Abstract
Tissue plasminogen activator (tPA) is the only FDA-approved treatment for acute stroke, but its use remains limited. Progesterone (PROG) has shown neuroprotection in ischemia, but before clinical testing, we must determine how it affects hemorrhagic transformation in tPA-treated ischemic rats. Male Sprague–Dawley rats underwent middle cerebral artery occlusion with reperfusion at 4.5 hours and tPA treatment at 4.5 hours, or PROG treatment intraperitoneally at 2 hours followed by subcutaneous injection at 6 hours post occlusion. Rats were killed at 24 hours and brains evaluated for cerebral hemorrhage, swelling, blood–brain barrier (BBB) permeability, and levels of matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor level (VEGF), and tight junction (TJ) proteins. We also evaluated PROG's efficacy in preventing tPA-induced impairment of transendothelial electrical resistance (TEER) and TJ proteins under hypoxia/reoxygenation in the endothelial cells. Delayed tPA treatment induced significant hemorrhagic conversion and brain swelling. Treatment with PROG plus tPA ameliorated hemorrhage, hemispheric swelling, BBB permeability, MMP-9 induction, and VEGF levels compared with controls. Progesterone treatment significantly prevented tPA-induced decrease in TEER and expression of occludin and claudin-5, and attenuated VEGF levels in culture media subjected to hypoxia. The study concluded that PROG may extend the time window for tPA administration in ischemic stroke and reduce hemorrhagic conversion.
Keywords: hemorrhage, MMP-9, progesterone, stroke, tPA, VEGF
Introduction
Thrombolytic therapy with tissue plasminogen activator (tPA) is currently the only FDA-approved treatment for acute ischemic stroke, but its use is limited to patients who can be treated within 4.5 hours of ischemic onset.1 Beyond this window, tPA treatment has a high risk-to-benefit ratio due to its deleterious effects in the ischemic brain, including neurovascular cell death, blood–brain barrier (BBB) disruption, and hemorrhagic complications. Cerebral hemorrhage is the greatest hazard with tPA treatment and occurs in 5% to 10% of patients.2 Emerging data show that the development of hemorrhagic transformation is associated with tPA's signaling action in the neurovascular unit3 and subsequent BBB disruption.4 Preventing endothelial cell injury and BBB breakdown associated with tPA treatment may contribute to better neuroprotection and increased overall efficacy for tPA reperfusion therapy.
A growing literature shows that the steroid hormone progesterone (PROG) has neuroprotective effects in several stroke models.5, 6, 7, 8 In addition to animal studies and two positive phase II clinical trials,9, 10 an NIH-sponsored, phase III, 31+ center clinical trial for traumatic brain injury is underway across the United States and a second phase III trial is taking place in the United States and 21 other countries (http://clinicaltrials.gov/ct2/show/record/NCT00822900 and http://www.synapse-trial.com). Although PROG may turn out to be an attractive potential therapeutic candidate for stroke as well, in the aftermath of so many failed stroke clinical trials, it is likely that the FDA will require more stringent preclinical testing before any new therapy for stroke is approved for clinical trial. It is therefore critical to determine how PROG will interact with tPA. Will combination with PROG increase or decrease the risk of bleeding and hemorrhagic conversion?
One strategy to improve outcome after ischemic injury is to prevent BBB breakdown caused by tPA treatment. Stabilizing the BBB after a stroke would substantially improve the overall efficacy of tPA reperfusion therapy. We know that after cerebral ischemia, matrix metalloproteinases, a family of zinc-binding proteolytic enzymes, participate in the disruption of the BBB by degrading the major components of basal lamina surrounding the microvessels.11 Several studies suggest a strong relationship between metalloproteinase-9 (MMP-9) induction and tPA-induced hemorrhage both in animal models of ischemic injury12, 13 and in stroke patients,14 suggesting that MMP-9 inhibition may be an important strategy to prevent hemorrhagic complications induced by delayed tPA treatment after ischemia.
Progesterone's neuroprotective effects include reducing inflammation, BBB permeability, and edema across a variety of animal and human injury models.15 We recently showed that treatment with PROG and its metabolite allopregnanolone alleviated increased MMP-9 expression and inhibited acute vascular endothelial growth factor level (VEGF) upregulation and BBB disruption in a permanent ischemic stroke model.6 Based on these findings, we hypothesized that PROG would prevent tPA-associated hemorrhagic transformation in an experimental stroke and an in vitro BBB model through inhibition of the VEGF–MMP-9 pathway. We used a rat transient middle cerebral artery occlusion (tMCAO) model to evaluate whether PROG could reduce tPA-associated hemorrhagic complications at 24 hours after ischemia/reperfusion. We also assessed whether PROG could decrease tPA-induced vascular permeability and the dissociation of tight junction (TJ) proteins and affect VEGF secretion in bEnd.3 cells, an immortalized brain endothelial cell line.
Materials and Methods
Animals
Male Sprague–Dawley rats, approximately 3 months old (300 to 350 g) at the time of surgery, were obtained from Charles River (Wilmington, MA, USA) and used as subjects. All animals were housed in an AAALAC approved Research Animal Facility with a temperature-, humidity-, and light-controlled environment, and placed under a 12-hour reverse light–dark cycle. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the Guide for the Care and Use of Laboratory Animals, and all other applicable regulations, policies, and procedures, were followed and approved by Emory University Institutional Animal Use and Care Committee (protocol #DAR-2001411). The experiments are reported here in accordance with the ARRIVE guidelines.
Transient Focal Ischemia
Male Sprague–Dawley rats were anesthetized with isoflurane (5% for surgical induction, 2% to 2.5% for maintenance) in NO2:O2 (70%:30%) during surgical procedures. Body temperature was monitored continuously with a rectal probe and maintained at 37.5°C±0.5°C using a heating lamp. Transient focal cerebral ischemia was induced by the intraluminal filament occlusion model using silicon-coated 4-0 nylon filament as described previously.5, 6, 7, 8 After 4-hour 30-minute occlusion, reperfusion was accomplished by withdrawal of the monofilament. For monitoring MCAO and reperfusion, cerebral blood flow was assessed by laser Doppler flowmetry using a probe fixed to the skull above the territory of the right MCA (core cortex: 2 mm posterior and 6 mm lateral to the bregma). Rats subjected to MCAO with less than 40% of baseline laser Doppler flowmetry were randomly assigned to receive drug treatments. Sham animals were anesthetized, an incision was made, and the fascia cleared to expose the bregma at the top of the head. After this a midline neck incision was made, and the common carotid and internal carotid arteries were isolated and exposed. Then the incision was sutured closed.
