Abstract
Reinforcement systems are believed to drive synaptic plasticity within neural circuits that store memories. Recent evidence from the fruit fly suggests that anatomically distinct dopaminergic neurons ultimately provide the key instructive signals for both appetitive and aversive learning. This dual role for dopamine overturns the previous model that octopamine signaled reward and dopamine punishment. More importantly, this anatomically segregated double role for dopamine in reward and aversion mirrors that emerging in mammals. Therefore, an antagonistic organization of distinct reinforcing dopaminegic neurons is a conserved feature of brains. It now seems crucial to understand how the dopaminergic neurons are controlled and what the released dopamine does to the underlying circuits to convey opposite valence.
Introduction
Learning provides animals with the ability to adapt their behaviour according to past and present circumstance. The presence of new and unexpected events in the environment, as well as the absence of predicted events are believed to be signaled in the brain by modulatory dopamine (DA) releasing neurons using a popular reinforcement learning mechanism [1,2]. In doing so these DA neurons provide an ongoing update of the environment and tune synaptic activity in the underlying neural circuitry so that current and future behaviour is optimal. Understanding whether such a reinforcement learning model represents a genuine and conserved mechanism of behavioural control in the nervous system, and if so how it operates, is of great interest and will require analyses across phyla. The enhanced cellular resolution offered by studying the genetically tractable and numerically less complex fruit fly brain provides considerable potential to understand DA neuron functions at the molecular, cellular, physiological and small neural network level.
Dopamine signals aversive reinforcement
Drosophila DA was previously thought to signal aversion and octopamine (OA), the invertebrate analog of norepinephrine, attraction [3,4]. Early studies disrupting DA neuron output during appetitive and aversive olfactory conditioning concluded that DA was exclusively required for aversive reinforcement [3]. Subsequently, using optogenetics, a subset of 12 DA neurons was identified in the PPL1 cluster (Figure 1a) whose activation paired with odour presentation could implant aversive memories [5]. Similar studies using thermogenetics to trigger other subsets of DA neurons in PAM (Figure 1b) and the PPL1 group produced effective negative reinforcement [6,7•]. In addition blocking these neurons impaired the formation of electric-shock reinforced aversive memory. It is therefore clear that certain DA neurons in PPL1 and PAM can provide negative valence if engaged during odour presentation [5-7•] (Figure 2a).
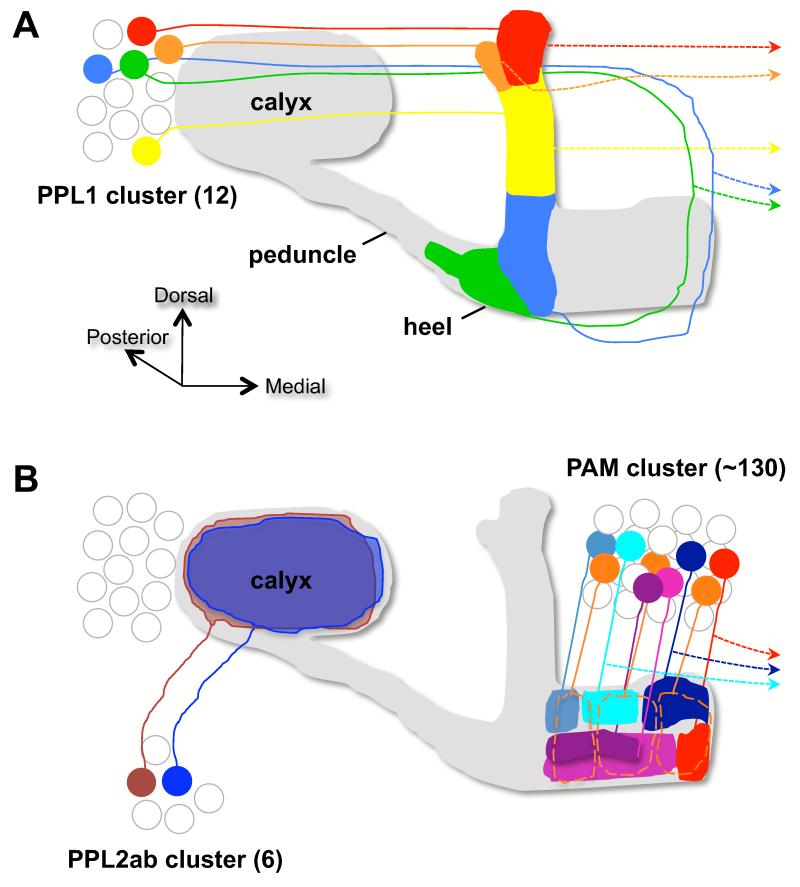
Figure 1. Innervation of the mushroom body by DA neurons.
