Abstract
Exercise has been found to alter pain sensitivity with a hypoalgesic response (i.e., diminished sensitivity to pain) typically reported during and/or following high intensity exercise. Most of this research, however, has involved the testing of men. Thus, the purpose of the following investigation was to examine changes in pain perception in women during and following exercise. Seventeen healthy female subjects (age 20.47±.87; VO2 peak 36.77± 4.95) volunteered to undergo pain assessment prior to, during, and after a graded exhaustive VO2 peak cycling challenge. Heart Rate (HR) and Oxygen Uptake (VO2) were monitored along with electro-diagnostic assessments of Pain Threshold (PT) and Pain Tolerance (PTOL) at: 1) baseline (B), 2) during exercise (i.e., 120 Watts), 3) at exhaustive intensity (VO2 peak), and 4) 10 minutes into recovery (R). Data were analyzed using repeated measures ANOVA to determine differences across trials. Significant differences in PT and PTOL were found across trials (PT, p = 0.0043; PTOL p = 0.0001). Post hoc analyses revealed that PT were significantly elevated at VO2 peak in comparison to B (p = 0.007), 120 Watts (p = 0.0178) and R (p = 0.0072). PTOL were found to be significantly elevated at 120 Watts (p = 0.0247), VO2 peak (p < 0.001), and R (p = 0.0001) in comparison to B. In addition, PTOL were found to be significantly elevated at VO2 peak in comparison to 120 Watts (p = 0.0045). It is concluded that exercise-induced hypoalgesia occurs in women during and following exercise, with the hypoalgesic response being most pronounced following exhaustive exercise.
Key Points.
Exercise-induced hypoalgesia (i.e., elevated PT and PTOL) was found to occur in women during and following exercise, with the hypoalgesic response being most pronounced during exhaustive exercise.
Key Words: Nociception, cycling, hypoalgesia, pain tolerance threshold
Introduction
Exercise Induced Hypoalgesia (EIH) is characterized by a temporary alteration in pain perception associated with exercise (Cook et al., 2000; Cook and Koltyn, 2000; Koltyn, 2000). Typically, investigators have found a hypoalgesic response (i.e., diminished pain sensitivity) to occur either during and/or following exercise (Cook and Koltyn, 2000; Koltyn, 2002; O’Connor and Cook, 1999). Different aspects of pain have been examined in these studies with the majority of studies reporting increases in pain thresholds (i.e., point at which a noxious stimulus first becomes painful) during and/or following exercise (Droste et al., 1991; Kempainen et al., 1985; 1990; 1998; Pertovaara et al., 1984). A few investigators have reported decreases in pain ratings (i.e., ratings of pain intensity) during and/or following exercise (Gurevich et al., 1994; Koltyn et al., 1996; 1998; 2001), but less is known regarding changes in pain tolerance (i.e., point at which an individual is not willing to endure further noxious stimulation) during and following exercise.
A number of different exercise protocols have been used in the studies that have been conducted in this area. Some investigators have used exercise protocols involving incremental increases in workloads (Droste et al., 1988; Kemppainen et al., 1985; 1990; 1998; Pertovaara et al., 1984) while other investigators have prescribed a specific workload to participants for the exercise session (Guieu et al., 1992; Gurevich et al., 1994). In addition, several investigators have used an exercise protocol in which participants self-selected the exercise intensity (Fuller and Robinson, 1993; Janal et al., 1984; Sternberg et al., 2001). Inconsistent results have been found for studies that let participants self-select the exercise intensity. More consistent EIH responses have been found for studies that used a protocol involving exercise prescribed at a percentage of maximal oxygen uptake (e.g., 60-75%). In addition, exercise protocols involving incremental increases in workloads to exhaustion have consistently revealed EIH to occur at the higher workloads, with the exception of an increase in pain thresholds at a lower workload (e.g., 100 W) in a study by Kemppainen et al. (1990).