Experimental Groups and In Vivo Drug Treatment
The rats were quarantined for 7 days before the experiment and housed in individual cages in a room maintained at 21 to 25°C, 45% to 50% humidity, a 12 to 12-hour light–dark cycle and free access to pellet chow and water. There were four groups (n=7–8/group) including sham-operated controls. We calculated the starting sample sizes to be at least six animals/group to reject the null hypothesis (H0) at P<0.05 with a power of 0.80. Animals underwent tMCAO or sham surgery and were randomly assigned to one of the treatments with either vehicle (saline), tPA alone, or a combination of PROG (8 mg/kg)+tPA. Previous studies have shown 8 mg/kg to be the optimally effective dose in a stroke model.5, 6, 7, 8 Progesterone was given intraperitoneally 2 hours post occlusion for faster absorption followed by a subcutaneous injection for slower and sustained absorption at 6 hours post occlusion. Physiologic saline with tPA (Genentech, San Francisco, CA, USA) was administered (10% bolus, 90% continuous infusion within 30 minutes) to the rats via femoral vein beginning 10 minutes before reperfusion at 5 mg/kg, a dose shown to have optimal fibrinolytic activity with lower mortality.16
Measurement of Brain Swelling
Brain swelling was estimated 24 hours post-occlusion by measuring the hemispheric areas of 2-mm thick brain slices using ImageJ software (NIH, Bethesda, MD, USA). The extent of swelling was calculated using the following equation: extent of brain swelling=(volume of ipsilateral hemisphere—volume of contralateral hemisphere)/volume of contralateral hemisphere.
Measurement of Infarction
At 24 hours after the induction of tMCAO, the rats were killed and perfused with phosphate-buffered saline (PBS). Their brains were removed immediately and brain tissue was cut into six serial 2 -mm coronal sections. Sliced brain tissues were immersed in a 2% solution 2,3,5-triphenyltetrazolium chloride in PBS at 37°C for 15 minutes and captured for quantification of infarct volume. An edema index was calculated by dividing the total volume of the left hemisphere by the total volume of the right hemisphere. The actual infarct volume adjusted for edema was calculated by dividing the infarct volume by the edema index. Infarct volume was expressed as a percentage of the contralateral area for each section. A person masked to the treatment groups conducted the measurement and evaluations.
Hemoglobin Assay
After 2,3,5-triphenyltetrazolium chloride staining and scanning, the hemispheric brain tissue was homogenized with PBS and centrifuged for 30 minutes (13,000 g). Then, 200 μL reagent (QuantiChrom Hemoglobin Assay Kit, BioAssay Systems, Hayward, CA, USA) was added to 50 μL supernatant. After 15 minutes, optical density was measured at 400 nm with a spectrophotometer.17
Cell Culture and Materials
An immortalized mouse brain endothelial cell line (bEnd.3) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco's modified Eagle medium (ATCC) supplemented with 10% fetal bovine serum (ATCC). The bEnd.3 cells were seeded onto the luminal side of the filter (0.4 μm pore size; Corning, Lowell, MA, USA) coated with fibronectin (5 μg/mL) in 12-well plates for the transendothelial electrical resistance (TEER) test; and in 100-mm dishes for Western blots. Cell cultures were incubated at 37°C in 5/95% mixture of CO2 and atmospheric air, and the medium was replaced every 3 days (see below for measurement details).
In Vitro Drug Treatment
Cells were treated with various concentrations (0.1 to 40 μmol/L) of PROG (Sigma-Aldrich, St Louis, MO, USA) or 20 μg tPA for 24 hours before hypoxia/reperfusion (H/R). In all cases, drug treatments continued throughout the H/R period. Progesterone was dissolved in DMSO (Sigma) and further dilutions (0.1%) were made with culture medium.
Normoxia and Hypoxia/Reperfusion Study
Normoxic cells were transferred into a serum-free medium of glucose-containing (4.5 g/L) phenol red-free Dulbecco's modified Eagle medium. To mimic ischemic conditions in vitro, bEnd.3 cells were exposed to 6-hour hypoxia and 3-hour reoxygenation with or without the administration of tPA. We used a tPA dose of 20 μg/mL, based on the finding that such a concentration can be observed in blood.18 In brief, confluent bEnd.3 cells were subjected to an ischemic injury by transferring cultures to glucose-free medium (Dulbecco's modified Eagle medium without glucose) pre-equilibrated with 95% N2 and 5% CO2. Cells were then incubated in a humidified airtight chamber equipped with an air lock and flushed with 95% N2 and 5% CO2. The oxygen concentration was⩽0.1% as monitored by an oxygen analyzer (Biospherix, Redfield, NY, USA). Reoxygenation was initiated by adding glucose-containing (4.5 g/L) phenol red-free Dulbecco's modified Eagle medium for 3 hours at 37°C in 95% air and 5% CO2.
Cell and Tissue Processing for Immunoblotting
Peri-infarct cortical tissue was processed for protein analysis. Tissues were homogenized in T-per (Pierce, Rockford, IL, USA) containing protease inhibitor cocktail (P8340, Sigma). Homogenates were centrifuged for 15 minutes at 13,000 g. Forty micrograms of total protein was separated on 8% to 12% gel and transferred onto polyvinylidene difluoride (Millipore, Billerica, MA, USA) membranes at 300 mA for 2 hours. To measure the level of immunoglobulin G (IgG), the membranes were incubated overnight at 4°C with anti-rat IgG conjugated to biotin. bEnd.3 cells were subjected to 6-hour hypoxia/3-hour reoxygenation and then lysed on ice in 100 μL of RIPA buffer. Total cell lysates were boiled and then electrophoresed in 12% SDS-PAGE acrylamide gels, transferred onto polyvinylidene difluoride membranes, and incubated for 1 hour in TBS-T (Tris-buffered saline and 0.1% tween 20) containing 5% bovine serum albumin. Membranes were then incubated overnight at 4°C with primary antibodies against occludin (Invitrogen, Camarillo, CA, USA; 1:1,000), claudin-5 (Invitrogen; 1:1,000), and ZO-1 (Invitrogen; 1:1,000), washed in TBS-T, and incubated for 1 hour at room temperature with corresponding peroxidase-conjugated secondary antibodies (1:5,000; KPL, Gaithersburg, MD, USA). All blots were stripped and re-incubated with β-actin antibodies (Sigma; 1:5,000) as a loading control. The peroxidase reaction was developed with an ECL-plus detection kit (Amersham BioSciences, Piscataway, NJ, USA). Intensity of the bands was measured by Image Gauge version 4.0 program (FUJI Film, Tokyo, Japan).
