Abstract
Background:
Extramural venous invasion (EMVI) is a poor prognostic factor in rectal cancer and identified on magnetic resonance imaging (MRI) (mrEMVI). The clinical relevance of improvement in mrEMVI following neoadjuvant therapy is unknown. This study aimed to demonstrate that regression of mrEMVI following neoadjuvant chemoradiotherapy (CRT) results in improved outcomes and mrEMVI can be used as an imaging biomarker
Methods:
Retrospective analysis of prospectively collected data was conducted examining the staging and post-treatment MRIs of patients who had presented with EMVI-positive rectal cancer. All patients had undergone neoadjuvant CRT and curative surgery. Changes in mrEMVI were graded with a new MRI-based TRG scale–mr-vTRG; and related to disease-free survival (DFS). The study fulfilled Reporting Recommendations for Tumour Marker Prognostic Studies criteria for biomarkers.
Results:
Sixty-two patients were included. Thirty-five patients showed more than 50% fibrosis of mrEMVI (mr-vTRG 1-3); 3-year DFS 87.8% and 9% recurrence. Twenty-seven patients showed less than 50% fibrosis (mr-vTRG 4-5); 3-year DFS 45.8% with 44% recurrence – P<0.0001. On multivariate Cox-regression, only mr-vTRG 4-5 increased risk of disease recurrence – HR=5.748.
Conclusion:
Patients in whom there has been a significant response of EMVI to CRT show improved DFS. Those patients with poor response should be considered for intensive treatment. As an imaging biomarker in rectal cancer, mrEMVI can be used.
Keywords: rectal cancer, venous invasion, EMVI, neoadjuvant chemoradiotherapy, follow-up, recurrence
Extramural venous invasion (EMVI) is defined as the presence of tumour cells in the vasculature beyond the muscularis propria. Its manifestation produces locally advanced tumours, which penetrate more deeply into the mesorectum and beyond; and is a known marker of poor survival outcomes and disease recurrence (Talbot et al, 1980; Knudsen et al, 1983; Freedman et al, 1984). Despite its association with poor prognosis, it is not treated independently or with consistency from other T3 tumours with regard to oncological treatment decisions.
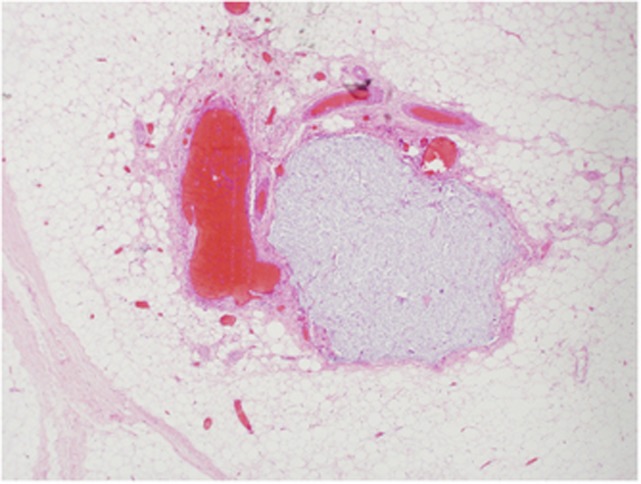
Traditionally, EMVI has been detected on histopathological analysis of surgical resection specimens (Figure 1). However, relying on pathological identification has been shown to result in substantial under-reporting (Messenger et al, 2012); a limitation of historical studies that could account for the large range of incidence of venous invasion (8–81%) cited in the literature (Brown, 1938; Seefeld and Bargen, 1943; Madison et al, 1954; Dukes and Bussey, 1958; Talbot et al, 1980; Rich et al, 1983; Jass et al, 1986; Sasaki et al, 1987; Minsky et al, 1988). The most likely reason for this is the lack of a consistent pathological definition and technique in reporting venous invasion, particularly the lack of use of elastic tissue stains, which can help distinguish lymphatic from venous invasion (Messenger et al, 2012).
Figure 1.
Histopathological detection of EMVI (H and E stain).
