Abstract
The anterior limb of the internal capsule (ALIC) is a white matter structure, the medial portion of which includes the anterior thalamic radiation (ATR) carrying nerve fibers between thalamus and prefrontal cortex. ATR abnormalities have a possible link with cognitive abnormalities and negative symptoms in schizophrenia. We aimed to study the fiber integrity of the ATR more selectively by isolating the medial portion of the ALIC using region-of-interest based methodology. Diffusion-tensor imaging was used to measure the anisotropy of total ALIC (tALIC) and medial ALIC (mALIC) in 39 schizophrenia and 33 control participants, matched for age/gender/handedness. Relationships between anisotropy, psychopathology, and cognitive performance were analyzed. Compared to controls, schizophrenia participants had 4.55% lower anisotropy in right tALIC, and 5.38% lower anisotropy in right mALIC. There were no significant group anisotropy differences on the left. Significant correlations were observed between right ALIC integrity and relevant domains of cognitive function (e.g., executive function, working memory). Our study suggests an asymmetric microstructural change in ALIC in schizophrenia involving the right side, which is only minimally stronger in mALIC, and which correlates with cognitive impairment. Microstructural changes in the ALIC may be linked to cognitive dysfunction in schizophrenia.
Keywords: Schizophrenia, Anterior Thalamic Radiation, Internal Capsule, DTI, Diffusion Tensor Imaging, ROI, Thalamus, anisotropy, working memory, executive function
1. INTRODUCTION
Dysfunction of the thalamus has been associated with the pathophysiology of schizophrenia due in part to its dense reciprocal connections with the cerebral cortex (Andreasen, 1997; Byne et al., 2009). Many structural studies have shown decreased size of the thalamus in schizophrenia (Wright et al., 2000; Konick and Friedman, 2001), although negative studies have also been reported (Portas et al., 1998). In our previous studies, using deformation-based shape analysis, thalamic abnormalities were localized to regions that included the anterior extremes of the structure (Csernansky et al., 2004; Harms et al., 2007). The anterior and mediodorsal nuclei have been associated with cognitive functions (Van der Werf et al., 2003), and postmortem studies have shown a reduction in neuronal population in these regions among individuals with schizophrenia (Young et al., 2000). The anterior thalamic nucleus receives hippocampal afferents and projects information to the anterior cingulate cortex, and can influence the encoding of new stimuli (Mitchell et al., 2002; Van der Werf et al., 2003). The mediodorsal thalamic nucleus has reciprocal connections with the prefrontal cortex, and structural and functional abnormalities in this area have been linked to dysfunction in executive processes pertaining to declarative memory (Van der Werf et al., 2003).
A major efferent tract of the thalamus is the anterior limb of the internal capsule (ALIC). The ALIC carries two major fiber systems: the anterior thalamic radiation (ATR) and the frontopontine tract (Kahle et al, 2002). To a lesser degree thalamo-striate and striato-striate fibers are also present (Axer and Keyserlingk, 2000; Cunningham, 1903) in the ALIC. The ATR consists of fibers between mediodorsal thalamic nuclei and the frontal cortex, and fibers between anterior thalamic nuclei and the anterior cingulate cortices (Kahle et al., 2002; Zhou et al., 2003). Frontopontine fibers are descending cortical fibers, which have motor functions (Hendelman, 2006). Although the distinct fiber systems intermingle to a degree in the ALIC (Axer and Keyerslingk, 2000, Axer et al., 1999), the anterior thalamic radiation fibers tend to course medial to the frontopontine tracts in at least some deep brain regions superior to the anterior commissure (Wright and Locke, 1971; Meyer et al., 1947; Wakana et al. 2004. At more inferior regions near the level of the anterior commissure, the ALIC consists almost exclusively of anterior thalamic radiation fibers (Spalteholz, 1923).
In recent years an increasingly valuable tool for evaluating the integrity of the ALIC and other white matter tracts has been diffusion tensor imaging (DTI) (Basser et al., 1994). DTI measures the diffusivity of water molecules within living brain and can characterize the organization of underlying tissue structure using scalar measures of anisotropy (Basser, 1999; Conturo et al., 1996). In healthy white matter, water is constrained to diffuse primarily along the axis of the fibers resulting in a high anisotropy, while in gray matter diffusion is less directional yielding a relatively low anisotropy (Pierpaoli et al., 1996; Le Bihan et al., 2001). Anisotropy refers to the extent to which water diffusion is direction-dependent within the tissue microstructure, and is considered a marker of white matter integrity. Therefore DTI can evaluate the integrity and directionality of white matter tracts in vivo. Previous DTI studies of schizophrenia have generally shown decreased anisotropy in various white matter structures as well as a loss of normal left-right asymmetry (Kanaan et al., 2005; Kubicki et al., 2007). The anisotropy of the ALIC has been previously reported to be decreased in schizophrenia using both voxel based morphometry (Sussmann et al., 2009; Munoz Maniega et al., 2008) and region-of-interest (ROI) based methods (Zou et al., 2008; Mitelman et al., 2007; Sussmann et al., 2009). In addition, reduced anisotropy has been found in unaffected siblings of patients (Munoz Maniega et al., 2008).
