Abstract
Background:
Black ethnic groups have a higher breast cancer mortality than Whites. American studies have identified variations in tumour biology and unequal health-care access as causative factors. We compared tumour pathology, treatment and outcomes in three ethnic groups in young breast cancer patients treated in the United Kingdom.
Methods:
Women aged ⩽40 years at breast cancer diagnosis were recruited to the POSH national cohort study (MREC: 00/06/69). Personal characteristics, tumour pathology and treatment data were collected at diagnosis. Follow-up data were collected annually. Overall survival (OS) and distant relapse-free survival (DRFS) were assessed using Kaplan–Meier curves, and multivariate analyses were performed using Cox regression.
Results:
Ethnicity data were available for 2915 patients including 2690 (91.0%) Whites, 118 (4.0%) Blacks and 87 (2.9%) Asians. Median tumour diameter at presentation was greater in Blacks than Whites (26.0 mm vs 22.0 mm, P=0.0103), and multifocal tumours were more frequent in both Blacks (43.4%) and Asians (37.0%) than Whites (28.9%). ER/PR/HER2-negative tumours were significantly more frequent in Blacks (26.1%) than Whites (18.6%, P=0.043). Use of chemotherapy was similarly high in all ethnic groups (89% B vs 88.6% W vs 89.7% A). A 5-year DRFS was significantly lower in Blacks than Asians (62.8% B vs 77.0% A, P=0.0473) or Whites (62.8 B% vs 77.0% W, P=0.0053) and a 5-year OS for Black patients, 71.1% (95% CI: 61.0–79.1%), was significantly lower than that of Whites (82.4%, 95% CI: 80.8–83.9%, W vs B: P=0.0160). In multivariate analysis, Black ethnicity had an effect on DRFS in oestrogen receptor (ER)-positive patients that is independent of body mass index, tumour size, grade or nodal status, HR: 1.60 (95% CI: 1.03–2.47, P=0.035).
Conclusion:
Despite equal access to health care, young Black women in the United Kingdom have a significantly poorer outcome than White patients. Black ethnicity is an independent risk factor for reduced DRFS particularly in ER-positive patients.
Keywords: breast cancer, prognosis, ethnicity
Although the overall incidence of invasive breast cancer remains lower in Black women than White women, the risk of developing breast cancer is higher in Blacks than Whites in women aged 45 and under (Newman and Alfonso, 1997; Harding and Rosato, 1999; Ward et al, 2004, Chlebowski et al, 2005; Smigal et al, 2006; Jack et al, 2009). There is now substantial evidence that Black women with breast cancer have a poorer prognosis than non-Black patients (Joslyn and West, 2000; Jatoi et al, 2003; Carey et al, 2006; Grann et al, 2006; Albain et al, 2009). This disparity in outcome is widening with time (Menashe et al, 2009). Whether ethnicity is an independent prognostic factor remains controversial.
Many studies have attributed the inferior outcomes in Black women to an increased incidence of adverse biological features. Blacks have an increased incidence of larger and higher-grade tumours with more lymph node involvement than Whites (Elledge et al, 1994; Dignam, 2000; Joslyn and West, 2000; Carey et al, 2006; Bowen et al, 2008). Blacks are also more likely to have ER-negative tumours than Whites, and there are reports of an increased percentage of triple-negative (ER/PR/Her2-) and basal cell tumours in this ethnic group (Carey et al, 2006; Bauer et al, 2007). The mean age of diagnosis is lower in Blacks than Whites, and this may partially explain the increased incidence of aggressive biological features in some non-age-matched studies (El-Tamer and Wait, 1999; Newman and Alfonso, 2007).
Most studies have been confounded by other factors, with most published data derived from American populations where access to diagnostic health care and treatment is affected by economic status and may vary between different ethnic groups (Bickell et al, 2006). Lower uptake of screening and lower rates of chemotherapy use in Blacks compared with other ethnic groups have been reported, with an association between health insurance status and receipt of chemotherapy (Freedman and Yea, 2011). Variations in other social and cultural factors between ethnic groups may also promote differential outcomes (Gerend and Pai, 2008).
Published data on the effect of ethnicity on breast cancer in the United Kingdom are limited but demonstrate a similar effect of ethnicity on outcome as the American studies, (Wild et al, 2006; Bowen et al, 2008; Jack et al, 2009). In their retrospective study of 102 Black and 191 White British women, Bowen et al (2008) observed a higher frequency of grade 3 tumours, lymph node-positive disease, negative oestrogen receptor and progesterone receptor status and triple-negative tumours in Black women than White women, with significantly worse survival in Blacks than Whites for patients with small (<2.0) tumours only. The cancer registry based analysis of Jack et al (2009) also reported significantly worse overall survival in Black African women than White women after adjustment for age, stage and treatment (HR: 1.24). This variation was less marked when breast cancer specific mortality was examined (HR: 1.09).
The POSH study is a prospective observational study of patients aged less than 41 years with breast cancer, diagnosed and treated in the United Kingdom (Eccles et al, 2007). This cohort of almost 3000 patients diagnosed and treated within the 21st Century represents, to the best of our knowledge, the largest prospective study of young breast cancer patients to date. All patients were managed within the National Health Service (NHS), and therefore had equal access to diagnostic, surgical and oncology services. Screening for breast cancer is not offered to women below age 40 years in the United Kingdom, thus removing this potentially confounding factor. Here we report the pathology and treatment of these patients according to their ethnic origin and compare outcome in White, Black and Asian patients.
Patients and methods
POSH is a multicentre prospective observational cohort study of young women diagnosed with breast cancer in the United Kingdom between 2000 and 2008, (http://www.southampton.ac.uk/medicine/research/posh.page).
The detailed study protocol was published in 2007 (Eccles et al, 2007). This study received approval from the South West Multicentre Research Ethics Committee (MREC 00/6/69).
Patients
Female patients were recruited from 127 UK hospitals. Patients were eligible if diagnosed with invasive breast cancer between 01 January 2000 and 31 January 2008 at an age of 40 years or younger. Potential recruits were identified within 12 months of initial diagnosis. All patients received treatment according to local protocols. Written consent was obtained.
Study variables and data sources
Details of personal characteristics, tumour pathology, disease stage and treatment received were collected from medical records. Pathology and imaging data have been verified with copies of original reports from sites. For patients treated with neo-adjuvant chemotherapy, initial tumour diameter was derived from radiological reports. Family history and personal risk factors were collected using a questionnaire completed by participants at recruitment. Ethnicity was self-reported, and patients were subsequently categorised into ethnic and racial categories according to National Institute of Health reporting guidelines, (NIH policy on reporting race and ethnicity data: subjects in clinical research 8-2001 http://grants1.nih.gov/grants/guide/notice-files/NOT-OD-01-053.html). Patients were categorised as Black if they reported ‘Black British', ‘African', ‘Black Caribbean', ‘Caribbean' or ‘West Indian' ethnicity and Asian if they reported, ‘Asian', ‘British Asian', ‘Asian-Pakistani' or ‘Indian subcontinent' ethnicity.
Detailed clinical follow-up data, including date and site of disease recurrence, were obtained from medical records at 6 months, 12 months and at yearly intervals post diagnosis until death or loss to follow-up. Patients were flagged in the NHS Medical Research Information Service to facilitate automatic notification of date and cause of death. This paper presents analyses conducted on follow-up data received until 11 April 2012.
Tumour receptor status data
ER, PR and HER2 receptor status of primary tumours was primarily determined from routine diagnostic pathology tests. Hormone receptor levels equivalent to an Allred score of ⩾3 were categorised as positive. Tissue microarray, (TMA) data from central pathology review at St. Bartholomew's Hospital, London has been performed on 1336 randomly selected tumour samples. TMA results for ER, PR and HER2 receptor status have been used to corroborate clinical data or supplement missing data points on receptor status for these 1336 patients. BRCA1/2 mutation testing is in progress.
