Abstract
Melatonin is a naturally occurring indolamine with mild antioxidant properties that is neuroprotective in perinatal animals. There is limited information on its effects on preterm brain injury. In this study, 23 chronically instrumented fetal sheep received 25 minutes of complete umbilical cord occlusion at 101 to 104 days gestation (term is 147 days). Melatonin was administered to the ewe 15 minutes before occlusion (0.1 mg/kg bolus followed by 0.1 mg/kg per hour for 6 hours, n=8), or the equivalent volume of vehicle (2% ethanol, n=7), or saline (n=8), or maternal saline plus sham occlusion (n=8). Sheep were killed after 7 days recovery in utero. Fetal blood pressure, heart rate, nuchal activity, and temperature were similar between groups. Vehicle infusion was associated with improved neuronal survival in the caudate nucleus, but greater neuronal loss in the regions of the hippocampus, with reduced proliferation and increased ameboid microglia in the white matter (P<0.05). Maternal melatonin infusion was associated with faster recovery of fetal EEG, prolonged reduction in carotid blood flow, similar neuronal survival to vehicle, improved numbers of mature oligodendrocytes, and reduced microglial activation in the white matter (P<0.05). Prophylactic maternal melatonin treatment is partially protective but its effects may be partly confounded by ethanol used to dissolve melatonin.
Keywords: asphyxia, brain, ethanol, melatonin, neuroprotection, preterm fetus
Introduction
Birth asphyxia is relatively common with preterm birth, and remains a significant cause of neonatal death and neurodevelopmental delay.1 Excess free radical production during asphyxia and early reperfusion are associated with lipid peroxidation, nucleic acid damage, and mitochondrial dysfunction that promote cell death.2 Melatonin is a naturally occurring indolamine involved in circadian rhythm, which also has antioxidant properties.3, 4 It readily crosses the human and ovine placentae making it an attractive option for prophylactic treatment of fetuses at high risk of perinatal hypoxia.4 Melatonin given before and immediately after hypoxia–ischemia is neuroprotective in postnatal rodents.5, 6, 7, 8, 9 Postnatally, high-dose (5 mg/kg per hour over 6 hours) melatonin given immediately after hypoxia–ischemia in term piglets augmented protection from therapeutic hypothermia on both magnetic resonance spectroscopy markers of anaerobic stress, and histopathology.10
In contrast, there have been few studies of antenatal treatment and relatively limited large-animal evidence that prophylactic low-dose melatonin can protect against hypoxic–ischemic injury in the preterm fetus. In fetal sheep, Miller et al11 showed that maternal prophylactic melatonin (1 mg total) given before 10-minute umbilical cord occlusion at term-equivalent was associated with reduced brain lipid peroxidation, neuronal death, microglial activation, and astrogliosis.11, 12 In preterm fetal sheep at 0.6 gestation (Term=147 days) fetal infusion of high-dose (20 mg/kg) melatonin for 6 hours from shortly after umbilical cord occlusion was associated with reduced apoptosis and microglia in the white matter, although cell survival was not quantified.13
A further important consideration is that as melatonin is hydrophobic, ethanol has often been used as a diluent. Ethanol adversely affects the developing brain.14 However, it can also improve outcome in models of adult stroke.15 Thus, there is potential for confounding the effects of melatonin. Postnatally, very high-dose melatonin (10 mg/kg) dissolved in ethanol was associated with hypotension and increased inotrope requirements after hypoxia–ischemia in term piglets; it is unknown whether the melatonin or ethanol or both mediated this adverse effect.10 For a prophylactic treatment strategy, it is important to use much lower doses to avoid risks of compromising fetal adaptation to severe insults or, indeed, any adverse effects in infants at low risk of injury.
In the present study, we tested the hypothesis that prophylactic maternal low-dose melatonin is neuroprotective after profound asphyxia in fetal sheep at 0.7 of gestation (Term=147 days), and the secondary hypothesis that the vehicle, low-dose ethanol, is deleterious. Brain development at this age is broadly consistent with 28 to 32 weeks in humans, before the development of cortical myelination.16
Materials and Methods
All procedures were approved by the Animal Ethics Committee of The University of Auckland under the New Zealand Animal Welfare Act, and the Code of Ethical Conduct for animals in research established by the Ministry of Primary Industries, Government of New Zealand. Twenty three Romney/Suffolk fetal sheep were operated on at 98 to 99 days of gestation (term=147 days). Food, but not water was withdrawn 18 hours before surgery. Ewes were given 5 ml of streptocin (procaine penicillin (250,000 IU/mL) and dihydrostreptomycin (250 mg/mL); Stockguard Labs, Hamilton, New Zealand) intramuscularly for prophylaxis 30 minutes before the start of surgery. Weight was recorded for calculation of drug doses. Anesthesia was induced by intravenous injection of propofol (5 mg/kg; AstraZeneca, Auckland, New Zealand), and general anesthesia maintained using 2% to 3% isoflurane in O2. A 20-gauge intravenous catheter was placed in a maternal front leg vein and the ewes were placed on a constant infusion saline drip to maintain maternal fluid balance. Ewes were ventilated and the depth of anesthesia, maternal heart rate, and respiration were constantly monitored by trained anesthetic staff.
All surgical procedures were performed using sterile techniques. After a maternal midline abdominal incision and exteriorization of the uterus and either the top or bottom half of the fetus, catheters were placed in the left fetal femoral artery and vein, right brachial artery and vein, and the amniotic sac. An ultrasonic blood flow probe (size 3S; Transonic Systems, Ithaca, NY, USA) was placed around the left carotid artery to measure carotid blood flow (CaBF) as an index of global cerebral blood flow.17 A second probe (2R) was placed around the femoral artery contralateralto the catheter to measure femoral blood flow (FBF). Two pairs of electroencephalogram (EEG) electrodes (AS633-5SSF, Cooner Wire, Chatsworth, CA, USA) were placed on the dura over the parasagittal parietal cortex (5 mm and 10 mm anterior to bregma and 5 mm lateral) and secured with cyanoacrylate glue. A reference electrode was sewn over the occiput. A pair of electrodes was sewn into the nuchal muscle to record electromyography (EMG) activity as an index of fetal body movements. A further pair of electrodes was sewn over the fetal chest to measure the fetal electrocardiogram. An inflatable silicone occluder was placed around the umbilical cord of all fetuses (In Vivo Metric, Healdsburg, CA, USA). All fetal leads were exteriorized through the maternal flank and a maternal long saphenous vein was catheterized to provide access for postoperative care and euthanasia. Eighty milligrams gentamicin (Rousell, Auckland, New Zealand) was administered into the amniotic sac before closure of the uterus.
Postoperatively, all sheep were housed in separate metabolic cages with access to water and food ad libitum, together in a temperature-controlled room (16±1°C, humidity 50±10%) with a 12-hour light–dark cycle. A period of at least 4 days postoperative recovery was allowed before experiments commenced, during which time antibiotics were administered to the ewe daily for four days intravenously (600 mg benzylpenicillin sodium; Novartis, Auckland, New Zealand, and 80 mg gentamicin). Fetal catheters were maintained patent by continuous infusion of heparinized saline (20 U/mL at 0.15 mL/hour) and the maternal catheter maintained by daily flushing. Light proof tubing was used to cover the maternal venous catheter.
Melatonin (Sigma-Aldrich, Auckland, New Zealand) was prepared by dissolving 100 mg in 2 mL of pure ethanol and then adding sterile normal saline to a total volume of 100 mL (i.e. 1 mg/mL melatonin in 2% ethanol). The total amount of maternal ethanol infused over the 6-hour period was 0.66±0.03 g in the vehicle+occlusion group and 0.71±0.02 g in the melatonin+occlusion group (nonsignificant).