The goal of the current study was to determine if selective measurement of the anterior thalamic radiation may provide increased sensitivity to detect anisotropy changes in schizophrenia relative to the ALIC as a whole. We also sought to determine if those changes have a relationship with specific symptoms and cognitive features of schizophrenia. We hypothesize that weighting the anisotropy measurements to the anterior thalamic radiation by measuring the more medial part of the ALIC will yield a more significant reduction in schizophrenia subjects than when measuring the total ALIC. This hypothesis is based on the premise that anterior thalamic radiation, and not frontopontine fibers, are disrupted in schizophrenia, due to decreased neuronal density in thalamic nuclei that project through the ATR (Young et al., 2000). Also, since the anterior thalamic radiation is associated with memory encoding and executive functioning (as described above), we hypothesize a relationship of fiber integrity to cognitive performance.
2. METHODS
2. 1. SUBJECTS
The study was approved by the local Institutional Review Board (IRB). Informed consent was obtained in all subjects. Two groups of subjects were recruited by advertising in local area psychiatric clinics and in the community: schizophrenia probands (SCZ; n=39) and healthy controls (CON; n=33). From an initial group of potential subjects that fit inclusion criteria (see below), the final group of study subjects were those from whom diffusion tensor image scans were generated and whose MR scans did not have significant artifact (e.g., movement). This cohort of subjects overlapped with those subjects used in our previous studies of thalamic structure (Csernansky et al, 2004; Harms et al, 2007), but is a subset of the subjects from those studies, since DTI scans were not collected in all subjects.
The demographic and clinical profiles of the subject groups are summarized in TABLE 1. All subjects were diagnosed using DSM-IV criteria on the basis of a consensus between a research psychiatrist and a Masters level research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al., 1995). Subjects were excluded if they had neurologic disorders, unstable medical disorders, head injury with loss of consciousness, contraindication to MRI (e.g., metal implant or claustrophobia), or if they met DSM-IV criteria for substance abuse or dependence within the three months preceding assessment. Handedness was determined in all subjects as the hand used for writing.
TABLE 1.
Demographic and clinical profiles.a
| Characteristics | SCZ n=39 | CON n=33 | F or χ2 | p |
|---|---|---|---|---|
|
| ||||
| Age | 38.0 (12.6) | 33.4 (16.7) | 1.7 | 0.19 |
| Gender – N (%) | ||||
| Female | 13 (33.3) | 13 (39.4) | 0.28 | 0.59 |
| Male | 26 (66.7) | 20 (60.6) | ||
| Race – N (%) | ||||
| African American | 23 (59.0) | 9 (27.3) | 7.4 | 0.03* |
| Caucasian | 15 (38.5) | 23 (69.7) | ||
| Hispanic | 1 (2.6) | 1 (3.0) | ||
| Handedness – N (%) | ||||
| Right | 33 (89.2) | 28 (87.5) | 0.05 | 0.83 |
| Left | 4 (10.8) | 4 (12.5) | ||
| Parental Socioeconomic Statusb | 3.8 (0.8) | 3.4 (0.9) | 3.84 | 0.054 |
| Illness duration (yrs) | 17.1 (14.2) | n/a | n/a | n/a |
| Positive Psychotic Symptoms (SAPS)c | ||||
| Hallucinations | 1.25 (1.7) | n/a | n/a | n/a |
| Delusions | 2.08 (1.8) | n/a | n/a | n/a |
| Bizarre Behavior | 0.68 (0.9) | n/a | n/a | n/a |
| Positive Thought | 1.59 (1.3) | n/a | n/a | n/a |
| Negative Psychotic Symptoms (SANS)c | ||||
| Affective Flattening | 1.10 (1.2) | n/a | n/a | n/a |
| Alogia | 0.97 (1.0) | n/a | n/a | n/a |
| Avolition/Apathy | 2.18 (1.2) | n/a | n/a | n/a |
| Anhedonia/Asociality | 2.21 (1.5) | n/a | n/a | n/a |
| Attention | 1.76 (1.4) | n/a | n/a | n/a |
Values are means (standard deviation) unless stated otherwise. F values were calculated using one-way ANOVA across groups. The χ2 value is the result of a chi-square comparison.
Socioeconomic status ranges from 1–5, with higher values indicating lower socioeconomic status.
SAPS and SANS scores range from 1–5, with higher values indicating stronger positive (SAPS) or negative (SANS) symptoms.
n/a=not applicable.
Statistically significant (p<0.05) between-group differences.
The SCZ participants were clinically stable; the global severity of their symptoms had remained unchanged for at least 2 weeks prior to participation in the study. The majority of SCZ subjects were currently on atypical antipsychotic drugs. The CON participants had no lifetime history of Axis I psychotic or major mood disorders (i.e. major depression or bipolar disorder) or any first-degree relative with a psychotic disorder.
2. 2. ASSESSMENT OF PSYCHOTIC SYMPTOMS
Measures of specific domains of psychopathology were derived in two ways. First, measures of lifetime history of psychopathology were derived for delusions, hallucinations, thought disorganization and negative symptoms using selected item scores extracted from the SCID-I, as previously described (Mamah et al., 2008). Second, measures of current psychopathology were derived from subscores of the Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen and Olsen, 1982). Mean individual subscores of SAPS and SANS assessments are listed on TABLE 1.