Statistical analysis
Details of the target sample size (3000) are reported in the protocol (Eccles et al, 2007). The statistical analysis was conducted according to a pre-specified plan and as recommended by STROBE (STrengthening the Reporting of Observational Studies in Epidemiology) guidelines (von Elm et al, 2007). Analyses were performed in STATA v11.2 on records with complete data (levels of missingness were reported). Summary statistics were used to describe the cohort. Where appropriate, Pearson's chi-squared or Mann–Whitney tests were performed in order to identify whether there were any specific differences in the characteristics between ethnic categories.
Overall survival (OS) and distant relapse-free survival (DRFS) were assessed using Kaplan–Meier curves. These were defined as time from date of invasive breast cancer diagnosis to death from any cause (OS) and to distant relapse or death from breast cancer (DRFS). Patients who had not experienced an event at the time of analysis were censored at their date of last follow-up. Multivariate analyses using Cox regression were performed according to the pre-specified analysis plan to adjust for the effect of confirmed prognostic factors (tumour grade, total tumour diameter, nodal status, ER status and body mass index) on DRFS in the different ethnic groups. The proportionality assumption was assessed by inspecting the Nelson-Aalen plots and Schoenfeld residuals and was satisfied in each case.
Results
The POSH study recruited 3095 patients across England (2695), Scotland, Wales and Northern Ireland. After excluding 139 trial participants (Figure 1), 2956 patients were included in this analysis.
Figure 1.
Flow Diagram for the Prospective Study of Outcomes in Sporadic and Hereditary Breast Cancer (POSH).
Patient characteristics and presentation
Self-reported ethnicity was available for 2915 (98.6%) patients. Of these 2902 reported a single ethnic/ racial category; 2690 (92.7%) were classified as White/ Caucasian, 106 (3.7%) as Black, 86 (3.0%) as Asian; and 20 (0.7%) were from ‘other' ethnic groups. Thirteen patients reported mixed ethnicity: eight Black/Caucasian, three Caribbean/White, one Caribbean/Irish and one Chinese/White. In view of the small number of mixed ethnicity patients, these patients were categorised as Black (12) or Asian (1) for the purpose of further analyses. Patients from ‘other' ethnic groups (n=20) were excluded from further analyses.
Table 1 demonstrates patient demographics and breast cancer risk factors in White, Black and Asian ethnic groups. Median age at diagnosis of breast cancer was significantly lower in Asians than Whites (35 years A vs 36 years W, P=0.001) or Blacks (35 years A vs 36 years B, P=0.0472). Median body mass index was significantly higher in Black patients than Whites (26.9 kg m−2 B vs 24.6 kg m−2 W, P<0.001) and Asians (26.9 kg m−2 B vs 24.1 kg m−2 A, P<0.001). The proportion of patients with at least one child was 71.6% of Whites, 69.0% of Blacks and 79.1% of Asians, with no statistically significant differences between groups. The median number of children in patients who had at least one child was significantly higher in Blacks than Whites (P=0.011). Symptomatic presentation accounted for 98% (2900) of the trial cohort, and mode of presentation was similar in all ethnic categories.
Table 1. Patients' characteristics and risk factors.
| Characteristic | Alla: n=2956 (100.0%) | W: n=2690 (91.0%) | B: n=118 (4.0%) | A: n=87 (2.9%) | P-value‡‡ |
|---|---|---|---|---|---|
|
Median (range, IQR), number of patients | |||||
| Age at diagnosis, in years | 36 (18–40, 33–38), 2956 | 36 (18–40, 34–38), 2690 | 36 (18–40, 33–38), 118 | 35 (23–40, 32–37), 87 | W vs B: P=0.463 (NS) |
| W vs A: P<0.001 | |||||
| |
|
|
|
|
B vs A: P=0.0472 |
| Duration of follow-up, in months |
60.5 (1.4–136.1, 45.4–75.4), 2956 |
60.8 (1.4–136.1, 46.5–76.3), 2690 |
47.8 (8.1–95.7, 34.1–70.8), 118 |
56.0 (8.0–102.0, 38.2–71.0), 87 |
— |
| Age at menarche, in years | 13 (8–18, 12–14), 2956 | 13 (8–18, 12–14), 2690 | 12 (8–17, 12–14), 118 | 13 (9–18, 12–13), 87 | W vs B: P=0.560 (NS) |
| W vs A: P=0.391 (NS) | |||||
| |
|
|
|
|
B vs A: P=0.921 (NS) |
| Body mass index, in kg m−2 | 24.6 (14.7–59.5, 22.1–28.4), 2842 | 24.6 (14.7–59.5, 22.1–28.4) , 2610 | 26.9 (18.9–49.1, 23.5–31.2) , 113 | 24.1 (17.2–39.0, 21.3–26.5), 79 | W vs B: P<0.001W vs A: P=0.0401 |
| Missing/unknown |
114 (3.9%) |
80 (3.0%) |
5 (4.2%) |
8 (9.2%) |
B vs A: P<0.001 |
| Age at first birth, in years | 27 (13–40, 23–30), 2080 | 27 (13–40, 23–30), 1898 | 26 (14–37, 20–30), 79 | 25 (16–36, 22–29), 65 | W vs B: P=0.200 (NS) |
| W vs A: P=0.154 (NS) | |||||
| Missing/unknown |
876 (29.6%) |
792 (29.4%) |
39 (33.1%) |
22 (25.3%) |
B vs A: P=0.965 (NS) |
| Number with children |
2097 (71.6%) |
1912 (71.6%) |
80 (69.0%) |
68 (79.1%) |
W vs B: P=0.545 (NS) |
| Number without children, n(%) | 834 (28.5%) | 760 (28.4%) | 36 (31.0%) | 18 (20.9%) | W vs A: P=0.128 (NS) |
| Missing/unknown |
25 (0.9%) |
18 (0.7%) |
2 (1.7%) |
1 (1.2%) |
B vs A: P=0.921 (NS) |
| Number of children – median (range, IQR), n for patients with ⩾1 child |
2 (1–8, 1–2), 2097 |
2 (1–8, 1–2), 1912 |
2 (1–4, 2–3), 80 |
2 (1–5, 2–3), 68 |
W vs B: P=0.011W vs A: P=0.087 (NS)B vs A: P=0.688 (NS) |
|
Number of patients (%) | |||||
|
Presentation: | |||||
| Symptomatic | 2900 (98.6%) | 2645 (98.6%) | 116 (98.3%) | 86 (100.0%) | W vs B: P=0.770 (NS) |
| Screen detected | 30 (1.0%) | 26 (1.0%) | 1 (0.9%) | 0 (0%) | W vs A: P=0.548 (NS) |
| Other | 12 (0.4%) | 11 (0.4%) | 1 (0.9%) | 0 (0%) | B vs A: P=0.479 (NS) |
| Missing/unknown |
14 (0.5%) |
8 (0.3%) |
0 (0%) |
1 (1.2%) |
|
|
Age at diagnosis, in years | |||||
| 18 to 25 | 46 (1.6%) | 39 (1.5%) | 2 (1.7%) | 4 (4.6%) | W vs B: P=0.880 (NS) |
| 26 to 30 | 269 (9.1%) | 240 (8.9%) | 13 (11.0%) | 9 (10.3%) | W vs A: P=0.003 |
| 31 to 35 | 900 (30.5%) | 810 (30.1%) | 35 (29.7%) | 37 (42.5%) | B vs A: P=0.109 (NS) |
| 36 to 40 |
1741 (58.9%) |
1601 (59.5%) |
68 (57.6%) |
37 (42.5%) |
W vs B: P<0.001 |
|
Use of contraceptive pill | |||||
| Ever | 2598 (87.9%) | 2421 (90.0%) | 80 (67.8%) | 49 (56.3%) | W vs A: P<0.001 |
| Never |
358 (12.1%) |
269 (10.0%) |
38 (32.2%) |
38 (43.7%) |
B vs A: P=0.093 (NS) |
|
Smoker | |||||
| Ever | 1455 (50.8%) | 1368 (52.4%) | 47 (41.2%) | 16 (18.8%) | W vs B: P=0.020 |
| Never | 1408 (49.2%) | 1243 (47.6%) | 67 (58.8%) | 69 (81.2%) | W vs A: P<0.001 |
| Missing/unknown |
93 (3.2%) |
79 (2.9%) |
4 (3.4%) |
2 (2.3%) |
B vs A: P=0.001 |
|
Menopausal status | |||||
| Premenopausal | 2885 (99.6%) | 2634 (99.6%) | 114 (100.0%) | 86 (98.9%) | W vs B: P=0.788 (NS) |
| Perimenopausal | 5 (0.2%) | 4 (0.2%) | 0 (0%) | 1 (1.2%) | W vs A: P=0.090 (NS) |
| Postmenopausal | 7 (0.2%) | 7 (0.3%) | 0 (0%) | 0 (0%) | B vs A: P=0.251 (NS) |
| Missing/unknown |
59 (2.0%) |
45 (1.7%) |
4 (3.4%) |
0 (0%) |
W vs B: P=0.126 (NS) |
|
No. of patients with first or second degree relatives with breast cancer | |||||
| First degree | 418 (14.7%) | 382 (14.7%) | 18 (15.8%) | 5 (6.0%) | W vs A: P=0.003 |
| Second degree |
554 (19.4%) |
521 (20.0%) |
14 (12.3%) |
9 (10.8%) |
B vs A: P=0.091 (NS) |
|
No. of relatives with breast cancer | |||||
| 0 | 1874 (65.8%) | 1696 (65.2%) | 82 (71.9%) | 69 (83.1%) | W vs B: P=0.076 (NS) |
| 1 | 702 (24.6%) | 651 (25.0%) | 21 (18.4%) | 12 (14.5%) | W vs A: P=0.006 |
| 2 | 199 (7.0%) | 189 (7.3%) | 5 (4.4%) | 1 (1.2%) | B vs A: P=0.168 (NS) |
| >2 | 75 (2.6%) | 67 (2.6%) | 6 (5.3%) | 1 (1.2%) | |
| Missing/unknown | 106 (3.6%) | 87 (3.2%) | 4 (3.4%) | 4 (4.6%) | |
Abbreviations: A=Asian; B=Black; IQR=Inter-Quartile Range; NS=not significant; W=White.
