Abstract
Background
In view of the massive rural-to-urban migration in Nigeria, investigations on transmission of urinary schistosomiasis were carried out in urban and semi-urban communities in Nike Lake area of Enugu State, Nigeria.
Methods
Urine samples of school children were tested for micro-haematuria using reagent strips followed by microscopic examination for Schistosoma haematobium eggs. Water contact sites were also identified and sampled for snails.
Results
The overall prevalence of S. haematobium eggs in school children was 4.64%. The mean intensity of infection was 1.14 ± 0.41 eggs/10ml urine. Males had insignificantly higher prevalence and intensity of S. haematobium infection than females. The youngest age group (4-7 years) had no infection. The prevalence of micro-haematuria (6.2%) was higher than that of microscopy, and this correlated positively with prevalence (r = 0.65, P < 0.01) and intensity (r = 0.50, P < 0.01) of the infection. Potential intermediate host of human shistosome collected were: Bulinus globosus, B. senegalensis and Biomphalaria pfeifferi. However, only B. globosus shed cercariae of S. haematobium, with a snail infection rate of 0.73%. Transmission was in the dry season coinciding with the drying of wells.
Conclusion
The results revealed that urinary schistosomiasis is prevalent, and that B. globosus and not B. truncatus as previously reported is the main intermediate host of urinary schistosomiasis in this part of Enugu State.
Keywords: Prevalence, S. haematobium, Bulinus globosus, Micro-haematuria, Nigeria
Introduction
Schistosomiasis is one of the prevalent human parasitic diseases in the world, second only to malaria in terms of socio-economic and public health importance. It is endemic in 76 countries in the tropics and subtropics, and is estimated that there are in excess of 200million people infected, with children being at particular risk (1). In sub-Saharan Africa, Nigeria has the largest number of disability-adjusted life years (DALY) lost to schistosomiasis (2). The current control strategy recommended by the WHO is to control morbidity due to schistosomiasis by mass drug administration (MDA) with praziquantel, targeting mainly school-age children and adult at high risk of infection (3). To ensure success of MDA programme in Nigeria, there is need to update information regarding the prevalence and distribution of urinary schistosomiasis in the country (4).
In Enugu State Nigeria, records of urinary schistosomiasis are mostly from rural areas. A prevalence rate of 79% was reported for S. haematobium infection in Amangunze village in Enugu State, Nigeria in 1989 by (5), with Bulinus truncatus as the intermediate host. In 1994 and 1995, the prevalence of the infection in Amangunze ranged from 46.6 to 76.0% (6). In Obollo-Eke, a rural community in the State, the prevalence of S. haematobium infection was 17.5% while the prevalence of haematuria was 15.6%. The snail host species was not highlighted in the study (7).
In view of the current massive rural-to-urban migration in Nigeria, our study reports the status of urinary schistosomiasis in urban and semi-urban parts of Enugu State, Nigeria.
Material and Methods
Study area
Nike Lake area is Nkwo Nike the headquarters of Enugu East Local government area (L.G.A) of Enugu State, Nigeria. Nkwo Nike lies between longitudes 70 36’ and 70 41'E and latitudes 60 31’ and 60 36'N and is made up of two urban (Amorji and Trans-Ekulu) and four semi-urban communities (Ibagwa, Mbuluowehe, Edem and Amokpo). Nkwo Nike has tropical rain forest vegetation. The climate is humid and the humidity is highest between March and November. The area has two weather conditions of rainy and dry seasons. The average rainfall during rainy season is around 400 mm. One characteristic geographical feature of Nkwo Nike is the presence of a Lake called Nike Lake situated in Amorji. Close to the Lake is the popular Nike Lake resort. From the six communities, three communities were randomly chosen, Amorji, Ibagwa and Amopko. Sources of water in these communities are water from tankers, who get water from bore-hole, and wells but these wells dry up in dry season. Petty trading, civil service and farming are the main occupation of those living in these communities. There is no treatment history and knowledge of urinary schistosomiasis in Nkwo Nike.