Most of this research, however, has involved the testing of men so it is currently unclear whether these results can be generalized to women. The general pain literature suggests that men and women differ in pain perception (Craft, 2003), but very little research has been conducted examining EIH in women (Koltyn et al., 2001; Sternberg et al. 2001). Further research is needed in this area. Therefore, the primary purpose of the following investigation was to examine changes in pain perception among women during and following exercise.
Methods
Seventeen division III varsity female athletes (7 Basketball, 5 Soccer, 3 Volleyball, 1 Softball, 1 Field Hockey) were recruited to participate in this investigation. All of the women were screened using a healthy history questionnaire and reported being in good health and free from injury. In addition, all of the women indicated that they had not taken any prescription or over the counter medications in the past 48 hours. Prior to data collection, each woman completed a document of informed consent and received a comprehensive verbal description of all the procedures along with an opportunity to ask questions. The research protocol and all associated documents were reviewed and approved by the Gettysburg College Institutional Review Board for the ethical treatment of human subjects.
Data collection began with anthropometric measurements of height, weight and body composition. Height and weight were measured using a balance beam scale (Detecto, Webb City, MO) and were recorded in centimeters and kilograms respectively. Body composition was estimated using Lange skin-fold calipers (Beta Technology Corp., Cambridge, MD) and a three-site formula (triceps, thigh and supraillium) previously described by Jackson and Pollock (1985). To avoid the hormonal variations associated with the menstrual cycle, we only tested our subjects between the 5th and 14th day after the onset of their last menses. A heart rate monitor (Polar US, Lake Success, NY) with conduction gel was adjusted, fitted and then strapped around the subject. The seat post of the stationary cycle (Monark, Sweden) was then adjusted so that each woman had a 5 degree bend at the knee during the bottom phase of the pedal stroke.
Once the woman was sitting comfortably on the cycle ergometer, she was then fitted with a neoprene face-mask that held the breath by breath neumotac apparatus which was connected to the metabolic cart (Medgraphics, St. Paul, MN). Sampling was reported in 30 sec intervals throughout the duration of the test and a time-down report of oxygen uptake and heart rate was printed after each test. A standard mercury sphygmomanometer was used to monitor blood pressure and a telemetry sensor from the metabolic cart was attached to the cycle to detect signals from the heart rate monitor.
The subject was then prepped for a neuroselective electrodiagnostic sensory nerve evaluation using a Neurometer® (Neurotron, Baltimore, MD) to assess pain perception. This machine has been used widely since 1986 for the assessment of nociceptive nerve function in a variety of populations (Katims, 1998; Raj et al., 2001). The device delivers an atraumatic electrical stimulus to a set of gold-plated electrodes (Raj et al., 2001). The stimulus created is delivered directly to the nerve fiber bypassing the nerves end-organs and it is not influenced by skin thickness, subcutaneous fat or temperature (Katims, 1998). The reliability and validity of this machine has been described elsewhere (Katims, 1998). For this investigation, we chose the median nerve of the right index finger as the site for assessment. The index finger was chosen because it was away from the active tissue of the legs. In so doing, we have attempted to minimize the potential for simultaneous afferent impulses being received at the spinal cord, thus limiting the potential influence for gate-control differences. An example of the electrodiagnostic pain assessment site can be found in Figure 1.
Figure 1.

Electrodiagnostic pain set-up.
The protocol used in this study for inducing a quantifiable controlled pain stimulus incorporated a sinusoidal continuous electrical stimulus at 5 Hz which typically stimulates the small diameter unmyelinated nocioceptive ‘C’ fibers associated with ‘slow-pain’ (Katims, 1998). The stimulus increased in intensity every second by 10 mA until the woman could no longer tolerate the pain. Therefore, the duration of the pain stimulus was determined by the time it took to reach pain tolerance, which was typically less than 1 minute.
Two separate pain perception variables were measured during each pain assessment. Pain Threshold (PT) was recorded when the tingling current first became painful. A verbal command of ‘Pain’ was used by the subject to indicate when PT was achieved. Pain Tolerance (PTOL) was recorded by the assessment device when the subject could no longer tolerate the painful current and the test was stopped. A verbal command of ‘Stop’ was used to tell the researcher to terminate the test.