2.3. ASSESSMENT OF NEUROCOGNITION
Cognitive function was assessed using a battery of neuropsychological tests. The raw scores from the individual neuropsychological tests were first z-scored (against a sample of 216 subjects, including controls, individuals with schizophrenia, and their siblings that have participated in studies at our center). Selected clusters of z-scores were then averaged to yield four broad cognitive domains (executive function, episodic memory, working memory and crystallized intelligence). For executive function, episodic memory, and working memory, the domain clusters were composed of the same items as described in Harms et al (2007; also see Delawalla et al., 2006). Crystallized intelligence (Kay, 2005) was assessed using scores from the WAIS-III Vocabulary subtest.
2.4. IMAGE ACQUISITION AND PROCESSING
DTI data were acquired in study participants over a 3-year period from 2004–2006 using the same MRI scanner, pulse sequence, and acquisition protocol. The DTI acquisition was incorporated into ongoing MRI studies of schizophrenia which began in 2001. All MRI scanning was performed using a Siemens Magnetom Vision 1.5T imaging system.
The DTI methods were tailored to anisotropy imaging of smaller white-matter structures such as the ALIC. Specifically, diffusion-tensor images were acquired using a custom in-house single-shot echo planar imaging (EPI) DTI pulse sequence designed for higher spatial resolution. Tetrahedral-perpendicular tensor encoding was employed (Shimony et al., 1999) using b values of 37, 333, and 1000 s/mm2. This encoding scheme has a low matrix condition number (i.e., low noise) (Skare et al., 2000; Hasan et al., 2001). The sequence was designed to use the resonant circuit to clip the sinusoidal gradient waveforms in order to perform EPI readout during a plateau, while using the early sinusoidal upslope to rapidly reach the readout plateau. Sampling on a plateau enabled on-line reconstruction on the scanner.
In the initial pulse sequence optimization phase of the study, the optimal repetition time (TR) for maximal SNR was analytically determined, assuming repeat scanning and signal averaging at a fixed scan time. Taking into account the on-line image reconstruction time, the optimal TR was found to be 2.5 s. To optimize isotropic voxel size, we then acquired human brain data with varied voxel size and number of scan repeats. (An isotropic voxel size was chosen to provide effective measurement of ROI means and volumes). An isotropic voxel size of 2.0 mm was found to be a good trade-off between smaller voxels that have low SNR (causing overestimation of anisotropy due to noise bias, or alternatively requiring an impractical amount of signal averaging) versus larger voxels that have partial volume averaging (causing underestimation of anisotropy due to surrounding gray matter, and causing coarse ROI sampling of the ALIC). Acquisition of 7 contiguous slices with a 2.0-mm slice thickness provided the necessary anatomical coverage (see below), while yielding a nearly optimal TR of 2.62 s. Acquisition of 12 scan repeats provided sufficient signal averaging for anisotropy imaging of the ALIC with minimal noise bias, and yielded a 5.6-min scan time allowing the DTI scan to be incorporated into to the overall MRI scan session. The echo time (TE) was 108 ms (with centered echoes and fat saturation) for acquisition of a 256×256 mm field of view, oversampled with a 128×200 matrix.
Subjects were positioned with their canthomeatal line perpendicular to the table. DTI scans were acquired such as to generate seven contiguous 2.0-mm slices (with no slice gaps) in a 14 mm axial slab containing the ALIC. Because the ALIC has a characteristic anatomical relationship to the adjacent anterior commissure (AC), the slices were positioned relative to the AC identified on a high-resolution sagittal scout scan. The bottom of the second-lowest slice was positioned at the inferior border of the AC, such that the AC coursed within the plane of this slice, while the lowest slice was immediately inferior to the AC.
Rigorous scanner quality control was administered over the 3-year data acquisition period, including quarterly RF room maintenance, and increased frequency of vendor QA testing and tuning. There were no vendor software or hardware changes during this period. These procedures provided very stable imaging conditions for this study.
Images were processed by realignment of all slices to the b~0 T2-weighted EPI image (I0 image) to correct for eddy-current effects and movement. The I0 image and diffusion-weighted images (DWIs) were then averaged across the 12 scans to increase SNR. From the averaged I0 and DWIs, anisotropy images were computed using the parameter Aσ (Conturo et al., 1996). Aσ ranges from 0 to 1, and has a linear response over that range. Aσ is related to fractional anisotropy (FA) (Basser., 1995) by a nonlinear numerical expression (Hasan and Narayana, 2003). Accordingly, Aσ has a linear partial-volume effect (Shimony et al., 1999) when imaging small white-matter structures surrounded by gray matter (e.g., ALIC). All anisotropy measurements are reported as Aσ.
