Abstract
Background:
Patients with stage I testicular seminoma are typically diagnosed at a young age and treatment is associated with low relapse and mortality rates. The long-term risks of adjuvant radiotherapy in this patient group are therefore particularly relevant.
Methods:
We identified patients and obtained treatment details from 12 cancer centres (11 United Kingdom, 1 Norway) and ascertained second cancers and mortality through national registries. Data from 2629 seminoma patients treated with radiotherapy between 1960 and 1992 were available, contributing 51 151 person-years of follow-up.
Results:
Four hundred and sixty-eight second cancers (excluding non-melanoma skin cancers) were identified. The standardised incidence ratio (SIR) was 1.61 (95% confidence interval (CI): 1.47–1.76, P<0.0001). The SIR was 1.53 (95% CI: 1.39–1.68, P<0.0001) when the 32 second testicular cancers were also excluded. This increase was largely due to an excess risk to organs in the radiation field; for pelvic–abdominal sites the SIR was 1.62 (95% CI: 1.43–1.83), with no significant elevated risk of cancers in organs elsewhere. There was no overall increase in mortality with a standardised mortality ratio (SMR) of 1.06 (95% CI: 0.98–1.14), despite an increase in the cancer-specific mortality (excluding testicular cancer deaths) SMR of 1.46 (95% CI: 1.30–1.65, P<0.0001).
Conclusion:
The prognosis of stage I seminoma is excellent and it is important to avoid conferring long-term increased risk of iatrogenic disease such as radiation-associated second cancers.
Keywords: seminoma, radiotherapy, carboplatin, metastasis
Approximately 80% of patients with testicular seminoma present with stage I disease and the traditional management for many decades has been orchidectomy followed by adjuvant radiotherapy to abdominal lymph nodes (Hamilton et al, 1986; Zagars and Babaian, 1987; Fossa et al, 1989). An overview of 16 series including 2603 patients identified a relapse rate after radiotherapy of 4.4% and a seminoma mortality rate of 2.1% (Zagars, 1996). As the majority of patients are treated in young adult life and do not need chemotherapy, they represent a group in whom the long-term radiotherapy risks are relevant and are measurable where population health records are available. Surveillance studies suggest that about 18–20% of these patients have subclinical abdominal node metastases (Horwich et al, 1992; von der Maase et al, 1993; Warde et al, 1993) and thus have the potential to gain from adjuvant radiotherapy. As the great majority of patients are cured, there is concern over radiation carcinogenesis (van Leeuwen et al, 1993; Horwich and Bell, 1994; Travis et al, 1997; Richiardi et al, 2007; Hemminki et al, 2010) and over other possible late radiation effects (Zagars et al, 2004).
Previous large cohort studies of long-term cancer risk in patients cured of testicular cancer have been based mainly on Cancer Registry cases where diagnostic, staging and treatment data may be recorded less accurately than in the radiotherapy centre, so that potential confounding factors such as chemotherapy may be underestimated, and the extent of radiation fields may not have been recorded. An exception was the report from Zagars et al (2004) on 453 patients treated for early-stage seminoma with radiotherapy at the MD Anderson Cancer Center between 1951 and 1999, which found a life-shortening effect of radiotherapy when comparing a cured population of seminoma patients with age-matched male US population. Specific excess mortalities were found because of second cancers and cardiac disease and appeared after 15 years of follow-up.
Patients with stage I seminoma represent a group with low cancer recurrence risk, a long prognosis and a relatively low exposure to diagnostic ionising radiation, and are thus an ideal group in which to define the risks of radiotherapy. Following award of funding from the UK Medical Research Council and approval from the Royal Marsden Research Ethics Committee (Protocol No. 1131), we therefore undertook an analysis of second cancer risks in men with stage I seminoma identified and treated in 1 of 11 radiotherapy centres in the United Kingdom or one in Norway diagnosed between 1960 and 1992 when management policies in these centres were stable.
Materials and Methods
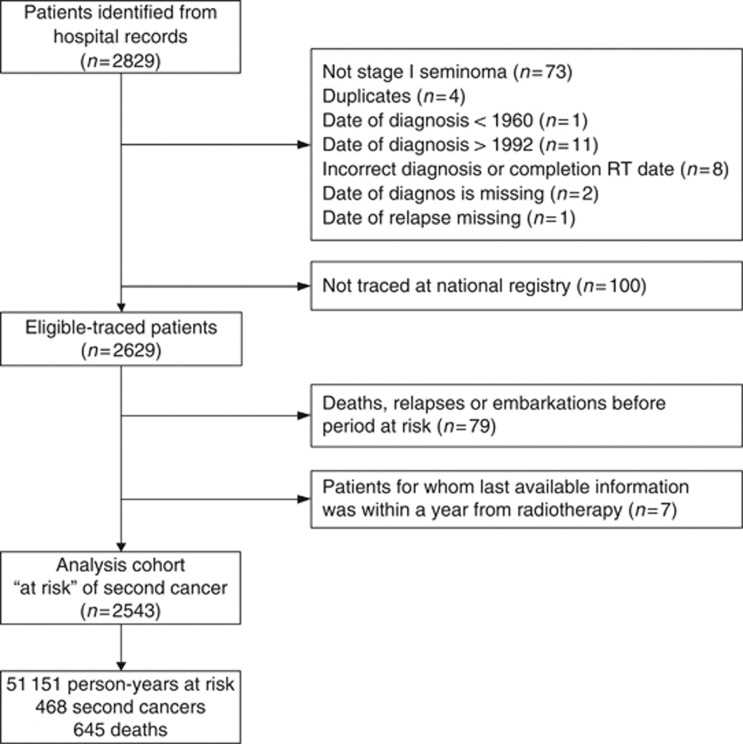
Two thousand eight hundred and twenty-nine patients were initially identified (UK 2058, Norway 771). Radiotherapy details were collected retrospectively from the treating radiotherapy centre. One hundred patients were excluded on review as they did not have stage I seminoma (n=73), were duplicates (n=4), were diagnosed outside the study period (n=12) or had missing/inconsistent dates of diagnosis, treatment or relapse (n=11) (Figure 1). Radiotherapy was administered from anterior and posterior portals with dosage specified at the mid-plane. The most common doses were 30 Gy, or 35/36 or 40 Gy given over 3–4 weeks, and for purposes of analysis doses were considered into three groups relating to these clusters. Overall follow-up was calculated from the date of diagnosis to death and was censored at 31 December 2007.