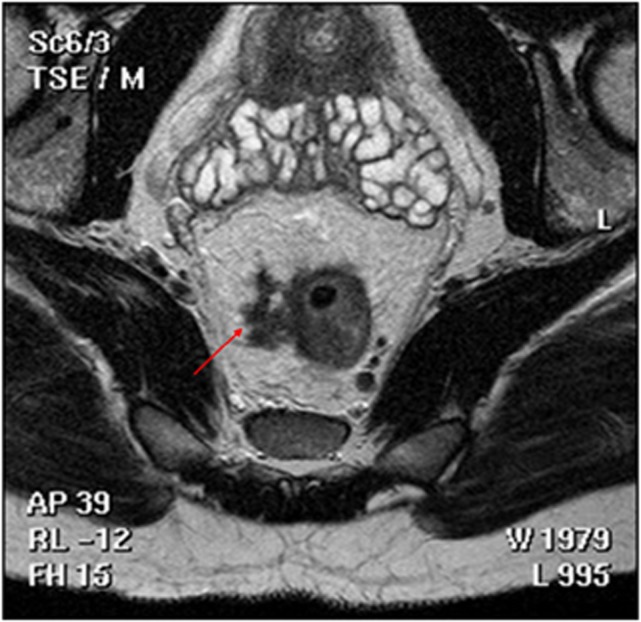
Recent reports have shown that EMVI can be readily identified on magnetic resonance imaging (MRI) – mrEMVI; both before and after neoadjuvant therapy (Mercury Study Group, 2006; Smith et al, 2008a, 2008b; Patel et al, 2011). Indeed, MRI may be superior to routine histopathology analysis of the resection specimens in identifying EMVI, particularly if it is not specifically sought or if technique is limited (Messenger et al, 2012). Magnetic resonance imaging has the advantage of demonstrating vascular anatomy in vivo and thus tumour invasion can be readily identified. Therefore, recording the presence or absence of mrEMVI has become routine in our institution since first described and validated (Smith et al, 2008a; Figure 2).
Figure 2.
MRI showing radiological features of EMVI.
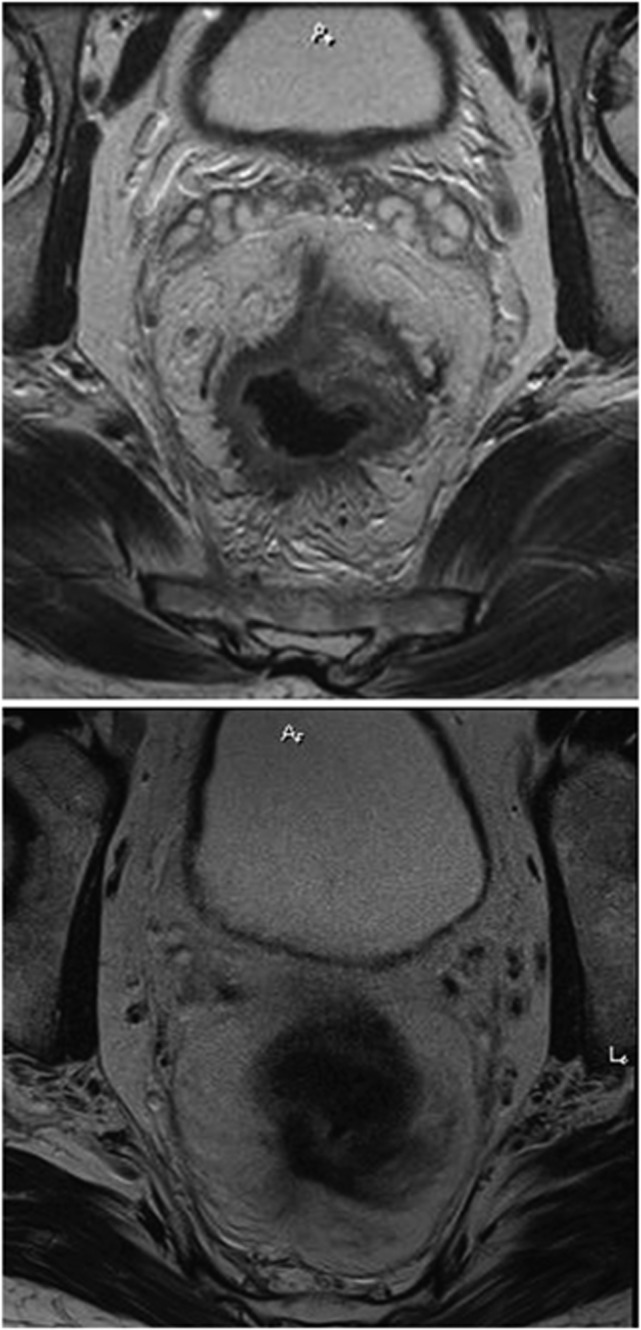
Extramural venous invasion may have a role in pre-operative risk-stratification and further, influence decisions with regard to adjuvant chemotherapy or more intensive neoadjuvant treatment. Extramural venous invasion responds to neoadjuvant chemoradiation therapy (CRT) by causing vessel fibrosis which can be detected on MRI (Figure 3). These radiological changes may result in improved survival outcomes. The prognostic effect of MRI-detected EMVI (mrEMVI) has previously been examined (Smith et al, 2008a); however, the effect of CRT on the morphology and subsequent clinical outcomes are unknown. The potential to grade regression of EMVI following neoadjuvant treatment means that it could be used as an imaging biomarker to measure effectiveness of such treatment.