(a) The protocerebral posterior lateral (PPL) 1 cluster. The Butcher’s cut illustration is edited from [19]. The image shows one neuron projecting to the tip of the α lobe (red), one to the tip of the α′ tip (orange), one to the upper stalk of the vertical lobes (MB-V1 neuron, yellow), one to the lower stalk and junction region (MB-MV1 neuron, blue) and one to the heel and distal peduncle (MB-MP1 neuron, green). All neurons shown have a projection to a similar zone on the contralateral MB (dotted lines with arrowhead). The number of neurons in each class has not been determined but there appears to be at least two MB-MP neurons per PPL1 cluster [6,18].
(b) The PPL2ab and protocerebral anterior medial (PAM) clusters. The illustration summarizes data described in [6,12••,15••,49]. At least two neurons from the PPL2ab cluster innervate the ipsilateral MB calyx (brown and blue). PAM DA neurons project to discrete zones of the horizontal β, β′ and γ MB lobes (marked with orange dotted lines for γ). The aversive MB-M3 neurons ramify on the tip of the β lobe (red). Several of the PAM DA neurons also have a projection to the contralateral MB (dotted line with arrowhead). Cell body position is not stereotyped and diagrams are not intended to be anatomically accurate.
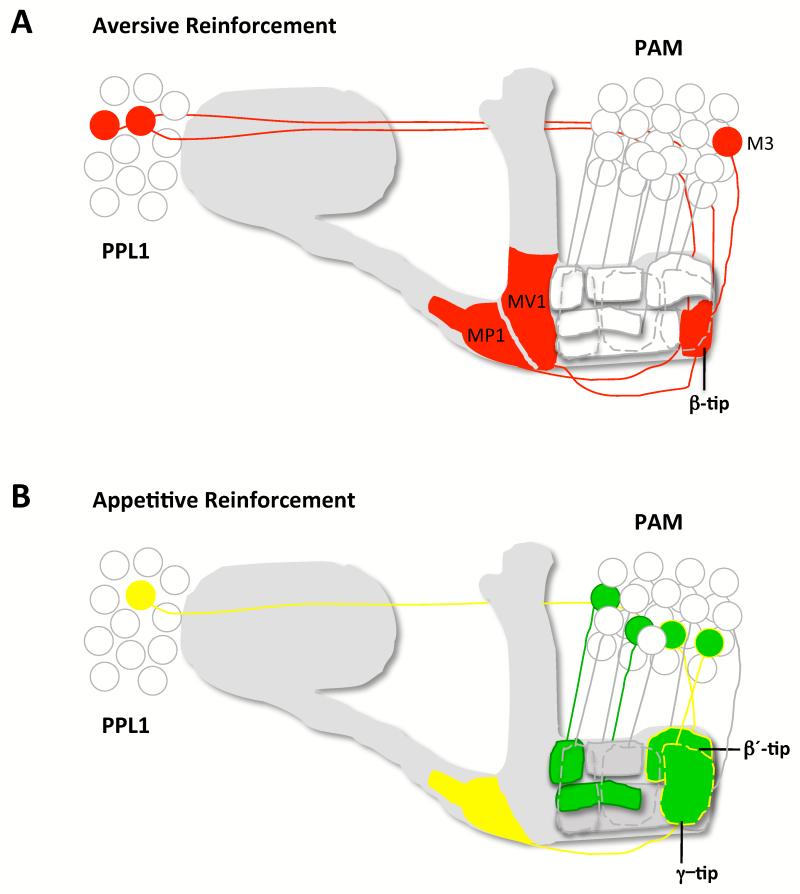
Figure 2. Models for the involvement of DA neurons in aversive and appetitive reinforcement.