Figure 1.
Cohort summary.
Second cancers and deaths were ascertained by matching patients to national registries. In the United Kingdom, cancers were coded to ICD10 and in Norway to ICD7 up to 1992 and ICD10 thereafter. In the United Kingdom, patient details were sent to the NHS Information Centre's Medical Research Information Service for matching to national cancer registry data and ‘flagging' to provide details of cancer registrations, deaths and other exits from the NHS system (e.g. emigration). One hundred of the 2031 eligible UK patients could not be traced at the registry. These were in general born earlier (median 1938 vs 1964 Mann–Whitney test, P=0.003) and diagnosed earlier (median 1978 vs 1984, P<0.0001) than the rest of the UK cohort but there was no difference in age at diagnosis (37.1 vs 36.9, P=0.62). In Norway, the status of all 698 eligible patients to end 2007 was ascertained by the cancer registry.
For each subject, person-years at risk of developing a subsequent cancer, by 5-year age group and calendar year were estimated. To exclude potential second cancers missed at diagnosis of the primary tumour or those not potentially due to radiotherapy exposure, the at-risk period was deemed to commence 1 year after the start of radiotherapy. Patients who relapsed, died or were lost to follow-up at the hospital site before this date are excluded from all person-years based analyses. All patients were left-censored on 1 January 1971, which is the first date for which UK national cancer registration data are available, resulting in a loss of 1195 person-years of follow-up. Patients were right-censored at known relapse of germ cell cancer (obtained from hospital records), date of emigration (from registry) or death. Surviving patients were censored on 31 December 2007. Risk of solid cancers at common sites and at sites within/close to the radiation field were explored. For analyses relating to time since diagnosis of seminoma, subjects were allocated at each point in their follow-up to the analytic category applicable for that time.
Expected numbers of deaths and second cancers incident in the cohort were calculated for each cancer site by multiplying age-, sex- and calendar year-specific person-years at risk in the cohort by the corresponding death and cancer registration rates in the general population of England and Wales (Cancer Registrations MB1 series, ONS) or Norway (Engholm et al, 2010) as appropriate. Causes of death were categorised as cancer (ICD10 C00-C97), diseases of the circulatory system (ICD10 I00-I99), including heart and cerebrovascular disease, or other.
The ratio of observed to expected numbers of deaths or cancers, and the standardised incidence/mortality ratios (SIR/SMR) were then calculated, with likelihood-based 95% confidence intervals (CIs). All significance levels cited are two-sided. All analyses were conducted in STATA v.11.2 (StataCorp LP, College Station, TX, USA).
Results
The eligible cohort comprised 2629 men who had had orchidectomy for stage I seminoma and were then treated at 1 of 12 radiotherapy centres (Figure 1). Median age at diagnosis was 37.2 years (interquartile range 31.3–44.7) and median overall follow-up was 21.8 years (interquartile range 17.5–27.5 years).
Age and year of diagnosis as well as radiotherapy treatment details of the analysis cohort (n=2543) are given in Table 1. Ninety-one per cent (2314) of patients had radiotherapy to abdominal and pelvic lymph nodes. A further 6.3% (161) had only para-aortic node radiotherapy and only 1% of the total had any thoracic or neck irradiation in addition to abdominal fields. Of the 88 eligible patients excluded from the analysis cohort (Figure 1), 41 were diagnosed before 1971.
Table 1. SIR for all cancers excluding non-melanoma skin and testis by age at diagnosis, year of diagnosis, radiotherapy volume, dose and machine.