Immunostaining of Occludin and F-Actin in Hypoxia/Reperfusion-Treated bEnd.3 Cells
The bEnd.3 cells grown to confluence on coverslips were subjected to various treatments. For immunostaining, cells were washed three times with PBS, fixed in 3% paraformaldehyde for 10 minutes, permeabilized with 0.25% Triton X-100 in PBS for 10 minutes, and blocked with 10% BSA and 0.1% Triton X-100 in PBS for 1 hour. After washing with PBS, the monolayers were incubated with a primary antibody for occludin (Invitrogen; 1:200), MMP-9 (Abcam; Cambridge, MA, USA), and Alexa Fluor 488 phalloidin (Invitrogen; 1:400) overnight at 4°C. The cell monolayers were then washed three times with PBS and incubated with Alexa Fluor 488 anti-rabbit and orange anti-mouse (Molecular Probes, Eugene, OR, USA) for 1 hour. After washing in PBS, the coverslips were mounted on glass slides with Vectashield with 4′,6-Diamidino-2-phenylindole.
Active Metalloproteinase-9 Assay
The specific enzymatic activity of active MMP-9 was measured with Fluorimetric Sensolyte 490 (AnaSpec, San Jose, CA, USA) to monitor an energy transfer from an excited fluorescent donor and a quenching acceptor following manufacturer's instructions. The samples (100 μg) were placed in a 96-well plate containing 50 μL of assay buffer. The proteolytic resonance energy transfer peptide was expressed as a fold-change in fluorescence intensity at excitation of 355 nm/emission of 460 nm.
Transendothelial Electrical Resistance Measurement
Electrical resistance across the membrane was measured with a Millicell ERS Voltohmmeter (Millipore). The fibronectin-coated transwell inserts were placed in 12-well plates containing culture medium and then used to measure background resistance. The resistance measurements of these blank filters were then subtracted from those of filters with cells. The values are shown as Ω × cm2 based on culture inserts.
Statistical Analysis
Based on a delta-value of 1.25, we calculated the sample sizes and power needed to reject the null hypothesis to achieve >80% power to detect a 50% difference. The number of rats per group at these criteria was determined to be at least six to reject the null hypothesis (H0) at the 0.05 level at a power of 0.8. The parameters were analyzed using one-way analysis of variance followed by the Tukey post hoc test. All data are presented as mean±s.e.m. All tests were considered statistically significant at P values ⩽0.05.
Results
Progesterone's Effect on Hemorrhagic Transformation, Brain Swelling, and Infarct Volume After Transient Middle Cerebral Artery Occlusion with Tissue Plasminogen Activator
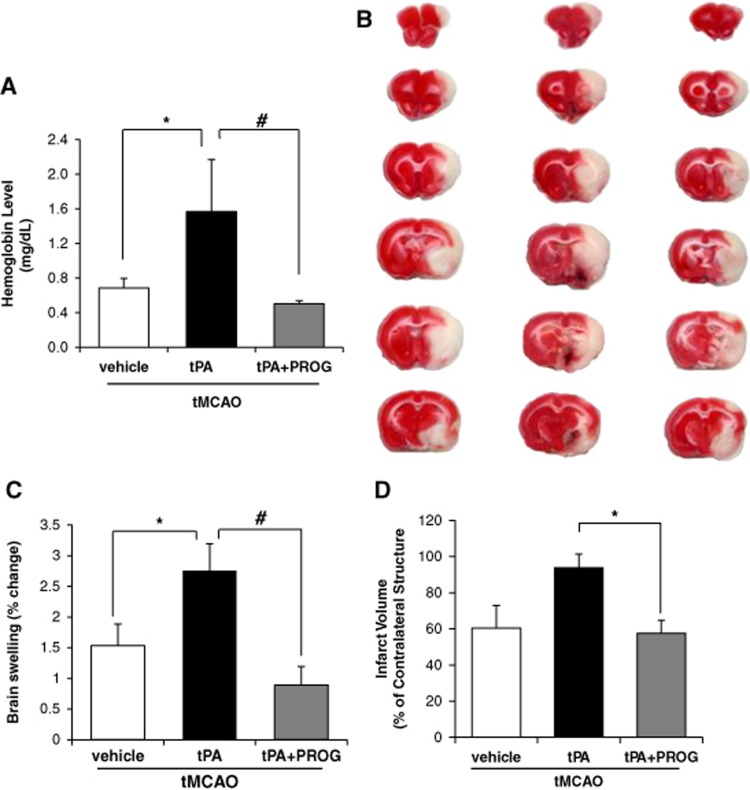
To examine whether the combination of PROG with tPA influences the incidence and severity of cerebral hemorrhage, we evaluated hemorrhage volume in ischemic brain with a spectrophotometric hemoglobin assay 24 hours after ischemia. In the vehicle group, we did not detect any significant hemorrhagic transformation; however, delayed tPA treatment after ischemia significantly increased the incidence of hemorrhage compared with saline-treated rats. In contrast, the rats treated with tPA and PROG showed significantly reduced hemoglobin concentrations compared with tPA alone (Figure 1A). Furthermore, the ischemic hemisphere of rats treated with delayed tPA showed significantly increased brain swelling compared with saline-treated rats, whereas tPA combined with PROG treatment significantly reduced hemispheric swelling (Figure 1C) compared with tPA alone. Infarct volume in rats infused with tPA after tMCAO did not differ from those in saline-treated rats at 24 hours after stroke (Figure 1D) but combined tPA and PROG treatment reduced the infarct volume by 59% compared with tPA alone.
