Abstract
An in vitro transcription system, dependent on catabolite gene activator protein (CAP), utilizing a 200 base-pair restriction fragment, has been used to show that the initiation site of the wild-type Escherichia coli lac mRNA, and that of two mutants, 8d and ps, are identical to that previously reported for the CAP-independent promoter mutant UV5. Order of addition experiments are used to show that the binding of lac repressor to the operator is competitive with that of the RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) to the promoter, thus demonstrating functional overlap of the operator and promoter sites.
Full text
PDF
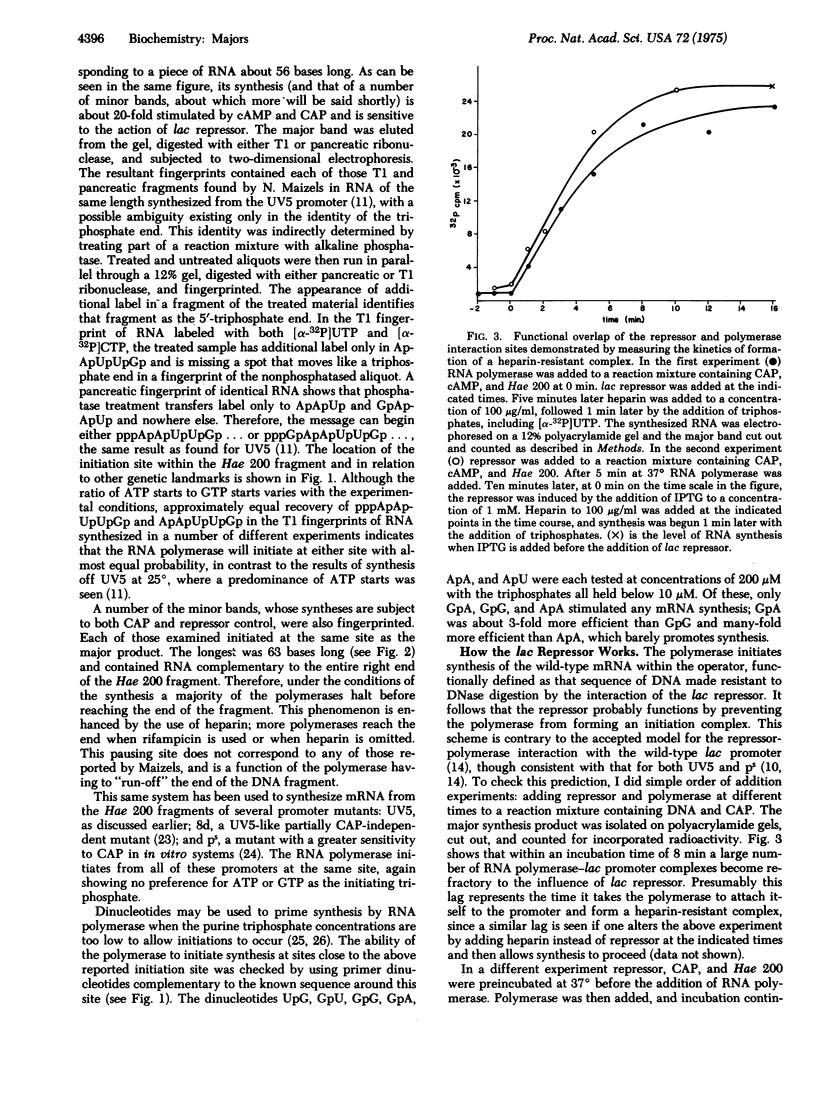


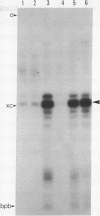
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R., Grodzicker T., Beckwith J. Cyclic adenosine monophosphate-independent mutants of the lactose operon of Escherichia coli. J Bacteriol. 1973 May;114(2):652–655. doi: 10.1128/jb.114.2.652-655.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., de Crombrugghe B., Anderson W. B., Gottesman M. E., Pastan I., Perlman R. L. On the mechanism of action of lac repressor. Nat New Biol. 1971 Sep 15;233(37):67–70. doi: 10.1038/newbio233067a0. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe E., Chen B., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. Role of cyclic adenosine 3',5'-monophosphate and the cyclic adenosine 3',5'-monophosphate receptor protein in the initiation of lac transcription. J Biol Chem. 1971 Dec 10;246(23):7343–7348. [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. doi: 10.1073/pnas.68.8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maizels N., Maxam A. Sequences of controlling regions of the lactose operon. Cold Spring Harb Symp Quant Biol. 1974;38:845–855. doi: 10.1101/sqb.1974.038.01.087. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. II. The kinetics of the binding reaction. J Mol Biol. 1972 Sep 28;70(2):187–195. doi: 10.1016/0022-2836(72)90532-3. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Ippen K., Scaife J. G., Beckwith J. R. The promoter-operator region of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):413–420. doi: 10.1016/0022-2836(68)90395-1. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Studies on the mechanism of action of the gal repressor. J Biol Chem. 1973 Sep 10;248(17):5937–5942. [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Pirrotta V. Sequence of the OR operator of phage lambda. Nature. 1975 Mar 13;254(5496):114–117. doi: 10.1038/254114a0. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Newby R. F., Bourgeois S. lac repressor--operator interaction. II. Effect of galactosides and other ligands. J Mol Biol. 1970 Jul 28;51(2):303–314. doi: 10.1016/0022-2836(70)90144-0. [DOI] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Lee F. D., Yanofsky C. Interaction of the trp repressor and RNA polymerase with the trp operon. J Mol Biol. 1975 Feb 15;92(1):93–111. doi: 10.1016/0022-2836(75)90093-5. [DOI] [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]