| Number of second cancers observeda (N=436) | Number of patients at riska (N=2543) | Person-years at riska,b (N=51 151) | Median follow-up (years)b | SIR | 95% CI | |
|---|---|---|---|---|---|---|
|
Age at diagnosis of initial seminoma (years)c | ||||||
| <30 | 44 | 509 | 10 730 | 21.8 | 2.90*** | 2.16–3.90 |
| 30–39 | 140 | 1012 | 21 191 | 21.9 | 1.94*** | 1.64–2.28 |
| 40–49 | 159 | 643 | 13 202 | 21.3 | 1.55*** | 1.32–1.81 |
| 50–59 | 64 | 255 | 4454 | 19.4 | 1.05 | 0.83–1.34 |
| 60+ |
28 |
116 |
1457 |
14.8 |
0.90 |
0.62–1.30 |
|
Year of diagnosis of initial seminoma | ||||||
| 1960–1965 | 51 | 125 | 2941 | 32.6 | 1.71*** | 1.30–2.25 |
| 1966–1970 | 60 | 196 | 4856 | 31.1 | 1.48** | 1.15–1.91 |
| 1971–1975 | 61 | 224 | 5953 | 32.5 | 1.41* | 1.10–1.82 |
| 1976–1980 | 95 | 415 | 9694 | 27.7 | 1.74*** | 1.42–2.13 |
| 1981–1985 | 89 | 603 | 12 261 | 23.5 | 1.45** | 1.18–1.79 |
| 1986–1990 | 65 | 699 | 11 603 | 19.0 | 1.44* | 1.13–1.84 |
| 1991–1992 |
15 |
281 |
3843 |
15.9 |
1.36 |
0.82–2.26 |
|
Treated volume | ||||||
| Para aortic nodes only | 10 | 161 | 2661 | 17.3 | 0.91 | 0.49–1.69 |
| Para aortic+pelvic nodes ±femoral nodes/scrotum | 253 | 1828 | 35 021 | 20.4 | 1.46*** | 1.29–1.66 |
| Whole abdomen | 164 | 486 | 12 064 | 28.3 | 1.74*** | 1.49–2.02 |
| Mediastium/neck (in addition to any of the above) |
4 |
25 |
577 |
26.2 |
1.46 |
0.55–3.90 |
|
Radiotherapy machine | ||||||
| Linear accelerator | 242 | 1795 | 33 762 | 19.8 | 1.52*** | 1.34–1.72 |
| Telecobalt |
17 |
123 |
2466 |
21.3 |
1.15 |
0.71–1.85 |
|
Radiotherapy dose (Gy)d | ||||||
| ⩽31 | 153 | 1401 | 25 265 | 19.0 | 1.31** | 1.12–1.54 |
| 32–38 | 163 | 740 | 16 544 | 23.8 | 1.62*** | 1.39–1.89 |
| >38 | 116 | 353 | 8481 | 27.6 | 1.83*** | 1.53–2.20 |
Abbreviations: CI=confidence interval; SIR=standardised incidence ratios.
***P⩽0.0001.
**P<0.001.
*P<0.01.
Column totals within each subgroup variable do not sum to totals due to missing data.
Person years is calculated from 1 year after radiotherapy and is left censored on 1 January 1971; median follow-up is calculated from the date of diagnosis.
Test for trend across groups: P⩽0.0001.
Test for trend across groups: P=0.006.
During 51151 person-years at risk in 2543 patients, there were 468 second cancers (excluding non-melanoma skin cancers (NMSCs)) reported in 403 men. Six hundred and forty-five men were reported to have died, 66 men were censored at relapse and 15 emigrated, leaving 1709 alive without second cancer at the time of data cutoff.
Overall, the SIR for second cancer incidence (excluding NMSC) was 1.61 (95% CI: 1.47–1.76, P<0.0001). If second testicular cancers were also excluded from analysis, the SIR was 1.53 (95% CI: 1.39–1.68, P<0.0001) giving an absolute excess risk of 29.4 cancers per 10 000 person-years.
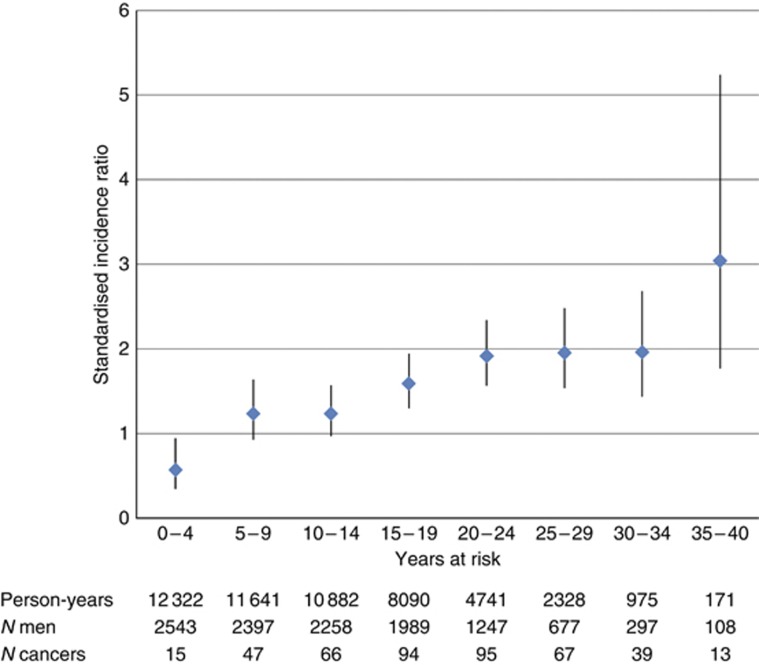
The SIR decreased with age at diagnosis (Table 1, test for trend across five age groups: P<0.0001). There was no obvious relationship between SIR and attained age. The SIR increased with time after radiotherapy (Figure 2, trend: P<0.0001); the significant increased risk is observed 15 years after radiotherapy onwards, although the apparent greater risk after 35 years is based on small numbers of men at risk and of events. Analysis of SIR by radiotherapy treatment variables (Table 2) provided some evidence of a radiation dose effect (P for trend across three groups=0.006), with the SIR for <32 Gy at 1.31 (1.12–1.54) compared with the dose range >38 Gy at 1.83 (1.53–2.20) giving an IRR of 1.39 (1.10–1.77). Although an increased risk was not shown in the 161 patients whose radiotherapy was confined to a para-aortic field, the incident number of second cancers and the person-years at risk were low, generating wide confidence intervals. In addition, para-aortic fields and lower radiation doses were techniques used in more recently treated patients, in whom the full second cancer risk may not yet be fully apparent. The median dose decreased significantly over year of diagnosis groups from 36 Gy between 1960 and 1980 to 34 Gy between 1981 and 1985 and 30 Gy between 1986 and 1992 (Kruskal–Wallis test with 6 d.f.: P<0.0001); hence, there is some confounding between dose and duration of follow-up, although a significant trend in SIR by years at risk was still seen in an analysis restricted to the subset of 1401 patients receiving <32 Gy.