Includes patients in an Other or Missing/unknown Ethnic group.
††P-values from Pearson's chi-squared test between Ethnic groups and each categorical variable (excluding Other Ethnic groups and missing/unknown data).
‡‡P-values from Mann–Whitney test between Ethnic groups and each continuous variable (excluding Other Ethnic groups and missing/unknown data).
Tumour pathology
Median total tumour diameter was significantly greater in Blacks than Whites (26.0 mm B vs 22.0 mm W, P=0.0103), and multifocal tumours were more frequent in Blacks (43.4%) than Whites (28.9%, P=0.002) Table 2. The median total tumour diameter for unifocal disease was signicantly smaller than the median total tumour diameter of patients with multifocal disease (<0.001). There was an increased frequency of grade 3 tumours in Blacks (68.1%) compared with Whites (60.4%), and a higher proportion of Blacks had positive nodal involvement than Whites (56.1% B vs 50.8% W) but these differences were not significant. Both Blacks and Asians had a higher frequency of ER-negative tumours than Whites (37.6% B, 42.5% A, 33.5% W), but this was not statistically significant. There were no significant differences in the frequency of HER 2 overexpressing tumours between ethnic groups. However, the frequency of ER/PR/HER2-negative tumours was significantly higher in Blacks (26.1%) than Whites (18.6%, P=0.043). Data for tumour grade, histological type, nodal status, presence of metastases and ER status were missing in 0–4.6% of cases only. Data for tumour distribution, PR status and HER2 status were missing more frequently, in up to 16.1%, 20.5% and 13.7% of cases, respectively.
Table 2. Tumour characteristics.
| Alla: n=2956 (100.0%) | W: n=2690 (91.0%) | B: n=118 (4.0%) | A: n=87 (2.9%) | P-value†† | |
|---|---|---|---|---|---|
|
Number of patients %) | |||||
|
Histological grade | |||||
| 1 | 163 (5.7%) | 147 (5.6%) | 1 (0.9%) | 10 (11.8%) | W vs B: P=0.055 (NS) |
| 2 | 972 (33.8%) | 891 (34.0%) | 35 (30.0%) | 24 (28.2%) | W vs A: P=0.045 |
| 3 | 1742 (60.6%) | 1586 (60.4%) | 77 (68.1%) | 51 (60.0%) | B vs A: P=0.004 |
| Missing/unknown |
79 (2.7%) |
66 (2.5%) |
5 (4.2%) |
2 (2.3%) |
|
|
Histological type | |||||
| Ductal | 2556 (87.6%) | 2320 (87.3%) | 101 (87.8%) | 81 (94.2%) | W vs B: P=0.882 (NS) |
| Lobular | 134 (4.6%) | 126 (4.7%) | 5 (4.4%) | 1 (1.2%) | W vs A : P=0.260 (NS) |
| Ductal and Lobular | 78 (2.7%) | 74 (2.8%) | 2 (1.7%) | 1 (1.2%) | B vs A : P=0.445 (NS) |
| Other | 149 (5.1%)λλ | 137 (5.2%) | 7 (6.1%) | 3 (3.5%) | |
| Not gradeda/missing/unknown |
39 (1.3%) |
33 (1.2%) |
3 (2.5%) |
1 (1.2%) |
|
|
Distribution of cancer | |||||
| Localised | 1873 (70.2%) | 1741 (71.1%) | 56 (56.6%) | 46 (63.0%) | W vs B: P=0.002 |
| Multifocal | 797 (29.9%) | 707 (28.9%) | 43 (43.4%) | 27 (37.0%) | W vs A: P=0.133 (NS) |
| Missing/unknown |
286 (9.7%) |
242 (9.0%) |
19 (16.1%) |
14 (16.1%) |
B vs A: P=0.395 (NS) |
|
Pathological T stage (all patients) | |||||
| T0 | 73 (2.5%) | 63 (2.4%) | 4 (3.4%) | 3 (3.5%) | W vs B: P<0.001 |
| T1 | 1411 (47.9%) | 1300 (48.5%) | 40 (33.9%) | 42 (48.3%) | W vs A: P=0.370 (NS) |
| T2 | 1167 (39.6%) | 1064 (39.7%) | 45 (38.1%) | 36 (41.4%) | B vs A: P=0.014 |
| T3 | 189 (6.4%) | 167 (6.2%) | 18 (15.3%) | 2 (2.3%) | |
| T4 | 6 (0.2%) | 5 (0.2%) | 0 (0%) | 1 (1.2%) | |
| Tis | 21 (0.7%) | 18 (0.7%) | 1 (0.9%) | 1 (1.2%) | |
| Tx | 77 (2.6%) | 62 (2.3%) | 10 (8.5%) | 2 (2.3%) | |
| Missing/unknown |
12 (0.4%) |
11 (0.4%) |
0 (0%) |
0 (0%) |
|
|
N stage | |||||
| N0 | 1417 (48.9%) | 1302 (49.2%) | 50 (43.9%) | 40 (48.2%) | W vs B: P=0.260 (NS) |
| N1 | 1484 (51.2%) | 1342 (50.8%) | 64 (56.1%) | 43 (51.8%) | W vs A: P=0.850 (NS) |
| Missing/unknown |
55 (1.9%) |
46 (1.7%) |
4 (3.4%) |
4 (4.6%) |
B vs A: P=0.547 (NS) |
|
M stage | |||||
| M0 | 2860 (97.5%) | 2613 (97.6%) | 111 (94.9%) | 84 (96.6%) | W vs B: P=0.069 (NS) |
| M1 | 74 (2.5%) | 65 (2.4%) | 6 (5.1%) | 3 (3.5%) | W vs A: P=0.545 (NS) |
| Missing/unknown |
22 (0.7%) |
12 (0.5%) |
1 (0.9%) |
0 (0%) |
B vs A: P=0.563 (NS) |
|
ER Statusb | |||||
| Positive | 1947 (66.1%) | 1782 (66.5%) | 73 (62.4%) | 50 (57.5%) | W vs B: P=0.358 (NS) |
| Negative | 997 (33.9%) | 898 (33.5%) | 44 (37.6%) | 37 (42.5%) | W vs A: P=0.080 (NS) |
| Missing/unknown |
12 (0.4%) |
10 (0.4%) |
1 (0.9%) |
0 (0%) |
B vs A: P=0.477 (NS) |
|
PR Statusb | |||||
| Positive | 1342 (56.5%) | 1215 (56.8%) | 65 (58.0%) | 40 (50%) | W vs B: P=0.793 (NS) |
| Negative | 1033 (43.5%) | 925 (43.2%) | 47 (42.0%) | 40 (50%) | W vs A: P=0.230 (NS) |
| Missing/unknown |
581 (19.7%) |
550 (20.5%) |
6 (5.1%) |
7 (8.1%) |
B vs A: P=0.270 (NS) |
|
HER2 Statusb | |||||
| Positive | 717 (28.1%) | 657 (28.3%) | 22 (20.2%) | 22 (29.7%) | W vs B: P=0.065 (NS) |
| Negative | 1839 (72.0%) | 1664 (71.7%) | 87 (79.8%) | 52 (70.3%) | W vs A: P=0.789 (NS) |
| Missing/unknown |
400 (13.5%) |
369 (13.7%) |
9 (7.6%) |
13 (14.9%) |
B vs A: P=0.138 (NS) |
|
TNT Statusc | |||||
| TNT | 537 (19.0%) | 478 (18.6%) | 30 (26.1%) | 19 (23.2%) | W vs B: P=0.043 |
| Not TNT | 2296 (81.0%) | 2099 (81.5%) | 85 (73.9%) | 63 (76.8%) | W vs A: P=0.291 (NS) |
| Missing/unknown |
123 (4.2%) |
113 (4.2%) |