Figure 3.
MRI showing EMVI response to chemoradiation.
This study aimed to demonstrate that improvement in the degree of mrEMVI following neoadjuvant CRT results in improved survival outcomes. Further, by relating the radiological improvement in EMVI to better outcomes in terms of disease recurrence and time to recurrence this would suggest that mrEMVI could be used as a predictive imaging biomarker.
Materials and Methods
A retrospective analysis of prospectively collected data was conducted examining the staging and post-treatment MRI scans of patients who had presented with primary rectal cancer and MRI evidence of EMVI between January 2006 and January 2012. Patients eligibility included mrEMVI on baseline staging imaging followed by treatment with neo-adjuvant CRT followed by curative ‘total mesorectal excision' (Heald and Ryall, 1986) surgery as decided by the specialist multidisciplinary team. All patients were offered and underwent adjuvant chemotherapy after surgery. Patients below the age of 18 years and those with synchronous tumours or metastases on presentation were excluded. The study fulfilled the relevant Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK) criteria (Mcshane et al, 2005).
Chemoradiotherapy regime
All patients underwent long-course preoperative therapy for MRI-identified potential circumferential resection margin (CRM) involvement and for MRI-predicted T3 tumours with adverse features, such as extramural venous invasion, extramural spread greater than 5 mm, or N2 disease. Radiotherapy consisted of 45–54 Gy delivered to the primary tumour and pelvic lymph nodes at risk. Concurrent CRT was given with a fluoropyrimidine. Treatment response was assessed by MRI and surgery was undertaken at 6–8 weeks after CRT.
Post-operative chemotherapy regime
Patients who are scheduled for adjuvant chemotherapy are given combination chemotherapy. The policy of our institution is to routinely give patients with stage III disease adjuvant chemotherapy in addition to patients who demonstrate high-risk features on baseline imaging and therefore require pre-operative therapy. For patients with stage II disease following pre-operative treatment, a discussion is had between patient and oncologist regarding the benefits of further adjuvant treatment.
Twelve weeks of adjuvant chemotherapy were administered. Oxaliplatin (130 mg m−2) was delivered every 3 weeks, and capecitabine was administered orally at a dose of 2000 mg m−2 per day divided into two split doses for 14 days followed by 7 days of rest repeated every 3 weeks. Dose adjustment was made in the event of toxicity.
Radiological mr-staging
The local policy of our institution is for all patients with rectal cancer to routinely undergo local staging with MRI and for those patients who have CRT to have further MRI scans to assess treatment response. Each patient underwent a minimum of two MRI scans, before and after CRT, which were available for review. Where patients had undergone more than one post-CRT MRI scan, the most recent scan immediately before surgery was reviewed for the purposes of analysis. A senior radiologist (GB) blinded to the clinical data reviewed all MRI images for staging before and after CRT.
mrT-stage was categorised into either ‘good' or ‘poor' based on the extent of tumour spread into the mesorectum. ‘T-good' included all tumours staged as T3b or better, whereas T3c or worse were recorded ‘T-poor' (Appendix 1 – T3 sub-staging). mrN stage was categorised as either positive or negative, and mrCRM was considered positive if the tumour edge was within 1 mm of the CRM. These characteristics were recorded pre-treatment and post-treatment (mr- and ymr-stage).
ymr-EMVI status was recorded as either positive or negative – positive status was a score of 3 or 4 on the MRI-EMVI grading system (Table 1). Further MRI assessment for the extent and site of EMVI was recorded in addition to the degree of regression. This was categorised as large-vessel mrEMVI if tumour invasion was identified into superior, middle or inferior rectal veins; or small-vessel disease if mrEMVI was seen into non-anatomical vessels. A novel EMVI-specific regression grading scale (mr-vTRG) was used to categorise the degree of EMVI regression. The criteria for the mr-vTRG score is shown in Table 2.The treatment response was measured by the degree of fibrosis present in the extramural vasculature.