Figure 2.
Standardised incidence ratio for all cancers excluding non-melanoma skin and testis (with 95% CI) by years at risk.
Table 2. SIRs for common cancers sites and cancer sites within the radiation field.
| Site | Second cancers observed | Second cancers expected | SIR | 95% CI |
|---|---|---|---|---|
|
All (excl non-melanoma skin) |
468 |
291.6 |
1.61*** |
1.47–1.78 |
| Testis |
32 |
3.4 |
9.45*** |
6.68–13.36 |
|
All non-testisa |
436 |
285.4 |
1.53*** |
1.39–1.68 |
| Prostate |
80 |
60.3 |
1.33* |
1.07–1.65 |
| Colorectal |
55 |
41.6 |
1.32* |
1.02–1.72 |
| Colon | 30 | 23.8 | 1.26 | 0.88–1.80 |
| Rectum (and anus) |
25 |
18.0 |
1.39 |
0.94–2.05 |
| Bladderb |
49 |
19.9 |
2.46*** |
1.86–3.25 |
| Bladder and ureter/renal pelvisb |
53 |
20.4 |
2.60 *** |
1.99–3.40 |
| Pancreas |
26 |
8.3 |
3.14*** |
2.13–4.60 |
| Stomach |
26 |
13.5 |
1.93** |
1.31–2.83 |
| Kidney |
8 |
8.9 |
0.90 |
0.45–1.80 |
| Liver |
2 |
2.7 |
0.75 |
0.19–3.00 |
| Penis |
2 |
1.0 |
1.96 |
0.49–7.82 |
|
Abdopelvicc |
252 |
155.7 |
1.62*** |
1.43–1.83 |
| Lung |
61 |
50.5 |
1.21 |
0.94–1.55 |
| Melanoma |
15 |
9.9 |
1.52 |
0.91–2.51 |
| Oesophagus |
10 |
7.6 |
1.31 |
0.71–2.44 |
| Leukaemiad |
9 |
5.11 |
1.76 |
0.92–3.38 |
| Soft tissuee | 4 | 1.6 | 2.53 | 0.95–6.75 |
Abbreviations: CI=confidence interval; SIR=standardised incidence ratios.
***P<0.0001.
**P<0.01.
*P<0.05. Figures in bold type for totals and subtotals.
Other cancers diagnosed but not listed elsewhere on table include: brain and CNS (n=12); lymphomas (10); head and neck (8); larynx (5); other respiratory/intrathoracic (5); mesothelioma (3); peritoneum (3); thyroid (2); small intestine (2); other/unspecified biliary tract (2); bone (1); eye (1); secondary cancers (17); other/ill-defined sites (8); unspecified/not known (6).
In ICD7 (to which Norwegian data were coded), bladder cancer (ICD7 181) included cancers of the ureter and renal pelvis. Data from England and Wales were coded to ICD9 and ICD10 in which these cancer sites are separately coded.
Includes all sites in the abdominal–pelvic region listed above.
UK data only because of difficulties mapping ICD7 coding.
ICD7 197; ICD9 171 and 176.1; ICD10 C49 and C46.1.
Risk of specific second cancers are shown in Table 2. There were significantly elevated risks for bladder cancer (SIR 2.46, 95% CI: 1.86–3.26), pancreatic cancer (SIR 3.14, 95% CI: 2.13–4.60) and stomach cancer (SIR 1.93, 95% CI: 1.31–2.83). For abdominal pelvic sites combined, the risk was 1.62 (95% CI: 1.43–1.83). There was no significant elevated risk of solid cancers in organs outside the radiation field, for example, lung: SIR 1.21 (95% CI: 0.94–1.55) and oesophagus: SIR 1.31 (95% CI: 0.71–2.44).
With regard to overall mortality in the cohort, there was no overall increase compared with age- and sex-matched population figures with a standardised mortality ratio of 1.06 (95% CI: 0.98–1.14), despite an increase in the cancer-specific mortality. The SMR for all cancers other than testis cancer was 1.46 (95% CI: 1.30–1.65, P<0.0001), with 264 deaths observed compared with 180 expected, and for circulatory system deaths was 0.80 (95% CI: 0.70–0.92, P=0.002) (Table 3).