3 (2.5%) |
5 (5.8%) |
B vs A: P=0.641 (NS) |
|
Median (range, IQR), number of patients | |||||
| Maximum tumour diameterd in mm (all patients) |
|
|
|
|
W vs B: P=0.0103 |
| median (IQR, range), n | 22 (15–33, 0–199), 2763 | 22 (15–33, 0–199), 2527 | 26 (15–50, 1–110), 103 | 26 (15–35, 0.15–98), 80 | W vs A: P=0.762 (NS) |
| Missing/unknown |
193 (6.5%) |
163 (6.1%) |
15 (12.7%) |
7 (8.1%) |
B vs A: P=0.0940 (NS) |
|
No. of positive axillary lymph nodes recovered (all patients) | |||||
| median (IQR, range), n |
2 (1–5, 1–50), 1495 |
2 (1–5, 1–50), 1352 |
3 (1–7, 1–19), 65 |
1 (1–4, 1–20), 43 |
W vs B: P=0.496 (NS) |
|
No. of positive axillary lymph nodes recovered (all patients), n(%) | |||||
| 1–3 | 952 (63.7%) | 859 (63.5%) | 41 (63.1%) | 32 (74.4%) | W vs A: P=0.0143 |
| 4–9 | 357 (23.9%) | 324 (24.0%) | 14 (21.5%) | 9 (20.9%) | B vs A: P=0.0169 |
| 10+ | 186 (12.4%) | 169 (12.5%) | 10 (15.4%) | 2 (4.7%) | W vs B: P=0.754 (NS) |
| Total | 1495 (100.0%) | 1352 (100.0%) | 65 (100.0%) | 43 (100.0%) | W vs A: P=0.220 (NS) |
| Missing/unknown | 59 (2.0%) | 50 (1.9%) | 4 (3.4%) | 4 (4.6%) | B vs A: P=0.204 (NS) |
Abbreviations: ER=oestrogen receptor; HER2=human epidermal growth factor receptor 2; IQR=inter-quartile range; PR=progesterone receptor; TNT=triple negative.
Includes patients in an other or missing/unknown ethnic group.
Includes data from TMA as well as primary POSH data.
Includes patients with an ER negative, HER2-negative and PR-negative status.
Maximum tumour diameter includes ductal carcinoma in situ.
††P-values obtained from the Pearson's chi-squared test between ethnic groups and each categorical variable (excluding other ethnic groups and missing/unknown data).
‡‡P-values obtained from the Mann–Whitney test between ethnic groups and each continuous variable (excluding Other Ethnic groups missing/unknown data).
Treatment
Most patients 98·6% (2915) had surgical treatment (Table 3). Rates of breast conserving surgery were lower in Blacks than Whites or Asians (39.8% B vs 48.1% W vs 48.3% A). Use of chemotherapy in early breast cancer patients was similarly high in all ethnic groups (89% B vs 88.6% W vs 89.7% A), but a higher proportion of Blacks received neo-adjuvant chemotherapy than Whites or Asians (23.7% B vs 14.8% W vs 20.7% A). The numbers of patients receiving anthracycline/taxane chemotherapy were similar in each ethnic group. Missing data for trastuzumab and hormonal therapy including ovarian suppression precluded the use of chi-squared tests to compare the proportions of ethnic categories receiving these treatments.
Table 3. Treatment details.
| Alla: n=2956 (100.0%) | W: n=2690 (91.0%) | B: n=118 (4.0%) | A: n=87 (2.9%) | P-value†† | |
|---|---|---|---|---|---|
|
Number of patients (%) | |||||
|
Definitive surgery | |||||
| Breast conserving surgery | 1409 (47.7%) | 1294 (48.1%) | 47 (39.8%) | 42 (48.3%) | W vs B: P<0.001 |
| Mastectomy | 1497 (50.7%) | 1355 (50.4%) | 64 (54.2%) | 44 (50.6%) | W vs A: P=0.978 (NS) |
| Nodal surgery only | 9 (0.3%) | 6 (0.2%) | 2 (1.7%) | 0 (0%) | B vs A: P=0.255 (NS) |
| No surgery | 39 (1.3%) | 33 (1.2%) | 5 (4.2%) | 1 (1.2%) | |
| Missing/unknown |
2 (0.1%) |
2 (0.1%) |
0 (0%) |
0 (0%) |
|
|
Chemotherapy timing | |||||
| Adjuvantb | 2152 (72.8%) | 1985 (73.8%) | 77 (65.3%) | 60 (69.0%) | W vs B: P=0.007 |
| Neo-adjuvant | 460 (15.6%) | 397 (14.8%) | 28 (23.7%) | 18 (20.7%) | W vs A: P=0.221 (NS) |
| Palliative | 54 (1.8%) | 46 (1.7%) | 5 (4.2%) | 3 (3.5%) | B vs A: P=0.942 (NS) |
| Not applicable |
290 (9.8%) |
262 (9.7%) |
8 (6.8%) |
6 (6.9%) |
|
|
Chemotherapy regimen | |||||
| Anthracycline &/or taxane | 2642 (89.4%) | 2405 (89.4%) | 110 (93.2%) | 80 (92.0%) | W vs B: P=0.239 (NS) |
| Otherc | 24 (0.8%) | 23 (0.9%) | 0 (0%) | 1 (1.2%) | W vs A: P=0.653 (NS) |
| None |
290 (9.8%) |
262 (9.7%) |
8 (6.8%) |
6 (6.9%) |
B vs A: P=0.505 (NS) |
|
Adjuvant trastuzumab | |||||
| Yes | 363 (12.3%) | 332 (12.3%) | 9 (7.6%) | 11 (12.6%) | — |
| Other treatment period/no/missing/unknown |
2593 (87.7%) |
2358 (87.7%) |
109 (92.4%) |
76 (87.4%) |
|
|
Adjuvant radiotherapy | |||||
| Yes | 2358 (79.8%) | 2160 (80.3%) | 87 (73.7%) | 67 (77.0%) | — |
| No/missing/unknown |
598 (20.2%) |
530 (19.7%) |
31 (26.3%) |
20 (23.0%) |
|
|
ER-positive patients only |
Alla: n=1947 (100.0%) |
W:
n=1782 (91.5%) |
B:
n=73 (3.8%) |
A:
n=50 (2.6%) |
|
|
Adjuvant hormone treatment | |||||
| Yes | 1725 (88.6%) | 1591 (89.3%) | 59 (80.8%) | 42 (84.0%) | — |
| No/missing/unknown |
222 (11.4%) |
191 (10.7%) |
14 (19.2%) |
8 (16.0%) |
|
|
Ovarian suppression (in any treatment period) Medical (LHRH agonist) | |||||
| Yes | 655 (33.6%) | 605 (34.0%) | 21 (28.8%) | 11 (22.0%) | — |
| No/missing/unknown |
1292 (66.4%) |
1177 (66.1%) |
52 (71.2%) |
39 (78.0%) |
|
|
Irradiation | |||||
| Yes | 11 (0.6%) | 11 (0.6%) | 0 (0%) | 0 (0%) | — |
| No/missing/unknown |
1936 (99.4%) |
1771 (99.4%) |
73 (100.0%) |
50 (100.0%) |
|
|
Oophorectomy | |||||
| Yes | 324 (16.6%) | 307 (17.2%) | 5 (6.9%) | 5 (10.0%) | — |
| No/missing/unknown | 1623 (83.4%) | 1475 (82.8%) | 68 (93.2%) | 45 (90.0%) | |
Abbreviations: A=Asian; B=Black; NS=not significant; W=White.