Both models are updated from those in [19]. (a) DA neurons representing aversive reinforcement. Studies [5-7] suggest that neurons in PPL1 convey negative reinforcement whereas live-imaging data [49] corroborate that DA neurons innervating the lower stalk and junction are strongly activated by shock. In this illustration a selection of DA neurons innervating these MB zones and the MB-M3 neurons on the tip of the β lobe are activated (red).
(b) DA neurons representing appetitive reinforcement. DA neurons in PAM innervate many discrete zones in the β, β′ and γ lobes [7,12••]. Ca2+ imaging of sugar-evoked activity suggests only some zones receive appetitive reinforcement[7]. Those DA neurons innervating the β′ and γ lobe tips are modulated by OA through OAMB [12] (yellow outline). OA-dependent reinforcement also requires OCTβ2R in the aversive MB-MP1 neurons (yellow). Some of the PAM DA neurons on the β′ and γ lobe tips are also required to mediate the OA-independent reinforcing effects of nutrient value.
Octopamine signals appetitive reinforcement from sweet taste
A role for OA in reward came from seminal studies in the honeybee that showed that electrical stimulation of a single octopaminergic neuron, or injection of OA into the honeybee antennal lobes or MB calyx, could replace the presentation of a sucrose reward in olfactory conditioning of the proboscis extension reflex [8,9]. Consistent with the bee studies, Tbh mutant flies that lack octopamine cannot form appetitive memory [3]. Additionally, optogenetic activation of OA neurons formed appetitive olfactory memories in Drosophila larvae [10].
Recent work utilizing a variety of natural sugars as reinforcers in learning revealed that sweet taste and nutrient value represent parallel appetitive reinforcement pathways in fruit flies [11•]. Acutely blocking output from OA neurons disrupted learning with arabinose, a sugar that tastes sweet but that the flies cannot metabolize [12••]. However, blocking OA neurons lacked consequence when flies were conditioned with sweet and nutritious sucrose. It therefore appears that OA only provides the transient reinforcing properties of the sweet taste of sugar and that nutrient value provides additional reinforcement and bypasses the requirement for OA. Consistent with this model, only short-lived memories are formed if adult flies are ‘artificially conditioned’ by pairing odour presentation with OA neuron activation [12••]. So how does OA act and what neurons mediate the reinforcing effects of nutrient value?
Dopamine signals appetitive reinforcement of sweet taste and nutrient value
Analysis of dumb flies mutant for the DopR dopamine receptor provided the first indication that dopamine was also involved in fly reward learning [13]. Mutant dumb flies are defective in both aversive and appetitive learning and the defect can be rescued by restoring DopR expression to the mushroom bodies (MB) [13,14•]. Strikingly, appetitive memory could not be implanted with OA neuron stimulation in dumb mutant flies suggesting that DA signaling is functionally downstream of OA in appetitive memory processes [12••].
The prior conclusion that DA was only required for aversive learning was drawn from studies using a TH-GAL4 line that does not express in all of the fly’s DA neurons [3,5]. Remarkably, we now know that among the DA neurons that are omitted by TH-GAL4 are those in the PAM cluster that signal rewarding reinforcement [12••,15••] (Figure 2b).
Two studies independently identified GAL4 driver lines that express in large numbers of PAM DA neurons that are not included in the TH-GAL4 cohort [12••,15••]. Pairing odour exposure with activation of these PAM DA neurons formed robust appetitive memory, even in flies lacking OA. Importantly, blocking PAM DA neurons disrupted appetitive learning with sweet only sugar [12••] and persistent appetitive memory formation with nutritious sugar [12••,15••]. Therefore PAM DA neurons lie downstream of OA signals and appear to represent both the short-term and long-term reinforcing effects of nutritious sugar [12••].
Further analysis revealed that the short-term reinforcing effects of OA go through the PAM-DA neurons (Figure 2b). Memory cannot be formed with OA neuron activation in flies that lack the α-adrenergic-like OA receptor OAMB and knocking the Oamb gene down specifically in PAM-DA neurons impairs conditioning with the sweet-only sugar arabinose, but not with nutritious sucrose [12••]. Lastly, the OAMB receptor couples to the release of intracellular Ca2+ [16,17] and OA application to the brain evoked a robust increase in cytosolic Ca2+ in PAM-DA neurons [12••] consistent with a direct action of OA through OAMB.