Table 3. Standardised mortality ratios.
| Cause of death | Observed deaths | Expected deaths | SMR | 95% CI |
|---|---|---|---|---|
| All causes |
645 |
610.0 |
1.06 |
0.98–1.14 |
| All cancers |
292 |
180.7 |
1.62*** |
1.44–1.81 |
| Circulatory system |
206 |
255.9 |
0.80** |
0.70–0.92 |
| Other non-cancer | 147 | 173.4 | 0.85* | 0.72–1.00 |
Abbreviations: CI=confidence interval; SMR=standardised mortality ratios.
***P<0.0001.
**P<0.01.
*P<0.05.
Discussion
We have sought to increase the accuracy of risk estimates of the long-term hazards of adjuvant radiotherapy by analysing a group treated at young adult age, who would be unlikely to have confounding risks from either chemotherapy or significant levels of diagnostic radiation, and in whom initial diagnosis, staging and treatment details were derived from the radiotherapy centre directly. These risks have a direct bearing on current management strategy in stage I seminoma of the testis since adjuvant radiotherapy is still widely used (Osswald et al, 2009; Vossen et al, 2012).
We have found that moderate dose infradiaphragmatic RT for stage I seminoma was associated with an increased risk of developing a second (non-testicular germ cell) cancer, with an SIR of 1.53 (95% CI: 1.39–1.68). Increased risks were for organs in the radiation field. A weakness of our analysis of the impact of reducing radiation dose and field size is that follow-up was shorter in men with these modifications. There was an associated increased cancer mortality of the same order of magnitude as the increase in incidence, but we did not detect an impact of the 264 cancer deaths (non-testicular) on overall mortality risk when comparing the cohort of 2543 patients followed for an average of 20.1 years at risk with matched population figures. This appeared to be due to a reduced risk of death from circulatory and other non-cancer causes. We have not determined the reason for this but postulate a possible impact of health advice such as smoking cessation at the time of diagnosis of the testicular malignancy. In addition, since reports both from the United Kingdom and the United States of America have suggested that testicular cancer has been more common in higher socioeconomic or higher affluence groups (Swerdlow et al, 1991; Van den Eeden et al, 1991; Toledano et al, 2001), it may be that an SMR based on whole population controls might underestimate an additional mortality risk from radiotherapy; however, socioeconomic data on testicular cancer incidence is not available for Norway. It has been suggested that radiotherapy might increase the risk of a subsequent cardiac event (Huddart et al, 2003) but recent other analyses have not confirmed this risk (Van den Belt-Dusebout, 2007; Beard et al, 2013)
Although an increased risk of second cancer is recognised in patients cured of testicular cancer, neither the baseline risk nor any increase in this due to either radiotherapy or chemotherapy have been quantified. An additional weakness of our study is that ascertainment bias cannot be excluded as the irradiated men would be likely to remain on medical follow-up for years after treatment. The suspicion that radiotherapy may be causal is strengthened by the particular organ sites of increased risk in relation to the radiation field. Analysis of a southeast England population included 5555 patients with seminoma and found they had excess risks of colon, pancreas, bladder and soft-tissue cancers, as well as leukaemia (Robinson et al, 2007). Studies of testicular cancer survivors suggest an overall relative risk increase of 1.4–2.8 compared with population controls (Fung et al, 2012). Travis et al (2005) reported a large population-based study from 14 Tumour Registries in Europe and North America, analysing the risks of second cancers in 40 576 testicular cancer survivors. They demonstrated a cumulative risk of 36% by the age of 75 years for patients diagnosed with seminoma at 35 years of age and 31% for a diagnosis of NSGCT compared with 23% for the matched general population. There was an association between the relative risk for secondary malignancy and time since diagnosis. For those more than 10 years from radiotherapy alone the relative risk was 2.0 (95% CI: 1.9–2.2). Infradiaphragmatic radiotherapy was associated particularly with increased risk of in-field tumours. Similarly, in a nationwide cohort of testicular cancers from The Netherlands, subdiaphragmatic radiotherapy was associated with a 2.6-fold increase in second cancers (van den Belt-Dusebout et al, 2007), whereas there were no significant excesses in patients treated only by surgery. Only limited numbers of patients with seminoma treated by surgery alone and then surveillance have been analysed for second malignancy risk, and when sufficient have been followed for a long period, these will form an good control group to help quantify treatment effects.
There is therefore concern that the increased risk of developing second cancer following adjuvant radiotherapy appears roughly equivalent to the probability of preventing seminoma recurrence in young men with stage I seminoma, especially as there are alternatives to adjuvant radiotherapy, as discussed below. As about one in four men in Western societies will develop cancer (other than non-NMSC), as a rough approximation (ignoring competing risks) our relative risk figures suggest a lifetime increase in cumulative risk of second cancer from 25 to 37%. These estimates are similar to the increased incidence figures reported by Travis et al (2005).
Radiotherapy techniques in testicular seminoma have been modified as the men in this cohort were treated, cutting both dose and volume to reduce toxicity, and changes have been tested in randomised trials to confirm no loss of efficacy (Fossa et al, 1999; Jones et al, 2005). These demonstrated the effectiveness of restricting the field to just the para-aortic region, and of reducing the dose to 20 Gy. A report from the German Testicular Cancer Study Group on 721 men treated to the para-aortic region to a dose of 26 Gy indicated a 95.8% (95% CI: 94.2–97.4) 5-year disease-free survival (Classen et al, 2004). There is some evidence, based on an analysis of both Hodgkin's lymphoma and testicular cancers, that second cancer risks can be reduced by limiting the mean organ radiation dose (van den Belt-Dusebout et al, 2009). It is likely that risk for secondary cancers due to current radiotherapy will be lower than that identified in our analysis, but not eliminated completely (Zwahlen et al, 2008), but as the reduced dose trial was only published as recently as 2005, longer follow-up will be needed to quantify any benefit.