Includes patients in an Other or Missing/unknown Ethnic group.
Excluding any treatment for M1 disease.
For example, CMF or anything not containing an anthracycline or taxane.
††P-values from Pearson's chi-squared test between ethnic groups and each categorical variable (excluding other ethnic groups and missing/unknown data).
‡‡P-values from Mann–Whitney test between ethnic groups and each continuous variable (excluding Other Ethnic groups and missing/unknown data).
Follow-up and survival
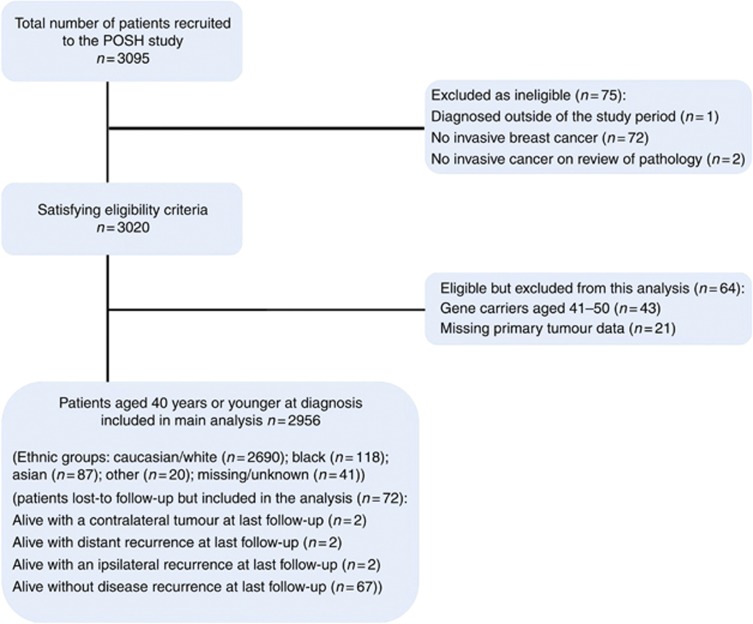
At the time of analysis, length of follow-up ranged from 1 month to 11 years (median 5 years). Only 72 patients (2·4%) had been lost to follow-up. Isolated local relapse events were rare with ipsilateral relapses occurring in 3.0% of Whites, 3.4% of Blacks and 1.2% of Asians, and contralateral tumours occurring in 2.1% of Whites, 0.0% of Blacks and 3.5% of Asians. Kaplan–Meier (KM) survival curves are plotted in Figures 2 and 3. The estimated 5-year OS for the entire POSH cohort was 81.9% (95% CI: 80.3–83.3%) and DRFS 76.6 (74.9–78.1%, table 4). The 5-year OS for Black patients, 71.1% (95% CI: 61.0–79.1%), was significantly lower than that of Whites (82.4%, 95% CI 80.8–83.9%, W vs B: P=0.0160). The 5-year OS for Asian patients was between that of Whites and blacks and not significantly different from either of these ethnic groups (78.7%, 95% CI 66.7–86.7%). A 5-year DRFS was significantly lower in Blacks 62.8% (95% CI: 52.1–71.8%) than both Whites (77.0%, 95% CI: 75.3–78.6%) and Asians (77.0%, 95% CI: 65.1–85.3% W vs B: P=0.0053; B vs A: P=0.0473). There was no significant difference in a 5-year DRFS between Whites and Asians (W vs A: P=0.991).
Figure 2.
Kaplan–Meier (A) OS and (B) DRFS estimates for Caucasian/White, Black and Asian patients.
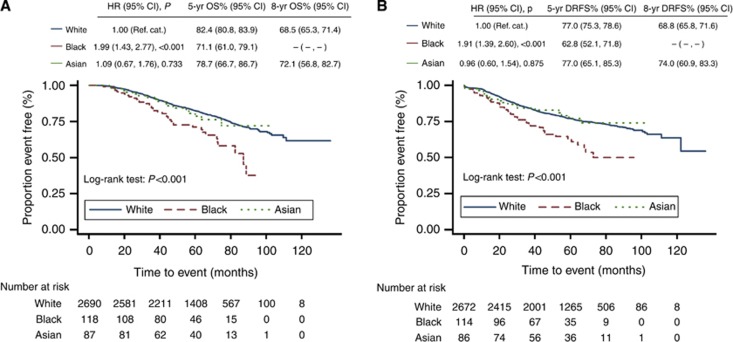
Figure 3.
Kaplan–Meier DRFS estimates for Caucasian/White, Black and Asian (A) ER-negative patients and (B) ER-positive patients.
Table 4. OS and DRFS estimates at 5 and 8 years.
|
Kaplan–Meier estimates |
Comparisons |
||||||
|---|---|---|---|---|---|---|---|
| All | W | B | A | W vs B (W—B) | W vs A (W—A) | B vs A (B—A) | |
| Group of patients | A 5-year OS% (95% CI), Number at risk, | A 5-year OS% difference (95% CI), P-value | |||||
| All patients |
81.9 (80.3, 83.3), 1511 |
82.4 (80.8, 83.9), 1408 |
71.1 (61.0, 79.1), 46 |
78.7 (66.7, 86.7), 40 |
11.3 (2.1, 20.5), P=0.0160 |
3.7 (−6.2, 13.7), P=0.471 |
−7.5 (−20.9, 5.9), P=0.273 |
| |
A 8-year OS% (95% CI), Number at risk, | A 8-year OS% difference (95% CI), P-value | |||||
| All patients |
67.6 (64.5, 70.4), 149 |
68.5 (65.3, 71.4), 145 |
− (−, −), 0 |
72.1 (56.8, 82.7), 2 |
— |
−3.6 (−16.8, 9.6), P=0.607 |
— |
| |
A 5-year DRFS% (95% CI), Number at risk, | A 5-year DRFS % difference (95% CI), P-value | |||||
| All patients |
76.5 (74.8, 78.1), 1353 |
77.0 (75.3, 78.6), 1265 |
62.8 (52.1, 71.8), 35 |
77.0 (65.1, 85.3), 36 |
14.2 (4.2, 24.2), P=0.0053 |
0.1 (−10.0, 10.2), P=0.991 |
−14.2 (−0.1, −28.2), P=0.0473 |
| ER negative |
72.5 (69.5, 75.3), 444 |
73.0 (69.8, 75.9), 411 |
58.0 (40.4, 72.0), 12 |
80.1 (62.6, 90.1), 17 |
15.0 (−1.3, 31.4), P=0.0716 |
−7.2 (−20.8, 6.5), P=0.307 |
−22.2 (−43.0, −1.3), P=0.0368 |
| ER Positive |
78.5 (76.5, 80.4), 907 |
79.1 (77.0, 81.0), 852 |
65.2 (51.0, 76.1), 23 |
74.5 (56.9, 85.8), 19 |
13.9 (1.1, 26.7), P=0.0326 |
4.5 (−9.9, 18.9), P=0.551 |
−9.4 (−28.4, 9.7), P=0.340 |
| Triple Negative |
73.3 (69.1, 77.0), 214 |
72.8 (68.4, 76.8), 191 |
65.8 (43.9, 80.8), 10 |
94.7 (68.1, 99.2), 10 |
7.1 (−12.0, 26.1), P=0.478 |
−21.9 (−32.8, −11.0), P<0.001 |
−29.0 (−50.1, −7.8), P=0.0073 |
| |
A 8-year DRFS% (95% CI), Number at risk, | A 8-year DRFS % difference (95% CI), P-value | |||||
| All patients |
68.3 (65.5, 71.0), 130 |
68.8 (65.8, 71.6), 126 |
− (−, −), 0 |
74.0 (60.9, 83.3), 2 |
— |
−5.2 (−16.7, 6.3), P=0.382 |
— |
| ER negative |
68.1 (63.8, 72.1), 43 |
68.6 (64.1, 72.6), 42 |
− (−, −), 0 |
− (−, −), 0 |
— |
— |
— |
| ER positive |
68.3 (64.5, 71.8), 87 |
68.8 (64.8, 72.4), 84 |
− (−, −), 0 |
74.5 (56.9, 85.8), 2 |
— |
−5.8 (−20.5, 9.0), P=0.454 |
— |
| Triple negative | 70.5 (65.5, 74.9), 15 | 69.9 (64.6, 74.5), 14 | − (−, −), 0 | − (−, −), 0 | — | — | — |
Abbreviations: W=White; A=Asian; B=Black; CI=confidence interval; DRFS=distant recurrence-free survival; OS=overall survival.