2.5. OUTLINING REGIONS OF INTEREST
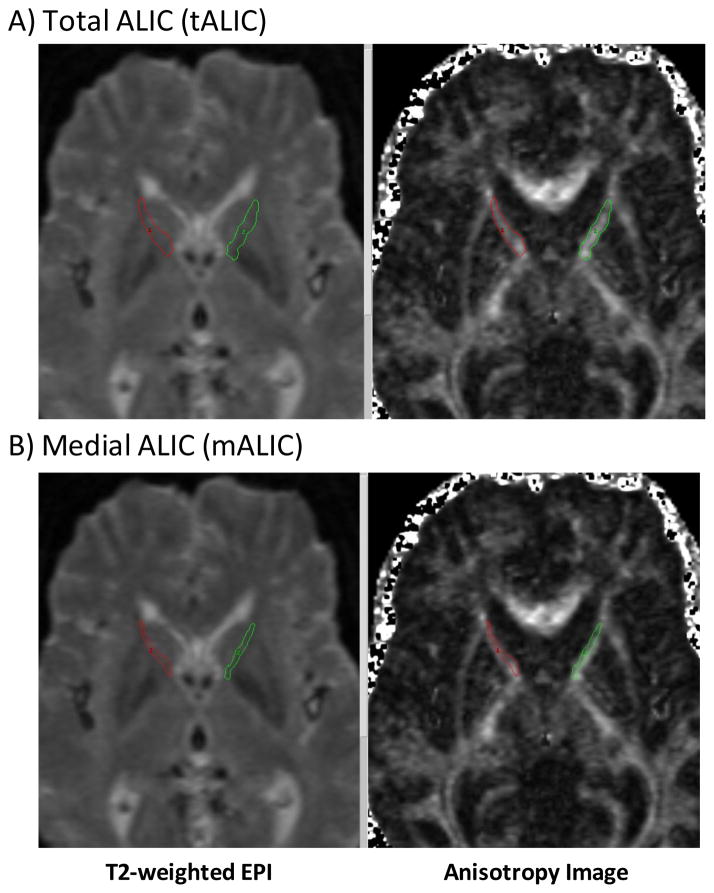
Regions of interest (ROI) were outlined using Analyze AVW™ software (Mayo Foundation, Rochester, Minnesota) on the anisotropy images. The T2-weighted EPI (I0) images (averaged across 12 scans) were displayed alongside anisotropy images to help guide ROI outlining. All ROIs were outlined by manual tracing on the anisotropy images, with the position confirmed on the I0 images (Fig. 1). The same operator, who was blinded to diagnostic group, traced all ROIs. Of the seven slices generated, only four slices were outlined: the third to the sixth axial slice (numbered from inferior to superior). Thus, ROIs were placed in the slices immediately superior to the anterior commissure (the latter coursing through slice #2). First, the total anterior limb of the internal capsule (tALIC) was outlined in its entirety, with its rostral border formed by the beginning of the external capsule, and its caudal border formed by a line continuous with the lateral border of the posterior limb of the internal capsule (Fig. 1A). Second, the medial portion of the ALIC (mALIC) was outlined by bisecting out the medial part of the tALIC (Fig. 1B) in all four slices except the most inferior slice (slice #3). On this most inferior slice, the tALIC was not medially bisected because the ALIC at this level is almost exclusively composed of anterior thalamic radiation fibers (Axer and Keyerslingk, 2000, Axer et al., 1999). On this slice, the entire width of the ALIC was included in the measurement of mALIC.
Figure 1. Outlining the anterior limb of the internal capsule (ALIC).
The figures show examples of axial brain slices with respective regions of interest for A) total ALIC (tALIC) and B) medial ALIC (mALIC). The mALIC was defined as the medial half of the ALIC in the superior three of four slices in which the ALIC was defined, plus the entire ALIC in the most inferior slice. Only one slice is shown in the figure.
2.6. DATA ANALYSIS
Mean anisotropy was computed across the four slices to generate the total mean anisotropy value for each region. Group differences in mean anisotropy and ROI volumes were assessed using one-way ANOVA with hemisphere as a repeated factor (SAS, SAS Institute, Cary NC). Bivariate correlations between anisotropy and subscores of psychopathology or neurocognitive domain scores were assessed using Pearson’s statistics (SAS). As anisotropy was hypothesized to be inversely related to the presence and severity of clinical abnormality, one-tailed tests were used to derive p-values. Because there was no hypothesis concerning volumes, comparison of ROI volumes utilized two-tailed tests.
3. RESULTS
3.1. ROI VOLUMES
For each subject, the volume of the specific ROIs outlined in each axial section were summed across sections to calculate the total ROI volumes. This calculation was used as a quality-control check on the ROI tracing procedure, and to test for differences in tALIC volume between groups. Because anisotropy images demonstrate the full thickness of the internal capsule compared to conventional T1- or T2-weighted images (Shimony et al., 1999), we assessed the tALIC volume using the volumes of the tALIC ROI outlines drawn on anisotropy images. Analysis of volumes using repeated measures ANOVA revealed no main effect of group (p=0.3) and no group-by-hemisphere interaction (p>0.8) for either tALIC or mALIC, although the main effect of hemisphere was significant for both ROIs (p<0.0001). Mean volumes of the tALIC ROIs were 324.7 mm3 (control) and 333.9 mm3 (schizophrenia) on the right (p=0.5), and 277.4 mm3 (control) and 288.5 mm3 (schizophrenia) on the left (p=0.35). Medial volumes of mALIC ROIs were 195.3 mm3 (control) and 203.9 mm3 (schizophrenia) on the right (p=0.37), and 171.8 mm3 (control) and 178.8 mm3 (schizophrenia) on the left (p=0.4). The percent of the tALIC ROI volume that was designated as mALIC was 60.15% (control) and 61.07% (schizophrenia) on the right, and 61.93% (control) and 61.98% (schizophrenia) on the left, indicating that the mALIC ROI was consistently defined by splitting the tALIC. (The percentage is > 50% due to the entire tALIC being assigned to mALIC on the lowest slice).