There are alternatives to radiotherapy in the management of stage I seminoma postorchidectomy. These include surveillance, a policy that reserves further treatment for those who relapse, or the use of adjuvant chemotherapy rather than radiotherapy. With surveillance in unselected patients about 15–20% will have recurrent disease, very predominantly in para-aortic lymph nodes, and virtually all recurrences are treated successfully (Warde et al, 2002; Daugaard et al, 2003; Martin et al, 2006; Cummins et al, 2010). Single-agent carboplatin has been evaluated as adjuvant treatment postorchidectomy. A large multicentre randomised trial in 1447 men followed for a median of 6.5 years (Oliver et al, 2011) compared with adjuvant radiotherapy with a single dose of carboplatin at a dose calculated to achieve an area under the concentration × time curve of 7 mg ml−1 × min. Relapse-free rates at 5 years were 94.7% for carboplatin (96.1% for those treated with the recommended dose) vs 96.0% for radiotherapy. Early results suggest that adjuvant carboplatin offers a high cure rate and little toxicity (Powles et al, 2008; Oliver et al, 2011). However, some regard the data supporting the adjuvant carboplatin option to be as yet too preliminary (Bosl and Patil, 2011).
The Swedish Norwegian Testicular Cancer Study Group undertook a prospective observational study between 2000 and 2006 in stage I seminoma (Tandstad et al, 2011), initially with options of either adjuvant radiotherapy to a dose of 25.2 Gy in 14 fractions, or with surveillance; subsequently, after 2003, adjuvant carboplatin (one cycle) was an alternative adjuvant treatment. There was a 14.3% relapse rate in the 512 patients on surveillance; retreatment was mainly with combination chemotherapy and there was only one second relapse. Two patients died, one was treatment-related and one was coincidental. Of the 481 patients treated with adjuvant RT, 4 (0.8%) relapsed at a median of 1.1 years, and 1 has needed treatment for a second relapse. Of 188 patients treated with adjuvant carboplatin, 7 (3.9%) relapsed at a median of 1.8 years; all seven were treated with etoposide/cisplatinum and there had not been any further relapse at the time of analysis.
A selective approach based on risk factors (Warde et al, 2002) with either surveillance for low-risk patients or with adjuvant carboplatin for higher risk patients has also been evaluated. For example, the Spanish Germ Cell Cooperative Group have reported a trial in 227 patients with stage I seminoma followed for a median of 34 months (Aparicio et al, 2011), in which 19% had tumours larger than 4 cm, 11% had rete testis involvement and a further 33% had both these risk factors. Those 74 with two risk factors received two cycles of adjuvant carboplatin, and only one relapsed. The remainder were managed by surveillance and 15 (9.8%) relapsed. Follow-up in this study is still short, and the specific prognostic model has not been validated (Chung et al, 2010).
In conclusion, the overall cure rate in patients with stage I seminoma is very high whether initial management postorchidectomy is by surveillance or by adjuvant radiotherapy or by adjuvant carboplatin. Thus, it is important and feasible to seek to avoid conferring a long-term increased risk of iatrogenic disease such as radiation-associated second cancers.
Acknowledgments
Lead staff at the following hospitals provided data (number of patients within parentheses): S Fossa, Oslo University Hospital, Radiumhospitalet, Norway (698); W Jones, Cookridge Hospital, Leeds, UK (300); A Horwich, DP Dearnaley, R Huddart, Royal Marsden Hospital, Sutton, UK (289); JT Roberts, Newcastle General Hospital, UK (241); G Rustin, Mount Vernon Hospital, Northwood, UK (214); DJ Cole and NP Rowell, Churchill Hospital, Oxford, UK (211); MV Williams, Addenbrooke's Hospital, Cambridge, UK (193); M Sokal, Nottingham City Hospital, UK (137); RTD Oliver, St Bartholomew's Hospital, London, UK (114); AL Houghton, Northampton General Hospital, UK (103); M Mason, Velindre Hospital, Cardiff, UK (65); JR Owen, Cheltenham General Hospital, UK (64). We also thank James Morden, Clinical Trials and Statistics Unit Institute of Cancer Research and staff at the Norway Cancer Registry and the UK NHS Medical Research Information Service. This work was undertaken in The Royal Marsden NHS Foundation Trust, which received a proportion of its funding from the NHS Executive; we acknowledge NHS funding to the NIHR Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was supported by the Institute of Cancer Research (ICR), and Cancer Research UK (CRUK) Grant Numbers C46/A10588 to the ICR Section of Radiotherapy and C1491/A9895 to the ICR Clinical Trials and Statistics Unit. Initial patient registration and baseline data collection was carried out by the Medical Research Council (MRC) Clinical Trials Unit; work at the participating sites was supported by funding from the MRC through its Cancer Therapy Committee.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Presented at 2010 ASCO Annual meeting, Poster Discussion Session, Genitourinary (Non prostate) Cancer (abstract no. 4538).
