Abstract
Background
Giardia duodenalis is one of the most important human enteric parasites throughout the world. Clinical symptoms of this parasite vary from asymptomatic infection to chronic diarrhea. Still it is not clear, whether different types of pathogenesis are due to different strains of organism or to variable host factors. The purpose of this study was to investigate possible correlation of clinical symptoms with assemblages among symptomatic and asymptomatic cases collected from southwest of Iran.
Methods
Fecal samples were collected from 100 symptomatic and asymptomatic cases, which were positive for G. duodenalis. The samples were subjected to semi-nested PCR and RFLP for gdh gene.
Results
Among symptomatic patients, 54% had mixed genotypes AII and BIII, 28% and 18% of samples indicated assemblages BIII and AII, respectively. In contrast, among asymptomatic cases, 64%, 26% and 10%samples had mixed genotypes, BIII and AII assemblages, respectively. Statistical analysis using Chi- Square test showed that there was no significant correlation between assemblage and clinical symptoms in current study.
Conclusion
High prevalence of mixed infection in both groups may affect this conclusion, therefore further study in more details are necessary to clarify these finding. Additionally, it is important to carry out investigations regarding human host factors as well.
Keywords: Giardia duodenalis, Glutamate dehydrogenase (gdh), Semi-nested PCR, PCR-RFLP, Iran
Introduction
Giardia duodenalis is a protozoan parasite of the small intestine found in human and a wide range of mammalian hosts (1, 2). It is estimated that in Asia, Africa and Latin America, about 200 million peoples are now suffering from giardiasis with some 500,000 new cases reported annually. The prevalence of Giardia infection has been reported 5-23 percent in different urban and rural parts of Iran (3, 4). Clinical symptoms of G. duodenalis infection are highly variable and some patients develop clinical giardiasis while others remain asymptomatic (5, 6). Symptomatic cases usually are accompanied with weakness, weight loss, occasionally watery diarrhea, funky stool, steatorrhea, abdominal cramp, bloating, belching, nausea, vomiting and malabsorption syndrome (7). The risk factors for giardiasis are poorly understood and undoubtedly include host factors and may be strain variation of the parasite (6).
Molecular studies such as PCR-RFLP and sequence analysis of housekeeping genes have shown that G. duodenalis is a species complex comprising eight major assemblages (A to H) (6, 8–10). According to available data assemblages A and B have been reported in human (8, 11). Assemblage A has been classified into subgroups I and II (8). Assemblages B consist of two subgroups III and IV. Assemblages AII and BIV appears to be human-specific (11). Genotyping techniques such as PCR-RFLP are used to be a simple, sensitive and powerful analytical tool and it has been successfully used by a number of researchers for Giardia genotyping and gdh (glutamate dehydrogenase) gene is proving useful for genotyping (12, 13). Recently a few and insufficient studies have been performed to determine the genotype and its correlation with clinical symptoms but they have had conflicting results. Assemblage A was more prevalent in asymptomatic infection (14, 15), but it can cause clinical giardiasis (16–19). There was no correlation between assemblages and clinical symptoms (20–22). Current research designed to investigate possible correlation of G. duodenalis assemblages with clinical symptoms among human infected cases.
Materials and Methods
Samples collection and purification of cysts
This study was carried out from September 2011 to July 2012. Fecal samples were collected from one hundred human cases of G. duodenalis. The samples were collected from human cases less than 15 years old (as children) and more than 15 years old as adults, referred to Ahvaz health centers, southwest of Iran. These samples were divided into two groups: 50 fecal samples from symptomatic patients (25 fecal samples from adult patients, 25 from children suffering from giardiasis). In addition, 50 fecal samples were collected from asymptomatic cases divided into two groups as adults and children. The study population included 65 males and 35 females, ranging from 4 to 65 years old. A questionnaire was filled for all cases compromising demographic information and clinical symptoms including: fever, abdominal pain, abdominal cramps, flatulence, weight loss, nausea, vomiting and fatty diarrhea. Patients who had more than four of these signs or symptoms were considered as symptomatic and cases that do not have any signs and symptoms were considered as asymptomatic. All of symptomatic children and adults cases were visited by pediatric or gastroenterologist respectively, and all suspected cases with etiology other than giardiasis were excluded. The fecal samples were analyzed by wet smear stained with Lugol's iodine, formalin ether (23). All symptomatic patients who were infected with other intestinal parasites or bacterial infection were also excluded from the study. The cysts were purified and concentrated from the faeces by sucrose density gradient centrifugation and washed with sterile distilled water and then stored at -20 °C until used (24). The intensity of infection was estimated by average cyst count per high power field (HPF/ ×40) of light microscope. Samples scoring were divided into three categories: 1-5 (1+), 6-10 (2+) and more than 10 cysts (3+).
