul P. W. (2011) 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol. Metab. 22, 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dieudonn M. N., Leneveu M. C., Giudicelli Y., Pecquery R. (2004) Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 286, C655–C661 [DOI] [PubMed] [Google Scholar]