Abstract
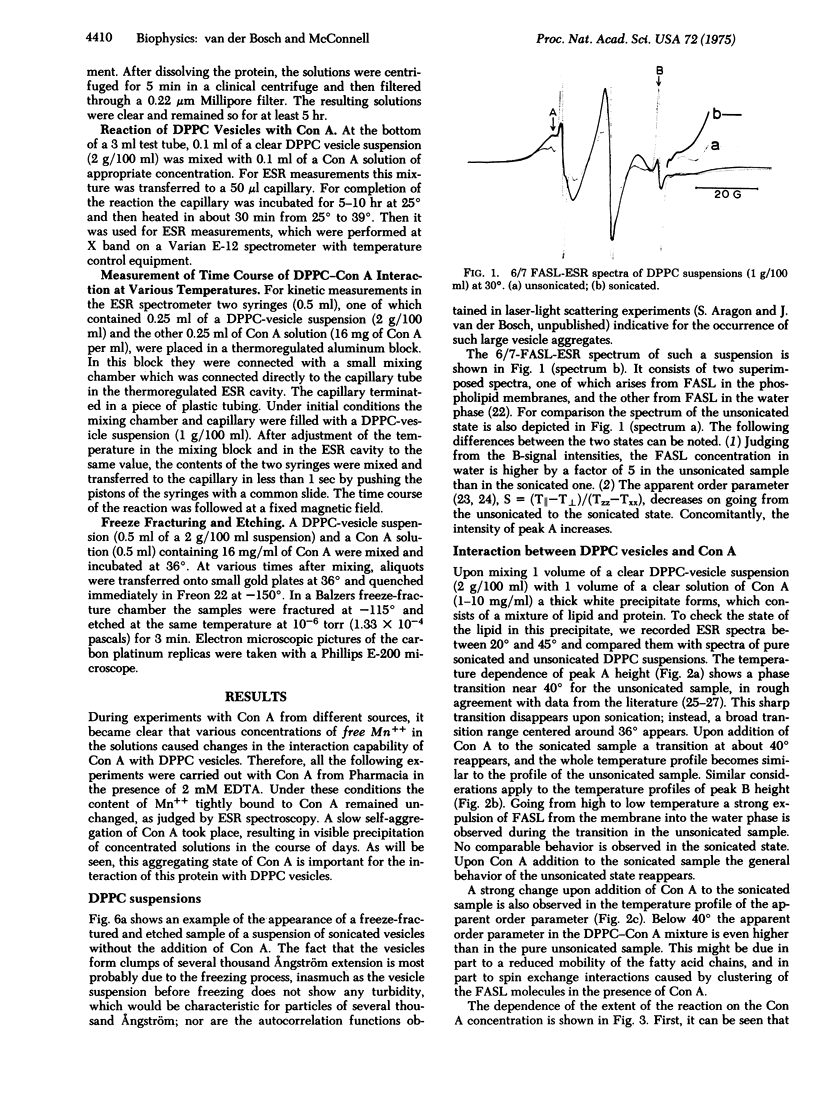
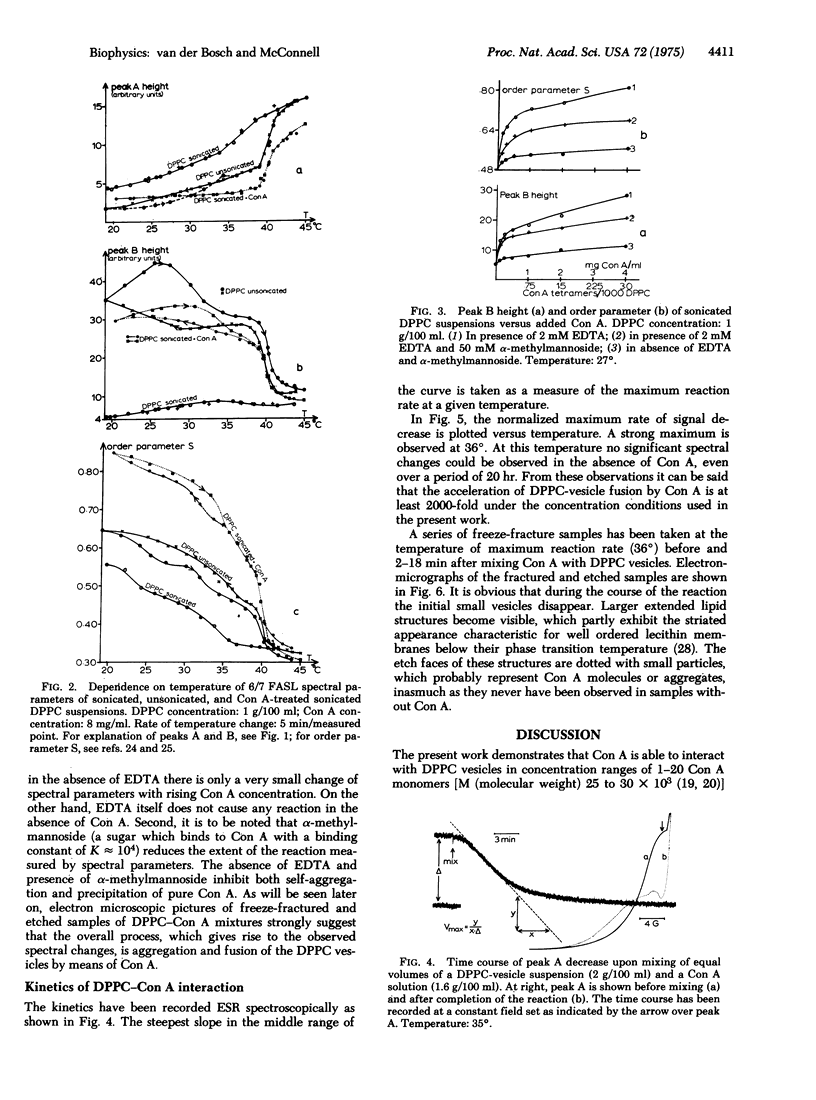
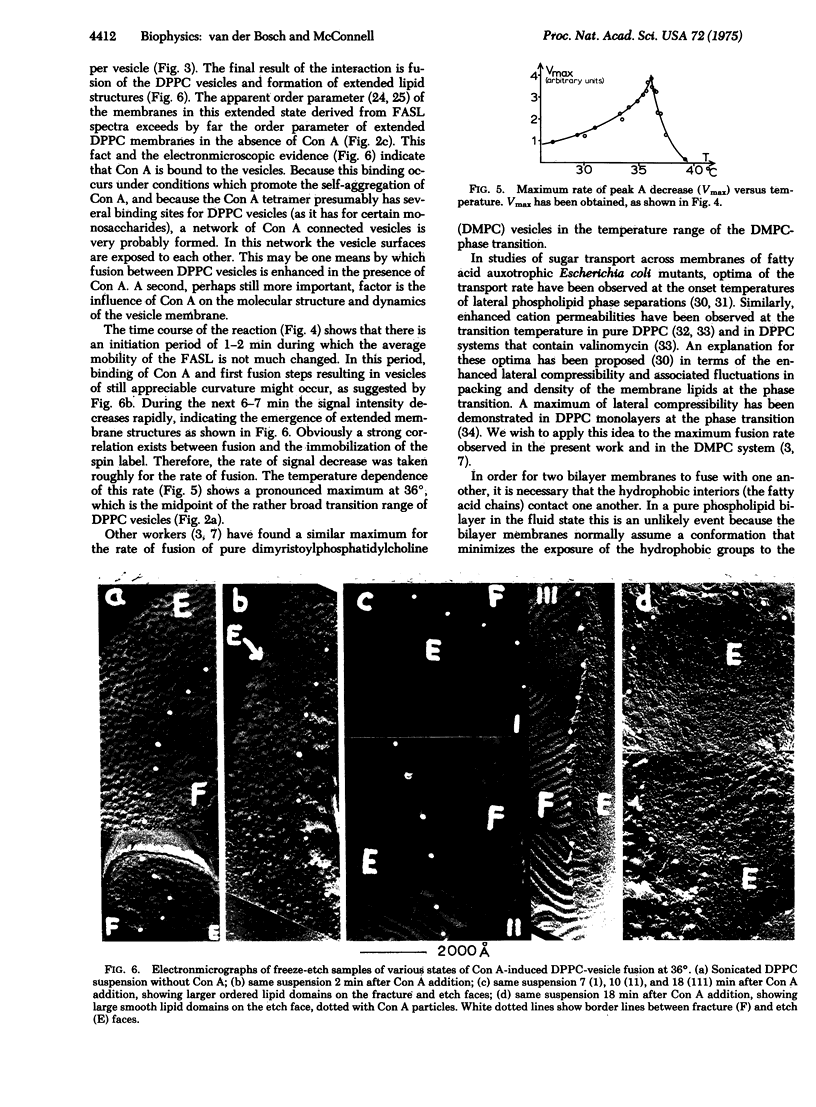
The temperature dependence of fatty acid spin label resonance spectra and freeze fracture micrographs of sonicated dipalmitoylphosphatidylcholine vesicles in the absence and presence of concanavalin A demonstrate a strong interaction of concanavalin A with these lipid membranes, which results in fusion of the vesicles. The rate of this reaction as followed with use of magnetic resonance exhibits a pronounced maximum at 36 degrees, the midpoint of the phase transition range of dipalmitoylphosphatidylcholine vesicles. This maximum is discussed in terms of structural fluctuations, which are maximal in the phase transition range of the membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barratt M. D., Laggner P. The pH-dependence of ESR spectra from nitroxide probes in lecithin dispersions. Biochim Biophys Acta. 1974 Aug 21;363(1):127–133. doi: 10.1016/0005-2736(74)90011-x. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr, Wang J. L., Cunningham B. A., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. III. Structure of the monomer and its interactions with metals and saccharides. J Biol Chem. 1975 Feb 25;250(4):1513–1524. [PubMed] [Google Scholar]
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Butler K. W., Tattrie N. H., Smith I. C. The location of spin probes in two phase mixed lipid systems. Biochim Biophys Acta. 1974 Sep 23;363(3):351–360. doi: 10.1016/0005-2736(74)90074-1. [DOI] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Fusion of phospholipid vesicles with viable Acholeplasma laidlawii. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1238–1240. doi: 10.1073/pnas.70.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. W., Wu S. H., McConnell H. M. Lateral phase separations in binary lipid mixtures: correlation between spin label and freeze-fracture electron microscopic studies. Biochim Biophys Acta. 1974 Sep 6;363(2):151–158. doi: 10.1016/0005-2736(74)90055-8. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Letter to the editor: Fusion of Sendai viruses with model membranes. J Mol Biol. 1974 Aug 15;87(3):625–628. doi: 10.1016/0022-2836(74)90107-7. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. L., Prestegard J. H. Fusion of fatty acid containing lecithin vesicles. Biochemistry. 1975 Apr 22;14(8):1790–1795. doi: 10.1021/bi00679a035. [DOI] [PubMed] [Google Scholar]
- Lau A. L., Chan S. I. Nuclear magnetic resonance studies of the interaction of alamethicin with lecithin bilayers. Biochemistry. 1974 Nov 19;13(24):4942–4948. doi: 10.1021/bi00721a010. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Fox C. F. A comparison of characteristic temperatures for transport in two unsaturated fatty acid auxotrophs of Escherichia coli. J Supramol Struct. 1973;1(6):535–544. doi: 10.1002/jss.400010608. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Ohnishi S. Membrane fusion. Transfer of phospholipid molecules between phospholipid bilayer membranes. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1509–1516. doi: 10.1016/0006-291x(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Martin F., MacDonald R. Liposomes can mimic virus membranes. Nature. 1974 Nov 8;252(5479):161–163. doi: 10.1038/252161a0. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Lipid bilayer phase transition: density measurements and theory. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3443–3444. doi: 10.1073/pnas.70.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Huang L., Wey C. Interaction of phospholipid vesicles with cultured mammalian cells. Nature. 1974 Nov 8;252(5479):166–167. doi: 10.1038/252166a0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Mayhew E., Poste G., Smith S., Vail W. J. Incorporation of lipid vesicles by mammalian cells provides a potential method for modifying cell behaviour. Nature. 1974 Nov 8;252(5479):163–166. doi: 10.1038/252163a0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E. Fusion of mammalian cells by unilamellar lipid vesicles: inflluence of lipid surface charge, fluidity and cholesterol. Biochim Biophys Acta. 1973 Sep 27;323(1):23–42. doi: 10.1016/0005-2736(73)90429-x. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E., Vail W. J. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim Biophys Acta. 1974 May 30;352(1):10–28. doi: 10.1016/0005-2736(74)90175-8. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Graham D. E., Hauser H. Lateral compressibility and penetration into phospholipid monolayers and bilayer membranes. Nature. 1975 Mar 13;254(5496):154–156. doi: 10.1038/254154a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Prestegard J. H., Fellmeth B. Fusion of dimyristoyllecithin vesicles as studied by proton magnetic resonance spectroscopy. Biochemistry. 1974 Mar 12;13(6):1122–1126. doi: 10.1021/bi00703a011. [DOI] [PubMed] [Google Scholar]
- Puszkin S., Kochwa S. Regulation of neurotransmitter release by a complex of actin with relaxing protein isolated from rat brain synaptosomes. J Biol Chem. 1974 Dec 10;249(23):7711–7714. [PubMed] [Google Scholar]
- Rash J. E., Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol. 1973 Jan;30(1):166–186. doi: 10.1016/0012-1606(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. IV. Atomic coordinates, hydrogen bonding, and quaternary structure. J Biol Chem. 1975 Feb 25;250(4):1525–1547. [PubMed] [Google Scholar]
- Satir B. Ultrastructural aspects of membrane fusion. J Supramol Struct. 1974;2(5-6):529–537. doi: 10.1002/jss.400020503. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Träuble H. Phasenumwandlungen in Lipiden. Mögliche Schaltprozesse in biologischen Membranen. Naturwissenschaften. 1971 Jun;58(6):277–284. doi: 10.1007/BF00624732. [DOI] [PubMed] [Google Scholar]
- Wu S. H., McConnell H. M. Lateral phase separations and perpendicular transport in membranes. Biochem Biophys Res Commun. 1973 Nov 16;55(2):484–491. doi: 10.1016/0006-291x(73)91112-1. [DOI] [PubMed] [Google Scholar]
- van der Bosch J., Schudt C., Pette D. Influence of temperature, cholesterol, dipalmitoyllecithin and Ca2+ on the rate of muscle cell fusion. Exp Cell Res. 1973 Dec;82(2):433–438. doi: 10.1016/0014-4827(73)90362-5. [DOI] [PubMed] [Google Scholar]



