Abstract
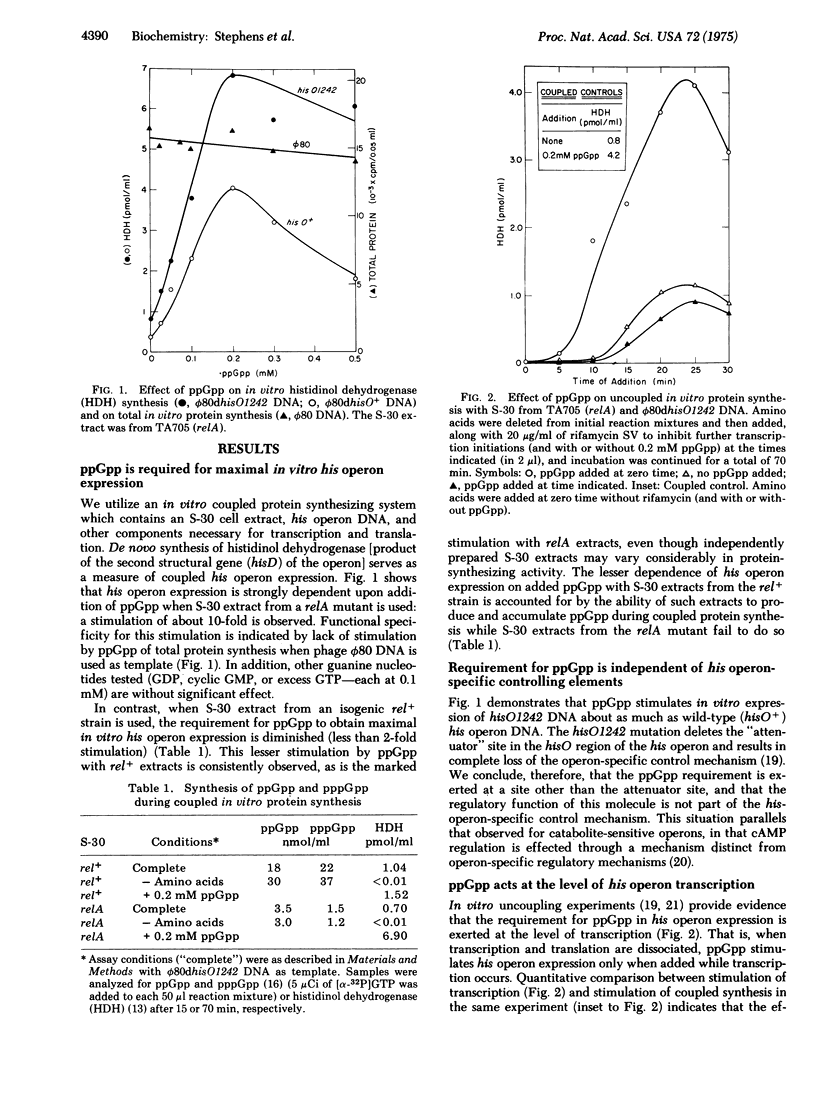
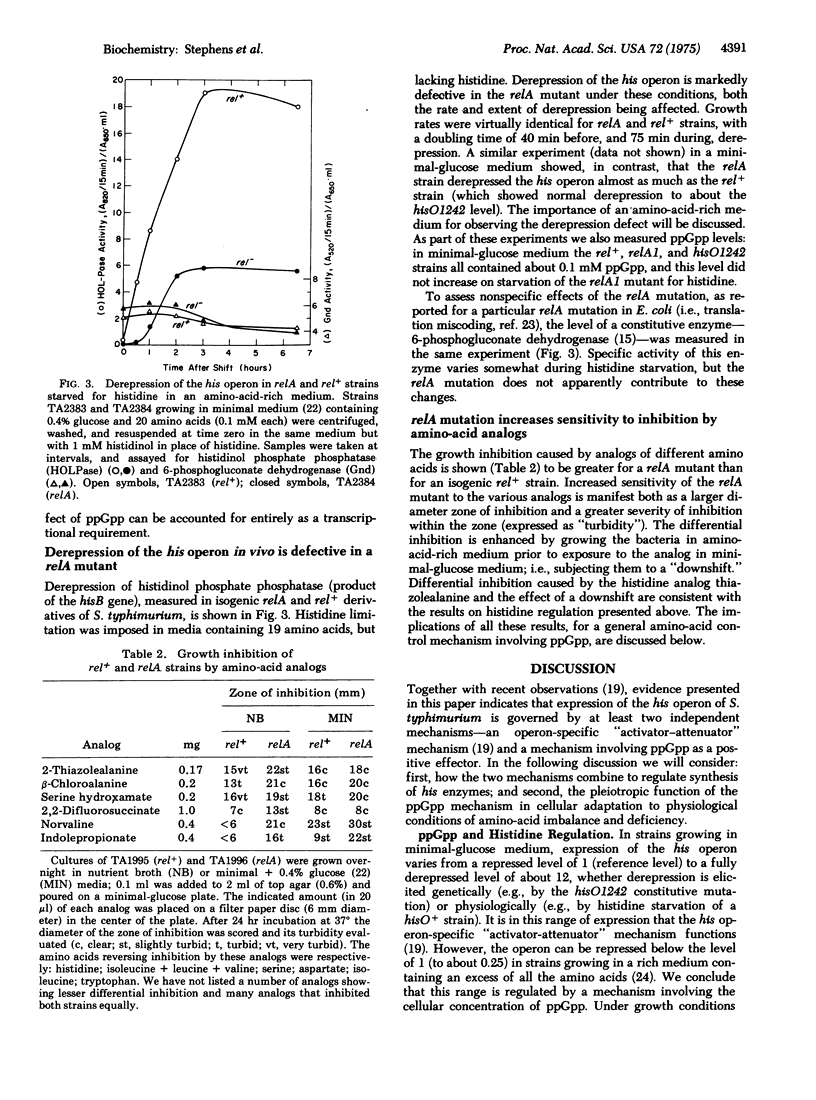
Maximal expression of the histidine operon of Salmonella typhimurium in a coupled in vitro transcription-translation system is strongly dependent upon addition of guanosine 5'-diphosphate 3'-diphosphate (ppGpp). This requirement for ppGpp is exerted at the level of transcription through a mechanism distinct from the his-operon-specific regulatory mechanism. In vivo derepression of the his operon is markedly defective when histidine starvation is imposed on a relA mutant--unable to rapidly increase synthesis of ppGpp--growing in amino-acid-rich medium. Increased sensitivity of relA mutants to growth inhibition by a number of amino-acid analogs suggests that ppGpp is generally important in adjusting expression of amino-acid-producing systems. Analysis of these findings leads us to propose that ppGpp is a positive effector in a system that enables the cell to balance endogenous amino-acid production with environmental conditions of amino-acid availability, and to compensate efficiently for transient changes in these conditions. We propose a unifying theory of the role of ppGpp as the general signal molecule (alarmone) in a "super-control" which senses an amino-acid deficiency and redirects the cell's economy in response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Cyclic 3', 5'-adenosine monophosphate phosphodiesterase mutants of Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1081–1090. doi: 10.1128/jb.122.3.1081-1090.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Camakaris J., Pittard J. Repression of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) and enzymes of the tryptophan pathway in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):406–414. doi: 10.1128/jb.107.2.406-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Control of RNA synthesis in Escherichia coli. I. Amino acid dependence of the synthesis of the substrates of RNA polymerase. J Mol Biol. 1968 Jul 14;34(2):317–330. doi: 10.1016/0022-2836(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Cashel M. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem. 1974 Jan;57(1):100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Cieslà Z., Salvatore F., Broach J. R., Artz S. W., Ames B. N. Histidine regulation in Salmonella typhimurium. XVI. A sensitive radiochemical assay for histidinol dehydrogenase. Anal Biochem. 1975 Jan;63(1):44–55. doi: 10.1016/0003-2697(75)90187-6. [DOI] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Gottesman M., Pastan I., Varmus H. E., Emmer M., Perlman R. L. Regulation of lac mRNA synthesis in a soluble cell-free system. Nat New Biol. 1971 Mar 10;230(10):37–40. doi: 10.1038/newbio230037a0. [DOI] [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., von Meyenburg K. Synthesis and turnover of basal level guanosine tetraphosphate in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):304–309. [PubMed] [Google Scholar]
- Hall B., Gallant J. Defective translation in RC - cells. Nat New Biol. 1972 May 31;237(74):131–135. doi: 10.1038/newbio237131a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1104–1111. doi: 10.1016/s0006-291x(74)80092-6. [DOI] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Martin R. G. Polarity in relaxed strains of Salmonella typhimurium. J Mol Biol. 1968 Jan 14;31(1):127–134. doi: 10.1016/0022-2836(68)90060-0. [DOI] [PubMed] [Google Scholar]
- Murray M. L., Klopotowski T. Genetic map position of the gluconate-6-phosphate dehydrogenase gene in Salmonella typhimurium. J Bacteriol. 1968 Apr;95(4):1279–1282. doi: 10.1128/jb.95.4.1279-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J Mol Biol. 1972 Aug 14;69(1):103–118. doi: 10.1016/0022-2836(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Schumacher G., Ehring R. RNA-directed cell-free synthesis of the galactose enzymes of Escherichia coli. Mol Gen Genet. 1973 Aug 28;124(4):329–344. doi: 10.1007/BF00267662. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Tong B. Construction of phi80 dhis carrying Salmonella typhimurium histidine operon mutations. J Bacteriol. 1974 Dec;120(3):1223–1226. doi: 10.1128/jb.120.3.1223-1226.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Protein turnover in amino acid-starved strains of Escherichia coli K-12 differing in their ribonucleic acid control. J Biol Chem. 1969 Nov 25;244(22):6304–6306. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]