Experimental Procedures
Recordings
Fetal mean arterial blood pressure (MAP, Novatrans II, MX860; Medex, Hilliard, OH, USA), corrected for maternal movement by subtraction of amniotic fluid pressure, fetal heart rate derived from the electrocardiogram, CaBF, FBF, EEG, and EMG were recorded continuously from−24 hours to 168 hours after umbilical cord occlusion. The blood pressure signal was collected at 64 Hz and low pass filtered at 30 Hz. The nuchal EMG signal was band-pass filtered between 100 Hz and 1 kHz, the signal was then integrated using a time constant of 1 sec. The analog fetal EEG signal was low pass filtered with the cut-off frequency set with −3 dB point at 30 Hz, and digitized at 256 Hz (using analog to digital cards, National Instruments, Austin, TX, USA). The intensity and frequency were derived from the intensity spectrum signal between 0.5 and 20 Hz. For data presentation, the total EEG intensity (power) was normalized by log transformation (dB, 20 × log (intensity)), and data from left and right EEG electrodes were averaged to give mean total EEG activity. The 90% spectral edge of the EEG, i.e., the frequency below which lay 90% of the EEG intensity, was calculated from the spectra. Quantitative EEG measurements for each waveform were performed to quantify power in the delta (0 to 3.9 Hz), theta (4 to 7.9 Hz), alpha (8 to 12.9 Hz), and beta (13 to 22 Hz) bands. This involved calculating power spectra (by fast Fourier transform) of the EEG on sequential epochs, using a 10-second Hanning-window to minimize spectral leakage.18 Data were collected by computer and stored to disk for off-line analysis.
Experimental protocol
Experiments were conducted at 103 to 104 days gestation. Fetuses were randomly assigned to melatonin+occlusion (n=7), vehicle+occlusion (n=8), or saline+occlusion (n=8). Sham occlusion fetuses received no occlusion (n=8). Melatonin or the same volume of vehicle (2% ethanol) or normal saline was administered to the maternal saphenous vein 15 minutes before umbilical cord occlusion as a 0.1 mg/kg bolus followed by a 0.1 mg/kg per hour infusion for 6 hours. The total amount of maternal melatonin received over the 6-h period in the melatonin+occlusion group was 4.5 ±0.1 mg.
Fetal asphyxia was induced by rapid inflation of the umbilical cord occluder for 25 minutes with sterile saline of a defined volume known to completely inflate the occluder and totally compress the umbilical cord, as determined in pilot experiments with a Transonic flow probe placed around an umbilical vein. Successful occlusion was confirmed by observation of a rapid onset of bradycardia with a rise in mean arterial blood pressure, and by pH and blood gas measurements. The duration of occlusion was chosen to represent an acute, severe, near-terminal insult that could be survived without postasphyxial cardiac support and is associated with severe subcortical neuronal loss.18 All occlusions or sham occlusions were undertaken around 0900 hours.
Fetal arterial blood was taken at 30 minutes before occlusion, 5 and 17 minutes during asphyxia, and then 10 minutes, 1, 2, 4, 6, 24, 48, 72, 96, 120, 144, and 168 hours postasphyxia for pH and blood gas determination (Ciba-Corning Diagnostics 845 blood gas analyzer and co-oximeter, MA, Walpole, USA) and for glucose and lactate measurements (YSI model 2300, Yellow Springs, OH, USA). Samples at −30 minutes before occlusion, at +6 hours, and then daily samples from the day after occlusion at 0900 were taken for plasma cortisol, ACTH and melatonin concentration determination. After the last blood sample, ewes and fetuses were killed by an intravenous overdose of pentobarbitone sodium (9 g) to the ewe (Pentobarb 300; Chemstock International, Christchurch, New Zealand).
Hormone assays
Cortisol levels were measured by liquid chromatography-tandem mass spectrometry method as previously described.19 Mean inter- and intra-assay CV values for cortisol were 5.8% and 6.0%, respectively. Adrenocorticotropic hormone levels in the plasma samples were measured in duplicate using a 125I RIA kit (24130, DiaSorin, Stillwater, MN, USA) validated for ovine maternal and fetal plasma. The intra-assay coefficient of variation was 9.7% and the inter-assay coefficient was 12.8%. The mean ACTH assay sensitivity was 9.7 pg/mL and samples showing less than this value were assigned this value for analysis.
Fetal plasma melatonin concentration was measured by RIA using a commercial kit (Labor Diagnostika Nord GmbH KG, Germany; Melatonin Research RIA Kit Cat# BA R-3900) after the manufacturer's instructions. The inter- and intra-assay coefficients were less than 10% sensitivity was 1.3 pg/mL. Because of a freezer accident, melatonin could not be measured at +6 hours.
Histopathology
The fetal brains were perfusion fixed with 10% phosphate-buffered formalin. Slices (10-μm thick) were cut using a microtome (Leica Jung RM2035, Leica Microsystems, Albany, New Zealand). Slides were dewaxed in xylene and rehydrated in decreasing concentrations of ethanol. Slides were washed in 0.1 mol/L phosphate-buffered saline (PBS). Antigen retrieval was performed using the pressure cooker method with citrate buffer. Endogenous peroxidase quenching was performed by incubation in 1% H2O2 in methanol for anti-neuronal nuclei monoclonal antibody (NeuN), Iba-1, CNPase and Ki-67, and PBS for Olig-2. Blocking was performed in 3% normal horse serum for NeuN and Iba-1 and normal goat serum for Olig-2, CNPase and Ki-67 for 1 hour at room temperature. Sections were labeled with 1:400 mouse NeuN (Chemicon International, Temecula, CA, USA), 1:400 Olig-2 (Chemicon International, Olig-2, a marker for oligodendrocytes at all stages of the lineage20), 1:200 Ki-67 (Dako, NSW, Australia), 1:200 goat anti-Iba-1 (Abcam, Hamilton, New Zealand) and 1:200 mouse anti-CNPase (Abcam) overnight at 4°C. Sections were incubated in biotin-conjugated secondary 1:200 horse anti-mouse (NeuN) or 1:200 goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) in 3.5% normal horse serum of normal goat serum for goat. Slides were then incubated in ExtrAvidin (1:200, Sigma-Aldrich) in PBS for 2 hours at room temperature and then reacted in diaminobenzidine tetrachloride (Sigma-Aldrich). The reaction was stopped by washing in PBS, the sections dehydrated and mounted. For fluorescent double labeling, dewaxing, rehydrating, and antigen retrieval were performed as described above. Sections were blocked in 3% normal goat serum for 1 hour at room temperature. Sections were incubated with either 1:400 rabbit anti-Olig-2 or 1:50 anti-platelet derived growth factor receptor alpha (a marker of oligodendrocyte progenitor cells)21 and 1:200 mouse anti-Ki-67 in 3% normal goat serum at 4°C overnight. Sections were washed in PBS and incubated with 1:200 biotinylated goat anti-mouse IgG (Vector Laboratories) for 3 hours at room temperature. Sections were washed and incubated with 1:200 streptavidin Alexa 488 and 1:200 donkey anti-rabbit Alexa 568. Sections were then washed and mounted.
Brain regions of the forebrain used for analysis included the mid-striatum (comprising the caudate nucleus and putamen), and the frontal subcortical white matter (comprising the intragyral, IGWM, and periventricular, PVWM, regions) on sections taken 23 mm anterior to stereotaxic zero. The cornu ammonis of the dorsal horn of the anterior hippocampus (divided into CA1/2, CA3, CA4, and dentate gyrus) were assessed on sections taken 17 mm anterior to stereotaxic zero. Neuronal (NeuN), oligodendrocyte (Olig-2, CNPase) and microglial (Iba-1) changes, and proliferation (Ki-67) were scored on stained sections by light microscopy at × 40 magnification on a Nikon 80i microscope with NIS Elements Br 4.0 software (Nikon Instruments, Melville, NY, USA) using seven fields in the striatum (four in the caudate nucleus, three in the putamen), two fields in the white matter (one intragyral, one periventricular) and one field in each of the hippocampal divisions. Microglia were counted as cells with Iba-1 immunostaining. For assessment of activated microglia Iba-1 cells showing an ameboid morphology with no cell processes were counted.22 For each animal, average scores across both hemispheres from two sections were calculated for each region. Counts were made by an assessor masked to treatment group.