Use of a multivariate model to adjust DRFS for total tumour diameter, grade, nodal status and patient body mass index (BMI) in all patients confirms that Black ethnicity is a significant independent marker of poor prognosis with a hazard ratio of 1.50 (95% CI: 1.06–2.13, P=0.023, Table 5) compared with Whites. Separate multivariate analyses of ER-negative and positive tumours indicate that the independent prognostic power of Black ethnicity is no longer significant in ER-negative tumours when adjustments are made for total tumour diameter, grade, nodal status and patient BMI (HR: 1.31 Blacks, 95% CI: 0.73–2.36, P=0.369), although the direction of effect is still the same. However, in ER-positive patients, Black ethnicity remains an independent marker of poor prognosis (HR:1.60, 95% CI: 1.03–2.47, P=0.035).
Table 5. Multivariate Analyses—DRFS.
| Unadjusteda | Adjustedb | ||||||
|---|---|---|---|---|---|---|---|
|
Group of patients |
Ethnic category |
Nc |
HR (95% CI) |
P-value |
Nd |
HR (95% CI) |
P-value |
| All patients | W | 2872 | 1 (Reference cat.) | — | 2581 | 1 (Reference cat.) | — |
| B | 1.91 (1.39, 2.60) | <0.001 | 1.50 (1.06, 2.13) | 0.023 | |||
| |
A |
|
0.96 (0.60, 1.54) |
0.875 (NS) |
|
0.85 (0.48, 1.51) |
0.578 (NS) |
| ER-negative patients only | W | 974 | 1 (Reference cat.) | — | 862 | 1 (Reference cat.) | — |
| B | 1.73 (1.04, 2.87) | 0.034 | 1.31 (0.73, 2.36) | 0.369 (NS) | |||
| |
A |
|
0.82 (0.40, 1.65) |
0.575 (NS) |
|
0.76 (0.31, 1.85) |
0.546 (NS) |
| ER-positive patients only | W | 1888 | 1 (Reference cat.) | — | 1716 | 1 (Reference cat.) | — |
| B | 2.03 (1.36, 3.02) | <0.001 | 1.60 (1.03, 2.47) | 0.035 | |||
| |
A |
|
1.04 (0.55, 1.94) |
0.910 (NS) |
|
0.90 (0.42, 1.90) |
0.776 (NS) |
| Triple negative patients only | W | 524 | 1 (Reference cat.) | — | 477 | 1 (Reference cat.) | — |
| B | 1.39 (0.71, 2.73) | 0.340 (NS) | 1.18 (0.52, 2.69) | 0.693 (NS) | |||
| A | 0.19 (0.03, 1.33) | 0.094 (NS) | 0.28 (0.04, 2.03) | 0.209 (NS) | |||
Abbreviations: A=Asian; B=Black; CI=Confidence Interval; DRFS=Distant Recurrence-Free Survival; W=White.
Univariate analyses: results obtained by fitting a Cox model with ethnic grouping as the only covariate.
Multivariate analyses: results obtained by fitting a Cox model with ethnic grouping as a covariate and adjusting for body mass index, tumour grade, size and nodal status.
Number of Caucasian/White, Black and Asian patients.
Number of Caucasian/White, Black and Asian patients with complete data for body mass index, tumour grade, size and nodal status.
Discussion
The primary aim of the POSH prospective, multicentre study is to determine whether the prognosis of patients with breast cancer is altered by inherited genetic factors. The presenting characteristics, pathology, treatment and survival of this large cohort of early-onset breast cancer patients diagnosed and treated in the first decade of this century have recently been published (Copson et al, 2013); genetic analysis of the study cohort is in progress. The POSH study cohort provides a unique opportunity to compare the outcomes of different ethnic groups in an age group that is not eligible for breast screening and in a population that receives entirely public funded health care, thus eliminating these potentially confounding socio-economic factors. We present here the clinical course of Blacks, Asians and Whites recruited to this study.
Our data confirm conclusions from retrospective studies that Blacks have a tendency towards more biologically aggressive tumours with significantly larger tumours and increased incidence of triple-negative tumours, and trends towards increased frequency of grade 3 and node-positive tumours (Elledge et al, 1994; Dignam, 2000; Joslyn and West, 2000; Carey et al, 2006; Bowen et al, 2008). Our finding of a higher incidence of multifocal tumours in Blacks than Whites is in agreement with other data (Litton et al, 2007). The incidence of multifocal disease in all ethnic groups of this cohort was higher than in some comparable series; this is likely to reflect the fact that multi-focality was defined pathologically following surgery in this study, rather than radiologically before surgery. The presence of multifocal disease was not incorporated into our multivariate analysis as both the definition of multi-focality and the independent prognostic effect of this feature over and above total tumour diameter remain controversial (Coombs and Boyages, 2005).
All of our cohort were diagnosed and treated within the UK NHS according to local protocols and therefore had equal access to standard therapies. There was no difference in receipt of chemotherapy for early breast cancer between ethnic groups, unlike the data from Bickell et al (2006) who reported use of appropriate chemotherapy in only 67% of Blacks compared with 78% of Whites. The increased use of neo-adjuvant rather than adjuvant chemotherapy in Blacks in our cohort is likely to be due to the increased frequency of larger tumours in this ethnic group. Use of anthracyline/taxane combination chemotherapy was similar in each ethnic category, in contrast to Griggs et al (2007a) who reported increased use of non-standard chemotherapy in American Blacks . There are also reports that Blacks are more likely to receive reduced dose chemotherapy and to discontinue chemotherapy prematurely (Griggs et al, 2007b; Hershman et al, 2009). Such treatment modifications could reflect higher rates of co-morbidities in Blacks compared with Whites, although Blacks do not have increased rates of neutropenic complications despite lower baseline white blood counts (Tammemagi et al, 2005; Hershman et al, 2009). We currently have insufficient data to compare chemotherapy dose density in our cohort. However, increased mortality in Blacks compared with Whites enroled in SWOG chemotherapy studies has been demonstrated despite similar relative dose intensity of adjuvant chemotherapy (Hershman et al, 2009).
As anticipated, the major cause of death in this cohort was breast cancer. Our data show clearly that both DRFS and OS were significantly lower in Blacks than Whites. This is in agreement with the findings of a number of previous USA and UK studies (Joslyn and West, 2000; Jatoi et al, 2003; Carey et al, 2006; Grann et al, 2006; Wild et al, 2006; Jack et al, 2009; Albain et al, 2009). Data on the association between Asian ethnicity and breast cancer prognosis are more limited, but our data are consistent with other studies showing no significant difference in the outcome of Whites and Asians (Wild et al, 2006).