Table 1. MRI characteristics of EMVI (mrEMVI).
| MRI score | Morphology features on MRI | MRI status |
|---|---|---|
| 0 |
Pattern of tumour extension through the rectal wall is not nodular; no adjacent vessels |
Negative |
| 1 |
Minimal extramural stranding; no adjacent vessels |
Negative |
| 2 |
Stranding in proximity of vessels but no tumour signal in normal calibre lumen |
Negative |
| 3 |
Intermediate signal in lumen of vessels; slight vessel expansion |
Positive |
| 4 | Irregular vessel contour; definite tumour signal | Positive |
Abbreviations: EMVI=extramural venous invasion; MRI=magnetic resonance imaging; mrEMVI=MRI-defined EMVI.
Table 2. mr-vTRG scale.
| mr-vTRG score | Post-treatment mrEMVI features |
|---|---|
| 1 |
Tumour signal replaced by vessel fibrosis |
| 2 |
50–75% fibrosis of tumour signal |
| 3 |
25–49% fibrosis of tumour signal |
| 4 |
Less than 25% fibrosis of tumour signal |
| 5 | Minimal fibrosis of tumour signal within lumen |
Abbreviation: mrEMVI= magnetic resonance imaging-defined extramural venous invasion.
Fibrosis in contradistinction to tumour is characterised on MRI as dense low (dark/black) signal intensity rather than the nodular intermediate (relatively brighter/grey) signal intensity of tumour. This is best seen on post-CRT T2-weighted MRI, the areas of fibrosis have very low signal intensity, whereas areas of residual tumour have intermediate signal intensity. The signal intensity of fibrosis is similar to that of the muscularis propria, and signal intensity of residual tumour is similar to that of baseline tumour.
Histopathology staging
Final histopathology staging (yp-stage) included T-stage, N-stage, EMVI status and CRM involvement.
Statistical analysis
The response of EMVI to CRT was measured using the mr-vTRG scale as described above. For the purposes of analysis, patients were categorised into two groups based on whether there had been more or less than 50% fibrosis seen within the lumen of the affected vessels – mr-vTRG1-3 (good mr-venous responder) and mr-vTRG4-5 (poor mr venous responder). Survival outcomes included recurrence rates, site of recurrences, and 3-year disease-free survival (DFS). Differences in staging characteristics between groups were analysed using Fisher's exact test and Chi-squared, where appropriate. Survival curves for DFS were calculated using the Kaplan–Meier product limit method; differences between survival curves for mr-vTRG levels were tested for significance using the Mantel-Cox log-rank test. An event was radiological or pathological detection of recurrent/metastatic disease or death from any cause. Time to event was recorded from the start of treatment date. Multivariate analysis was performed using Cox regression for time to recurrence. A P-value of <0.05 was considered significant. Hazard ratios were recorded with 95% confidence intervals. Statistical analysis was performed on Excel and SPSS 19.0 (Chicago, IL, USA).
Results
A total of 88 patients met the inclusion criteria of which 62 were included for analysis. Reasons for exclusion were metastatic disease on presentation, incomplete neoadjuvant treatment and EMVI not evident on MRI review. Twenty-one patients were female and forty-one male. The median age was 68 (range 28–87) years.
Baseline mr-staging
The baseline MRI staging characteristics are shown in Table 3. Sixty patients (97%) were staged as mrT-poor and forty-four patients (71%) also had MR-defined nodal disease at the time of diagnosis. In all 62 patients, mrEMVI was present; of these, 50 patients (81%) had evidence of large-vessel mrEMVI. The height of the lower edge of the tumour from the anal verge was measured on MRI for each patient – 21 low rectal (0–5 cm); 15 mid-rectal (6–10 cm) and 26 upper rectal (>10 cm). The CRM was considered threatened on MRI if the tumour margin spread to within 1 mm – same as histopathology criteria. Thirty-one patients (50%) had a positive mrCRM.