After the initial prepping procedures, a familiarization pain test was given to allow the subject to experience the unique electrical transcutaneous pain stimulus. This initial test also allowed the subject to become comfortable with the verbal commands associated with indicating each type of pain. Familiarization testing has been used extensively when inducing electrical transcutaneous pain (Katims, 1998). During the pain testing procedure the subjects rested their hands on the handle bars of the cycle ergometer. In between pain assessments the women were allowed to grasp the handlebars with both hands. A ten-minute rest period was given after the familiarization pain test.
Data collection began after 10 minutes of quiet rest by obtaining baseline measures of PT, PTOL, relative oxygen consumption, heart rate and blood pressure. The metabolic cart collected data continuously until the end of the protocol. Once the baseline data was recorded, the subject was asked to warm-up by pedaling the cycle ergometer at 60 rpm’s at a resistance of 30 Watts for 4 minutes. After the fourth minute the resistance was increased by 30 Watts every minute until the subject reached 120 Watts. After a full minute of pedaling at this resistance, a second set of cardiovascular and pain assessments were obtained while the subject continued to pedal in order to examine changes in pain perception during exercise. A resistance of 120 Watts was chosen in an effort to provide a ‘moderately difficult’ cardiovascular challenge that has been previously demonstrated in active females (Lee and Nieman, 1990).
After the assessment at 120 Watts, the resistance was increased again by 30 Watts every minute until the subject could no longer maintain 60 rpm’s or they verbally indicated volitional failure (VO2 peak). Ratings of Perceived Exertion (RPE) scores (1-10) (Pollock et al., 1998) were obtained every minute throughout the exercise protocol to help the investigators anticipate the achievement of VO2 peak. Immediately after the VO2 peak was achieved, another set of cardiovascular and pain measures were taken while the subject continued to pedal at 60 RPM’s with little resistance (30 Watts). After the final exercise assessments were recorded, the subject was asked to sit on the cycle without pedaling for a ten-minute recovery period, which concluded with a final set of cardiovascular and pain assessments.
Data analyses
The PT and PTOL data were individually normalized by dividing the raw scores for each subject by their own respective baseline scores taken prior to exercise. This method of normalizing electrical pain stimulus data has been used by others studying EIH (Droste, 1992; Kemppainen et al., 1990; Kosek and Ekholm, 1995). The data were then analyzed using repeated measures ANOVA to determine differences. When differences were indicated, a Fisher Protected Least Significant Difference post hoc analysis was used to determine differences among the variables. An a priori p-value of 0.05 was considered statistically significant. Post hoc power analysis for the main effects was performed for PT and PTOL and revealed a power of 0.82 for PT and 0.99 for PTOL, respectively, given 4 measurements with a sample size of 17 subjects and an alpha of 0.05.
Results
Reliability of pain responses
Data from the familiarization pain test were compared to baseline data to examine whether alterations in pain perception occurred as a result of pre-test exposure to the noxious electrical stimulus. Because these two tests were conducted under the same conditions in an effort to establish a valid baseline score, the normalization process was not used in this analysis. When the Raw PT pain scores from the familiarization test (198 ± 73) were compared to Raw baseline scores (207 ± 90) with a repeated measures ANOVA there were no significant differences between the trials for PT (p = 0.732). When the Raw PTOL pain scores from the familiarization test (428 ± 221) were compared to Raw baseline scores (431 ± 261) with a repeated measures ANOVA there were no significant differences between the trials for PTOL (p = 0.963). Intra-class correlations between the familiarization and baseline scores were significant for both PT (r = 0.737; p = 0.001) and PTOL (r = 0.963; p = 0.001). Thus, it appeared that pre-test exposure to the noxious electrical stimulus did not significantly influence the subsequent pain perception assessment.
Descriptive data
The mean age of the subjects was 20.5 ± 0.9 years, height 1.69 ± 0.08 m, weight 67.4 ± 9.0 kg, body fat % 29.8 ± 2.0. Descriptive data for the cardiovascular measurements of heart rate, oxygen uptake, systolic pressure and diastolic pressure can be found in Table 1.