3.2. ANISOTROPY
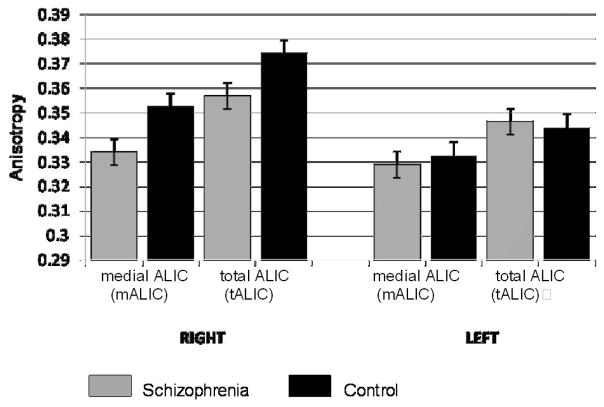
Graphs comparing mean anisotropy between groups in each ROI are shown in Fig. 2. Mean Aσ values of the tALIC were 0.374 (control) and 0.357 (schizophrenia) on the right (−4.55%), and 0.344 (control) and 0.346 (schizophrenia) on the left (+0.58%). There was a significant main effect of hemisphere on tALIC mean Aσ (right>left, F[1,70]=22.6, p<0.0001). The main effect of group was not significant (F[1,70]=0.9, p=0.36). However, because the group-by-hemisphere interaction was significant (F[1,70]=5.2, p=0.026), we conducted a group comparison separately by hemisphere and found a significant effect of group status on mean anisotropy of the tALIC on the right side (F[1,70]=4.4, p=0.04), but not the left (p=0.8). Including race as a covariate in the analysis showed near identical results.
Figure 2. Anisotropy of the ALIC.
The figure shows mean anisotropy of the entire ALIC (tALIC) and the medial ALIC (mALIC) in schizophrenia and control subjects.
Mean Aσ values of the mALIC were 0.353 (control) and 0.334 (schizophrenia) on the right (−5.38%), and 0.333 (control) and 0.329 (schizophrenia) on the left (−1.20%). The main effect of hemisphere was again significant for the mALIC (F[1,70]=8.8, p=0.004). The main effect of group was not significant (F=[1,70]=1.9, p=0.18), but because the group-by-hemisphere interaction trended toward significance (F=[1,70]=3.2, p=0.077), we conducted a post-hoc group comparison separately by hemisphere. This analysis found that mean anisotropy in the mALIC was significantly reduced in the schizophrenia group on the right (F[1,70]=4.9, p=0.03), but not the left (p=0.7). Including race as a covariate in the analysis did not change the results.
3.3. RELATIONSHIPS OF ANISOTROPY TO PSYCHOPATHOLOGY
Using lifetime psychopathology measures derived from the SCID, there were no significant correlations observed in SCZ subjects between hallucinations, delusions or thought disorganization and mean anisotropy values in any ROI. Among the subtests obtained from the SAPS and SANS, the only significant relationships were the attention subtest (SANS) with the right tALIC (r= −0.29; p=0.038) and with the right mALIC (r= −0.34; p=0.017) in SCZ subjects.
3.4. RELATIONSHIP OF ANISOTROPY TO PSYCHOPATHOLOGY AND NEUROCOGNITION
Using lifetime psychopathology measures derived from the SCID, there were no significant correlations observed in SCZ subjects between hallucinations, delusions or thought disorganization and mean anisotropy values in any ROI. Among the subtests obtained from the SAPS and SANS, the only significant relationships were the attention subtests (SANS) with the right tALIC (r= −0.29; p=0.038) and with the right mALIC (r= −0.34; p=0.017) in SCZ subjects.
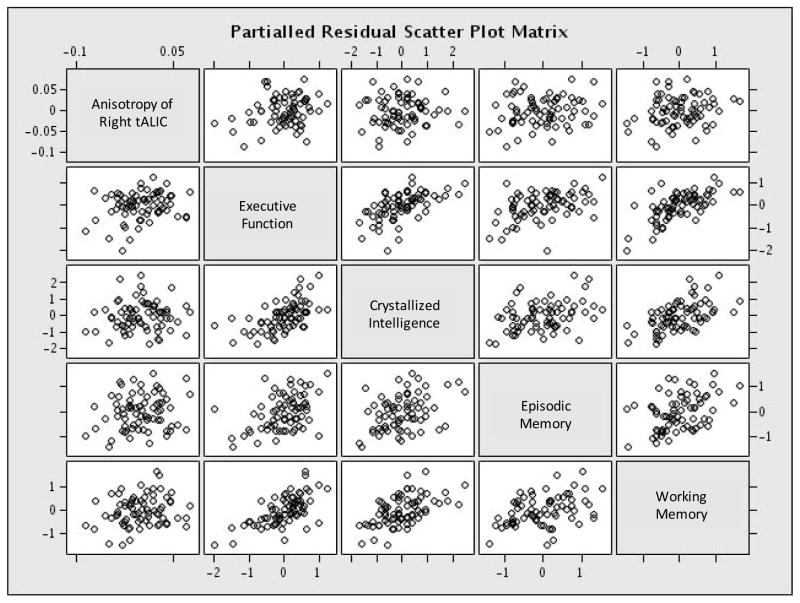
Partial correlation analysis (controlled for group status), including both subject groups, was used to evaluate the relationship between mean anisotropy and four cognitive domains (i.e. executive function, episodic memory, working memory and crystallized intelligence). Significant relationships were observed for anisotropy of the right mALIC with executive function (r=0.23; p=0.03), as well as a trend-level correlation with working memory (r=0.16; p=0.095). Right tALIC values similarly correlated significantly with executive function (r=0.23; p=0.03), and showed trend-level correlations with both working memory (r=0.19; p=0.06) and episodic memory (r=0.16; p=0.1). Graphs of the relationship between the right tALIC and the cognitive domains are shown in Fig. 3.