Study population and subject selection
The study is a cross-sectional study conducted from January to March, 2012. Ethical permission was obtained from the Enugu State Educational Board. Informed consent was obtained from parent or guardian of school children one week before sample collection. The community primary school in the three communities was sampled. School children were randomly selected and only those 4-15years were recruited into the study. School children within this age group who declined after selection were excluded.
Sample collection and analysis
Urine samples were collected from school children in well labelled, clean, wide mouthed containers with screw caps between 10:00am and 2:00pm. The samples were immediately moved to the laboratory of the Department of Zoology and Environmental Biology, University of Nigeria. The presence of blood and protein in urine were determined using reagent strips (Medi-test Combi-9, manufactured by Machery-Hagel, Duren, Germany). Urine samples were gently agitated and 10ml drawn out using disposable syringe. The 10ml urine samples were concentrated by sedimentation for 30mins and supernatant carefully drawn without disturbing the sediment. The entire sediment was examined under the microscope and egg counts were recorded. Cases of schistosomiasis were defined as children with at least one S. haematobium egg on microscopic examination of urine. Intensities of S. haematobium infection was categorized as light (<50eggs/10ml urine) and heavy (≥50 eggs/ 10ml urine) infections (8). Infected children were given 40 mg/kg praziquantel.
Snail sampling
Water contact sites in these communities were identified and sampled for snails for 12months using fine mesh kitchen strainers. Where necessary, snails were hand-picked and precautions taken by using a pair of gloves. The snail sampling was done by two collectors for 30minutes and the number of snails collected were counted and recorded. The first site is Nike Lake, which approximately 0.5km from the community school and residential areas in Amorji. The Lake is approximately 2km from Ibagwa and 1.5km from Amokpo. The second site is Iheugba, a stream that dries up during dry season. This stream is approximately 10km away from Amorji, 1km way from Ibagwa and 7km away from Amokpo. Iyi-oku is a river, with a distance of approximately 0.2km from Ibagwa, 1.5km from Amorji and 4km from Amokpo. The fourth site is Ava, a river that flows very fast in rainy season and cuts across Amorji and Amokpo, and is 3km from Ibagwa. After collection, snails were taken to the laboratory where they were identified using the field guide to West African fresh water snails prepared by the Danish Bilharziasis Laboratory, Denmark (9). Schistosome intermediate host identified were screened for cercariae using the shedding and crushing methods. Using the shedding method, snails were placed individually into flat bottom glass vials containing clean water and exposed to light for four hours. Snails that did not shed cercariae were pressed and crushed between two microscope slides. Cercariae were identified as described by (10, 11).
Data analysis
Version 17.0 of the SPSS for windows software was used to analyse data. Age of school children was classed into three: 4-7years, 8-11years and 12-15years. Significant difference in the prevalence of infection by age and gender were determined using chi-square (X2) test. Significant differences in mean egg count between age groups and gender were determined using one-way analysis of variance (ANOVA) and t-test respectively. Relationship between presence and intensity of S. haematobium eggs with micro-haematuria was determined using Pearson Correlation. All analysis was done at P < 0.05.
Results
Parasitological findings
A total of 323 (9.02 ± 2.86 years) school children were examined, comprising 48.61% females (n= 157, 8.89 ± 2.71years) and 51.39% males (n = 166%, 9.14 ± 3.00 years). Children 4-7years of age were 16 (4.95%) while those 8-11 and 12-15years were 156 (48.30%) and 151 (46.75%) respectively.
Overall prevalence of urinary schistosomiasis was 4.64% (n = 15). The prevalence of the infection was insignificantly higher (Χ2=0.024, P =1.0) in males (4.8%, n = 8) than in females (4.5%, n = 7). Schistosoma haematobium infection was not detected in the age group 4-7years while the prevalence of the infection in those 8-11 and 12-15years was 4.5% (n = 7) and 5.3% (n = 8) respectively. The difference in prevalence in age groups was also not significant (X2=0.934, P = 0.627) (Table 1).
Table 1.