Table 3. Post-CRT MRI characteristics mr-vTRG 1-3 and mr-vTRG 4-5.
| |
|
Post-CRT |
|
|
|---|---|---|---|---|
| Staging characteristic | Baseline staging | Mr-vTRG 1-3 | Mr-vTRG 4-5 | P-value |
|
mrT-stage | ||||
| Good (mrT1-T3b) | 60 | 22 | 1 | <0.05 |
| Poor (>mrT3b) |
2 |
13 |
26 |
|
|
mrN stage | ||||
| 0 | 18 | 24 | 9 | <0.05 |
| 1/2 |
44 |
11 |
18 |
|
|
EMVI vessel type | ||||
| No evidence | 0 | 12 | 0 | >0.05 (Small and large vessel) |
| Small vessel | 12 | 3 | 1 | |
| Large vessel |
50 |
20 |
26 |
|
|
mrCRM status | ||||
| Negative | 31 | 30 | 10 | <0.05 |
| Positive | 31 | 5 | 17 | |
Abbreviations: CRM=circumferential resection margin; CRT=chemoradiotherapy; EMVI=extramural venous invasion; MRI=magnetic resonance imaging.
Post-treatment ymr-staging
ymr-Extramural venous invasion
The post-treatment MRI staging characteristics can be seen in Table 3. Thirty-five patients (56%) had more than 50% fibrosis of EMVI following CRT (‘good venous reposnders'). This included 12 patients where there was complete fibrosis of EMVI (mr-vTRG1), which is considered to be a change in EMVI status from positive to negative. Twenty-seven patients (44%) demonstrated less than 50% fibrosis of EMVI (‘poor venous responders').
In the good venous responder, 20 of 35 patients showed evidence of large-vessel EMVI; 2 patients had small vessel disease and 12 patients showed no evidence of EMVI. In the poor venous responders, 26 of 27 patients had large-vessel EMVI and only 1 patient with small-vessel disease.
Other staging characteristics
Following CRT, 13 of 35 (37%) patients were now staged as T-poor; 11 patients had nodal disease (31%); and 5 patients (14%) showed that the CRM was still threatened or involved in the good venous responders. Of the 27 poor venous responders, 26 were still T-poor (96%), 18 (67%) had nodal disease and 17 (63%) had an involved CRM.
Histopathology staging
The post-treatment MRI staging characteristics can be seen in Table 4. The majority of the good venous reposnders were staged as ypT3 (17/35). One patient had a pathological complete response with no residual tumour found; twelve patients were staged as ypT2 and five patients were ypT4. For nodal staging, 11 patients were node-negative and 24 patients had N1/N2 disease. The CRM was clear of tumour in all patients.
Table 4. Histopathology staging following surgery for mr-vTRG 1-3 or mr-vTRG 4-5.
| |
No of patients |
|
|
|---|---|---|---|
| Staging characteristic | Mr-vTRG 1-3 | Mr-vTRG 4-5 | P-value |
|
ypT stage | |||
| PCR | 1 | 1 | <0.05 |
| T2 | 12 | 1 | |
| T3 | 17 | 22 | |
| T4 |
5 |
3 |
|
|
ypN stage | |||
| 0 | 24 | 13 | 0.12 |
| 1/2 |
11 |
14 |
|
|
ypCRM status | |||
| Negative | 35 | 25 | NA |
| Positive | 0 | 2 | |
Abbreviations: CRM=circumferential resection margin; NA=not applicable.
In the poor venous responders group, one patient had a pathological complete response and there was one patient staged as ypT2. Twenty-two patients had ypT3 stage and three patients had ypT4. Fourteen patients had node-negative disease, whereas thirteen patients had N1/N2 disease. Two patients had a positive CRM.