Table 1.
Means (± SD) for the cardiovascular data at rest, 120 Watts, VO2 peak, and recovery.
| Workload | Variables | Means (± SD) |
|---|---|---|
| Rest | Heart Rate (bpm) | 73 (10) |
| Systolic Blood Pressure (mmHg) | 112 (11) | |
| Diastolic Pressure (mmHg) | 75 (9) | |
| 120Watts | VO2 (ml·kg-1·min-1) | 27.33 (3.66) |
| Heart Rate (bpm) | 152 (12) | |
| Systolic Blood Pressure (mmHg) | 150 (13) | |
| Diastolic Pressure (mmHg) | 68 (33) | |
| VO2 peak | VO2 (ml·kg-1·min-1) | 36.77 (4.95) |
| Heart Rate (bpm) | 179 (10) | |
| Systolic Blood Pressure (mmHg) | 156 (17) | |
| Diastolic Pressure (mmHg) | 59 (24) | |
| Recovery | Heart Rate (bpm) | 95 (10) |
| Systolic Blood Pressure (mmHg) | 116 (14) | |
| Diastolic Pressure (mmHg) | 72 (9) |
Pain threshold
Significant differences in pain thresholds were detected across trials (F1,14 = 5.077; p = 0.0043). Post hoc analysis revealed PT scores were significantly higher at VO2 peak in comparison to baseline (Table 2). The VO2 peak scores were also significantly higher than both the 120 Watts scores (p = 0.0178) and the recovery scores (p = 0.0072). No significant differences were found between baseline and 120 Watts (p = 0.2577), baseline and recovery (p = 0.498) and 120 Watts and recovery (p = 0.6727). Of the 17 subjects tested none of the subjects had a higher PT score at 120 Watts versus VO2 peak and only 3 subjects had a higher score during recovery. The results for pain thresholds are illustrated in Figure 2. In addition, a correlation analysis was performed to examine the association between Systolic Blood Pressure (SBP) and PT. Results indicated there was not a significant correlation between SBP and PT (r = 0.03).
Table 2.
Means (±SD) for pain threshold and pain tolerance scores.
| Baseline (1) |
120Watts (2) |
VO2 peak (3) |
Recovery (4) |
|
|---|---|---|---|---|
| Pain Threshold | 100 (0) 3 | 109.6 (27.1) 3 | 124.7 (25.5) 1, 2, 4 | 105.1 (21.8) 3 |
| Pain Tolerance | 100 (0) 2,3,4 | 116.3 (16.0) 1,3 | 133.6 (19.4) 1,2 | 127.2 (29.3) 1 |
Superscripts indicate p < 0.05 among the workloads.
Figure 2.
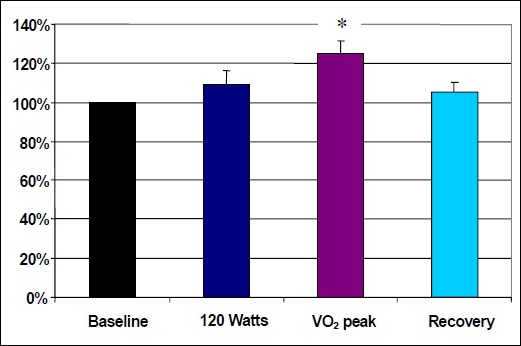
Normalized pain threshold scores and Standard Errors for Baseline, 120 Watts, VO2 peak and Recovery (scores are expressed as a % of Baseline). * p < 0.05 compared with Baseline, 120 Watts and Recovery.