Zonal organization of reinforcing DA neuron input to the MB
Relative valence is therefore conveyed to different regions along the processes of MB neurons by anatomically distinct DA neurons. The two types of PPL1 cluster DA neuron that can provide aversive reinforcement innervate the vertical MB lobe and heel regions [5-7•] (Figure 2a). In contrast, the larger group of 40-80 PAM cluster neurons that can provide appetitive reinforcement exclusively innervate the horizontal MB lobes [12••,15••] (Figure 2b). However, a model that segregates positive and negative valence between the MB neuron collaterals is too simple because one subset of PAM-DA neurons that can convey aversive reinforcement innervates the horizontal β-tip region [6] (Figure 2a). Therefore, aversive DA neurons are distributed between the PPL1 and PAM clusters whereas the PAM cluster contains DA neurons with opposing valence. Moreover, recent experiments suggest that OA-directed appetitive reinforcement also requires simultaneous modulation of the MB-MP1 neurons through a β-adrenergic like OA receptor OCT2βR [12••]. The MB-MP1 neurons can provide aversive value if artificially engaged [6] and have been shown to be hunger-modulated by Neuropeptide F (the fly analog of Neuropeptide Y, NPY) to control appetitive motivation [18]. It therefore seems likely that value is signaled in the MB through a complex concerted action of the different types of DA neurons [19].
What input pathways drive DA neurons?
The activity of the appropriate classes of fly DA neuron must be coordinated by input pathways. Therefore, delineating relevant afferent neurons should reveal the nuances of the functional organization of the fly DA system.
Work suggests that the MV1 and MP1 PPL1 neurons and the PAM-M3 neurons are all required to convey the reinforcing effects of electric shock [7•]. These neurons have dendrites in a similar brain area and could plausibly be driven by the same afferent neurons. However, we do not know the peripheral input pathways, or interneurons, that relay shock information to these DA neurons. Nor do we know whether the non-physiological shock stimulus will be represented in a specific pathway. Ultimately, it will be important to determine whether DA neurons signaling aversive value are engaged together by other more ecologically relevant aversive stimuli. Bitter tastants [20-22] and post-ingestive reinforcing effects of toxin [23•] may represent a good way forward.
OA neurons provide a critical input to DA neurons for appetitive reinforcement with OA simultaneously modulating positive PAM and negative MB-MP1 DA neurons through α- and β-adrenergic like receptors respectively [12••]. We do not know if OA modulates the activity of additional DA neurons and whether other appetitive reinforcers activate the same or different OA and DA neurons. Likewise, the input representing nutrient value to PAM-DA neurons does not involve OA and remains to be identified.
What does DA do to MB neuron synapses?
The zonal organization of reinforcing DA neurons on the MB (Figure 2) suggests that the underlying MB synapses are the substrate of memory-relevant synaptic plasticity. It is therefore predicted that the relevant output neurons will have processes within the MB in tight opposition to those of reinforcing DA neurons, maintaining the zonal architecture [5]. Anatomical work is consistent with this model [24]. However only the MB-V2 output are known to be important for behaviour [25••]. The <70 MB-V2 neurons have dendrites that densely innervate the MB α-stalk in the general vicinity of the MB-V1 DA neurons, that do not have a strong reinforcing role [7•,25••]. Nevertheless, MB-V2 output is required for retrieval of aversive memory [25••] consistent with memory being represented in the synapses between MB and MB-V2 neurons.
Identifying functionally relevant MB-V2 output neurons allows one to test the consequence of learning at MB neuron output synapses. Aversive learning decreased the odor-driven activation of MB-V2 neurons [25••]. Although it is perhaps surprising that learning depresses synaptic weight between the MB Kenyon cell (KC) and the MB-V2 output neurons, a comparable decrease has been describe at the KC-β-lobe output synapse in the locust brain following OA application [26••].
As their name suggests, locust β-lobe output neurons (bLN) are a group of 10-100 neurons that pool KC outputs in the MB beta lobe. Electrophysiological analyses revealed that individual bLNs are broadly tuned to odors and that the KC-bLN synapses exhibit Hebbian spike-timing dependent plasticity (STDP) [26••]. Importantly, OA application to the brain specifically depressed STDP marked, or odour-evoked, synapses. Moreover, even OA application that was delayed for up to one second after the STDP window caused synaptic depression. Therefore, STDP can temporarily ‘tag’ synapses making them susceptible to subsequent modulation by OA. Such an STDP mechanism that tags odour-activated KC-output synapses allows a temporally delayed and broadly targeted reinforcement signal to gain odour and synaptic specificity [27].