Data analysis
Off-line analysis of the physiologic data was performed using customized Labview programs. Carotid vascular conductance and femoral vascular conductance were calculated as mean arterial blood pressure/flow. The raw EEG was assessed for epileptiform activity. Seizures were identified visually and defined as the concurrent appearance of sudden, repetitive, evolving stereotyped waveforms in the EEG signal lasting more than 10 seconds and of an amplitude greater than 20 μV.23
Data were analyzed using SPSS 15.0 for windows (SPSS, Chicago, Il, USA) and JMP 8.0 (SAS Institute, Cary, NC, USA). For analysis of the recovery data after occlusion (from 1 to 168 hours post occlusion) the baseline period was taken as the mean of the 24 hours before occlusion. For between group comparisons two-way analysis of variance for repeated measures was performed. When statistical significance was found between groups for repeated measures analysis of variance in JMP post hoc contrast tests were used between groups. When significance was found between group and time for repeated measures, Tukey's pairwise comparisons were used to compare selected time points. For blood gas, electrolyte, biochemical, hormonal, and histologic data, analysis of variance was performed, with post hoc Tukey's pairwise comparisons when significance was found. When baseline differences were found, analysis of covariance was performed on subsequent results and Least-Squares difference post hoc tests performed as appropriate. If there was an effect of region and group then each region was tested individually. Non-parametric Mann–Whitney U-tests were used when appropriate. Alpha error was set as P<0.05. Data are presented as mean±s.e.m. unless otherwise stated.
Results
Baseline and Umbilical Cord Occlusion
All fetuses had baseline blood gases, acid-base status, and glucose–lactate values in the normal range by our laboratory standards. Umbilical cord occlusion was associated with marked fetal hypoxia, hypercarbia and acidosis (Table 1), bradycardia, hypotension, peripheral vasoconstriction, cerebral hypoperfusion, and EEG suppression. There were no differences between occlusion groups during or immediately after occlusion.
Table 1. Fetal arterial pH, blood gases, glucose, and lactate levels, before, during and after umbilical cord occlusion.
| Group | Baseline | 5 minute occlusion | 17 minute occlusion | +10 minutes | +1 hour | +2 hours | +4 hours | +6 hours | +24 hours | +48 hours | +72 hours | +96 hours | +120 hours | +144 hours | +168 hours |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | |||||||||||||||
| S | 7.39±0.01 | 7.03±0.02a | 6.84±0.01a | 7.16±0.01a | 7.30±0.01a | 7.35±0.01 | 7.42±0.01 | 7.41±0.01 | 7.38±0.01 | 7.39±0.01 | 7.40±0.01 | 7.41±0.01 | 7.40±0.00 | 7.40±0.00 | 7.39±0.01 |
| V | 7.37±0.01 | 7.04±0.02a | 6.85±0.02a | 7.16±0.01a | 7.30±0.02a | 7.37±0.02 | 7.42±0.01a | 7.41±0.01a | 7.36±0.01 | 7.37±0.00 | 7.39±0.00a | 7.38±0.01 | 7.38±0.01 | 7.43±0.04 | 7.37±0.01 |
| M | 7.37±0.01 | 7.03±0.02a | 6.84±0.02a | 7.15±0.01a | 7.28±0.01a | 7.34±0.01a | 7.39±0.01 | 7.40±0.01a | 7.39±0.01a | 7.37±0.01 | 7.38±0.01b | 7.37±0.01b | 7.36±0.01b | 7.36±0.01 | 7.35±0.01b,c |
| PCO2 (mmHg) | |||||||||||||||
| S | 48.1±0.7 | 107.0±5.0a | 142.1±5.1a | 54.6±2.1a | 42.5±0.9a | 43.7±1.3a | 43.0±0.5a | 47.3±0.7 | 46.9±0.5 | 47.9±1.1 | 47.1±0.8 | 46.3±1.6 | 47.6±1.5 | 49.1±1.0 | 48.6±1.7 |
| V | 48.7±0.9 | 90.7±4.3a | 117.4±6.8a,b | 50.5±1.9 | 42.3±0.6a | 42.3±1.1a | 41.6±0.6a | 47.2±1.4 | 45.6±1.3a | 46.9±2.4 | 45.6±1.1a | 47.6±1.2 | 49.3±0.9 | 49.3±1.3 | 48.2±0.6 |
| M | 51.8±1.5 | 98.5±5.1a | 139.3±8.2a | 52.8±2.8 | 45.9±2.2 | 43.0±3.5 | 45.4±1.8 | 48.7±0.8 | 49.1±0.7a | 50.5±2.0 | 49.8±1.1c | 49.5±1.2 | 51.4±2.0 | 50.8±1.8 | 53.7±1.8 |
| PO2 (mmHg) | |||||||||||||||
| S | 24.7±1.5 | 6.0±0.9a | 6.4±0.7a | 33.3±2.0a | 30.0±1.9a | 25.8±2.2 | 27.0±2.0 | 25.9±2.4 | 27.0±1.7 | 28.2±1.8a | 27.3±1.7 | 27.8±1.6a | 26.0±1.9 | 26.5±2.0 | 26.3±2.3 |
| V | 25.2±0.8 | 9.8±0.8a,b | 10.8±1.0a,b | 35.6±1.6a | 31.0±1.2a | 27.6±0.8a | 29.2±1.2a | 28.7±1.1a | 31.9±1.6a | 29.0±2.2 | 32.2±2.0a | 30.4±1.5a | 28.7±1.6 | 28.6±1.1a | 28.4±1.0a |
| M | 22.9±0.6 | 7.7±1.0a | 9.0±1.5a | 33.8±1.8a | 29.5±1.1a | 26.1±1.3a | 25.5±1.3 | 25.2±1.2a | 28.7±0.8a | 29.8±0.7a | 29.4±1.3a | 26.8±1.3a | 26.3±1.5 | 27.3±2.7 | 24.1±2.2 |
| Hb (g.dL−1) | |||||||||||||||
| S | 10.0±0.6 | 11.3±0.6a | 10.4±0.5a | 10.4±0.7 | 9.3±0.6 | 9.7±0.7 | 9.4±0.6a | 9.9±0.6 | 9.6±0.5 | 9.3±0.4 | 9.3±0.4 | 9.0±0.4 | 9.2±0.3 | 9.5±0.4 | 9.5±0.5 |