Survival analysis
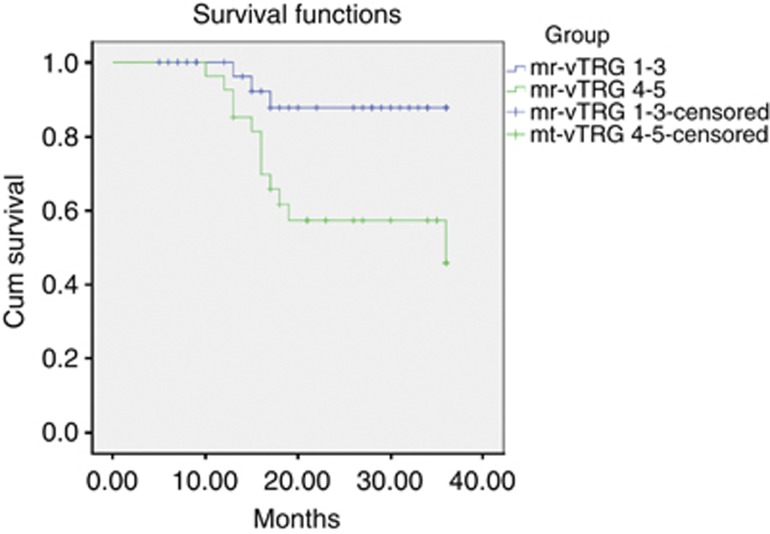
Only three patients (9%) developed recurrence from the good venous responders – two hepatic and one local recurrence. Three-year DFS was 87.8%. In comparison, 12 patients (44%) developed disease recurrence from the poor venous responders, with the majority being hepatic metastases – 7 hepatic, 3 pulmonary and 2 local. Three-year DFS was 45.8% (Graph 1). A Mantel-Cox log-rank test comparing survival showed a statistically significant difference – P=0.013.
Graph 1.
Kaplan–Meier curves for disease-free survival for patients mr-vTRG 1-3 and mr-vTRG 4-5.
On multivariate analysis of specific patient and tumour characteristics using Cox regression, only regression of EMVI by more than 50% (mr-vTRG1-3) was shown to be significant for improved DFS and recurrence – P=0.013. The hazard ratio of a patient developing recurrence following CRT and surgery if there was less than 50% fibrosis of EMVI was 5.748 (95% CI: 1.442–22.905).
Discussion
Extramural venous invasion is known to be a marker of poor prognosis (Talbot et al, 1980; Knudsen et al, 1983; Freedman et al, 1984). Extramural venous invasion is seen on MRI as a serpiginous extension of tumour signal within a vascular structure. This is best identified on T2-weighted images where the characteristic signal and morphology differentiates it from nodal disease (Smith et al, 2008b). It is accurately detected on MRI (mrEMVI) correlating with histopathology and allows for a more accurate estimation of the true prevalence of EMVI in rectal cancer – approximately 40% (Mercury Study Group, 2006; Smith et al, 2008a, 2008b; Taylor et al, 2011b). This can be seen in Table 5.
Table 5. Multivariate analysis of prognostic factors for disease-free survival following neoadjuvant chemoradiation and TME surgery.
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
|
Sex | |||
| Female | 1.000 | 0.527–4.149 | 0.458 |
| Male |
1.479 |
|
|
|
Height from anal verge (cm) | |||
| >5 | 1.000 | 0.248–1.620 | 0.341 |
| <5 |
0.634 |
|
|
|
EMVI regression | |||
| mrTRG 1-3 | 1.000 | 1.442–22.905 | 0.013 |
| mrTRG 4-5 |
5.748 |
|
|
|
ypT stage | |||
| T3b or better | 1.000 | 0.067–1.052 | 0.059 |
| T3c or worse |
0.265 |
|
|
|
ypN stage | |||
| N0 | 1.000 | 0.891–5.710 | 0.086 |
| N1/2 |
2.255 |
|
|
|
ypCRM status | |||
| Negative | 1.000 | 0.136–10.496 | 0.871 |
| Positive | 1.197 | ||
Abbreviations: CI=confidence interval; CRM=circumferential resection margin; EMVI=extramural venous invasion; TME=total mesorectal excision.
This study has shown that good regression of mrEMVI with more than 50% fibrosis is associated with significant improvement in DFS, independent of final pathological staging (T-stage, nodal status and CRM status). This finding was seen in 56% of patients initially presenting with mrEMVI. Three-year DFS was only 45.8% when there was less than 50% fibrosis (poor venous responders) compared with 87.8% in good venous responders. In addition, the recurrence rates were 9% for good mrEMVI vs 44% for poor mrEMVI responders. This is the first study to specifically stratify the tumour regression grade of EMVI following neoadjuvant treatment using REMARK criteria for investigating imaging biomarkers. These results would suggest that mrEMVI could be used as a predictive imaging biomarker and that it is worthwhile targeting patients with mrEMVI for further oncological treatment such as neo-adjuvant chemotherapy rather than just chemo-sensitisation to elicit further downstaging of mrEMVI, as those patients showing a significant degree of fibrosis of mrEMVI have improved survival outcomes.