Prevalence and intensity of S. haematobium infection among school children stratified by gender and age
| NO. examined | Positivity n (%) | Intensity n (%) | |||
|---|---|---|---|---|---|
| Gender | Mean (SD) egg/10ml urine | Heavy infection | light infection | ||
| Males | 166 | 8(4.8) | 1.22(0.60) | 2 (1.2) | 6 (3.6) |
| Females | 157 | 7(4.5) | 1.06 (0.56) | 1 (0.6) | 6 (3.8) |
| Age group(yr) | |||||
| 4-7 | 16 | 0(0) | 0 (0) | 0 (0) | 0(0) |
| 8-11 | 156 | 7(4.5) | 1.39 (0.71) | 2 (1.3) | 5 (3.2) |
| 12-15 | 151 | 8(5.3) | 0.99 (0.49) | 1 (0.7) | 7 (4.6) |
| Total | 323 | 15(4.6) | 1.14 (0.41) | 3 (0.9) | 12 (3.7) |
The mean ova count of school children was 1.14 ± 0.41 eggs/10ml urine. Although the mean ova count of males (1.22 ± 0.60 eggs/10ml urine) was more than that of the females (1.06 ± 0.56 eggs/10ml urine), there was no significant difference (P = 0.86) in their mean ova count. Children 8-11yrs had higher mean ova count than those 12-15yrs. However, no difference in mean ova count was observed statistically (P = 0.73). The proportion of school children with light infections was 3.7% while only 0.9% of the school children were heavily infected. Females (3.8%) had light infections than males (3.6%) while heavy infections were more prevalent in males (1.2%) than in females (0.6%). In the age groups, those 8-11 years had more heavy infection than those 12-15years while the reverse was observed with light infections (Table 1).
The prevalence of micro-haematuria (6.2%, n = 20) was higher than the prevalence of S. haematobium infection determined by microscopy. The prevalence of micro-haematuria in females and males were 6.4% (n = 10) and 6.0% (n = 10). Micro-haematuria was not detected in urine of children 4-7years. The prevalence of micro-haematuria in age group 8-11 and 12-15years was 8.3% (n = 13) and 4.6 (n = 7), respectively. Micro-haematuria correlated significantly with both the prevalence (r = 0.615, P<0.0001) and intensity (r = 0.5, P<0.0001) of S. haematobium eggs.
Snail sampling
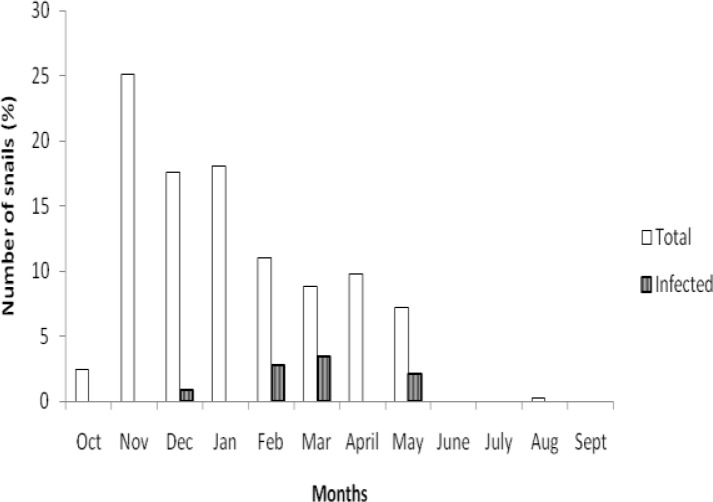
In Nike Lake, Bulinus globosus, B. senegalensis, Lymnaea natalensis and Biomphalaria pfeifferi were found. Snail composition in Ava River was L. natalensis and B. globosus. In Iyi-oku, Lanistes spp was the only snail found while in Iheugba stream no snail was found. A total of 677 B. globosus and 180 B. senegalensis were collected from the area. Only B. globosus shed Schistosoma cercariae, with snail infection rate of 0.73%. Infected snails were found in December, February, March and May (Fig. 1).
Fig. 1.
Monthly variation in the number of B. globosus collected and infected with S. haematobium in Nike Lake area of Enugu State
Discussion
The prevalence rate of 4.6% confirms that S. haematobium infection is also prevalent in this urban part of Enugu State although the prevalence and intensity is lower than previously reported in rural areas of the State. However, similar low prevalence has been reported in some primary schools in the urban city of Ibadan, south-western, Nigeria (12).