| V | 8.5±0.3 | 9.4±0.1a,b | 8.9±0.4 | 8.9±0.3a | 8.5±0.2 | 8.2±0.2 | 8.6±0.2 | 9.2±0.3a | 9.4±0.4a | 9.3±0.6 | 9.6±1.0 | 9.6±1.1 | 9.4±1.2 | 9.3±1.1 | 8.5±0.8 |
| M | 9.5±0.4 | 9.5±0.3b | 10.0±0.6 | 9.8±0.4 | 9.7±0.5 | 9.4±0.7 | 9.8±0.6 | 10.5±0.6a | 10.4±0.9 | 9.8±0.6 | 9.8±0.7 | 9.8±0.9 | 9.9±1.1 | 11.1±1.9 | 10.8±2.6 |
| Hct (%) | |||||||||||||||
| S | 29.6±1.9 | 33.2±1.9a | 30.5±1.5a | 30.7±2.1 | 27.5±1.7a | 28.8±2.1a | 27.7±1.6a | 29.2±1.6 | 28.2±1.4 | 27.3±1.1 | 27.3±1.0 | 26.3±1.1 | 27.0±0.9 | 27.2±1.2 | 28.3±1.5 |
| V | 25.5±0.6 | 27.5±0.6a,b | 26.2±1.2 | 26.0±0.8 | 25.1±0.6 | 24.0±0.5 | 25.4±0.5 | 27.1±1.0 | 27.6±1.2a | 27.4±1.7 | 28.3±2.8 | 28.3±3.2 | 27.6±3.5 | 27.4±3.3 | 25.2±2.2 |
| M | 28.0±1.2 | 28.0±0.9b | 29.3±1.8 | 28.8±1.4 | 28.5±1.4 | 27.8±2.0 | 29.0±1.6 | 31.0±1.9a | 30.6±2.4 | 29.0±1.8 | 29.2±2.0 | 28.8±2.7 | 29.0±3.2 | 32.5±5.7 | 31.8±7.6 |
| O2ct (mmol/L) | |||||||||||||||
| S | 4.0±0.4 | 0.5±0.0a | 0.4±0.0a | 4.7±0.1 | 4.2±0.2 | 4.1±0.2 | 4.3±0.2 | 4.4±0.2 | 4.4±0.2 | 4.4±0.3 | 4.3±0.2 | 4.2±0.1 | 4.1±0.2 | 4.2±0.3 | 4.1±0.3 |
| V | 3.7±0.1 | 0.5±0.0a | 0.4±0.0a | 4.2±0.1a | 4.0±0.1 | 3.8±0.1 | 4.2±0.1a | 4.6±0.2a | 4.7±0.2a | 4.7±0.2a | 4.7±0.3a | 4.5±0.3a | 4.1±0.3 | 4.2±0.3 | 4.1±0.2a |
| M | 3.9±0.2 | 0.5±0.0a | 0.5±0.0a | 4.6±0.2a | 4.5±0.1a | 4.1±0.2 | 4.3±0.2a | 4.6±0.3a | 5.0±0.3a | 4.8±0.2a | 4.6±0.2a | 4.4±0.2 | 4.0±0.2 | 4.1±0.3 | 3.5±0.2 |
| HCO3 (mmol/L) | |||||||||||||||
| S | 26.5±0.5 | 18.2±0.5a | 13.4±0.5a | 16.7±0.4a | 19.6±0.5a | 22.8±0.9a | 26.5±0.8 | 28.1±0.9 | 25.9±0.5 | 27.3±0.4 | 27.3±0.6 | 27.1±0.5 | 27.1±0.5 | 27.7±0.5 | 27.0±0.5 |
| V | 26.9±0.5 | 22.8±0.7a,b | 19.3±1.3a,b | 17.0±0.8a | 19.9±1.0a | 23.5±0.8a | 26.1±0.6a | 28.4±0.5a | 24.7±1.0a | 26.4±1.4 | 26.5±0.6 | 27.1±0.3 | 27.7±0.4 | 27.8±0.6 | 26.8±0.4 |
| M | 28.9±0.8 | 24.5±0.5a,b | 22.4±1.1a,b | 17.4±0.7a | 21.0±1.1a | 22.6±2.1a | 26.4±1.5 | 28.8±0.4 | 28.2±0.6c | 28.0±0.7 | 27.9±0.7 | 27.6±0.9 | 28.1±0.9 | 27.6±1.2 | 28.4±1.3 |
| BE (mmol/L) | |||||||||||||||
| S | 2.9±0.4 | −5.7±0.7a | −12.4±0.6a | −9.3±0.5a | −5.4±0.6a | −1.5±1.0a | 2.6±0.8 | 4.5±0.9a | 2.0±0.6a | 3.6±0.5 | 3.5±0.7 | 3.3±0.7 | 3.4±0.6 | 4.0±0.6 | 3.3±0.7 |
| V | 2.0±0.6 | −8.1±0.8a,b | −14.1±1.2a | −10.6±0.9a | −5.5±1.3a | −1.2±0.9a | 2.2±0.7 | 4.0±0.6 | −0.1±1.0 | 1.6±1.2 | 2.0±0.5 | 2.3±0.2 | 2.7±0.4 | 2.9±0.5 | 1.9±0.4 |
| M | 3.7±0.7 | −6.4±0.3a | −12.4±0.8a | −10.4±0.5a | −4.7±1.1a | −2.2±1.9a | 1.9±1.6 | 4.1±0.5 | 3.4±0.7c | 2.9±0.5 | 3.0±0.7 | 2.6±0.9 | 2.9±0.9 | 2.3±1.2 | 2.8±1.2 |
| Lactate (mmol/L) | |||||||||||||||
| S | 1.0±0.2 | 4.4±0.2a | 6.8±0.4a | 6.2±0.3a | 4.4±0.2a | 3.3±0.4a | 2.3±0.4a | 2.3±0.3a | 1.1±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 0.9±0.1a | 0.9±0.1a | 0.9±0.1a |
| V | 0.8±0.0 | 3.5±0.2a | 5.2±0.2a,b | 5.6±0.3a | 4.1±0.2a | 2.6±0.2a | 1.5±0.2a | 1.9±0.2a | 1.7±0.4 | 0.8±0.0 | 0.8±0.0 | 0.8±0.1 | 0.8±0.0 | 0.8±0.0 | 0.8±0.0 |
| M | 0.7±0.1 | 3.9±0.3a | 6.3±0.3a | 5.9±0.2a | 4.5±0.5a | 3.3±0.5a | 2.2±0.6a | 2.4±0.7a | 1.2±0.2a | 0.8±0.1 | 0.9±0.1a | 0.9±0.1 | 0.8±0.1 | 0.7±0.1 | 0.8±0.1 |
| Glucose (mmol/L) | |||||||||||||||
| S | 1.0±0.1 | 0.4±0.1a | 0.8±0.1 | 1.8±0.2a | 1.3±0.1a | 1.4±0.2a | 1.3±0.2a | 1.5±0.1a | 1.2±0.1 | 1.2±0.1a | 1.1±0.1 | 1.1±0.1 | 1.1±0.0 | 1.1±0.1 | 1.1±0.1 |
| V | 0.9±0.1 | 0.2±0.1a | 0.7±0.1a | 1.6±0.1a | 1.2±0.1a | 1.2±0.1a | 1.3±0.1a | 1.6±0.1a | 1.5±0.1a | 1.2±0.1a | 1.2±0.1a | 1.0±0.2 | 1.0±0.1 | 1.2±0.1 | 1.2±0.1 |
| M | 1.0±0.1 | 0.4±0.0a | 0.7±0.1 | 1.5±0.1a | 1.4±0.1a | 1.3±0.1a | 1.3±0.1a | 1.5±0.1a | 1.6±0.1a | 1.2±0.1a | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 | 1.0±0.1 | 1.0±0.2 |
S, saline+occlusion; V, vehicle+occlusion; M, melatonin+occlusion. Data are mean±s.e.m.
Compared with baseline. O2ct, arterial blood oxygen content; Hct, hematocrit
P<0.05 compared with S.
compared with V, by Tukey test.
Recovery
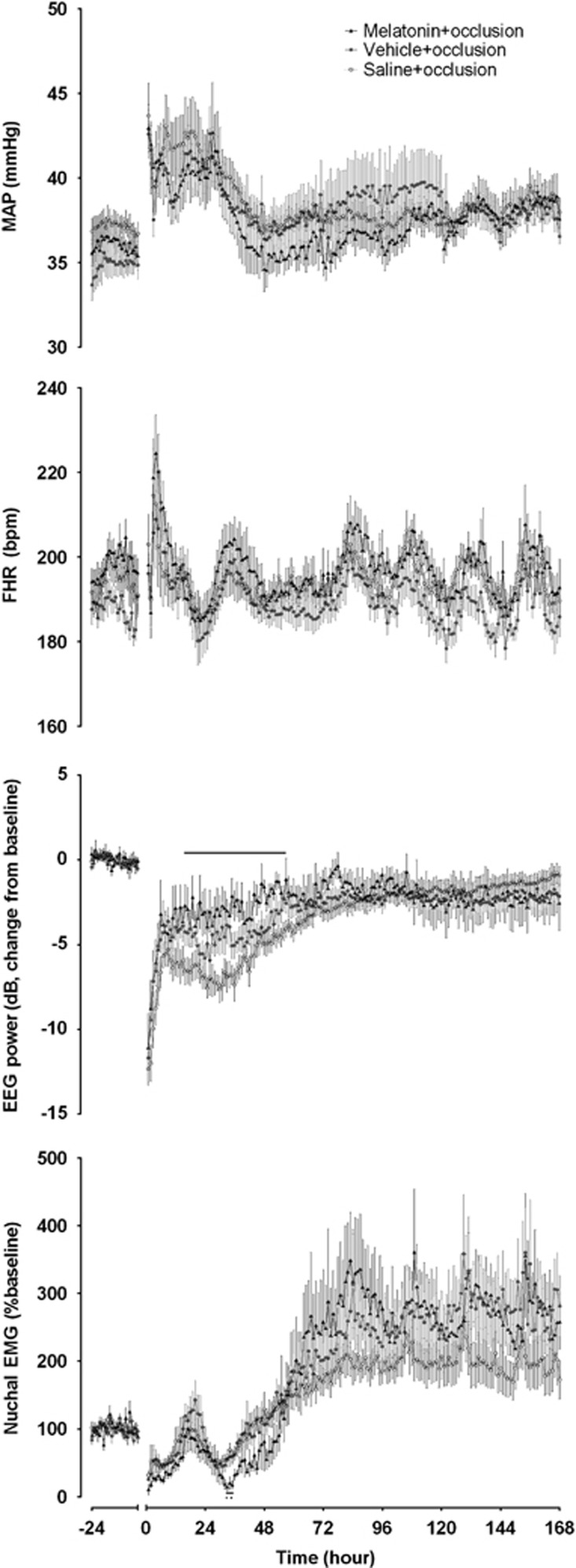
There was a significant effect of time (P<0.05), and group and time (P<0.05), but not group for mean arterial blood pressure, FHR, extradural temperature, and nuchal EMG (Figure 1). There were no significant differences on post hoc tests except briefly at 33 to 35 hours for nuchal EMG where melatonin+occlusion was significantly lower than the other two groups (P<0.05). Extradural temperature in the first 6 hours of recovery was not different between the saline+occlusion, vehicle+occlusion, and melatonin+occlusion groups; 39.3±0.1, 39.3±0.0, and 39.3±0.1°C, respectively.