Increasingly, using detailed MRI assessment enables more effective risk and therefore therapeutic stratification early in the patient treatment pathway (Mercury Study Group, 2006; Shihab et al, 2011; Taylor et al, 2011a). The most important recognised factors that influence neoadjuvant treatment are depth of tumour penetration and, in particular, the extent of spread into the mesorectum and beyond (T3-sub-stage and T4), and proximity of the tumour edge to the CRM (Shihab et al, 2011; Taylor et al, 2011c). The response to neoadjuvant treatment can be measured radiologically and histopathologically by the use of tumour regression grades (Mandard et al, 1994; Suarez et al, 2008). The regression grading enables an assessment of response in terms of cytological changes and stromal changes including fibrosis, which have been show to correlate well against outcomes (Rullier et al, 2001; Patel et al, 2011). Understanding the tumour response to treatment pre-operatively with mrTRG scores has the advantage of ensuring that surgery is oncologically successful and that further intensive treatment may be offered to those patients who have not shown an adequate response. However, both these scoring systems do not specifically account for the effect of treatment on MRI extramural venous invasion.
The consistent depiction on MRI of the larger veins such as the superior and inferior rectal veins aids identifying the type and size of vessel involved. Magnetic resonance imaging is therefore more accurate in identifying large-vessel EMVI than smaller-vessel disease (Smith et al, 2008a). We observed that the majority of patients had large-vessel disease initially and where there was less than 50% fibrosis of EMVI large-vessel disease predominated. This confirms the importance, first shown by Talbot, of large-vessel disease over smaller vessel (Talbot et al, 1981). Although small-vessel EMVI may be more difficult to identify both radiologically and histopathologically, it may in fact be of little clinical consequence.
Following CRT, 38 patients showed more than 50% fibrosis (mr-vTRG score of 1–3). Interestingly, of these 38 patients, 12 patients showed complete regression of mrEMVI and would be reported as EMVI-negative on MRI – no evidence of EMVI. Only one patient who had become mrEMVI-negative developed a recurrence – hepatic metastases. There was also improvement in all other prognostic factors as one would expect. As the study cohort all underwent CRT, it is, by definition, a high-risk group of patients. This can be seen by the baseline staging characteristics, which show that almost all patients were staged as T-poor. In our institution, not all T3 patients are universally irradiated and nodal disease alone is not an indication for neo-adjuvant therapy. In the mr-vTRG 1-3 group, the majority of patients was staged T3 but the CRM was negative in all patients. Although there were a few patients who were radiologically staged to have CRM involvement following CRT on MRI, this information would have been known to the surgeon allowing them to make appropriate surgical decisions to ensure a clear margin. In the poor venous responders, T3 tumours predominated and the CRM was involved in 3 patients out of 28. More than half of patients still had nodal disease, although this was not statistically relevant difference between the groups. Further, on multivariate analysis, nodal disease was not a significant factor and only mr-vTRG grade was significant.
These results suggest that EMVI regression is a prognostic indicator for disease recurrence in addition to being predictive as well. Patients who show little fibrosis of EMVI following CRT are more likely to develop metastatic disease. The hazard ratio of 5.748 is significant and suggests that if patients show minimal regression of EMVI following standard CRT that more intensive pre-operative chemotherapy to further treat EMVI may improve disease recurrence. These patients are at risk of developing metastases and should be considered for aggressive therapy pre- or post-operatively. This also raises the issue of more frequent follow-up as currently there is no universal consensus on rectal cancer surveillance after curative surgery. Although patients who are enrolled in trials have a defined follow-up strategy, the timing of imaging, serum CEA and colonoscopy varies from centre to centre. Patients who are identified as high risk of metastatic disease should be considered for more frequent follow-up, and in particular more frequent liver imaging.