DNA extraction
Prior to DNA extraction, cysts were freeze-thawed ten times at -80 °C and + 80 °C. DNA was extracted from purified samples by using the commercial QIAamp DNA Stool Mini Kit (Qiagen, Germany) according to the manufactures protocol. DNA samples were preserved at -20 °C until used.
PCR amplification
The amplification of the gdh gene (432 bp) was performed as a semi-nested PCR with a external forward primer GDHeF (TCA ACG TYA AYC GYG GYT TCC GT), internal forward primer GDHiF (CAG TAC ACC TCY GCT CTC GG), and reverse primer GDHiR (GTT RTC CTT GCA CAT CTC C as described by Read et al. (13). The primers were tested with positive DNA control. The positive control G. deudenalis subgroup was generously donated by Kerman University of Medical Sciences. Distilled water used as negative control. The PCR reaction mixture comprised 5 µl genomic DNA, 5 µl of 10X buffer (Fermentas, Lithuania), 1.5 µl (50mM) MgCl2, 1 µl (10 mM) dNTP mix, 0.3 µl (5U/ µl) Taq DNA polymerase (Fermentas, Lithuania), and 1 µl (12.5) pmol of each primer. The reactions were performed in 50 µl valumes. GDHeF and GDHiR were used in the primary PCR reaction. One microliter of PCR product from the primary reaction was added to the secondary PCR containing primers GDHiF and GDHiR. The DNA was amplified using iCycler, BioRad Thermal Cycler under the following condition: 1 cycle of 94 °C for 3 min, 56 °C for 1 min and 72°C for 2 min, followed by 35 cycles, 94 °C for 1min, 56 °C for 20 s and 72°C for 45 s. A final extension of 72 °C for 7 min and a 20 °C hold was used. The PCR products were electrophoresed on ethidium bromide stained 1% agarose gels (Roche, Germany).
PCR-RFLP of region of gdh gen
RFLP analysis was carried out by digesting 10 µl of PCR product. It was added to 1 X enzyme buffer, and 2 µl (10 U/ µl) BspL1 (Fermentas, Lithuania) or 2 µl (10 U/ µl) Rsa1 (Roche, Germany) for 16 h at 37 °C. The final valumes of Rsa1 and BspL1 were 25 and 30 µl, respectively. The BspL1 digestion was used for the distinction between AI or AII and B assemblages. Rsa1 digestion distinguished between subtypes BIII and BIV. Restriction fragments were separated in 3% high resolution grade agarose (Roche, Germany) stained with ethidium bromide. A 50 bp DNA ladder (Fermentas, Lithuania) was used as a size marker (13).
Statistical analysis
All data processing was carried out by SPSS software version 16. Chi square test was used to evaluate the relationship between variables. Mann-Whitney U test was used to investigate the relationship between intensity of infection and clinical symptoms. Logistic regression was used to calculate the odds ratio and 95% confidence interval. α = 0.05 were considered for statistical analysis.