Pain tolerance
Significant differences in pain tolerance were detected across trials (F1,14 = 9.387; p < 0.0001). Post hoc analysis revealed that PTOL scores were significantly higher at 120 Watts (p = 0.0.247), VO2 peak (p < 0.001), and recovery (p = 0.0001) in comparison to baseline. PTOL scores were also found to be significantly higher at VO2 peak in comparison to 120 Watts (p = 0.0045). Of the 17 subjects tested only 1 subject (5.8%) had a higher score at 120 Watts versus VO2 peak. Although approaching statistical significance, no difference was found between 120 Watts and recovery scores (p = 0.0679). In addition, a correlation analysis was performed to examine the association between Systolic Blood Pressure (SBP) and PTOL. Results indicated there was not a significant correlation between SBP and PT (r = 0.30). Finally, no significant differences were found between VO2 peak scores and recovery scores (p = 0.3098). The results for pain tolerances are illustrated in Figure 3.
Figure 3.
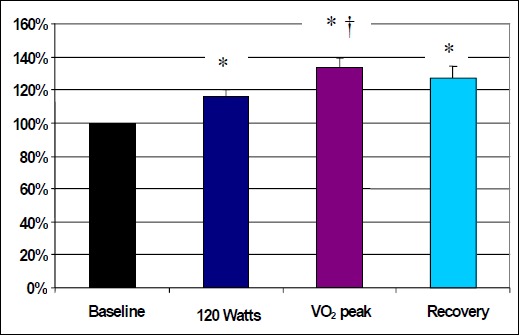
Normalized pain tolerance scores and Standard Errors for Baseline, 120 Watts, VO2 peak and Recovery (scores are expressed as a % of Baseline). * p < 0.05 compared with Baseline, † p < 0.05 compared with 120 Watts.
Discussion
The primary purpose of this investigation was to examine changes in pain perception in women during and following exercise. Results from this study indicated that EIH occurred in women during and following exercise, with the hypoalgesic response being most pronounced during exhaustive exercise. These results are in agreement with results from other investigations in which men were tested using protocols involving incremental increases in workloads (Droste et al., 1991; Kemppainen et al., 1985; 1990; 1998; Pertovaara et al., 1984). In addition, the results from this study add to the small literature on EIH in women (Sternberg et al., 2001; Koltyn et al., 2001). Specifically, results from the present study indicated that pain thresholds and pain tolerances were significantly elevated at VO2 peak. In addition, pain tolerances were found to be elevated during exercise (120 Watts), as well as 10 minutes following exercise.
The mechanisms responsible for EIH are poorly understood. Several researchers have hypothesized that proprioceptive and muscle afferents may be responsible for ‘overloading’ the nociceptive circuitry causing hypoalgesia (Hoffman et al., 2004; O’Connor and Cook, 1999). This hypothesis is related to the gate-control theory wherein the nervous system may prioritize the large diameter, fast-propagating fibers that are responsible for tactile and prorioceptive afferent input over the smaller unmylenated nociceptors (Porth, 2004). One of the unique aspects of the current investigation is that small unmylenated nociceptors were stimulated by providing a painful stimulus at a frequency of 5 hz which has been shown to be specific to ‘C’ pain fibers (Katims, 1998; O’Connor and Cook, 1999). The fact that pain differences emerged while using an inactive testing site, provides further evidence that central mechanisms may play a role in EIH.
Another possibility that has received some attention in the literature is that alterations in blood pressure (BP) associated with exercise may be related to alterations in pain perception. It has been reported that there is an interaction between pain modulatory and cardiovascular systems (Randich and Maixner, 1984). Examination of BP in the present study indicated that SBP was the highest when pain thresholds and pain tolerances were the highest, however, correlations between SBP and pain threshold and SBP and pain tolerance were not found to be significant. It is unclear why there was not a significant association between BP and pain perception in this study, but sample size may have been limited to detect significant associations between BP and pain perception. The sample size was determined based on the primary purpose of this study, which was to examine EIH in women, however, this sample size may have been too small to detect significant associations between BP and pain perception. Further research is needed to clarify the relationship between BP and pain perception in women.