Figure 1.
Progesterone reduces delayed tissue plasminogen activator (tPA)-induced increase in edema, hemorrhage, and infarct volume after transient middle cerebral artery occlusion (tMCAO). (A) Quantitative analysis of cerebral hemorrhage volume with spectrophotometric assay. (B) Representative coronal sections of 2,3,5-triphenyltetrazolium chloride-stained brain sections of rats from the transient ischemia group (vehicle, left), tPA group (center), and tPA plus progesterone group (right). (C) Quantitative analysis of brain swelling 24 hours after ischemic onset. (D) Quantitative analysis of infarct volume in saline (n=6), tPA (n=6), and tPA+PROG (progesterone) (n=6). *P<0.05 versus saline, #P<0.05 versus tPA plus PROG. Data are expressed as means±s.e.m.
Progesterone's Effect on Blood–Brain Barrier Disruption After Transient Middle Cerebral Artery Occlusion and on the Transendothelial Electrical Resistance of Hypoxia/Reperfusion bEnd.3 Cells
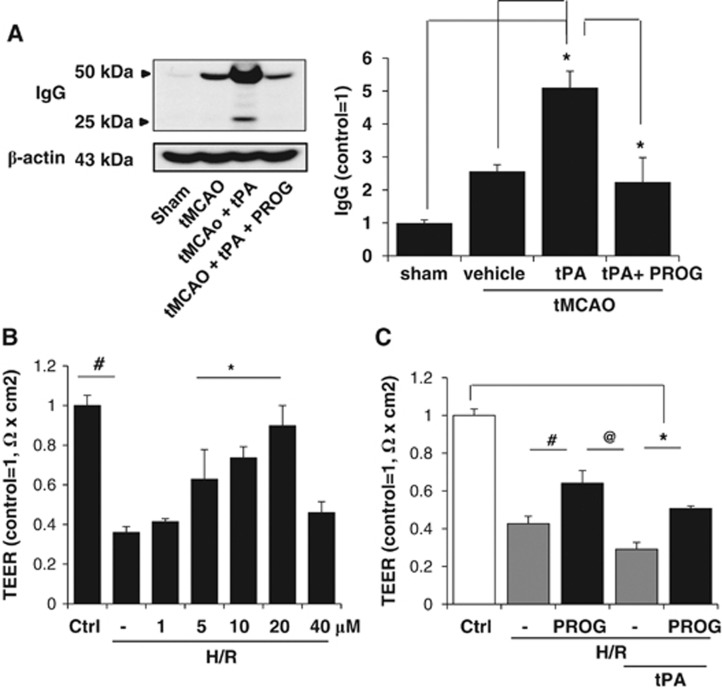
To evaluate BBB disruption, we assessed levels of light (25 kDa) and heavy (50 kDa) chains of serum IgG at 24 hours after cortical ischemic injury. The results of immunoblotting showed that IgG levels increased at 24 hours post injury, and increased twofold after delayed tPA treatment (Figure 2A). Administration of PROG attenuated this increase.
Figure 2.
Effect of progesterone (PROG) on blood–brain barrier (BBB) disruption after transient middle cerebral artery occlusion (tMCAO) with tissue plasminogen activator (tPA) in rats and on the transendothelial electrical resistance (TEER) in endothelial cell monolayers under hypoxia and reperfusion (H/R). (A) Western blot analysis measured the levels of immunoglobulin G (IgG). Combination therapy with tPA plus PROG prevented tPA-associated increase in IgG. (B) Transendothelial electrical resistance was decreased by H/R. The decrease was significantly prevented by pretreatment with 5, 10, and 20 μmol/L PROG. (C) Pretreatment with PROG (with observed maximum protective dose of 20 μmol/L) prevented tPA (20 μg/mL)-associated H/R-induced, reduction in TEER. #P<0.05 versus H/R plus PROG, @P<0.05 versus tPA alone. *P<0.05 versus tPA plus PROG (n=5–6). Data are expressed as means ±s.e.m.
Because BBB integrity is correlated with TEER,19 we measured TEER after H/R. Transendothelial electrical resistance values significantly decreased after H/R compared with normoxia (Figure 2B). We then tested the effect of PROG (in a dose range of 0.1 to 40 μmol/L) on TEER values after H/R and found a significant dose-dependent increase (1 to 20 μmol/L PROG) (Figure 2C). Treatment with tPA further decreased TEER values after H/R (Figure 2C). Transendothelial electrical resistance values of probes treated with PROG (20 μmol/L) were significantly higher after H/R with tPA compared with untreated probes (Figure 2C). An MTT assay quantifying cell death at 6-hour hypoxia/3-hour reoxygenation showed insignificant (12%) cell death (data not shown).
Progesterone's Effect on Tight Junction Expression After Transient Middle Cerebral Artery Occlusion with Tissue Plasminogen Activator and Hypoxia/Reperfusion with Tissue Plasminogen Activator
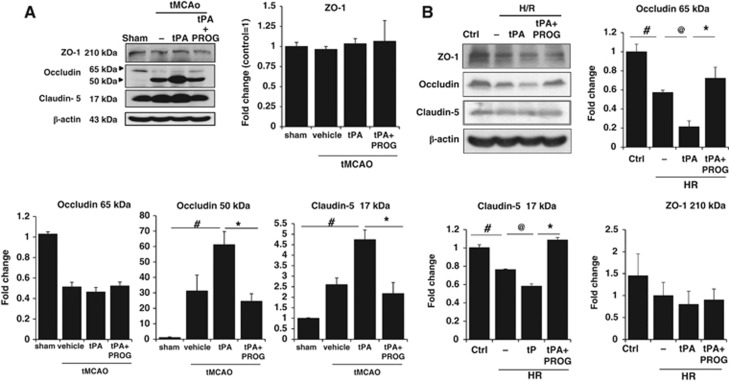
Using Western blot assays, we examined whether delayed tPA treatment after 24-hour ischemic injury can induce alteration of TJ proteins (occludin, claudin-5, and ZO-1). A reduction in the 65 kDa occludin band was seen in the ischemic hemisphere (Figure 3A). A concomitant increase in the occludin fragment at a molecular weight of 50 kDa was seen in the ischemic hemisphere and was further increased after delayed tPA treatment (Figure 3A). Combination therapy with tPA plus PROG significantly prevented the increase of 50 kDa occludin. Claudin-5 has a normal molecular weight of 22 kDa. When it is degraded, lower molecular weight fragments can be observed. Immunoblots on claudin-5 after delayed tPA treatment after ischemic injury showed a marked increase in 17 kDa compared with stroke animals without tPA (Figure 3A). Administration of PROG significantly prevented the increase in the lower molecular weight fragment.