Results
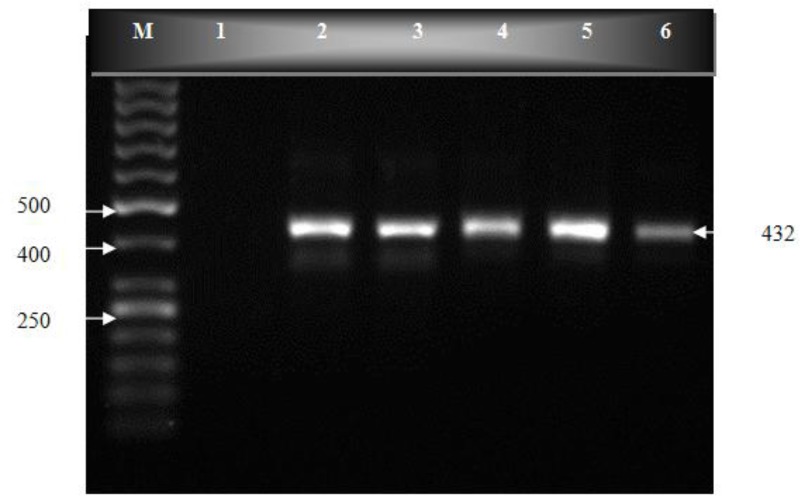
One hundred human fecal samples were amplified in semi-nested PCR (Fig. 1). PCR products were cut by BspL1 and Rsa1 restriction endonuclease, and the RFLP patterns of the digested products were studied (Figs. 2 and 3). The predicted fragment sizes are shown in Table 1. Fragments less than 50 bp were not included in the analysis, as they could not be reliably resolved on the gel and because the assemblages could be distinguished without the need to analyze these small bands.
Fig. 1.
PCR products on an ethidium bromide-stained 1% agarose gel. lane M, molecular weight marker (50 bp); lane 1, negative control; lane 2, positive control; lanes 3-6, PCR products from clinical samples
Fig. 2.
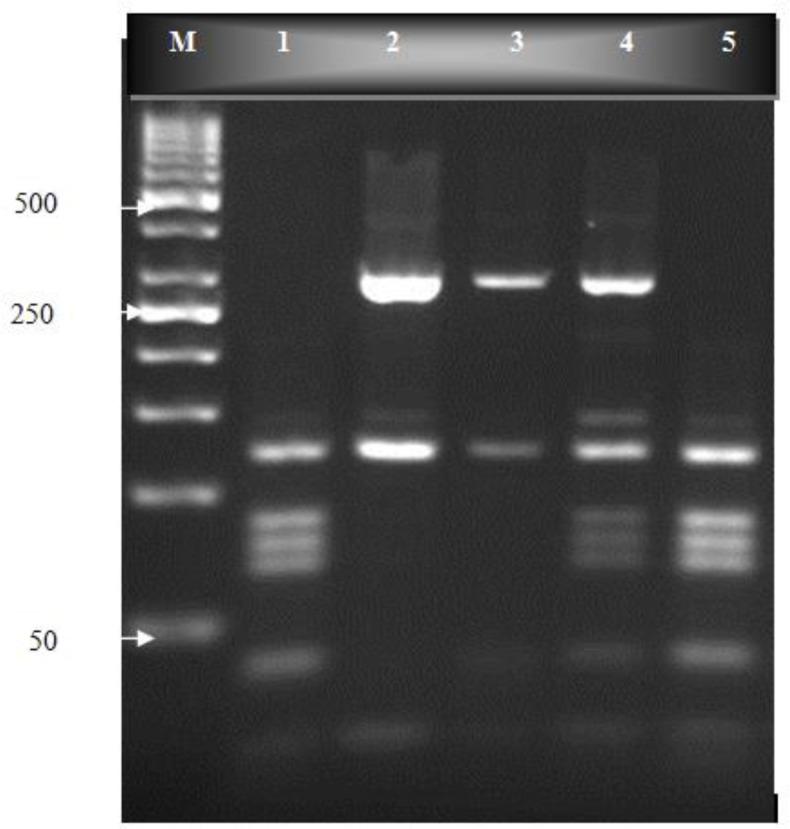
Bspl1 digestion of gdh-PCR products on an ethidium bromide-stained 3% high resolution grade agarose gel. Lane M, molecular weight marker (50 bp); lane 1, G. duodenalis positive control (genotype AII), lanes 2-3, genotype B; lane 4, mixed genotype AII and B; lane 5, genotype AII
Fig. 3.