The insignificant higher prevalence and intensity of infection in males than females still depict the male bias that exists in schistosome infection despite the low prevalence of infection. This may be attributed to higher cercarial exposure in males as opposed to any kind of sex differences in biological mechanism of resistance or egg output (13). Similar results of no significant difference in the prevalence of S. haematobium infection between males and females have been reported in neighbouring Anambra State by (14, 15).The zero prevalence found in school-children 4-7yr could be attributed to the fact that this age group only accompany the older ones to the open water bodies but are not actively involved in activities that take place at the water contact sites because of their age and fear of drowning.
The use of blood detected by reagent strip has been considered an alternative in the diagnosis of S. haematobium infection. As observed in the present study, blood in urine has been shown to have significant positive correlation with presence and intensity of the infection because the quantity of blood passed out increases with intensity of infection (16, 17). The higher prevalence of S. haematobium infection using micro-haematuria as diagnostic marker suggests the daily variation of S. haematobium egg excretion in infected individuals (18).
Bulinus truncatus was implicated as intermediate host of S. haematobium infection in Amangunze, Enugu State (5) but in the present study no B. truncatus was collected while the S. haematobium shedding snail host collected is B. globosus. The B. senegalensis collected did not shed Schistosoma cercariae. However, in Senegal River Basin, B. senegalensis has been identified as the intermediate host of urinary schistosomiasis (19). Consistent with the result from Amagunze village in Enugu State (5), the Biom. pfeifferi collected did not shed schistosome cercariae. The shedding of S. haematobium cercariae from Bulinus globosus was mostly in the dry season, which coincides with when wells dry up. It has been observed that the prevalence of digenean cercariae is usually higher in dry season than rainy season. Possibly, due to low volume of water in conjunction with increased density of snail host and intensified use of habitat by definitive host (20).
Conclusion
Urinary schistosomiasis is prevalent in Nike Lake area of Enugu State. Pipe-borne water source should be made functional especially during dry season. Furthermore, urban areas as this should not be neglected in MDA control programmes. There is also need for community health education on this neglected tropical disease in the area as most people have no knowledge of the disease, the intermediate host and the causative organism.
Acknowledgement
We are grateful to Chief Poly Emenike, CEO of Neros Pharmaceuticals, who funded and provided drugs for treating infected children. We are also grateful to Engr. and Dr S.N. Chikwendu for the support provided during the course of this work. We acknowledge the parents and teachers of community primary schools used for the study. We also appreciate the school children used in the study for their consent. The authors declare that there is no conflict of interest.
References
- 1.Southgate VR, Rollinson D, Tchuente TLA, Hagan P. Towards control of schistosomiasis in sub-Saharan Africa. J Helminthol. 2005;79:181–185. doi: 10.1079/joh2005307. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Kamath A. Neglected tropical diseases in Sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. Plos Negl Trop Dis. 2009;3(8):412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zang Y, MacArthur C, Mubila L, Baker S. Control of neglected tropical diseases needs a long-term commitment. BMC Medicine. 2010;8(67):2–9. doi: 10.1186/1741-7015-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson E, Picon D, Sturrock HJ, Sabasio A, Lado M, Kolaczineki J, Brooker S. The performance of haematuria reagent strips for the rapid mapping of urinary schistosomiais: field experience from Sudan. Trop Med Inter Health. 2009;14(2):1484–1487. doi: 10.1111/j.1365-3156.2009.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozumba NA, Christensen NO, Nwosu ABC, Nwaorgu OC. Endemicity, Focalitu and Seasonality of transmission of human schistosomiasis in Amagunze village, Eastern Nigeria. J Hel-minthol. 1989;63:206–212. doi: 10.1017/s0022149x00008993. [DOI] [PubMed] [Google Scholar]