Figure 3.
Effects of progesterone (PROG) on tight junction proteins after ischemia onset with tissue plasminogen activator (tPA), and after hypoxia/reperfusion (H/R) in brain endothelial cells. (A) Immunoblot images and quantitative data showing that, after tPA treatment (5 mg/kg, intravenous) in transient middle cerebral artery occlusion (tMCAO) rats, occludin and claudin-5 levels were decreased in peri-infarct cortex compared with sham, and increased after treatment with PROG. (B) Immunoblot images and quantitative data showing that administration of tPA (20 μg/mL) with H/R markedly decreased occludin and claudin-5 levels, and PROG significantly prevented that decrease in vitro. Data are expressed as means ±s.e.m. #P<0.05 versus Control, @P<0.05 versus tPA, *P<0.05 versus tPA plus PROG (n=6–7).
We further investigated the effect of PROG on TJ protein expression in brain endothelial cells using immunoblotting technique. Administration of tPA with H/R markedly decreased occludin and claudin-5 levels. Progesterone significantly prevented the decrease of occludin and claudin-5 levels (Figure 3B). There were no significant differences among groups in ZO-1 (Figure 3B).
The Effect of Progesterone on Metalloproteinase-9 Expression at 24 hours after Transient Middle Cerebral Artery Occlusion with Tissue Plasminogen Activator
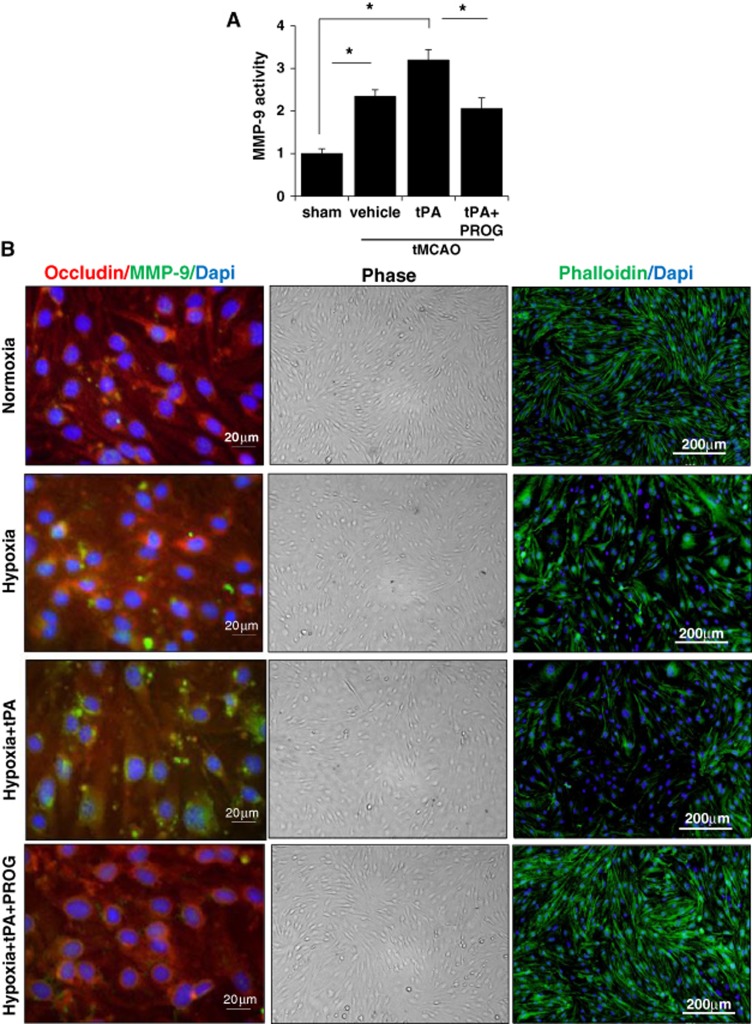
We evaluated MMP-9 activity after ischemia using fluorescence resonance energy transfer peptide. We found that MMP-9 activity at 24 hours after ischemia increased, especially in the tPA group (Figure 4A). Levels of MMP-9 activity in the tPA group were significantly higher than those in the control group. Levels of MMP-9 activity in the tMCAO group were also significantly higher than those in the control group. Progesterone treatment significantly attenuated MMP-9 levels. Metalloproteinase-9 has been shown to be capable of proteolytic degradation in a variety of pathologic conditions including stroke.14 To determine whether MMP-9 was involved in H/R-induced disturbance in occludin expression, we used immunofluorescence staining to visualize the changes in occludin and MMP-9 in H/R with or without tPA treatment and then looked at the effects of PROG. Consistent with the Western blot results, exposure of cells to H/R for 9 hours reduced the immunostaining of occludin, and the reduction was more prominent in tPA-treated H/R cells than in control bEnd.3 cells. Metalloproteinase-9 levels were increased in cells exposed to 6-hour hypoxia/3-hour reoxygenation and further increased in cells exposed to H/R with tPA. In contrast, these increases in MMPs were completely inhibited when cells were pretreated with PROG (Figure 4B).
Figure 4.