Figure 1.
Mean arterial blood pressure (MAP), fetal heart rate (FHR), EEG power, and nuchal electromyography (nuchal EMG) from 24 hour baseline before umbilical cord occlusion through to +168 hours after occlusion. Intra-occlusion data omitted. Open circles: saline+occlusion; closed circles: vehicle+occlusion; closed triangles: melatonin+occlusion. Data are hourly mean±s.e.m. Solid significance bar denotes P<0.05 for saline+occlusion versus melatonin+occlusion.
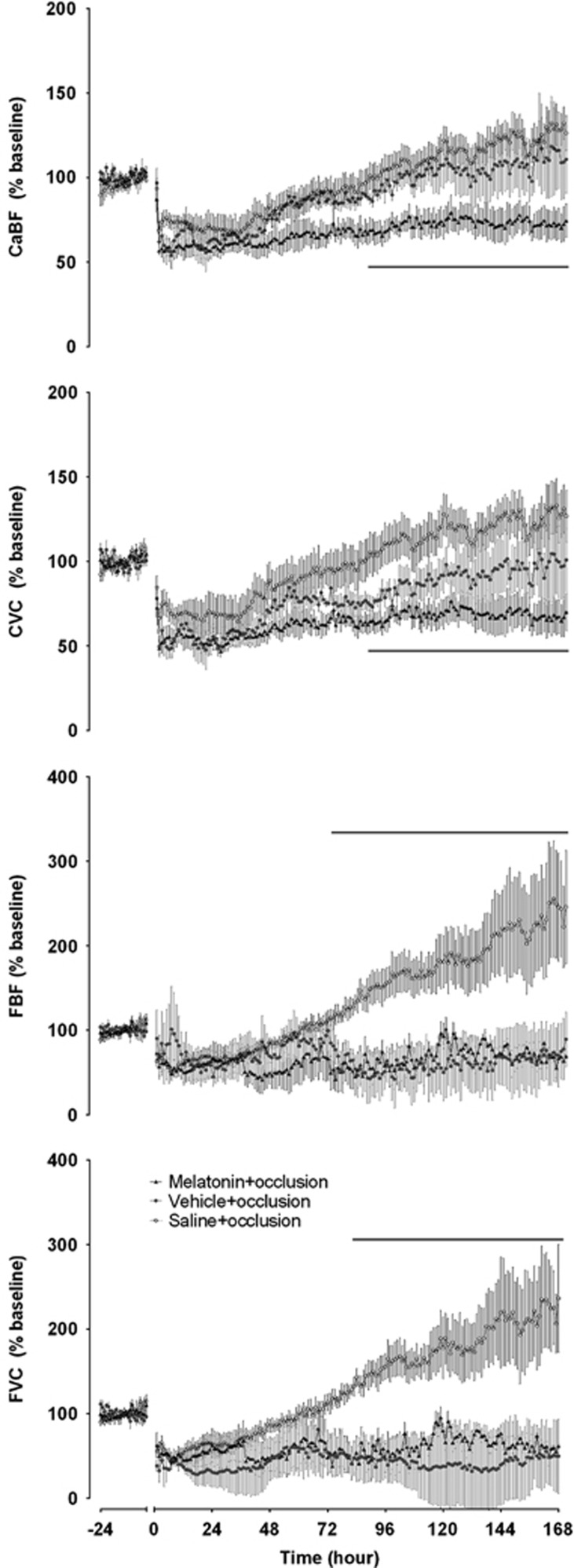
There were significant effects of time and group (P<0.05) for CaBF, carotid vascular conductance, FBF, and femoral vascular conductance (Figure 2). Post hoc contrast tests showed that the melatonin+occlusion group had a significantly lower CaBF, carotid vascular conductance, and femoral vascular conductance over the recovery period compared with the saline+occlusion group. Additionally, there was a statistically borderline reduction in CaBF compared with vehicle+occlusion (P=0.05). Post hoc, the melatonin+occlusion group had significantly lower CaBF and carotid vascular conductance compared with the saline+occlusion group from 87 to 168 hours. There were no individual differences between the vehicle+occlusion group and the other groups. Peripheral blood flow and femoral vascular conductance were significantly lower in the melatonin+occlusion group compared with the saline+occlusion group from 80 to 168 hours.
Figure 2.
Carotid artery blood flow (CaBF), carotid artery vascular conductance (CVC), femoral blood flow (FBF), and femoral vascular conductance (FVC) from 24 hours baseline before umbilical cord occlusion through to +168 hours after occlusion. Intra-occlusion data omitted. Open circles: saline+occlusion; closed circles: vehicle+occlusion; closed triangles: melatonin+occlusion. Data are hourly mean±s.e.m. Solid significance bar denotes P<0.05 for saline+occlusion versus melatonin+occlusion.
There was also a significant effect of time and group for EEG power and spectral edge. Electroencephalogram power in the melatonin+occlusion group recovered significantly faster than the saline+occlusion group, from 16 to 57 hours (Figure 1). There was no significant difference between the vehicle+occlusion group and the other two groups. There was no significant difference between the final EEG recoveries between groups. There were no significant post hoc differences for the spectral edge between groups. Similarly, there was significant effect of time and group for delta, theta, alpha, and beta power (Supplementary Figure 1). Post hoc, delta power was significantly higher in the melatonin+occlusion at 23 hours compared with both the vehicle+occlusion and saline+occlusion groups, and at 28 hours compared with the saline+occlusion group only. Alpha power was significantly greater at 2 hours in the melatonin+occlusion compared with the saline+occlusion group only. There were no post hoc differences for theta or beta power.
No seizures were observed in the sham occlusion group. There were no significant differences in the seizure period, median duration, or median power between occlusion groups (Supplementary Table 1). Seizures began significantly later in the melatonin+occlusion group than the saline+occlusion group, but not the vehicle+occlusion group. The total number and total duration were significantly lower in the vehicle+occlusion group compared with saline+occlusion, but not compared with melatonin+occlusion.
Biochemistry
Blood gas data showed significant differences between groups, with a lower PCO2 and lactate, and higher PO2 in the vehicle+occlusion group than the saline+occlusion group (Table 1), and a lower Hb and Hct, and higher HCO3− in the melatonin+occlusion and vehicle+occlusion groups than the saline+occlusion group during occlusion. pH was lower in the melatonin+occlusion group than saline+occlusion from 3 to 7 days. Serum electrolytes, liver function tests, thyroid function tests, and full blood counts are shown in Supplementary Table 2. Key differences were lower sodium during the recovery phase in the melatonin+occlusion and vehicle+occlusion groups than the saline+occlusion group. Transaminases were not elevated. T4 levels progressively increased in the recovery phase in all groups; the increase was earlier and greater in the melatonin+occlusion and vehicle+occlusion groups than the saline+occlusion group. There were no differences between groups for full blood counts. There was a significant leukocytosis in the saline+occlusion group at 6 hours, and in all groups at 24 hours compared with baseline (Supplementary Table 3). There was a combined lymphocytosis and neutrophilia in the saline+occlusion group, but only neutrophilia in the vehicle+occlusion and melatonin+occlusion groups.