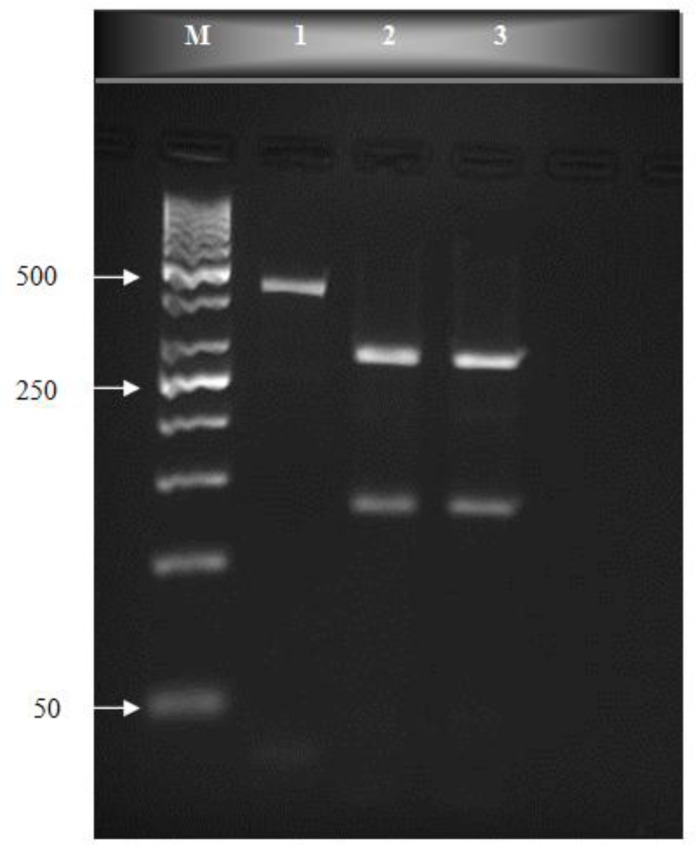
Rsa1 digestion of gdh-PCR products on an ethidium bromide-stained 3% high resolution grade agarose gel. lane M, molecular weight marker (50 bp); lane 1, PCR products (432 bp fragment); lanes 2-3, genotype B group BIII
Table 1.
Predicted fragment sizes (bp) of G. duodenalis assemblages after digesting with BspL1 and Rsa1
| Assemblage | Enzyme | Predicted fragment sizes | Diagnostic genotyping profile |
|---|---|---|---|
| AI | BspL1 | 16, 18, 39, 87, 123, 149 | 90, 120, 150 |
| AII | BspL1 | 16, 18, 39, 72, 77, 87, 123 | 40,70, 80, 90, 120 |
| B | BspL1 | 18, 39, 123, 291 | 120, 290 |
| BIII | RsaI | 2,133, 297 | 130, 300 |
| BIV | RsaI | 12, 430 | 430 |
Fifty nine percent had mixed genotypes AII and BIII, defined by the presence of DNA bands at 40, 70, 80, 90, 120 and 290 bp. Twenty seven (27%) cases had assemblages BIII, defined by the presence of DNA bands at 130 and 300 bp and fourteen (14%) cases had assemblage AII, determined by the presence of DNA bands at 70, 80, 90 and 120 bp (Figs. 2, 3 and Table 1). Among symptomatic samples 27 (54%) cases contained mixed genotypes, 14 (28%) samples contained assemblage BIII and 9 (18%) samples indicated assemblage AII. On other hand among the 50 asymptomatic cases 32 (64%) samples had mixed genotypes AII and BIII, 13 (26%) samples were BIII and 5 (10%) samples showed assemblage AII.
There was no statistically significant correlation between assemblages and clinical symptoms (κ2=1.6, df=2, P=0.45). Intensity rate of infection in symptomatic patients (median: 3.55) was also more significantly than the asymptomatic patients (median: 2.4) (P=0.013).