Our multivariate analyses indicate that the inferior outcomes of Blacks are not fully explained by an increased frequency of adverse pathological features. In particular, our separate analyses of ER-positive, ER-negative and triple-negative patients confirm previous reports that the poor prognosis of Blacks is not fully explained by the increased incidence of triple-negative tumours (Albain et al, 2009), although we cannot entirely exclude other confounding biological factors. Our analysis is based on biological features obtainable from routine histopathological review; it is feasible that our results could be explained by differences in tumour gene expression profiles. An excess of luminal B tumours (a subtype of ER-positive breast cancer defined by increased proliferation, relative resistance to chemotherapy compared with other highly proliferative breast cancers, and poor outcome with endocrine therapy) in Blacks could, for example, explain our findings, (Perou et al, 2000). Alternatively, it is well established that breast cancers diagnosed during or within a year of pregnancy tend to be more aggressive than cancers in nulliparous women (reviewed by Azim et al (2012)), and it is possible that our results could be explained by a higher number of pregnancy associated tumours in Blacks than other ethnic groups. Our data set does not permit us to make direct assessments about the number of pregnancy-related cancers in our cohort, as pregnancy was assessed as a risk factor for breast cancer rather than a potential prognostic factor, and we therefore lack data on the date of second and subsequent pregnancies. However, the fact that Blacks who had already started their families by the time of their diagnosis were more likely to have a larger number of children than Whites suggests that Blacks would have spent more time being pregnant before their cancer diagnosis and could therefore have been at a higher risk of pregnancy-related breast cancer.
Although previous publications have reported persistence of ethnicity as an independent prognostic factor in both ER-negative and positive patients after adjustment for other pathological factors, we found this in ER-positive patients only. Albain et al (2009) also found a greater hazard ratio in Black premenopausal ER-positive patients than ER-negative patients (1.74 vs 1.29), and Hershman et al (2009) commented that their finding of no interaction between race and tumour ER status should ‘not be overinterpreted' given their small sample size and the long survival of their non-age selected patients . The small number of events in our ER-negative Black patients may affect our ability to demonstrate an independent effect of ethnicity in ER-negative patients. However, our finding that Black ethnicity is particularly an independent prognostic factor particularly in young ER-positive patients could suggest that either the use or effectiveness of hormonal therapy may vary significantly between ethnic groups. Bickell et al (2006) reported significantly lower use of adjuvant hormonal therapy in Blacks (71%) than Whites (80%) in women treated in America. Unexpectedly, we also found a lower percentage of ER-positive Black patients (80.8%) treated with hormonal therapy than White patients (89.3%); however, we cannot confirm that this is a statistically significant difference owing to missing data. It has been reported that compliance with tamoxifen is significantly lower in non-Whites than Whites (Partridge et al, 2003). Reduced compliance with hormonal therapy remains a possible explanation for our observations, although we did not collect compliance data. Our finding that more oophorectomies were performed in White than Asian or Black women could reflect a higher proportion of identified BRCA mutations in our Whites than Black or Asian patients as reported elsewhere (Ademuyiwa and Olopade, 2003).
Pharmocogenetics may also have a role in ethnic populations with different genetic structure. Some CYP2D6 variants associated with ‘poor metabolism' of tamoxifen are more common in Blacks than other ethnic groups (Bradford, 2002; Gaedigk et al, 2002). However, it is currently controversial whether CYP2D6 genotype directly affects breast cancer survival in patients on adjuvant tamoxifen (Abraham et al, 2010). Albain et al (2009) reported inferior outcome in Blacks compared with Whites in other ‘sex-specific' cancers as well as breast cancer . This suggests that other hormonal influences could interact with genetic factors. However, studies of the association between polymorphisms of the CYP1A1 gene (involved in oestrogen metabolism) and risk of breast cancer in African-Americans have been inconclusive (Taioli et al, 1995; Bailey et al, 1998).
Many publications have examined the role of socio-economic factors in the presentation and outcome of malignancies. In a systematic review of mostly American studies, socio-economic position was been found to explain part of the variation in OS between ethnic groups but did not account for differences in breast cancer survival (McKenzie and Jeffreys, 2009). Other social issues may also cause disparities in breast cancer outcome in different ethnic groups. McKenzie and Jeffreys (2009) noted that, ‘although there is a lack of major systemic genetic differences between ethnic groups, there are extensive differences in lifestyle'. Their systematic review however found little evidence to indicate that smoking or alcohol use could explain the inferior survival of Blacks compared with Whites, unlike BMI which did explain some of this variation (McKenzie and Jeffreys, 2009).
Health systems such as the UK NHS are designed to provide equal access to health care; however, this does not automatically equate to equal use of health care (Forbes et al, 2011). Recent immigration is frequently associated with linguistic and cultural challenges, which may act as barriers to accessing health care. It has also been reported that Blacks are less aware of symptoms of breast cancer than other groups and are less likely to self-check (Forbes et al, 2011). Therefore, the increased average tumour size may reflect a cultural tendency to delay presentation, as all our patients were below the minimum age for breast cancer screening. However, there was no significant difference between the rates of nodal involvement in the different ethnic groups in our cohort. Details of follow-up routines were not collected in this study; however, the independent effect of ethnicity persists when limiting analyses to hospitals treating both White and Black patients (data not shown). Clearly, our cohort contains a much smaller number of Black patients than in many of the American published series. However, the proportion of Black patients in the POSH cohort is very similar to the English population as a whole (2.9%). English cancer registry data for 2007 includes ethnicity data on only 80% of patients but indicate that 3.8% of breast cancers diagnosed in under 50 year olds were in Black women (National Cancer Intelligence Network, 2011), suggesting that our data are representative of the premenopausal English population. The average age of diagnosis is lower in Black women than Caucasians in the United Kingdom as elsewhere (Bowen et al, 2008). We cannot exclude selection bias; patients who agree to participate in clinical trials may not fully represent the general population. However, previous work indicates these patients may be more compliant with treatment than non-trial patients (Antman et al, 1985).
Categorisation of patients into broad ethnic categories on the basis of self-reported ethnicity is a simplification of a very complex picture. We have not attempted to differentiate between Blacks of African and Caribbean descent because of small patient numbers. However, previous data suggest that there is disparity in the outcome of these two groups (Wild et al, 2006). The complexities of categorising ethnicity and the controversies associated with analysing data from individuals with mixed ethnicity have been highlighted previously (Agyemang et al, 2005; Aspinall, 2011); however, here we have been transparent in our management of these data. Place of birth may provide additional information about genetic ancestry (Ingleby, 2008).
Other limitations of this study include the low number of ER-negative patients in the Black and Asian groups. The target sample size of the POSH study (n=3000) was calculated to detect a 10% difference in event rates between BRCA mutation carriers and sporadic early-onset breast cancer patients. It is likely that this study was underpowered to demonstrate an independent effect of ethnicity in ER-negative patients. Incomplete data on HER2 status reflect the time course of recruitment to this study, with routine HER2 testing largely being introduced from 2005 onwards, while missing PR data are largely accounted for by the fact that some of the recruiting hospitals did not routinely assess PR status during the study period. In addition, inconsistencies in the reporting of trastuzumab use, hormonal therapy and ovarian suppression have resulted in missing data, which has precluded a formal analysis of the use of these treatments in different ethnic groups. We have also not attempted to classify the socio-economic status of patients, as the POSH study did not collect data on income or education.
However, the POSH cohort is the first prospective study of young breast cancer patients treated within the UK NHS and, unlike previous registry based retrospective series, includes extensive data on treatment as well as pathology. This analysis of the effect of ethnicity on breast cancer outcome is strengthened by our use of a pre-specified analysis plan and STROBE reporting guidelines. Ethnicity was also directly self-reported by study participants, rather than inferred from other information, and we have been explicit in our categorisation of ethnic groups. Our data also benefits from the fact that all patients received ‘modern' oncological therapies, in contrast to some historical registry studies.
We have recently published a comprehensive description of the entire POSH cohort, which confirms the poor medium term outcome of both ER-positive and ER-negative patients aged 40 years or under at the time of diagnosis (Copson et al, 2013). The cause for the poor outcome of young breast cancer patients currently remains controversial; the POSH study ultimately aims to determine whether this is in part due to underlying inherited genetic mutations.