Plasma cortisol, ACTH, and melatonin were similar between groups at baseline (Supplementary Figure 2). The post occlusion rise in cortisol and ACTH was significantly greater in the melatonin+occlusion and vehicle+occlusion groups than the saline+occlusion group. Plasma melatonin was significantly higher in the melatonin+occlusion group at 24 hours compared with baseline (0.04±0.00 versus 0.02±0.00 ng/mL, respectively) and compared with the vehicle+occlusion group (0.02±0.00 ng/mL, Supplementary Figure 2).
Post-Mortem Data and Histopathology
Lung and brain weight were significantly lower in the saline+occlusion and vehicle+occlusion groups but not in the melatonin+occlusion group compared with sham occlusion (Supplementary Table 4). Relative brain weight was significantly lower in the saline+occlusion group compared with the sham occlusion group. There were no significant differences in other organ weights or body weight between groups.
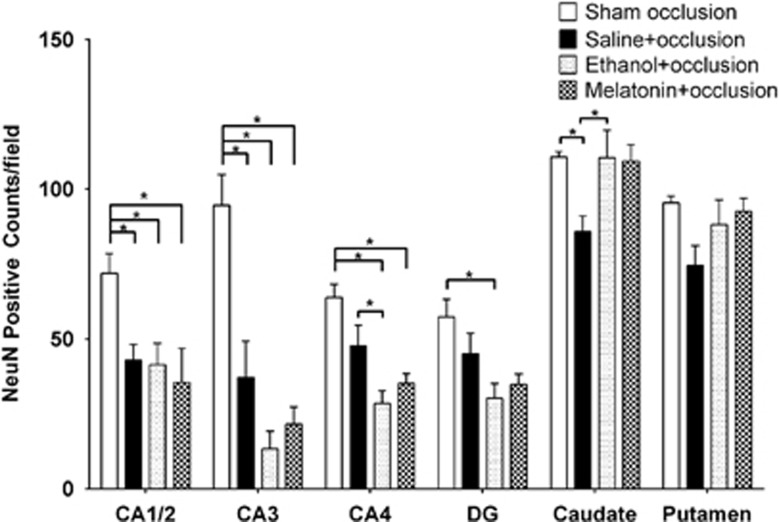
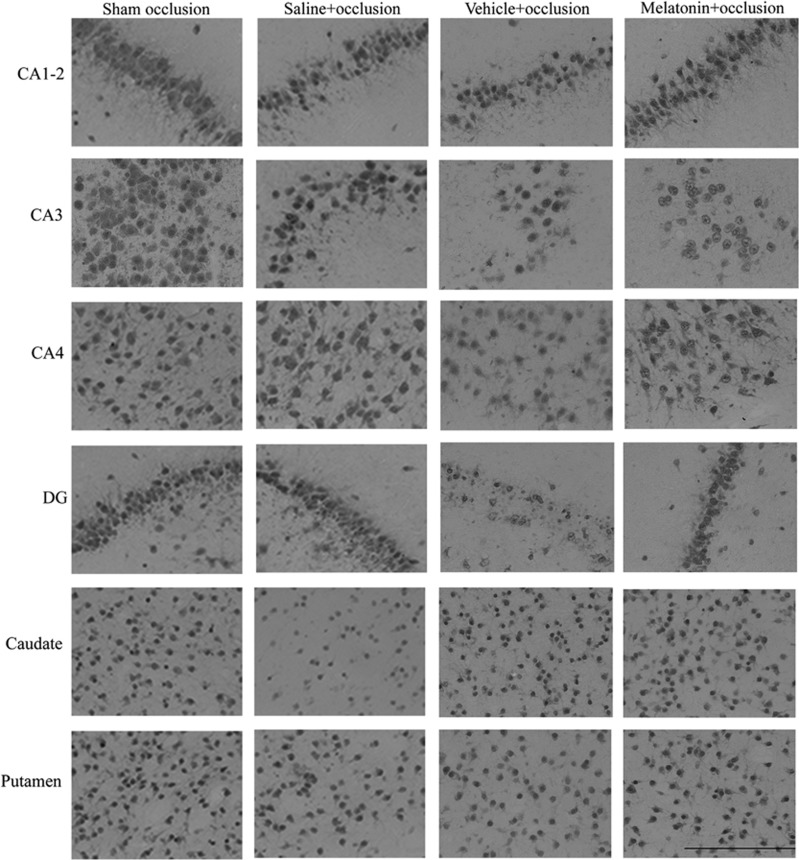
The number of NeuN-positive cells in the CA1-2 and CA3 region of the hippocampus was significantly lower in the saline+occlusion, vehicle+occlusion, and melatonin+occlusion groups compared with sham controls (Figures 3 and 4). There were no significant differences between occlusion groups in CA1-2. There were significantly fewer NeuN-positive cells in the CA4 region in the vehicle+occlusion and melatonin+occlusion groups compared with sham occlusion and in the vehicle+occlusion group compared with the saline+occlusion group. There were significantly fewer cells in the dentate gyrus in the vehicle+occlusion group compared with sham occlusion. There were significantly fewer cells in the caudate nucleus in the saline+occlusion group compared with the sham occlusion, vehicle+occlusion, and melatonin+occlusion groups. There were no significant differences in the putamen.
Figure 3.
Anti-neuronal nuclei monoclonal antibody (NeuN) cell counts in cornu ammonis 1–2 (CA1-2), CA3, CA4, dentate gyrus (DG), caudate nucleus, and putamen. Data are mean±s.e.m. Significance bars where P<0.05 after analysis of variance and post hoc Tukey's tests when overall significance was found. *P<0.05.
Figure 4.
Photomicrograph plate showing representative anti-neuronal nuclei monoclonal antibody (NeuN) staining imaged at × 40 magnification. Scale bar is 200 μm.
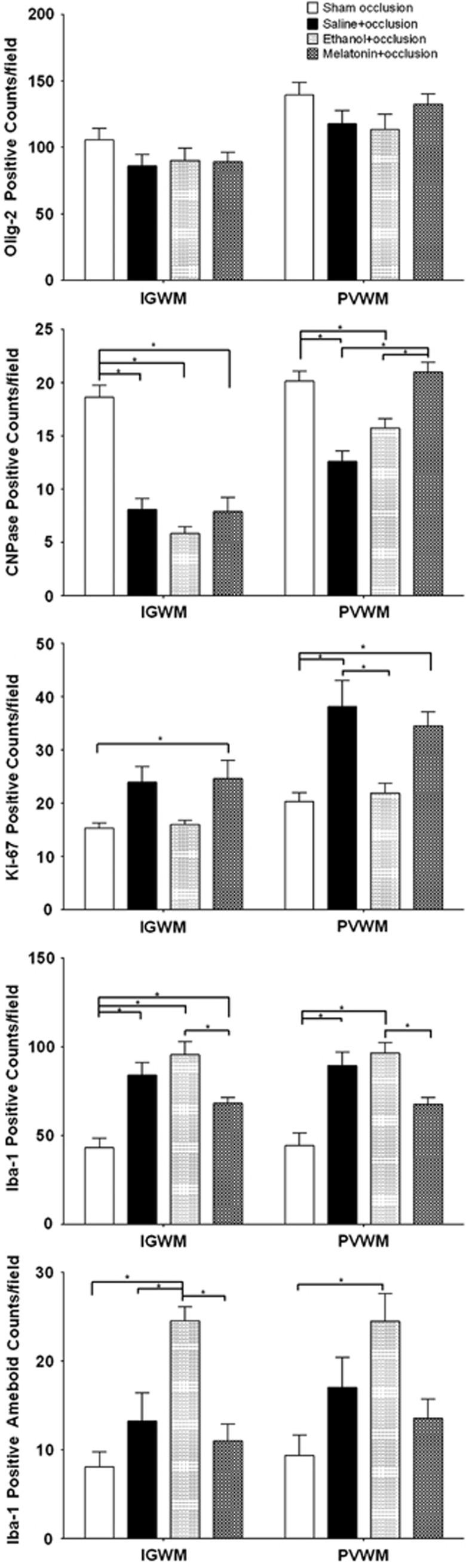
There were no significant differences in the number of Olig-2-positive cells between groups (Figures 5 and 6). There was a substantial reduction in numbers of immunopositive CNPase cells in the IGWM in all three occlusion groups compared with sham occlusion. In the PVWM, there were significantly fewer CNPase-positive cells in the saline+occlusion and vehicle+occlusion groups compared with the sham occlusion and melatonin+occlusion groups. There were significantly more Ki-67-positive staining cells in the melatonin+occlusion and saline+occlusion groups compared with the sham occlusion and vehicle+occlusion groups (Figures 5 and 6). Ki-67 immunostaining substantially co-localized with both Olig-2 and the oligodendrocyte progenitor cell marker, PDGFR-α (Supplementary Figures 3 and 4).