Conclusions
Results from this study indicated that exercise can temporarily reduce pain in women but this finding can only be generalized to the sample that was tested in this study (i.e., female athletes with average aerobic capacity). It is currently unclear whether these results generalize to athletes with a higher aerobic capacity or to non-athletes. Also, since the women tested in this study were healthy individuals with no reported chronic pain, it is unclear whether these results generalize to women experiencing various chronic pain conditions (e.g., arthritis, fibromyalgia, low back pain). It is conceivable that high intensity exercise may exacerbate an already existing painful condition, thus, further research is warranted to examine the impact of exercise on pain in women with existing chronic pain.
Acknowledgement
This project was funded by a Presidential Research Fellowship from Gettysburg College. We would like to thank the Provost’s Office at Gettysburg College for their ongoing support. We would also like to thank Mr. David Petrie for his help during the data collection process.
Biographies
Daniel G. DRURY
Employment
Assistant Professor, Gettysburg College.
Degree
MS,BS.
Research interests
Exercise Induced Hypoalgesia, Eccentric Muscle Physiology, New Product Testing.
E-mail: ddrury@gettysburg.edu
Katelyn GREENWOOD
Employment
Nursing Student.
Degree
BS.
Kristin J. STUEMPFLE
Employment
Associate Professor, Gettysburg College.
Degree
PhD Penn State- Hershey.
Research interests
Hyponatremia.
Kelli F. KOLTYN
Employment
Associate Professor, Univ. Of Wisconsin - Madison.
Degree
PhD.
Research interests
Exercise Induced Hypoalgesia.
References
- Borg G. (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Champaign, IL [Google Scholar]
- Cook D., Koltyn K. (2000) Pain and Exercise. International Journal of Sports Psychology 31, 256-277 [Google Scholar]
- Cook D., Oconnor P., Ray C. (2000) Muscle pain perception and sympathetic nerve activity to exercise during opoid modulation. American Journal of Physiology-Integrative and Comparitive Physiology 279, r1565-r1573 [DOI] [PubMed] [Google Scholar]
- Craft R. (2003) Sex differences in drug- and non-drug-induced analgesia. Life Sciences 72, 397-411 [DOI] [PubMed] [Google Scholar]
- Droste C. (1992) Transient hypoalgesia under physical exercise: Relation to silent ischemia and implications for cardiac rehabilitation. Annals of Academy of Medicine 21, 23-33 [PubMed] [Google Scholar]
- Droste C., Greenlee M., Schrek M., Roskamm H. (1991) Experimental pain thresholds and plasma beta-endorphin levels during exercise. Medicine Science in Sports and Exercise 23, 334-342 [PubMed] [Google Scholar]
- Droste C., Meyer-Blackenburg H., Greenlee M., Roskamm H. (1988) Effect of physical exercise on pain thresholds and plasma beta-endorphins in patients with silent & symptomatic myocardial ischemia. European Heart Journal 9, 25-33 [DOI] [PubMed] [Google Scholar]
- Fuller A., Robinson R. (1993) A test of exercise induced analgesia using signal detection theory and a within-subjects design. Perception of Motor Skills 76, 1299-1310 [DOI] [PubMed] [Google Scholar]
- Guieu R., Blin O., Pouget J., Serratrice G. (1992) Nocioceptive threshold and physical activity. Canadian Journal of Neurological Science 19, 69-71 [PubMed] [Google Scholar]
- Gurevich M., Kohn P., Davis C. (1994) Exercise induced analgesia and the role of reactivity in pain sensitivity. Journal of Sports Sciences 12, 549-559 [DOI] [PubMed] [Google Scholar]
- Hoffman M., Shepanski M., Ruble S., Valic Z., Buckwater J., Clifford P. (2004) Intensity and duration threshold for Aerobiv Exercise-Induced Analgesia to pressure pain. Archives of Physical Medcine and Rehabilitation 85, 1183-1187 [DOI] [PubMed] [Google Scholar]
- Jackson A., Pollock M. (1985) Practical assessment of body composition. Physician and Sports Medicine 13, 76-90 [DOI] [PubMed] [Google Scholar]