References
- Aparicio J, Maroto P, del Muro XG, Gumà J, Sánchez-Muñoz A, Margelí M, Doménech M, Bastús R, Fernández A, López-Brea M, Terrassa J, Meana A, del Prado PM, Sastre J, Satrústegui JJ, Gironés R, Robert L, Germà JR. Risk-adapted treatment in clinical stage I testicular seminoma: the third Spanish Germ Cell Cancer Group Study. J Clin Oncol. 2011;29 (35:4677–4681. doi: 10.1200/JCO.2011.36.0503. [DOI] [PubMed] [Google Scholar]
- Beard CJ, Travis LB, Chen MH, Arvold ND, Nguyen PL, Martin NE, Kuban DA, Ng AK, Hoffman KE. Outcomes in stage I testicular seminoma: a population-based study of 9193 patients. Cancer. 2013;119 (15:2771–2777. doi: 10.1002/cncr.28086. [DOI] [PubMed] [Google Scholar]
- Bosl GJ, Patil S. Carboplatin in clinical stage I seminoma: too much and too little at the same time. J Clin Oncol. 2011;29 (8:949–952. doi: 10.1200/JCO.2010.29.5055. [DOI] [PubMed] [Google Scholar]
- Chung PW, Daugaard G, Tyldesley S, Panzarella T, Kollmannsberger CK, Gospodarowicz MK, Warde PR.2010Prognostic factors for relapse in stage I seminoma managed with surveillance: a validation study J Clin Oncol 28(Suppl15s(abstract 4535). [Google Scholar]
- Classen J, Schmidberger H, Meisner C, Winkler C, Dunst J, Souchon R, Weissbach L, Budach V, Alberti W, Bamberg M, German Testicular cancer study Group Para-aortic irradiation for stage I testicular seminoma: results of a prospective study in 675 patients. A trial of the German testicular cancer study group (GTCSG) Br J Cancer. 2004;90 (12:2305–2311. doi: 10.1038/sj.bjc.6601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins S, Yau T, Huddart R, Dearnaley D, Horwich A. Surveillance in stage I seminoma patients: a long-term assessment. Eur Urol. 2010;57 (4:673–678. doi: 10.1016/j.eururo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Daugaard G, Petersen PM, Rørth M.2003Surveillance in stage I testicular cancer APMIS 111(176–83.. discussion 83–85. [DOI] [PubMed] [Google Scholar]
- Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, Køtlum JE, Olafsdóttir E, Pukkala E, Storm HH. NORDCAN – a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49 (5:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- Fossa SD, Aass N, Kaalhus O. Radiotherapy for testicular seminoma stage I: treatment results and long-term post-irradiation morbidity in 365 patients. Int J Radiat Oncol Biol Phys. 1989;16 (2:383–388. doi: 10.1016/0360-3016(89)90334-9. [DOI] [PubMed] [Google Scholar]
- Fossa SD, Horwich A, Russell JM, Roberts JT, Cullen MH, Hodson NJ, Jones WG, Yosef H, Duchesne GM, Owen JR, Grosch EJ, Chetiyawardana AD, Reed NS, Widmer B, Stenning SP. Optimal planning target volume for stage I testicular seminoma: A Medical Research Council Randomized Trial. Medical Research Council Testicular Tumor Working Group. J Clin Oncol. 1999;17 (4:1146. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- Fung C, Fossa SD, Beard CJ, Travis LB. Second malignant neoplasms in testicular cancer survivors. J Natl Compr Cancer Netw. 2012;10 (4:545–556. doi: 10.6004/jnccn.2012.0052. [DOI] [PubMed] [Google Scholar]
- Hamilton C, Horwich A, Easton D, Peckham MJ. Radiotherapy for stage I seminoma testis: results of treatment and complications. Radiother Oncol. 1986;6 (2:115–120. doi: 10.1016/s0167-8140(86)80017-2. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Liu H, Sundquist J. Second cancers after testicular cancer diagnosed after 1980 in Sweden. Ann Oncol. 2010;21 (7:1546–1551. doi: 10.1093/annonc/mdp562. [DOI] [PubMed] [Google Scholar]
- Horwich A, Alsanjari N, A'Hern R, Nicholls J, Dearnaley DP, Fisher C. Surveillance following orchidectomy for stage I testicular seminoma. Br J Cancer. 1992;65 (5:775–778. doi: 10.1038/bjc.1992.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A, Bell J. Mortality and cancer incidence following radiotherapy for seminoma of the testis. Radiother Oncol. 1994;30 (3:193–198. doi: 10.1016/0167-8140(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, Dearnaley DP. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21 (8:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- Jones WG, Fossa SD, Mead GM, Roberts JT, Sokal M, Horwich A, Stenning SP. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I Testicular Seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328) J Clin Oncol. 2005;23 (6:1200–1208. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Martin J, Chung P, Warde P. Treatment options, prognostic factors and selection of treatment in stage I seminoma. Onkologie. 2006;29 (12:592–598. doi: 10.1159/000096608. [DOI] [PubMed] [Google Scholar]
- Oliver RT, Mead GM, Rustin GJ, Joffe JK, Aass N, Coleman R, Gabe R, Pollock P, Stenning SP. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214) J Clin Oncol. 2011;29 (8:957–962. doi: 10.1200/JCO.2009.26.4655. [DOI] [PubMed] [Google Scholar]