This publication from the POSH study provides valuable confirmation that Black ethnicity is an independent marker of poor prognosis in this young age group. Further research is clearly required to establish whether the effect of Black ethnicity is indeed more marked in ER-positive than ER-negative disease, and if so whether this is related to the use or effectiveness of Tamoxifen. In addition, there is a need to clarify the contribution of socio-economic position, education and breast cancer awareness to outcome of early breast cancer in different ethnic groups in the United Kingdom. The fact that almost 50% of patients in all ethnic groups presented with tumours ⩾2.0 cm does of course raise questions about the need for screening in younger women. However, as robust evidence for screening mammography in this group does not exist, and there are demonstrable differences in breast awareness between different ethnic groups then improving education and understanding of breast cancer in these populations may be effective in reducing the differences observed in tumour size in this study (Forbes et al, 2011).
Conclusion
We present the first prospective study of young breast cancer patients in the United Kingdom to analyse outcome data according to ethnicity. Our results confirm that Black patients have an increased risk of breast cancer recurrence than Whites despite equal access to health care including adjuvant therapies. Black ethnicity is an independent indicator of poor prognosis in young women with invasive breast cancer, suggesting that current treatment approaches may be less effective in this population. Further studies are required to investigate this in more detail and to optimise the management of this patient group.
Acknowledgments
We acknowledge the POSH collaborators and all the patients who participated in this study. Funding for this study has been provided by The Wessex Cancer Trust, Cancer Research UK (grant refs A7572, A11699, C22524), the study is a National Cancer Research Network Portfolio study. RE acknowledges support from the NIHR to the Biomedical Research Centre At The Institute Of Cancer Research and Royal Marsden NHS Foundation Trust. (http://www.ncrn.org.uk/Portfolio/index.htm). Participating principal investigators are listed in Supplementary Document 1 and on the study website. http://www.southampton.ac.uk/medicine/research/posh.page
Appendix
POSH Steering group: Professor Diana Eccles, Dr Peter Simmonds, Professor Douglas G Altman, Dr Paul Pharoah, Professor Louise Jones, Professor Ros Eeles, Professor Gareth Evans, Professor Alistair Thompson, Professor Shirley Hodgson, Mr Hisham Hammad, Professor Tim Bishop, Dr Ruth Warren, Professor Fiona Gilbert, Professor Sunil Lakhani, Professor Andrew Hanby.
EC has received honoraria from Roche and RIC has received honoraria from GSK and Pfizer. RE has received educational support from Vista Diagnostics, Tepnel (now GenProbe), Illumina and Janssen-Cilag. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, Luccarini C, Shah M, Ingle S, Greenberg D, Earl HM, Dunning AM, Pharoah PD, Caldas C. CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res. 2010;12:R64. doi: 10.1186/bcr2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev. 2003;22:47–53. doi: 10.1023/a:1022259901319. [DOI] [PubMed] [Google Scholar]
- Agyemang C, Bhopal R, Bruijnzeels M. Negro, Black, Black African, African Caribbean, African American or what? Labelling African origin populations in the health arena in the 21st century. J Epidemiol Commun Health. 2005;59:1014–1018. doi: 10.1136/jech.2005.035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman K, Amato D, Wood W, Carson J, Suit H, Proppe K, Carey R, Greenberger J, Wilson R, Frei E., III Selection bias in clinical trials. J Clin Oncol. 1985;3:1142–1147. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- Aspinall PJ. The utility and validity for public health of ethnicity categorization in the 1991, 2001 and 2011 British Censuses. Public Health. 2011;125:680–687. doi: 10.1016/j.puhe.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Azim HA, Jr, Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012;38 (7:834–842. doi: 10.1016/j.ctrv.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Bailey LR, Roodi N, Verrier CS, Yee CJ, Dupont WD, Parl FF. Breast cancer and CYPIA1, GSTM1, and GSTT1 polymorphisms: evidence of a lack of association in Caucasians and African Americans. Cancer Res. 1998;58:65–70. [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–281. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229–243. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;9:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- Coombs NJ, Boyages J. Multi-focal and multi-centric breast cancer: Does each focus matter. J Clin Oncol. 2005;23:7497–7502. doi: 10.1200/JCO.2005.02.1147. [DOI] [PubMed] [Google Scholar]
- Copson E, Eccles B, Maishman T, Gerty S, Stanton L, Cutress RI, Altman DG, Durcan L, Simmonds P, Lawrence G, Jones L, Bliss J, Eccles D, POSH Study Steering Group Prospective Observational Study of Breast Cancer Treatment Outcomes for UK Women Aged 18-40 Years at Diagnosis: The POSH Study. J Natl Cancer Inst. 2013;105 (13:978–988. doi: 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- Dignam JJ. Differences in breast cancer prognosis among African American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- Eccles D, Gerty S, Simmonds P, Hammond V, Ennis S, Altman DG. Prospective study of Outcomes in Sporadic vs Hereditary breast cancer (POSH): study protocol. BMC Cancer. 2007;7:160. doi: 10.1186/1471-2407-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- El-Tamer MB, Wait RB. Age at presentation of African-American and Caucasian breast cancer patients. J Am Coll Surg. 1999;188:237–240. doi: 10.1016/s1072-7515(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Forbes LJ, Atkins L, Thurnham A. Breast cancer awareness and barriers to symptomatic presentation among women from different ethnic groups in East London. Br J Cancer. 2011;105:1474–1479. doi: 10.1038/bjc.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RA, Yea VKH. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Bradford LD, Marcucci KA, Leeder JS. Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther. 2002;72:76–89. doi: 10.1067/mcp.2002.125783. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- Grann V, Troxel AB, Zojwalla N, Hershman D, Hershman D, Glied SA, Jacobson JS. Regional and racial disparities in breast cancer-specific mortality. Soc Sci Med. 2006;62:337–347. doi: 10.1016/j.socscimed.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford, Dale DC, Lyman GH. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25 (18:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25:277–284. doi: 10.1200/JCO.2006.08.3063. [DOI] [PubMed] [Google Scholar]
- Harding S, Rosato M. Cancer incidence among first generation Scottish, Irish, West Indian and South Asian migrants living in England and Wales. Ethn Health. 1999;4:83–92. doi: 10.1080/13557859998218. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Unger JM, Barlow WE, Hutchins LF, Martino S, Osborne CK, Livingston RB, Albain KS. Treatment quality and outcomes of African American vs white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingleby JD. Early onset of breast cancer in Black British women: how reliable are the findings. Br J Cancer. 2008;99:986–987. doi: 10.1038/sj.bjc.6604625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack RH, Davies EA, Moller H. Breast cancer incidence, stage, treatment and survival in ethnic groups in South East England. Br J Cancer. 2009;100 (3:545–550. doi: 10.1038/sj.bjc.6604852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98:894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Litton JK, Eralp Y, Gonzalez-Angulo A, Broglio K, Uyei A, Hortobagyi G, Arun B. Multifocal breast cancer in women <35 years old. Cancer. 2007;110:1445–1150. doi: 10.1002/cncr.22928. [DOI] [PubMed] [Google Scholar]
- Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality:a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F, Jeffreys M. Do lifestyle or social factors explain ethnic/ racial inequalities in breast cancer. Epidemiol Rev. 2009;31:52–66. doi: 10.1093/epirev/mxp007. [DOI] [PubMed] [Google Scholar]
- National Cancer Intelligence Network Second all breast cancer report (2011 ) http://www.ncin.org.uk/publications/reports/default.aspx .
- Newman LA, Alfonso AE. Age-related differences in breast cancer stage at diagnosis between black and white patients in an urban community hospital. Ann Surg Oncol. 2007;4:655–662. doi: 10.1007/BF02303751. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- Taioli E, Trachman J, Chen X, Toniolo P, Garte SJ. A CYP1A1 restriction fragment length polymorphism is associated with breast cancer in African-American women. Cancer Res. 1995;55:3757–3758. [PubMed] [Google Scholar]
- Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- Wild SH, Fischbacher CM, Brock A, Griffiths C, Bhopal R. Mortality from all cancers and lung, colorectal, breast and prostate cancer by country of birth in England and Wales, 2001-2003. Br J Cancer. 2006;94:1079–1085. doi: 10.1038/sj.bjc.6603031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.