Figure 5.
Olig-2, CNPase, Ki-67, and Iba-1 cell counts in intragyral white matter (IGWM) and periventricular white matter (PVWM). Data are mean±s.e.m. Significance bars when P<0.05 after analysis of variance and post hoc Tukey's tests when overall significance was found. *P<0.05.
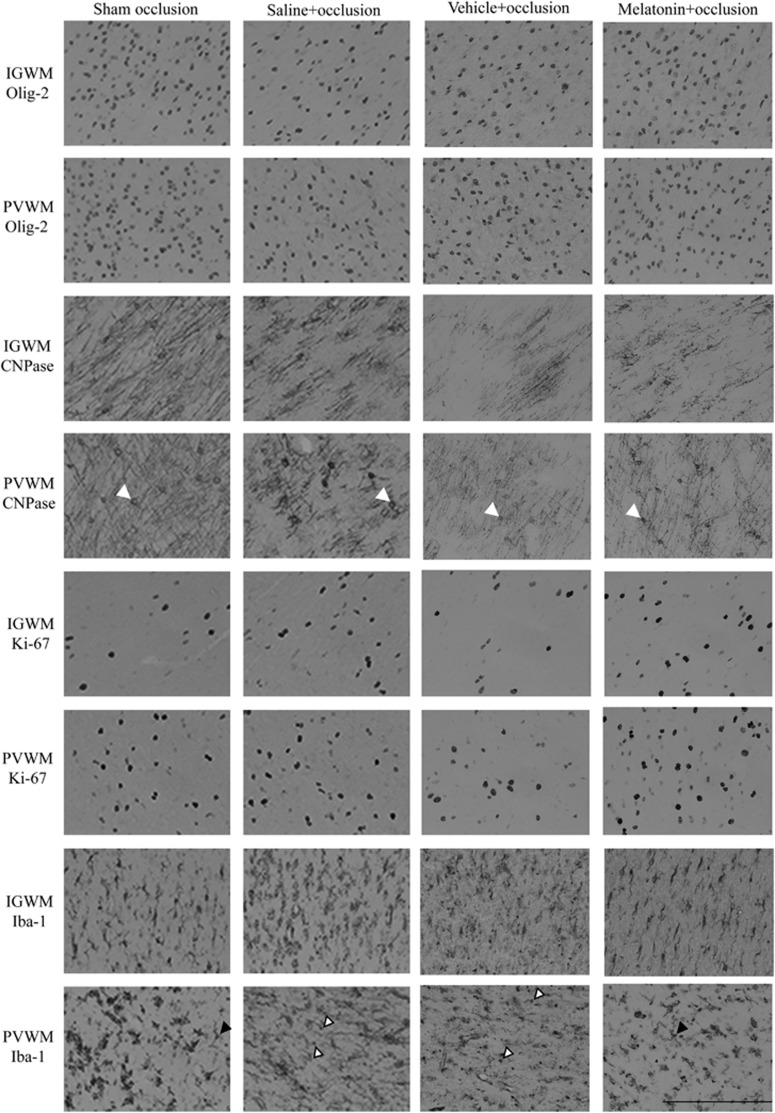
Figure 6.
Photomicrographs showing representative Olig-2, CNPase, Ki-67, and Iba-1 staining imaged at × 40 magnification. White arrows show examples of CNPase-positive cells. Black arrows show examples of resting microglia. White arrows with black outline show examples of ameboid microglia. Scale bar is 200 μm.
The total number of Iba-1-positive cells was significantly greater in all occlusion groups than sham occlusion (Figures 5 and 6). There were significantly fewer cells per field in the melatonin+occlusion group compared with the vehicle+occlusion group. There were significantly more Iba-1-positive cells with ameboid morphology in the vehicle+occlusion group compared with sham occlusion and melatonin+occlusion, and a borderline trend compared with saline+occlusion.
Discussion
The present study demonstrates that prophylactic maternal low-dose melatonin treatment was associated with multifaceted improvement after profound asphyxia in preterm fetal sheep, including faster fetal EEG recovery, delayed onset of seizures, improved survival of mature oligodendrocytes, increased proliferation, and reduced microglial activation in the periventricular white matter. Fetal melatonin levels were still elevated 24 hours after occlusion, consistent with its excellent transplacental passage.4 However, although maternal melatonin was associated with apparent improvement in neuronal survival in the striatum, but not the hippocampus, the vehicle, 2% ethanol was associated with a similar improvement in striatal neuronal survival, and with reduced duration of fetal seizures compared with maternal saline treatment. Ethanol vehicle was also associated with greater neuronal loss in the CA3 and CA4 regions of the hippocampus and reduced white matter proliferation, with greater induction of ameboid microglia. These findings strongly suggest potential for partial confounding of the effects of melatonin with those of ethanol. These differences were not associated with any differences in the fetal brain temperature between groups.24
Prolonged umbilical cord occlusion was associated with substantial fetal subcortical injury to white and gray matter. Intriguingly, there was no significant net loss of Olig-2-positive oligodendrocytes in the intragyral and periventricular white matter after 7 days recovery. In contrast, there was a marked loss of mature, CNPase-positive, oligodendrocytes, suggesting a shift to more immature forms of oligodendroglia. We have previously shown that after 3 days recovery at this gestation, occlusion was associated with loss of O4 immunostaining, a marker of immature oligodendrocytes but sparing of CNPase-positive cells.24, 25 Further, in the present study, we found a substantial increase in proliferation, with co-localization of Ki-67 with Olig-2-positive cells, and critically, with PDGFR-α positive oligodendrocyte progenitors. This combination of findings suggests that rather than a primary loss of mature oligodendrocytes, asphyxia was associated with initial loss of immature oligodendroglia, followed by intense restorative proliferation of oligodendrocyte progenitors that restored total number of cells, but with impaired lineage maturation. This pattern is consistent with the seminal findings of Back and colleagues26 of maturational arrest of oligodendrocytes in the neonatal rat after hypoxia–ischemia, and in premature infants with diffuse white matter injury.27
Ethanol is widely used as a vehicle for numerous studies, such as those using melatonin as a putative neuroprotective agent.11 It is an allosteric modulator of the inhibitory GABA receptor, increasing opening frequency and chloride flux,28 and increases GABAergic neurotransmission both indirectly and through GABA release from presynaptic terminals in many brain regions.29 Further, it can suppress activation of innate immunity through the toll-like receptor 4.30 These neuroinhibitory and anti-inflammatory effects may have contributed to improved neuronal survival in the striatum in the current study. In contrast, maternal ethanol was associated with reduced neuronal survival in the CA3 and 4 regions of the fetal hippocampus. The reason for these markedly different effects are unknown; however, it is striking that these changes were associated with such a small exposure to ethanol (a total of 0.66±0.03 g in the vehicle+occlusion group and 0.71±0.02 g in the melatonin+occlusion group), corresponding with approximately 7% of a standard 10 g drink over 6 hours. This is far less than the amount previously shown to cause fetal brain injury in isolation; for example, Daltiz et al14 reported white matter injury in late gestation sheep exposed to 1 g of ethanol per kg maternal weight for 3 consecutive days (corresponding with a total of ∼70 g per day).