Discussion
Human giardiasis is a global disease that is caused by two major genetic assemblages A and B, of G. duodenalis (1). In current study, assemblage BIII was detected in 28% and 26% of symptomatic and asymptomatic patients and assemblage AII was detected in 18% and 10% of the same samples. Studies conducted in different parts of Iran indicated, that the most prominent types of assemblages were AII and BIII but with different prevalence rates (25–29). Clinical symptoms of this parasite are highly variable. Host factors and parasite strains are probably involved in different types of pathogenesis (5, 30). Studies on the correlation between assemblages and clinical symptoms have controversial results.
In our study, there was no correlation between the clinical symptoms and assemblages. These results correspond with those from studies carried out in Fars Province in southern of Iran by Sarkari et al. (28). They mentioned that assemblages A and B caused similar clinical symptoms. In contrast, in Kerman, central south of Iran, assemblage B was more significantly common among symptomatic patients (26). Assemblage B was equally distributed among symptomatic and asymptomatic patients and there was no correlation between assemblage B and clinical symptoms (21). Similarly, there was no relation between clinical presentation and genotypes of G. duodenalis in Brazilian children (20). There was no significant correlation between assemblages and clinical symptoms in older ages greater than 5 years in Spain (31).
In contrast, in Saudi Arabia, Hamdan reported that there was a correlation between assemblage B and symptomatic infection (32). Similarly, Mohammed Mahdy et al. (15) and Pelayo et al found that assemblage B was more significantly common among symptomatic patients (33). Assemblage B was more present in symptomatic patients (34). In other hand, some researchers reported correlation between assemblage A and symptomatic infection, and assemblage B with asymptomatic cases (16–19). In our study, symptomatic patients had more intensity rate of cysts than asymptomatic cases which may indicate higher parasite activity in symptomatic patients.
To our best knowledge, this is the first study that divided studied populations into children and adults in equal numbers compromising symptomatic and asymptomatic groups. Some earlier studies also reported a similar dual infection, but according to our literature review, it seems that the rate of mixed infection with genotypes AII and BIII, in our report is higher than others (25, 28, 31, 32, 35–39). This higher rate of dual infection among symptomatic and asymptomatic cases which is not in agreement with most previous investigation may play a role as a confounding variable for such study. We do not have a clear explanation for this higher rate of mixed infection in the region. It seems further study are needed to provide a better interpretation of the occurrence of mixed infections. Additionally, it is important to carry out studies regarding human host factors and possible correlation with clinical symptoms.
Conclusion
According to current results and previous studies any correlation between giardiasis and assemblages remains unclear and further researches with more details are needed regarding clear interpretation.
Acknowledgements
This paper is prepared from thesis of Miss Elham Roointan and financial support was provided by Research center of Tropical and Infectious Diseases of Ahvaz Jundishapur University of Medical Sciences. We also thanks the health centers staffs and all patients who participated in the survey undertaken. The authors declare that there is no conflict of interest.