- Osswald M, Harlan LC, Penson D, Stevens JL, Clegg LX. Treatment of a population based sample of men diagnosed with testicular cancer in the United States. Urol Oncol. 2009;27 (6:604–610. doi: 10.1016/j.urolonc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Robinson D, Shamash J, Moller H, Tranter N, Oliver T. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol. 2008;19 (3:443–447. doi: 10.1093/annonc/mdm540. [DOI] [PubMed] [Google Scholar]
- Richiardi L, Scélo G, Boffetta P, Hemminki K, Pukkala E, Olsen JH, Weiderpass E, Tracey E, Brewster DH, McBride ML, Kliewer EV, Tonita JM, Pompe-Kirn V, Kee-Seng C, Jonasson JG, Martos C, Brennan P. Second malignancies among survivors of germ-cell testicular cancer: a pooled analysis between 13 cancer registries. Int J Cancer. 2007;120 (3:623–631. doi: 10.1002/ijc.22345. [DOI] [PubMed] [Google Scholar]
- Robinson D, Møller H, Horwich A. Mortality and incidence of second cancers following treatment for testicular cancer. Br J Cancer. 2007;96 (3:529–533. doi: 10.1038/sj.bjc.6603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, Douglas AJ, Huttly SR, Smith PG. Cancer of the testis, socioeconomic status, and occupation. Br J Ind Med. 1991;48 (10:670–674. doi: 10.1136/oem.48.10.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandstad T, Smaaland R, Solberg A, Bremnes RM, Langberg CW, Laurell A, Stierner UK, Ståhl O, Cavallin-Ståhl EK, Klepp OH, Dahl O, Cohn-Cedermark G. Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish Norwegian testicular cancer study group. J Clin Oncol. 2011;29 (6:719–725. doi: 10.1200/JCO.2010.30.1044. [DOI] [PubMed] [Google Scholar]
- Toledano MB, Jarup L, Best N, Wakefield J, Elliott P. Spatial variation and temporal trends of testicular cancer in Great Britain. Br J Cancer. 2001;84 (11:1482–1487. doi: 10.1054/bjoc.2001.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, Kohler BA, Pukkala E, Lynch CF, Andersson M, Bergfeldt K, Clarke EA, Wiklund T, Stoter G, Gospodarowicz M, Sturgeon J, Fraumeni JF, Jr, Boice JD., Jr Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89 (19:1429–1439. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD, Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97 (18:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- van den Belt-Dusebout AW, Aleman BM, Besseling G, de Bruin ML, Hauptmann M, van 't Veer MB, de Wit R, Ribot JG, Noordijk EM, Kerst JM, Gietema JA, van Leeuwen FE. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75 (5:1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, Hoekstra HJ, Ouwens GM, Aleman BM, van Leeuwen FE. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25 (28:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- Van den Eeden SK, Weiss NS, Strader CH, Daling JR. Occupation and the occurrence of testicular cancer. Am J Ind Med. 1991;19 (3:327–337. doi: 10.1002/ajim.4700190307. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, Stiggelbout AM, van den Belt-Dusebout AW, Noyon R, Eliel MR, van Kerkhoff EH, Delemarre JF, Somers R. Second cancer risk following testicular cancer: a follow-up study of 1,909 patients. J Clin Oncol. 1993;11:415–424. doi: 10.1200/JCO.1993.11.3.415. [DOI] [PubMed] [Google Scholar]
- von der Maase H, Specht L, Jacobsen GK, Jakobsen A, Madsen EL, Pedersen M, Rørth M, Schultz H. Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer. 1993;29A (14:1931–1934. doi: 10.1016/0959-8049(93)90446-m. [DOI] [PubMed] [Google Scholar]
- Vossen CY, Horwich A, Daugaard G, van Poppel H, Osanto S. Patterns of care in the management of seminoma stage I: results from a European survey. BJU Int. 2012;110 (4:524–531. doi: 10.1111/j.1464-410X.2011.10887.x. [DOI] [PubMed] [Google Scholar]
- Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20 (22:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- Warde PR, Gospodarowicz MK, Goodman PJ, Sturgeon JF, Jewett MA, Catton CN, Richmond H, Thomas GM, Duncan W, Munro AJ. Results of a policy of surveillance in stage I testicular seminoma. Int J Radiat Oncol Biol Phys. 1993;27 (1:11–15. doi: 10.1016/0360-3016(93)90415-r. [DOI] [PubMed] [Google Scholar]
- Zagars GK, Babaian RJ. Stage I testicular seminoma: rationale for postorchiectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13 (2:155–162. doi: 10.1016/0360-3016(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22 (4:640–647. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- Zagars GK.1996Management of stage 1 seminoma: radiotherapyIn Testicular Cancer, Investigation and Management Horwich A (eds)2nd edn99–121.Chapman & Hall Medical: London, Weinheim, New York, Tokyo, Melbourne, Madras [Google Scholar]
- Zwahlen DR, Martin JM, Millar JL, Schneider U. Effect of radiotherapy volume and dose on secondary cancer risk in stage I testicular seminoma. Int J Radiat Oncol Biol Phys. 2008;70 (3:853–858. doi: 10.1016/j.ijrobp.2007.10.007. [DOI] [PubMed] [Google Scholar]