One of the perceived limitations of this study is the use of a specialist gastrointestinal radiologist to stage the MRI scans. It is not always possible for patients' imaging studies to be reported by specialist radiologists and sub-specialisation has not been accepted in the United Kingdom as yet. However, it has been previously shown in the Mercury Study that technique and assessment of MRI can be standardised, particularly when a specific proforma-based system is adopted (Taylor et al, 2008, 2010). Further, this is a retrospective analysis, however, the data collected was done so from routine undertaken proforma reports, which mandate all the MRI staging assessment data described in this paper.
Conclusion
Neoadjuvant therapy is known to improve survival outcomes and downstage disease in terms of depth of tumour spread, regional lymph node and CRM involvement. There have been no studies of the effect of CRT on EMVI in terms of morphological change or effect on survival outcomes. This is the first study to quantify the radiological changes, which occur in EMVI following CRT and how they relate to survival outcomes. Patients in whom there has been more than 50% fibrosis and regression of EMVI show significant improvement in DFS. In patients where there has been less than 50% regression, consideration should be made for additional neoadjuvant therapy. The regression and fibrotic changes in EMVI following CRT are identifiable on MRI and by showing that mrEMVI treatment response correlates with improvement in disease recurrence, we suggest that it has a role as a predictive imaging biomarker.
Acknowledgments
This study is funded by NIHR BRC Royal Marsden Hospital Sutton.
Appendix 1
Table A1. T3 sub-stage classification.
|
T-stage |
Depth of extramural spread of tumour edge into the mesorectum (mm) |
| T3a |
<1.00 |
| T3b |
1.01–5.00 |
| T3c |
5.01–15.00 |
| T3d | >15.00 |
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Brown CF. Visceral metastasis from rectal carcinoma. Surg Gynecol Obstet. 1938;66:611–621. [Google Scholar]
- Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958;12:309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet. 1984;2:733–736. doi: 10.1016/s0140-6736(84)92636-9. [DOI] [PubMed] [Google Scholar]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, Todd IP. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Knudsen JB, Nilsson T, Sprechler M, Johansen A, Christensen N. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum. 1983;26:613–617. doi: 10.1007/BF02552975. [DOI] [PubMed] [Google Scholar]
- Madison MS, Dockerty MB, Waugh JM. Venous invasion in carcinoma of the rectum as evidenced by venous radiography. Surg Gynecol Obstetr. 1954;92:170–178. [PubMed] [Google Scholar]
- Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mcshane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercury Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger DE, Driman DK, Kirsch R. Developments in the assessment of venous invasion in colorectal cancer: implications for future practice and patient outcome. Hum Pathol. 2012;43:965–973. doi: 10.1016/j.humpath.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer. 1988;61:1417–1424. doi: 10.1002/1097-0142(19880401)61:7<1417::aid-cncr2820610723>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::aid-cncr2820520731>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rullier E, Goffre B, Bonnel C, Zerbib F, Caudry M, Saric J. Preoperative radiochemotherapy and sphincter-saving resection for T3 carcinomas of the lower third of the rectum. Ann Surg. 2001;234:633–640. doi: 10.1097/00000658-200111000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki O, Atkin WS, Jass JR. Mucinous carcinoma of the rectum. Histopathology. 1987;11:259–272. doi: 10.1111/j.1365-2559.1987.tb02631.x. [DOI] [PubMed] [Google Scholar]
- Seefeld PH, Bargen JA. The spread of carcinoma of the rectum: invasion of lymphatics, veins and nerves. Ann Surg. 1943;118:76–90. doi: 10.1097/00000658-194311810-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihab OC, Taylor F, Salerno G, Heald RJ, Quirke P, Moran BJ, Brown G. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18:3278–3284. doi: 10.1245/s10434-011-1776-2. [DOI] [PubMed] [Google Scholar]
- Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- Suarez J, Vera R, Balen E, Gomez M, Arias F, Lera JM, Herrera J, Zazpe C. Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis. 2008;10:563–568. doi: 10.1111/j.1463-1318.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–163. doi: 10.1111/j.1365-2559.1981.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- Taylor F, Mangat N, Swift IR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging 10 Spec no A. 2010. pp. S142–S150. [DOI] [PMC free article] [PubMed]
- Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study that recruited consecutive patients with rectal cancer. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, ST Rose S, Sebag-Montefiore DJ, Tekkis P, Brown G. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98:872–879. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR. Am J Roentgenol. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]