- Janal M., Colt E., Clark W., Glasman M. (1984) Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: Effects of Naloxone. Pain 19, 13-25 [DOI] [PubMed] [Google Scholar]
- Katims J. (1998) Electrodiagnostic functional sensory evaluation of the patient with pain: A review of the neuroselective current perception threshold and pain tolerance threshold. Pain Digest 8, 219-230 [Google Scholar]
- Kemppainen P., Hamalainen O., Kononen M. (1998) Different effects of physical exercise on cold pain sensitivity in fighter pilots with and without the history of acute in-flight neck pain attacks. Medicine in Science in Sports and Exercise 30, 577-582 [DOI] [PubMed] [Google Scholar]
- Kemppainen P., Paalasmaa P., Pertovaara A., Alila A., Johansson G. (1990) Dexamethasone attenuates exercise-induced dental analgesia in man. Brain Research 519, 329-332 [DOI] [PubMed] [Google Scholar]
- Kemppainen P., Pertovaara A., Huppaniemi T., Johansson G., Karonen S. (1985) Modification of dental pain and cutaneous thermal sensitivity by physical exertion in man. Brain Research 360, 33-40 [DOI] [PubMed] [Google Scholar]
- Koltyn K. (2000) Analgesia following exercise. Sports Medicine 29, 85-98 [DOI] [PubMed] [Google Scholar]
- Koltyn K. (2002) Exercise-induced hypoalgesia and intensity of exercise. Sports Medicine 32, 477-487 [DOI] [PubMed] [Google Scholar]
- Koltyn K., Arbogast R. (1998) Perception of pain after resistance exercise. British Journal of Sports Medicine 32, 20-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn K., Garvin A., Gardiner R., Nelson T. (1996) Perception of pain following aerobic exercise. Medicine in Science Sports and Exercise 28, 1418-1421 [DOI] [PubMed] [Google Scholar]
- Koltyn K., Trine M., Stegner A., Tobar D. (2001) Effect of isometric exercise on pain perception and blood pressure in men and women. Medicine in Science Sports and Exercise 33, 282-290 [DOI] [PubMed] [Google Scholar]
- Kosek E., Ekholm J. (1995) Modulation of pressure pain thresholds during and following isometric contraction. Pain 61, 481-486 [DOI] [PubMed] [Google Scholar]
- Lee J., Nieman D. (1990) A nomogram for calculation of aerobic capacity from cycle ergometry. Annals of Sports Medicine 5, 163-165 [Google Scholar]
- O’Connor P., Cook D. (1999) Exercise and pain: The neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exercise and Sports Science Reviews 29, 119-166 [PubMed] [Google Scholar]
- Pertovaara A., Huppaniemi T., Virtanen A., Johansson G. (1984) The influence of exercise on dental pain thresholds and the release of stress hormones. Physiology and Behavior 33, 923-926 [DOI] [PubMed] [Google Scholar]
- Pollock M., Gaesser G., Butcher J., Despres J., Dishman R., Franklin B., Garber C. (1998) The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibilityin health adults. Medicine in Science in Sports and Exercise 30, 975-991 [DOI] [PubMed] [Google Scholar]
- Porth C. (2004) Essentials of pathophysiology. Lippincott Williams & Wilkins, Baltimore [Google Scholar]
- Randich A., Maixner W. (1984) Interactions between cardiovascular and pain regulatory systems. Neuroscience and Biobehavioral Reviews 8, 343-67 [DOI] [PubMed] [Google Scholar]
- Raj P., Chado H., Angst M., Heaver J., Dotson R., Brandstater M., Johnson B., Parris W., Finch P., Shahani B., Dhand U., Mekhail N., Daoud E., Hendler N., Somerville J., Wallace W., Panchal S., Glusman S., Jay G., Palliyath S., Longton W., Irving G. (2001) Painless electrodiagnostic current perception threshold and pain tolerance values in CRPS subjects and healthy controls: A multicenter study. Pain Practice 1, 55-60 [DOI] [PubMed] [Google Scholar]
- Sternberg W., Bokat C., Kass L., Alboyadjian A., Gracely R. (2001) Sex dependent components of the analgesia produced by athletic competition. Pain 2, 65-74 [DOI] [PubMed] [Google Scholar]