Effects of progesterone (PROG) on metalloproteinase-9 (MMP-9) activity and actin cytoskeletal change. (A) Metalloproteinase-9 activity of the lysates from the control or ischemic cortices at 24 hours after ischemia measured by fluorometric assay using fluorescence resonance energy transfer peptides as substrates. Progesterone treatment significantly attenuated the tissue plasminogen activator (tPA)-induced increase in MMP-9 levels. #P<0.01 versus control group, *P<0.05 (n=6–7). (B) Triple immunofluorescent staining (left panel) of MMP-9 (green), occludin (red), and 4′,6-Diamidino-2-phenylindole (DAPI) (blue) after hypoxia/reperfusion (H/R) with tPA in brain endothelial cells. Digital photomicrographs (middle panel) of phase contrast images of confluent bEnd.3 cells under H/R with or without tPA in the presence or absence of 20 μmol/L PROG. Representative fluorescence microscopy images (right panel) of phalloidin staining (green) showing actin cytoskeleton reorganization under H/R and after tPA (20 μg/mL) application under H/R. The cells pretreated with 20 μmol/L PROG preserved the cell morphology and actin cytoskeletal component. tMCAO, transient middle cerebral artery occlusion.
The loss of TJ proteins implies significant changes in the endothelial cell morphology and monolayer integrity. Using phase contrast microscopy and phalloidin staining, we looked at the changes in cell surface morphology and F-actin induced by tPA under H/R conditions. Staining of F-actin with fluorescein phalloidin showed that endothelial cells exposed to H/R were less elongated and more rounded and ruffle-shaped than normal cells (Figure 4B). This effect was more pronounced with tPA administration. Comparison of cellular F-actin distribution with the surface morphology of the cells revealed a positive correlation between changes in surface morphology and decreased F-actin staining intensity. The cells pretreated with PROG were found to preserve the cell morphology and actin cytoskeletal component at the cell periphery.
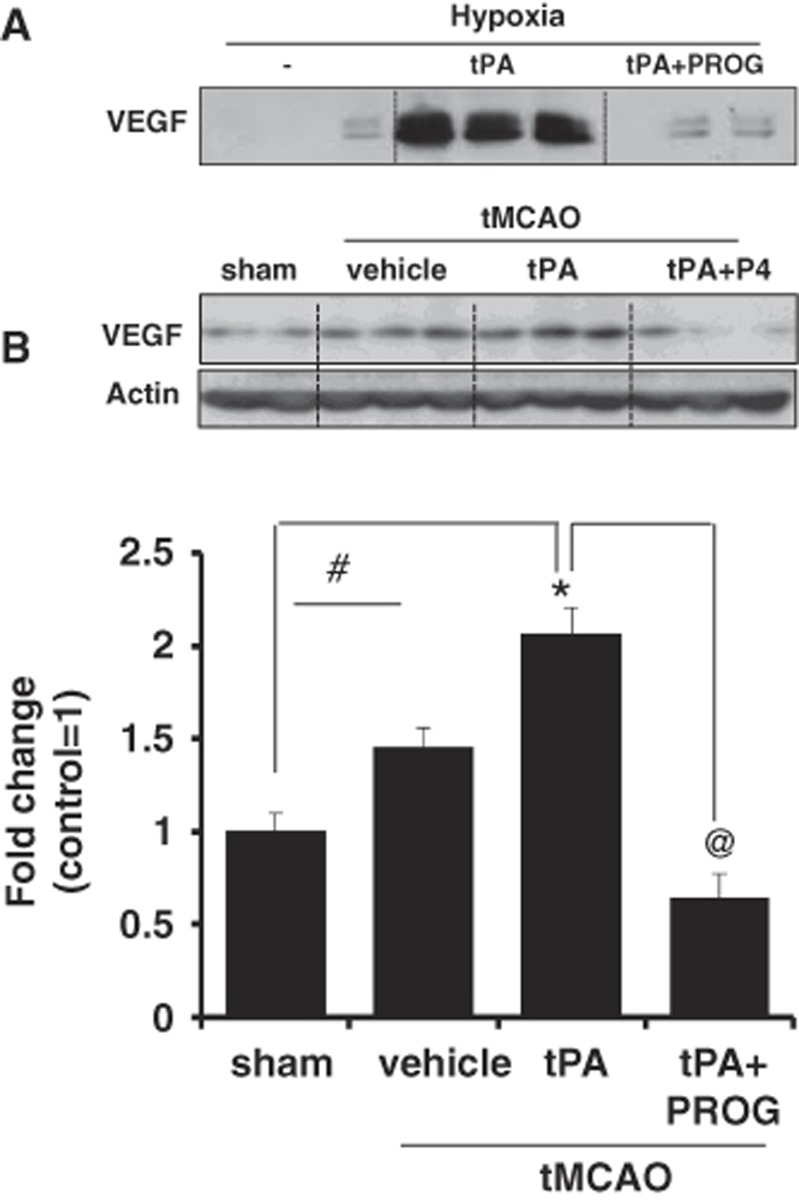
The Effect of Progesterone on Vascular Endothelial Growth Factor Levels at 24 hours after Transient Middle Cerebral Artery Occlusion with Tissue Plasminogen Activator
To explore the protective mechanisms of PROG on cerebral hemorrhage and BBB disruption after ischemia, we examined PROG's effect on the expression of VEGF after tPA treatment in vivo and in vitro. Vascular endothelial growth factor is a secreted mitogen associated with increased vascular permeability after stroke.20 Interestingly, VEGF levels in the cortical area as measured by Western blotting were significantly elevated in the ischemic group given tPA compared with the sham group. Progesterone treatment significantly reduced VEGF levels in the cortical area after delayed tPA treatment (Figure 5). Upregulation of VEGF expression after stroke has been reported in various cell types such as neurons, astrocytes, and endothelial cells.21 We further investigated the effect of PROG on VEGF level in brain endothelial cells using immunoblotting after 6-hour hypoxia with tPA. Tissue plasminogen activator plus hypoxia dramatically increased the levels of VEGF in the culture media. Progesterone prevented the increase of VEGF levels in the cell culture media.
Figure 5.
Effect of progesterone (PROG) on vascular endothelial growth factor (VEGF) level. (A) Immunoblot images and quantitative data showing VEGF levels in cell culture media. Application of tissue plasminogen activator (tPA) (20 μg/mL) with hypoxia increased the levels of VEGF in the culture media. Equal volumes of media were loaded in each lane. Progesterone (20 μmol/L) prevented the increase of VEGF levels in the cell culture media. (B) Immunoblot images and quantitative data showing VEGF levels in the brain tissue. Vascular endothelial growth factor levels in the cortical area were significantly elevated in the ischemic group given tPA (5 mg/kg, intravenous) compared with the sham group. Progesterone treatment (8 mg/kg) significantly reduced VEGF levels in the cortical area after delayed tPA treatment. Data are expressed as mean ±s.e.m. *P<0.05 versus sham, #P<0.05 versus saline, and @P<0.05 versus PROG. tMCAO, transient middle cerebral artery occlusion.