Given the recent evidence that OA works through DA [12••] and that DA ultimately signals aversion and reward, it seems possible that dopamine is responsible for both the aversive learning effect in fruit flies and the OA plasticity in the locust.
How informative is a comparison to the mammalian DA system?
DA has been classically associated with pleasure and addiction/motivation in mammals but it is now clear that DA also mediates aversion. Subsets of mammalian DA neurons respond to rewarding, rewarding and aversive stimuli or aversive stimuli [28-31]. In addition DA neurons are required for aversive learning [31,32].
Aversive Ventral Tegmental Area (VTA) DA neurons project to medial prefrontal cortex and onto GABAergic neurons in the rostromedial tegmental area. In contrast rewarding VTA DA neurons project to the nucleus accumbens [33,34••]. Some mammalian rewarding DA neurons are inhibited by aversive cues [35] by neurons in the lateral habenula that code a negative prediction error [36]. Furthermore, hypothalamic NPY/AgRP neurons in the mouse control motivation and rewarding DA neurons [37]. It is therefore clear that the fly and mammalian DA systems are similarly organized and so it seems likely that some lessons learned in flies will be relevant to DA reinforcement systems in mammals and vice versa.
Mammalian DA neuron inputs are largely glutamatergic and GABAergic. Aversive VTA DA neurons receive glutamatergic input from lateral habenula neurons. In contrast rewarding VTA DA neurons receive glutamatergic afferents from the laterodorsal tegmentum [33,34••,38]. Furthermore, GABAergic neurons in VTA are activated by aversive stimuli and encode reward expectancy [39••]. These VTA GABAergic neurons in turn inhibit the activity of rewarding DA neurons. So far such a detailed input is missing in the fly DA system.
Knowledge of input allows one to alter the activity of DA neurons. For example, NMDA glutamate receptor deletion from DA neurons in the mouse selectively impaired phasic burst firing without altering tonic activity [40]. Strikingly these mice had impaired reward and punishment learning while other DA-dependent behaviours were unaffected. Deleting a subunit of the GABA(A) receptor from DA neurons enhanced reward learning while leaving aversive learning intact suggesting an important role for DA neuron inhibition in appetitive reinforcement [41].
Work in mammals has revealed a complex interaction between STDP and DA [42]. DA receptor activation is often a prerequisite for STDP [43,44] and can set the conditions for plasticity. Most interestingly DA can widen the STDP window, decrease the number of spike pairings required to induce timing-dependent long-term potentiation (t-LTP), and can even sign reverse timing-dependent long-term depression (t-LTD) into t-LTP [45]. It could be that some MB output synapses are subject to OA/DA based repression whereas others are potentiated by OA/DA or DA alone. Earlier experiments suggested that only neurotransmission from the α′β′ subset of MB neurons is required during fly learning [46]. Uncoupling αβ and γ presynaptic release from postsynaptic neural activity lacked consequence [46-48] presenting a conundrum for a straightforward STDP model of fly learning in these neurons. It will therefore be critical to clarify the mechanisms of DA action and the synaptic rules of learning throughout the MB neuron ensemble.
Highlights.
Dopamine represents appetitive and aversive value in the fruit fly brain.
Dopamine neurons representing opposite value are anatomically segregated.
Octopamine conveys appetitive reinforcement of sweet taste through dopamine neurons.
A dual role for dopamine in appetitive and aversive value is conserved in mammals.