References
- 1.Adam RD. Biology of G. duodenalis. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, Xiao L. Zoonotic Potential and Molecular Epidemiology of Giardia Species and Giardiasis. Clin Microbiol Rev. 2011;24:110–40. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molavi G, Mir Ahmadi H, Rezaeian M, Beigom Kia E, Daryani N, Rokni MB, Golestan B, Shafiei R, Fereshtehnejad SM, Keramati MR. Excerpts from persian medical literature. The prevalence of intestinal parasites in tribal parts of Khuzestan province. Arch Iranian Med. 2009;12:97–9. [Google Scholar]
- 4.Thaherkhani H, Shariati S, Abdolahi N, Roshandel G. Clinical manifectations of giardiasis in Iran. J Clin Diag Res. 2009;3:1418–26. [Google Scholar]
- 5.Farthing MJ. The molecular pathogenesis of giardiasis. J Pediatr Gastroenterol Nutr. 1997;24:79–88. doi: 10.1097/00005176-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Thampson RCA. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. 2000;30:1259–67. doi: 10.1016/s0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 7.Aucott J. Nelson text book of pediatrics. 14th ed. WB Sanunders Co; 1996. [Google Scholar]
- 8.Thompson RCA, Monis PT. Variation in Giardia Implication for taxonomy and epidemiology. Adv Parasitol. 2004;58:69–137. doi: 10.1016/S0065-308X(04)58002-8. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RCA. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Lasek-Nesselquist E, Welch DM, Sogin ML. The identification of a new G. duodenalis assemblage in marine vertebrates and a preliminary analysis of G. duodenalis population biology in marine systems. Int J Parasitol. 2010;40:1063–74. doi: 10.1016/j.ijpara.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monis PT, Andrews RH, Mayrhofer G, Ey PL. Molecular systematics of the parasitic protozoan Giardia intestinalis. Mol Biol Evol. 1999;16:1135–44. doi: 10.1093/oxfordjournals.molbev.a026204. [DOI] [PubMed] [Google Scholar]
- 12.Monis PT, Mayrhofer G, Andrews RH, Homan WL, Limper L, Ey PL. Molecular genetic analysis of Giardia intestinalis isolates at the glutamate dehydrogenase locus. Parasitol. 1996;112:1–12. doi: 10.1017/s0031182000065021. [DOI] [PubMed] [Google Scholar]
- 13.Read CM, Monis PT, Thompson CA. Discrimination of all genotypes of G. duodenalis at the glutamate dehydrogenase locus PCR-RFLP. Infect Genet Evol. 2004;4:125–30. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.14- Homan W, Mank T. Human giardiasis: genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–26. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed Mahdy AK, Surin J, Wan KL, Mohd-Adnan A, Al-Mekhlafi MS, Lim YA. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 2009;112:67–70. doi: 10.1016/j.actatropica.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Read CM, Walters J, Robertson ID, Thompson RCA. Correlation between genotype of G. duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–31. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 17.Aydin AF, Bessirbellioglu BA, Avci IS, Tanykuksel M, Araz E, Pahsa A. Classification of G. duodenalis parasites in Turkey into Group A and B using restriction fragment length polymorphism. Diag Microbiol Infect Dis. 2004;50:147–51. doi: 10.1016/j.diagmicrobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infectionand diarrhea in Bangladesh. J Infect Dis. 2005;192:2171–73. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 19.Cordon PG, Saldon OCP, Vasquezas VF, Soto JR, Bordes LS, Moreno MS, Rosales MJ. Prevalence of enteroparasites and genotyping of G. duodenalis in Peruvian children. Parasitol Res. 2008;103:459–65. doi: 10.1007/s00436-008-1007-3. [DOI] [PubMed] [Google Scholar]
- 20.Kohli A, Bushin OY, Pinkerton RC, Houpt E, Newman RD, Sears CL, Lima AA, Guerrant RL. G. duodenalis assemblage, Clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg. 2008;102:718–25. doi: 10.1016/j.trstmh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajjampur SSR, Sankaran P, Kannan A, Sathyakumar k, Sarkar R, Gladstone B, Kang G. G. duodenalis Assemblages associated with diarrhea in children in south Indiaidentified by PCR-RFLP. Am J Trop Med. 2009;80:16–19. [PMC free article] [PubMed] [Google Scholar]