Discussion
The present study evaluated the important question of whether PROG treatment can decrease tPA-associated hemorrhage, brain swelling, BBB disruption, and mortality after delayed tPA treatment in an animal model of stroke and in an in vitro BBB model. Our results showed that delayed tPA treatment increased hemorrhage volume and brain swelling after ischemia (Figure 1A). These results are consistent with previous studies in mechanical and embolic animal stroke models.22, 23 In our hands, PROG treatment attenuated tPA-induced hemorrhage, brain swelling, BBB disruption, and infarct volume. We observed mortality rates of 7.14% and 27.78% in the saline- and tPA-treated groups, respectively, whereas no mortality was seen in the tPA-plus-PROG-treated rats.
Hematomas and brain edema reportedly worsen patient outcomes after intracranial hemorrhage. We showed here that delayed treatment with tPA resulted in edema and this condition was clearly attenuated by PROG treatment (Figure 1C). Our findings are consistent with previous work showing no infarct-attenuating effects of delayed tPA treatment after MCAO in mice and embolic stroke in rats. In our hands, tPA combined with PROG treatment reduced infarct, edema, and hemorrhagic volume.
Loss of BBB integrity soon after the onset of ischemia is believed to be a precursor to brain edema and hemorrhagic transformation.24 Accumulating evidence shows that tPA mediates the development of BBB permeability and accelerates the progression of ischemic damage. The presence of extravasated IgG is widely used as a marker for BBB disruption in neurovascular injury, including stroke.25 We found that tPA dramatically increases the level of IgG in the ischemic hemisphere and, as hypothesized, PROG treatment significantly reduced the tPA-induced extravasation of IgG (Figure 2A).
Ballabh et al26 have reported that brain endothelial cells are directly responsible for the formation of barriers and have more extensive TJs than endothelial cells in other tissues. Tight junctions lead to high TEER and low paracellular permeability.27 A reduction of TEER values, charge, and size selectivity indicates TJ disruption. In this study, TEER values in an in vitro BBB model were reduced after H/R (Figure 2B), suggesting that the opening of TJs and the loss of BBB function after H/R may contribute to the pathology of stroke. At a concentration of 20 μg/mL, tPA exacerbated the decrease in the TEER during H/R. Progesterone was effective in protecting BBB function in a dose-dependent manner (Figure 2B).
We next examined the effects of tPA plus PROG treatment on the alteration of TJs in vivo and in vitro. Occludin, claudin-5, and ZO-1 are the major structural proteins that make up the BBB and are sensitive markers for BBB disruption.28 We found that the fragmentation of occludin and claudin-5 was further increased by tPA and ischemia (Figure 3A). Our observation is consistent with a report by Yang et al29 that 60 kDa occludin and 17 kDa claudin-5 were increased at 24 hours after focal ischemia in rats. Our results are also consistent with findings that levels of occludin and the ratio of claudin-5 were significantly higher in patients with hemorrhagic transformation than in those without hemorrhagic transformation.30 In contrast, Ishiguro et al31 and Mishiro et al13 showed reduction of claudin-5 at 24 hours after ischemia and a reduction of occludin and ZO-1 at 48 hours after ischemia in mice treated with tPA, but these studies did not assess protein fragments. The discrepancy may also be attributable to the species used (rat as opposed to mouse) and the timing of killing.
Interestingly, in the present experiment in bEnd.3 cells, we observed that H/R plus tPA led to reduced expression of occludin (65 kDa) and claudin-5 but not ZO-1 (Figure 3B). Mishiro et al13 found a reduction of occludin and ZO-1 by tPA after H/R in human brain endothelial cells. The different pattern of results between our in vitro and in vivo studies could be attributable to the fact that TJ proteolytic fragments are directly released into the culture medium by protease activation after cell damage, a hypothesis supported by a report that occludin degradation products and several claudins increase in the basolateral medium after 30-minute incubation with methyl-β-cyclodextrin in MDCK cells.32 Nevertheless, in our stroke model, the alteration of TJs led to BBB disruption after ischemia or H/R plus tPA. It is noteworthy that treatment with PROG prevented the alteration of occludin and claudin-5 after delayed tPA treatment in ischemic animals and after H/R with tPA (Figure 3).