Figure 3. Relationship of right mALIC anisotropy with cognition.
The graphs are derived from partial correlation analysis that included both schizophrenia and control patients. The relationships shown include that between right mALIC and executive function (r=0.23, p=0.03), crystallized intelligence (r=0.07; p=0.6), episodic memory (r=0.16; p=0.1) and working memory (r=0.19; p=0.06). Cognitive relationships for the right tALIC were similar.
4. DISCUSSION
In this study, we found evidence for decreased anisotropy in the anterior limb of the internal capsule (ALIC) in patients with schizophrenia, compared to controls. Anisotropy decreases could be due to decreased myelination, neuronal fiber density, or directional coherence (Basser, 1999). A possible explanation for the right ALIC finding is decreased neuronal fiber density, which would be consistent with histopathologic observations showing decreased neuronal density in thalamic neurons that project through the ALIC (Young et al., 2000). Previous DTI studies in schizophrenia have also found decreased anisotropy and/or increased mean diffusivity of the ALIC (Oh et al, 2009; Sussmann et al., 2009; Munoz Maniega et al., 2008; Zou et al., 2008; Mitelman et al., 2007). Other studies have reported smaller volumes (Wobrock et al, 2009; Brickman et al, 2006; Lang et al, 2006; Zhou et al, 2003; Kito et al, 2009), or tract length (Buchsbaum et al, 2006), of the ALIC in schizophrenia, which were not found in our study. Altered myelin- and axonal-associated proteins have also been noted in the ALIC in schizophrenia (Beasley et al, 2009), which further suggests that impaired ALIC integrity is linked to disorder pathology. Previous findings of smaller mediodorsal and/or anterior thalamic nuclei in schizophrenia (Shimizu et al, 2008; Byne et al, 2002; Young et al, 2000; Hazlett et al, 1999) support the association of these nuclei with ALIC abnormalities. It is notable that not all DTI studies found abnormal anisotropy in schizophrenia and some have reported abnormalities in the posterior limb of the internal capsule (PLIC) (Cheung et al, 2008; Szeszko et al, 2005). Reduced volumes limited to the PLIC, rather than ALIC, have also been noted (Yoshihara et al, 2008). Unlike other studies of the ALIC anisotropy in schizophrenia (Sussmann et al., 2009; Munoz Maniega et al., 2008; Zou et al., 2008; Mitelman et al., 2007), our results show only unilateral abnormality involving the right side. Our finding of higher anisotropy on the right compared to the left in control subjects is consistent with previous studies (Park et al., 2004; Peled et al., 1998). There was a notable decrease in the left-right asymmetry of anisotropy in individuals with schizophrenia, compared to controls, consistent with the hypothesis of attenuated asymmetry in schizophrenia (Crow et al., 1989). Right greater than left asymmetry of thalamic volume has been previously reported in both controls and schizophrenia patients (Harms et al., 2007; Deicken et al., 2002), although other authors have noted different results (Csernansky et al., 2004). Interestingly, a selectively decreased metabolic rate of the right thalamus has also been reported in schizophrenia, with a loss of the normal right greater than left asymmetry (Buchsbaum et al., 1996). This may indicate that thalamic processes may be disrupted asymmetrically in schizophrenia.
It was notable that the overall mean anisotropy of the mALIC was lower than the tALIC in both groups. This could be due to biological factors such as a relatively decreased fiber density, myelination, or fiber directionality (Byne et al, 2006; Madler et al, 2008; Kubicki et al, 2005) in medial regions of the ALIC. As this difference between mALIC and tALIC was observed in both control and schizophrenia subjects, it does not appear to be pathologic. The tALIC consists of the anterior thalamic radiation, frontopontine tract, and to a lesser extent thalamostriate and striato-striate fibers (Kahle et al., 2002; Axer and Keyserlingk, 2000; Cunningham, 1903). While it has been reported that the anterior thalamic radiation (ATR) tends to run medial to the frontopontine tract in the ALIC (Wright and Locke, 1971; Meyer et al., 1947; Wakana et al., 2004), other studies have indicated a more complex intermingling of fiber systems (Axer and Keyserlingk, 2000; Axer et al., 1999). Descending fibers of the frontopontine tract are highly organized (Stieltjes et al, 2001), and may account for higher anisotropy in lateral regions of the ALIC. Our results show that more medial regions of the ALIC have a similar percentage (as well as statistical) difference in anisotropy between SCZ and CON as the ALIC as a whole. Comparing mALIC to tALIC anisotropy, we observed less than 1% difference in anisotropy decrease in schizophrenia. Thus, the difference between the mALIC and tALIC effects were not as strong as anticipated, possibly because the medial portion of the ALIC biologically has a significant microscopic intermingling of different fiber tracts. While variability in the tracing/positioning of the narrow mALIC ROI (Fig. 1) might lead to admixtures of structures other than the ATR, and dilute the effect seen in mALIC, the consistency of the mALIC ROI volume as a percent of the tALIC volume indicates that the mALIC ROI was consistently defined. The observation that effects occur in both mALIC and tALIC suggests that both the frontopontine tract system and ATR are affected in schizophrenia.