- 22.Breathnach AS, McHugh TD, Butcher PD. Prevalence and clinical correlations of genetic subtypes of G. duodenalis in an urban setting. Epidemiol Infect. 2010;138:1459–67. doi: 10.1017/S0950268810000208. [DOI] [PubMed] [Google Scholar]
- 23.Garcia LS. Diagnostic Medical Parasitology. 5th ed. Washington DC: American Society for Microbiology Press; 2007. [Google Scholar]
- 24.Barazesh A, Majidi J, Fallah E, Ghazanchaii A. Camparison of three different methods for concentration and purification of Giardia cyst. Yafte J. 2006;8:71–6. [Google Scholar]
- 25.Babaei Z, Oormazdi H, Akhlaghi L, Rezaie S, Razmjou E, Soltani-Arabshahi SK, Meamar AR, Hadighi R. Molecular characterization of the Iranian isolated of Giardia lamblia: application of the glutamate dehydrogenase gene. Iranian J Publ Health. 2008;37:75–82. [Google Scholar]
- 26.Etemadi S, Zia-Ali N, Babaei Z, Fasihi-Harandi M, Zia-Ali A, Salari Z, Kamyabi H. The Correlation between Clinical Signs and Genotypes of G. duodenalis Isolated from Patients with Giardiasis in Kerman City. J Kerman Univ Med Sci. 2011;18:330–38. [Google Scholar]
- 27.Hatam-Nahavandi k, Fallah E, Asgharzadeh M, Mirsamadi N, Mahdavipour B. Glutamate dehyrogenase and triose-phosphate-isomerase coding genes for detection and genetic cheracterization of Giardia lamblia in human feces by PCR and PCR-RFLP. Turk J Med Sci. 2011;41:283–89. [Google Scholar]
- 28.Sarkari B, Ashrafmansori A, Hatam GR, Motazedian MH, Asgari Q, Mohammadpour I. Genotyping of Giardia lamblia isolates from human in southern Iran. Trop Biomed. 2012;29:366–71. [PubMed] [Google Scholar]
- 29.Roointan ES, Rafiei A, Samarbaf-Zadeh AR, Shayesteh AA, Shamsizadeh A, Pourmahdi Borujeni M. Molecular identification of Giardia lamblia isolates from adult human cases in southwest of Iran. African J Biotech. 2013;12:901–6. [Google Scholar]
- 30.Ivanov AI. Giardia and Giardiasis. Bulg J Vet Med. 2011;13:65–80. [Google Scholar]
- 31.Sahagun J, Clavel A, Goni P, Seral C, Llorente MT, Castillo FJ. Correlation between the presence of symptoms and the G. duodenalis genotype. Eur J Clin Microbiol Infect Dis. 2008;27:81–3. doi: 10.1007/s10096-007-0404-3. [DOI] [PubMed] [Google Scholar]
- 32.Hamdan AM. Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi childern. Parasitol Res. 2011;108:1375–81. doi: 10.1007/s00436-010-2033-5. [DOI] [PubMed] [Google Scholar]
- 33.Pelayo L, Nunez F, Rojas L, Furuseth E, Gjerde B, Wilke H, Mulder B, Robertson L. Giardia infection in Cuban childern: the genotypes circulating in a rural population. Ann Trop Med Parasitol. 2008;102:585–95. doi: 10.1179/136485908X355247. [DOI] [PubMed] [Google Scholar]
- 34.Gelanew T, Lalle M, Hailu A, Pozio E, Caccio SM. Molecular characterization of human isolates of G. duodenalis from Ethiopia. Acta Trop. 2007;102:92–9. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Amar CF, Dear PH, Pedraza-Diaz S, Looker N, Linnane E, McLauchlin J. Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of G. duodenalis in human feces. J Clin Microbiol. 2002;40:446–52. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guy RA, Xiao C, Horgen PA. Real-time PCR assay for detection and genotype differentiation of G. duodenalis in stool specimens. J Clin Microbiol. 2004;42:3317–20. doi: 10.1128/JCM.42.7.3317-3320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yason J, Rivera W. Genotyping of G. duodenalis isolates among residents of slum area in Manila, Philippines. Parasitol Res. 2007;101:681–87. doi: 10.1007/s00436-007-0533-8. [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Janaki L, Petri WA, Houpt ER. Giardia intestinalis assemblages A and B infections in Nepal. Am J Trop Med Hyg. 2009;81:538–39. [PMC free article] [PubMed] [Google Scholar]
- 39.Tungtrongchitr A, Sookrung N, Indrawattana N, Kwangsi S, Ongrotchanaku J, Cjaicumpa W. Giardia intestinalis in Thailand: identification of genotypes. J Health Popul Nutr. 2010;28:42–52. doi: 10.3329/jhpn.v28i1.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]