Acknowledgements
I thank Wolf Huetteroth, Suewei Lin and David Owald for comments on the manuscript. Scott Waddell is funded by a Wellcome Trust Senior Research Fellowship in the Basic Biomedical Sciences, by MH081982 from the National Institutes of Health and by funds from the Gatsby Charitable Foundation and Oxford Martin School.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 2.Maia TV. Reinforcement learning, conditioning, and the brain: Successes and challenges. Cogn Affect Behav Neurosci. 2009;9:343–364. doi: 10.3758/CABN.9.4.343. [DOI] [PubMed] [Google Scholar]
- 3.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 5.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Aso Y, Herb A, Ogueta M, Siwanowicz I, Templier T, Friedrich AB, Ito K, Scholz H, Tanimoto H. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 2012;8:e1002768. doi: 10.1371/journal.pgen.1002768. [Using a thermogenetic approach this study defined differential roles for PPL1 and PAM dopamine neurons in aversive memory formation and stability.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 9.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 10.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 11•.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [Using natural sugars with different nutritional value as reinforcing stimuli revealed parallel reinforcing pathways in the fly. Sweet taste is sufficient to reinforce short-term memory but nutrient value is required for robust long-term memory.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012 doi: 10.1038/nature11614. [Using thermogenetic approaches octopaminergic neurons were found to only represent the short-term reinforcing effects of sweet taste. Strikingly, octopamine (OA) was shown to function via the α-adrenergic like receptor OAMB in a new subset of mushroom body targeted dopaminergic neurons. These dopamine (DA) neurons convey reinforcement of both sweet taste and nutrient value. OA dependent reinforcement was also shown to require parallel signaling through the β-adrenergic like receptor OCTβ2R in aversive MB-MP1 neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Qin H, Cressy M, Li W, Coravos JS, Izzi SA, Dubnau J. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [Restoring expression of the DopR receptor to only the γ neurons of the MB was sufficient to rescue the learning and long-term memory defects of dumb mutant flies. This work suggests an essential reinforcing role for DA signaling through the MB γ neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [Using thermogenetic approaches a new subset of mushroom body targeted dopaminergic neurons was shown to convey appetitive reinforcement from sugar. Ca2+ imaging confirmed that these neurons are activated when hungry flies eat sucrose.] [DOI] [PubMed] [Google Scholar]
- 16.Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balfanz S, Strunker T, Frings S, Baumann A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- 18.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- 21.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 23•.Wright GA, Mustard JA, Simcock NK, Ross-Taylor AA, McNicholas LD, Popescu A, Marion-Poll F. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr Biol. 2010;20:2234–2240. doi: 10.1016/j.cub.2010.11.040. [Using behavioral analysis in the bee this study demonstrates the delayed formation of robust aversive olfactory memory reinforced by ingestion of toxin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 25••.Sejourne J, Placais PY, Aso Y, Siwanowicz I, Trannoy S, Thoma V, Tedjakumala SR, Rubin GM, Tchenio P, Ito K, et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci. 2011;14:903–910. doi: 10.1038/nn.2846. [The cholinergic MB-V2 neurons are shown to be downstream of mushroom body neurons and to be required for retrieval of aversive memory. Conditioning was shown to decrease the odour-driven activity of MB-V2 neurons suggesting that learning depresses some MB neuron synapses. This paper taken with [26••] suggests that depression of MB synaptic efficacy might be a feature of insect learning.] [DOI] [PubMed] [Google Scholar]
- 26••.Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012;482:47–52. doi: 10.1038/nature10776. [The locust beta-lobe output neurons are shown to exhibit Hebbian spike-timing dependent plasticity. Following induction of STDP with delayed delivery of octopamine causes synaptic depression. This paper taken with [25••] suggests that depression of MB synaptic efficacy might be a feature of insect learning.] [DOI] [PubMed] [Google Scholar]
- 27.Ito I, Ong RC, Raman B, Stopfer M. Sparse odor representation and olfactory learning. Nat Neurosci. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem. 2011;18:136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [This study uses viral tracers, optogenetic stimulation, physiology and behavioural analyses to map functional input pathways to mouse ventral tegmental area (VTA) dopamine neurons. Stimulating input from the lateral habenula or laterodorsal tegmentum elicits conditioned place avoidance or conditioned place preference, respectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, Horvath TL. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 39••.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [This study used optogenetic techniques to differentiate between dopaminergic and GABAergic cell types in the mouse VTA by optical stimulation during recording. The GABAergic neurons were found to signal the expected reward value which is a critical element of a system that would calculate a reward prediction error.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, Palmiter RD. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not Everything: Neuromodulation Opens the STDP Gate. Front Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:13028–13033. doi: 10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 48.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 49.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]