Our results also suggested a relationship between white matter integrity in the right ALIC (and its medial portion) and cognitive performance. Among the domains of cognitive function, the most significant relationship was with executive function, and to a lesser degree working memory. Interestingly, there were no significant correlations of positive or negative psychopathology measures and the ALIC, with the exception of the attention subtest of the SANS. Taken together, this suggests a possible relationship of the integrity of the ALIC with cognitive, but not psychotic symptoms, at least in our cohort of subjects. This finding is consistent with the role of the ATR in cognition, since the ATR carries fibers from the thalamic nuclei to the prefrontal cortex, which is involved in executive function and planning complex behaviors (Van der Werf et al., 2003; Zoppelt et al., 2003; Floresco and Grace, 2003). Anterior thalamic nuclei process afferent information from the hippocampus, which is involved in working memory, and project mainly to anterior cingulate cortex (Mitchell et al., 2002; Van der Werf et al, 2003). Many of the other authors investigating the ALIC in schizophrenia (Sussmann et al., 2009; Zou et al., 2008; Mitelman et al., 2007; Munoz Maniega et al., 2008) did not study its relationship to cognition. Sussmann et al (2009) did not find a correlation between ALIC integrity and psychopathology using the Positive and Negative Symptom Scale (PANSS). However, Harms et al. (2007) found a weak relationship between working memory performance and shape abnormalities of the thalamus which involved its anterior surface. Results of our correlation analysis should be interpreted with caution as they were not controlled for multiple comparisons, which would have negated significant effects. Nonetheless, our finding a relationship between the integrity of ALIC anisotropy and executive function is consistent with prior work suggesting the importance of thalamic-prefrontal connections to executive control.
Since beginning the collection of our imaging data, acquisition schemes for obtaining diffusion imaging data have continued to evolve, which may improve the validity of results of DTI studies in the future (Vaessen et al, 2010; Cheng et al, 2010). A limitation to the current study is that it was not designed to adequately control for the effect of medications and recreational substances on our findings. Antipsychotic medications or the higher prevalence of substance use in schizophrenia patients may account for at least some of the abnormalities reported in the ALIC. However, the asymmetric abnormalities noted make it unlikely that these are chemically induced. Furthermore, ALIC abnormalities have been previously shown in antipsychotic-naïve schizophrenia patients (Zou et al., 2008). Future studies designed to explore the effects of specific antipsychotic medications and substances may explain the degree to which they may contribute to white matter abnormalities.
Acknowledgments
This research was funded by federal NIH grants P20 MH062130 and P50 MH071616 (Conte Center for the Neuroscience of Mental Disorders), R01 MH056584, and R01 NS039538.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC. The role of the thalamus in schizophrenia. Canadian Journal of Psychiatry. 1997;42:27–33. doi: 10.1177/070674379704200104. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen NC, Olsen S. Negative v positive schizophrenia. Archives of General Psychiatry. 1982;39:789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 4.Axer H, Keyserlingk DG. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. Journal of Neuroscience Methods. 2000;94:165–75. doi: 10.1016/s0165-0270(99)00132-6. [DOI] [PubMed] [Google Scholar]
- 5.Axer H, Lippitz BE, Keyserlingk DG. Morphological asymmetry in anterior limb of human internal capsule revealed by confocal laser and polarized light microscopy. Psychiatry Research: Neuroimaging. 1999;91:141–154. doi: 10.1016/s0925-4927(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysics Journal. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 8.Beasley CL, Dwork AJ, Rosoklija G, Mann JJ, Mancevski B, Jakovski Z, Davceva N, Tait AR, Straus SK, Honer WG. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res. 2009;109:159–66. doi: 10.1016/j.schres.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, Mitelman SA, Hazlett EA, Lincoln SJ, Newmark RE, Shihabuddin L. Internal capsule size in good-outcome and poor-outcome schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18:364–76. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- 10.Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, Ahmed S, Tang C. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann Gen Psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE., Jr PET and MRI of the thalamus in never-medicated patients with schizophrenia. American Journal of Psychiatry. 1996;153:191–9. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmotem assessment of thalamic nuclear volumes in subject with schizophrenia. Am J Psychaitry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathologica. 2009;117:347–68. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- 14.Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutaunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nuclei. Schizophrenia Research. 2006;85:245–53. doi: 10.1016/j.schres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Cheng YW, Chung HW, Chen CY, Chou MC. Diffusion tensor imaging with cerebrospinal fluid suppression and signal-to-noise preservation using acquisition combining fluid-attenuated inversion recovery and conventional imaging: comparison of fiber tracking. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2009.12.032. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–85. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 17.Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magnetic Resonance Medicine. 1996;35:399–412. doi: 10.1002/mrm.1910350319. [DOI] [PubMed] [Google Scholar]
- 18.Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry: A postmortem study and a proposal concerning the genetic basis of the disease. Archives of General Psychiatry. 1989;46:1145–50. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 19.Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. American Journal of Psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham DJ. Text-book of anatomy. Pentland; 1903. [Google Scholar]