A strong relationship between MMP-9 and tPA-induced cerebral hemorrhage and BBB disruption in ischemic stroke has been demonstrated in animals and humans.12, 13, 14 It is known that occludin contains extracellular MMP cleavage sites and is a direct substrate for MMPs.33 Our results support the finding that occludin expression is decreased by increased levels of MMP-9 after H/R with tPA (Figure 4B). Actin disassembly from cytoskeleton contributes to loss of endothelial cell barrier function34 because the localization of F-actin filament in endothelial and epithelial cell membrane is necessary for TJ formation. Madara et al,35 in particular, reported that the structural disruption between TJ protein occludin and actin filaments can lead to perturbation of paracellular permeability. The effects of PROG on these cytoskeletal changes complements and helps explain our observation on the differences of tPA-induced in vitro TEER changes under hypoxia. Recent research shows that a broad-spectrum MMP inhibitor prevented hemorrhagic complications and loss of TJs induced by tPA in mice.13 We found that delayed tPA treatment significantly increases MMP-9 activity compared with controls and this effect is inhibited by PROG treatment (Figure 4A). These data are consistent with our earlier study showing that PROG attenuated BBB dysfunction after permanent focal ischemia by reducing MMPs and the inflammatory response and by preventing the degradation of occludin and claudin-5.6 Moreover, PROG was shown to reduce early brain injury and mortality after subarachnoid hemorrhage induced by endovascular perforation by stabilizing the BBB and inhibiting MMP-9 activity.36
Vascular endothelial growth factor has been shown to be beneficial at a later stage of stroke by enhancing angiogenesis,21, 37 but in the acute stage, it is also known to increase microvascular permeability, causing increased edema and hemorrhage.37 Vascular endothelial growth factor also mediates tPA-associated activation of MMP-9 and the degradation of BBB components and these events, in turn, contribute to hemorrhagic transformation.22 After the onset of ischemia, the expression of VEGF is localized to the ischemic core, where leakage of the BBB has been reported to occur as early as 1 to 3 hours post-ischemia, reaching its highest level at 24 to 48 hours.21, 38 During the acute stage of stroke, intravenous or intracerebral VEGF given to rats within 1 hour after ischemia has been shown to increase BBB damage,20 and treatment with anti-VEGF antibody decreases BBB disruption in the ischemic cortex.39 Vascular endothelial growth factor's effects on vascular permeability are transduced by binding to receptors such as tyrosine kinase, VEGFR1, and VEGFR2. The activation of VEGFR2 through dimerization and phosphorylation leads to the activation of several intracellular signaling pathways including phospholipase Cγ, protein kinase C, ERK1/2, and Src. It was recently reported that PROG affords neuroprotection after permanent MCAO by inhibiting acute VEGF upregulation.40 Here, we investigated the role of VEGF in PROG-mediated attenuation of tPA-induced BBB dysfunction. Western blot analysis revealed that cerebral ischemia significantly increased VEGF expression compared with the sham group and rats treated with tPA showed an even greater increase of VEGF. Consistent with our previous findings in a permanent MCAO model,40 our results demonstrate that treatment with PROG significantly decreased the level of VEGF after stroke (Figure 5).
Furthermore, tPA treatment dramatically increased VEGF secretion in brain endothelial cells after 6-hour hypoxia in vitro. In keeping with our expectation, PROG prevented the secretion of VEGF into culture media after 6-hour hypoxia. We interpret our data to suggest that PROG may attenuate BBB dysfunction by inhibition of the VEGF–MMP-9 pathway.
Further investigations are needed to evaluate dose- and time-response relationships for combination PROG and tPA treatment in an animal stroke model more representative of the clinical population at risk for stroke. Our study used an in vitro ischemic model as well as young normotensive male subjects. This could lead to inexact across-the-board conclusions, as the dynamics of vascular damage by tPA and its remodeling by PROG treatment may very well be age- and sex-dependent. Much less is known about the effects of neurosteroid treatments for stroke in comorbid conditions in the presence of specific stroke risk factors such as aging, diabetes, chronic hypertension, and hypercholesteremia, where vascular protection may very well be compromised. Future studies should define both gender- and age-dependent responses to the combination treatment in both normal and comorbid subjects, and should be replicated across multiple laboratories as well in a larger cohort of animals.
Conclusions
Increasing evidence shows that PROG treatment can reduce cerebral hemorrhage, brain swelling, BBB disruption and mortality after delayed tPA treatment for ischemic stroke. Progesterone's inhibition of the tPA-induced increase in MMP-9 may explain some of the mechanisms underlying PROG's BBB protection. Our study suggests that PROG's neurovascular protective effects may support an important therapeutic strategy to reduce hemorrhagic complications. We propose that PROG merits further consideration as a neuroprotective partner for combination therapy with tPA to improve its safety and efficacy in stroke patients.
Acknowledgments
The authors thank Leslie McCann for her editorial assistance. We thank Drs Michael Frankel and David Wright for their encouragement and help with the study design.
DGS is entitled to royalty payment from BHR Pharmaceuticals related to research on progesterone and brain injury. His future financial interests may be affected by the outcome of this research. The terms of this arrangement have been reviewed and approved by Emory University, which receives the largest share of all financial benefits.
Footnotes
This work was supported by AHA SDG grant 11SDG5430002 to IS and NIH UO1 NS062676 to DGS. Partial funding for some of the assays used in this project was provided by BHR Pharma.
References
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:e1–e2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Mishiro K, Ishiguro M, Suzuki Y, Tsuruma K, Shimazawa M, Hara H. A broad-spectrum matrix metalloproteinase inhibitor prevents hemorrhagic complications induced by tissue plasminogen activator in mice. Neuroscience. 2012;205:39–48. doi: 10.1016/j.neuroscience.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties. Ann Emerg Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, et al. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Godfrey KR, Tanswell P, Bates RA, Chappell MJ, Madden FN. Nonlinear pharmacokinetics of tissue-type plasminogen activator in three animal species: a comparison of mathematical models. Biopharm Drug Dispos. 1998;19:131–140. doi: 10.1002/(sici)1099-081x(199803)19:2<131::aid-bdd87>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Li G, Simon MJ, Cancel LM, Shi ZD, Ji X, Tarbell JM, et al. Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann Biomed Eng. 2010;38:2499–2511. doi: 10.1007/s10439-010-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283–287. doi: 10.1016/j.expneurol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31:1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Aihara N, Tanno H, Hall JJ, Pitts LH, Noble LJ. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, et al. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12:215–222. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Manolescu BN. Breaking down barriers to identify hemorrhagic transformation in ischemic stroke. Neurology. 2012;79:1632–1633. doi: 10.1212/WNL.0b013e31826e9b9d. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Mishiro K, Fujiwara Y, Chen H, Izuta H, Tsuruma K, et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS One. 2010;5:e15178. doi: 10.1371/journal.pone.0015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas E, Barron C, Francis SA, McCormack JM, McCarthy KM, Schneeberger EE, et al. Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments. Exp Cell Res. 2010;316:353–365. doi: 10.1016/j.yexcr.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112:4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Boardman KC, Aryal AM, Miller WM, Waters CM. Actin re-distribution in response to hydrogen peroxide in airway epithelial cells. J Cell Physiol. 2004;199:57–66. doi: 10.1002/jcp.10451. [DOI] [PubMed] [Google Scholar]
- Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Hu Q, Chen J, Wu C, Gu C, Chen G. Progesterone attenuates early brain injury after subarachnoid hemorrhage in rats. Neurosci Lett. 2013;543:163–167. doi: 10.1016/j.neulet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and non-ischemic cerebral cortex. Neurol Res. 2005;27:864–868. doi: 10.1179/016164105X49418. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–450. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]