- 6.Ezewanji NE, Okafor FC. Protein excretion in urine during Schistosoma haematobium infection. Animal Res Inter. 2007;4(3):762–765. [Google Scholar]
- 7.Ogbonna CC, Dori GU, Nweze EI, Muoneke G, Nwankwo IE, Aputa N. Comparative analysis of urinary schistosomiasis among primary school children and rural farmers in Obollo-Eke, Enugu State, Nigeria: implications for control. Asian Pac J Trop Med. 2012;5(10):796–802. doi: 10.1016/S1995-7645(12)60146-1. [DOI] [PubMed] [Google Scholar]
- 8.Montresor A, Crompton DWT, Gyorkos TW, Savoli L. Helminth control in school-age children: a guide for managers of control programme; Geneva: World Health Organization; 2002. [Google Scholar]
- 9.Brown DS, Kristensen T.K. Denmark: Danish Bilharziasis Laboratory; 1993. A field guide to African freshwater snails. 1. West African species. [Google Scholar]
- 10.Cheng TC. General parasitology. 2nd ed. Singapore: Harcourt Brace & Company Asia PTE LTD; 1999. [Google Scholar]
- 11.Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snail with special reference to cercariae of veterinary importance. Acta Trop. 1984;41(2):181–202. [PubMed] [Google Scholar]
- 12.Okoli EI, Odaibo AB. Urinary schistosomiasis among school children in Ibadan, an urban community in South-western Nigeria. Trop Med Inter Health. 1999;4(4):308–315. doi: 10.1046/j.1365-3156.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- 13.Rudge JW, Stothard JR, Basanez M, Mgeni AF, Khamis IS, Khamis AN, Rollinson D. Micro-epidemiology of urinary schistosomiasis in Zanzibar: local risk factor associated with distribution of infections among school children and relevance for control. Acta Trop. 2008;105:45–54. doi: 10.1016/j.actatropica.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Emejulu AC, Alabaronye FF, Ezenwanji HMG, Okafor FC. Investigation into theprevalence of urinary schistosomiasis in the Agulu Lake area of Anambra State, Nigeria. JHelminthol. 1994;68:119–123. doi: 10.1017/s0022149x00013638. [DOI] [PubMed] [Google Scholar]
- 15.Amazigo UO, Anago-Amanze CI, Okeibunor JC. Urinary schistosomiasis among school children in Nigeria: consequences of indigenous beliefs and water contact activities. J Biosoc Sci. 1997;29:9–18. doi: 10.1017/s0021932097000096. [DOI] [PubMed] [Google Scholar]
- 16.Hammad TA, Gabr NS, Talaat MM, Orieby A, Shawky E, Strickland GT. Haematuria and proteinuria as predictors of Schistsoma haematobium infection. Am J Trop Med Hyg. 1997;57(3):363–367. doi: 10.4269/ajtmh.1997.57.363. [DOI] [PubMed] [Google Scholar]
- 17.Ugbomoiko US, Obiezue RNN, Ogunniyi TAB, Ofoezie IE. Diagnostic accuracy of different urine dipsticks to detect urinary schistosomiasis: a comparative study in five endemic communities in Osun and Ogun States, Nigeria. J Helminthol. 2009;83:203–209. doi: 10.1017/S0022149X08133570. [DOI] [PubMed] [Google Scholar]
- 18.Houmsou RS, Kela SL, Suleiman MM. Performance of micro-haematuria and proteinuria as measured by reagent strip in estimating intensity and prevalence of S. haematobium infections in Nigeria. Asian Pac J Trop Med. 2001;4(12):997–1000. doi: 10.1016/S1995-7645(11)60233-2. [DOI] [PubMed] [Google Scholar]
- 19.Picquet M, Ernould JC, Vercruysse J, Southgate VR, Mbaye A, Sambou B, Niang M, Rollison D. The epidemiology of human schistosomiasis in Senegal river basin. T Roy Soc Trop Med H. 1996;90:340–346. doi: 10.1016/s0035-9203(96)90501-5. [DOI] [PubMed] [Google Scholar]
- 20.Nkwengulola G, Kigadye ESP. Occurrence of digenean larvae in freshwater snails in the Ruru Basin, Tanzania. Tanz J Sci. 2005;31(2):23–30. [Google Scholar]