- 21.Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophrenia Research. 2002;58:135–44. doi: 10.1016/s0920-9964(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 22.Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, Csernansky JG. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophrenia Bulletin. 2006;32:525–37. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 24.Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. Journal of Neuroscience. 2003;23:2930–43. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their non-psychotic siblings. Journal of Neuroscience. 2007;27:13835–42. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magnetic Resonance Medicine. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- 27.Hasan KM, Parker DL, Alexander AL. Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magnetic Resonance Imaging. 2001;13:769–780. doi: 10.1002/jmri.1107. [DOI] [PubMed] [Google Scholar]
- 28.Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry. 1999;156:1190–9. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- 29.Hendelman W. Atlas of functional neuroanatomy. CRC Press; 2006. [Google Scholar]
- 30.Kahle W, Platzer W, Frotscher M, Leonhardt H. Color atlas and textbook of human anatomy. Thieme; 2002. [Google Scholar]
- 31.Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biological Psychiatry. 2005;58:921–9. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Kay J. Crystallized intelligence versus fluid intelligence. Psychiatry. 2005;68:9–13. doi: 10.1521/psyc.68.1.9.64189. [DOI] [PubMed] [Google Scholar]
- 33.Kito S, Jung J, Kobayashi T, Koga Y. Fiber tracking of white matter integrity connecting the mediodorsal nucleus of the thalamus and the prefrontal cortex in schizophrenia: a diffusion tensor imaging study. Eur Psychiatry. 2009;24:269–74. doi: 10.1016/j.eurpsy.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biological Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 35.Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies of schizophrenia. Journal of Psychiatry Research. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Honer WG. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res. 2006;87:89–99. doi: 10.1016/j.schres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 39.Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26:874–88. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Mamah D, Harms MP, Wang L, Barch D, Thompson P, Kim J, Miller MI, Csernansky JG. Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biological Psychiatry. 2008;64:111–20. doi: 10.1016/j.biopsych.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer A, Beck E, McLardy T. Prefrontal leukotomy: a neuroanatomical report. Brain. 1947;70:18–49. doi: 10.1093/brain/70.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AS, Dairymple-Alford JC, Christie MA. Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. Journal of Neuroscience. 2002;22:1922–8. doi: 10.1523/JNEUROSCI.22-05-01922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long association fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research. 2007;92:211–24. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Munoz Maniega S, Lymer GK, Bastin ME, Marjoram D, Job DE, Moorhead TW, Owens DG, Johnstone EC, McIntosh AM, Lawrie SM. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophrenia Research. 2008;106:132–9. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, Westin CF, Shenton ME. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–25. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–23. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA. Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Research. 1998;780:27–33. doi: 10.1016/s0006-8993(97)00635-5. [DOI] [PubMed] [Google Scholar]
- 48.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 49.Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, Kikinis R, Donnino R, Jolesz FA, McCarley RW. Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biological Psychiatry. 1998;43:649–659. doi: 10.1016/s0006-3223(97)00339-9. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu M, Fujiwara H, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr Res. 2008;101:331–8. doi: 10.1016/j.schres.2007.12.486. [DOI] [PubMed] [Google Scholar]
- 51.Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 52.Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17:391–8. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- 53.Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. Journal of Magnetic Resonance. 2000;147:340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- 54.Spalteholz W. Hand atlas of human anatomy. Lippincott; 1923. [Google Scholar]
- 55.Stieltjes B, Kaufman WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–35. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 56.Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Maniega SM, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11:11–8. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 57.Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162(3):602–5. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 58.Vaessen MJ, Hofma PA, Tijssen HN, Aldenkamp AP, Jansen JF, Backes WH. The effect and reproducibility of different clinical DTI gradient sets on small world brain connectivity measures. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.03.011. (in press) [DOI] [PubMed] [Google Scholar]
- 59.Van der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–62. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- 60.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Lee DY, Bailey E, Hartlein JM, Gado MH, Miller MI, Black KJ. Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with Tourette syndrome. Psychiatry Research. 2007;28:181–90. doi: 10.1016/j.pscychresns.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wobrock T, Gruber O, Schneider-Axmann T, Wolwer W, Gaebel W, Riesbeck M, Maier W, Klosterkotter J, Schneider F, Buchkremer G, Moller HJ, Schmitt A, Bender S, Schlosser R, Falkai P. Internal capsule size associated with outcome in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009;259:278–83. doi: 10.1007/s00406-008-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 64.Wright SJ, Jr, Locke S. Fiber systems of medial and anterior thalamus of Macaque. Journal of Comparative Neurology. 1971;143:313–22. doi: 10.1002/cne.901430304. [DOI] [PubMed] [Google Scholar]
- 65.Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biological Psychiatry. 2000;47:944–53. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biological Psychiatry. 2003;54:427–36. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 68.Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC. Diffusion tensor imaging study of the anterior limb of the internal capsule in neuroleptic-naïve schizophrenia. Academic Radiology. 2008;15:285–9. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]