Ethanol promotes GABA release in the hippocampus but not thalamus.29 There is some evidence that the GABA receptor is excitatory in the developing brain,31 and thus, speculatively, regional differences in inhibitory versus excitatory function could contribute to differential effects.32 Further, in the present study, ethanol was associated with an exaggerated increase in fetal plasma cortisol by 24 hours, which may have adverse effects on the brain. For example, in guinea pigs ethanol increases glucocorticoid concentration-induced glutamate release from the fetal hippocampus,33 and so may have worsened excitotoxicity.
Maternal ethanol exposure had no net effect on total oligodendrocytes or net loss of mature (CNPase positive) oligodendrocytes or induction of microglia in the fetal white matter tracts compared with saline. However, it was associated with marked suppression of proliferation at day 7 after occlusion. Consistent with the reduced proliferation, the increase in circulating neutrophils, lymphocytes, and monocytes was delayed after ethanol treatment. Invading monocytes are also stained with Iba-1, which may contribute to the increase in Iba-1-positive cells after occlusion. Despite this delay, ethanol was associated with greater induction of ameboid (activated) microglia. The role of microglia in recovery from injury is complex, with increasing evidence for cytotoxic responses in the early stages of injury, mediated by release of pro-inflammatory cytokines, followed by a phenotypic shift toward immune regulation, and injury resolution.34 The present findings would be consistent with initial suppression of inflammation but impairment of the shift towards protective phenotypes, and continuing impairment of white matter maturation.
Melatonin is well established to have potent antioxidant properties as shown, for example, by neural lipid peroxidation and hydroxyl radical production in fetal sheep.11, 12 Further, there is evidence that at least part of its direct neuroprotective, anti-apoptotic effects were mediated by binding to the MT1/MT2 membrane receptors after excitotoxic injury in neonatal mice.35 The apparent effects of maternal melatonin were not different from vehicle in gray matter in the present study. In the white matter, however, melatonin was associated with restoration of mature oligodendrocytes consistent with normal maturation, restoration of proliferation compared with vehicle, reduced total number of microglia compared with saline or ethanol, and alleviation of the exaggerated rise in numbers of ameboid forms associated with ethanol. Further studies would be valuable to assess whether these morphologic changes were associated with altered microglial toxicity; however, this outcome is highly consistent with previous reports of reduced microglial activation by melatonin in rodents and fetal sheep after hypoxia–ischemia.5, 11, 12 It is likely that these effects were related to suppression of the neural microglia. Maternal melatonin was associated with a reduced peripheral white cell response but its effects were not significantly different from ethanol.
Similar to previous studies, umbilical cord occlusion was associated with prolonged reduction in CaBF and suppression of EEG activity, which resolved after ∼48 to 72 hours.18 The early stages of this pattern primarily reflect active suppression of cerebral metabolism and activity.36 In the present study, maternal melatonin was associated with sustained reduction in carotid vascular conductance and blood flow that had not resolved after 7 days recovery. Melatonin has been associated with constriction of cerebral arteries in rodents,37 and can inhibit inducible nitric oxide synthase- and mitochondrial nitric oxide synthase-mediated injury in septic rodents.38 Whether this is also associated with reduced NO-mediated vasodilation is unknown. Carotid blood flow remained suppressed for most of the recovery period while EEG power, including high frequency activity, had returned to normal; the impact of this is unclear given the modest neuronal and oligodendrocyte protection, and no significant change in brain weight compared with sham occluded fetuses. We have previously shown that this paradigm is associated with progressive development of relative luxury perfusion after approximately 24 hours as measured by near-infrared spectroscopy.39 Thus, it is possible that ongoing suppression of CaBF may reflect a beneficial reduction in luxury perfusion.
Recovery of fetal EEG power was significantly faster after maternal melatonin than saline, with intermediate recovery after maternal ethanol infusion. Both clinically,40 and in fetal sheep,18 more rapid EEG recovery is associated with better outcomes. Thus, it is highly likely that this electrophysiological improvement reflects the combination of reduced white matter and striatal injury in the melatonin group, and in the striatum in the ethanol group. Further, in the present study, the onset of postasphyxial seizures was delayed after melatonin, and there were significantly fewer seizures in the ethanol group. It is unlikely that this is a direct inhibitory effect of ethanol per se as it would have been cleared by the ewe by this time, and may therefore indicate an effect of treatment.
In conclusion, prophylactic low-dose maternal melatonin treatment was associated with protection of fetal mature oligodendrocytes, increased proliferation and reduced microglial activation in white matter tracts, and delayed seizure onset. However, striatal neuroprotection appeared to be mediated by the ethanol vehicle. Given that ethanol had adverse effects on the hippocampus and on microglial activation, it is essential to develop an alternate, safe, vehicle for melatonin before clinical evaluation can be considered.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH grant R21 NS063141-01A1 (ST, AJG, LB), the Health Research Council of New Zealand, the Auckland Medical Research Foundation and the Lottery Health Grants Board of New Zealand. PPD was supported by the New Zealand Neurological Foundation W&B Miller Doctoral Scholarship.
Supplementary Material
References
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant. J Pediatr. 2012;160:e544. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini W, Carloni S, Perrone S, Bertrando S, Tataranno ML, Negro S, et al. The use of melatonin in hypoxic-ischemic brain damage: an experimental study. J Matern Fetal Neonatal Med. 2012;25:119–124. doi: 10.3109/14767058.2012.663232. [DOI] [PubMed] [Google Scholar]
- Villapol S, Fau S, Renolleau S, Biran V, Charriaut-Marlangue C, Baud O. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr Res. 2011;69:51–55. doi: 10.1203/PDR.0b013e3181fcb40b. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kuan YH, Chen HY, Chen TY, Chen ST, Huang CC, et al. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res. 2007;42:297–309. doi: 10.1111/j.1600-079X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, et al. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton LC, Abbass M, Dickinson H, Ireland Z, Walker DW. Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus) Dev Neurosci. 2009;31:437–451. doi: 10.1159/000232562. [DOI] [PubMed] [Google Scholar]
- Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yan EB, Castillo-Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27:200–210. doi: 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- Yawno T, Castillo-Melendez M, Jenkin G, Wallace EM, Walker DW, Miller SL. Mechanisms of melatonin-induced protection in the brain of late gestation fetal sheep in response to hypoxia. Dev Neurosci. 2012;34:543–551. doi: 10.1159/000346323. [DOI] [PubMed] [Google Scholar]
- Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, et al. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153–158. doi: 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- Dalitz P, Cock M, Harding R, Rees S. Injurious effects of acute ethanol exposure during late gestation on developing white matter in fetal sheep. Int J Dev Neurosci. 2008;26:391–399. doi: 10.1016/j.ijdevneu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang Y, Geng X, Asmaro K, Peng C, Sullivan JM, et al. Neuroprotective effect of acute ethanol administration in a rat with transient cerebral ischemia. Stroke. 2012;43:205–210. doi: 10.1161/STROKEAHA.111.629576. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. Foetal brain development in the sheep. Neuropathol Appl Neurobiol. 1979;5:103–114. doi: 10.1111/j.1365-2990.1979.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Hunter CJ, Bennet L, Power GG, Gunn AJ. Cerebral oxygenation during post-asphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab. 2005;25:911–918. doi: 10.1038/sj.jcbfm.9600087. [DOI] [PubMed] [Google Scholar]
- Keogh MJ, Drury PP, Bennet L, Davidson JO, Mathai S, Gunn ER, et al. Limited predictive value of early changes in EEG spectral power for neural injury after asphyxia in preterm fetal sheep. Pediatr Res. 2012;71:345–353. doi: 10.1038/pr.2011.80. [DOI] [PubMed] [Google Scholar]
- Keogh MJ, Bennet L, Drury PP, Booth LC, Mathai S, Naylor AS, et al. Subclinical exposure to low-dose endotoxin impairs EEG maturation in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2012;303:R270–R278. doi: 10.1152/ajpregu.00216.2012. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Faulkner S, Bainbridge A, Kato T, Chandrasekaran M, Kapetanakis AB, Hristova M, et al. Xenon augmented hypothermia reduces early lactate/N-acetylaspartate and cell death in perinatal asphyxia. Ann Neurol. 2011;70:133–150. doi: 10.1002/ana.22387. [DOI] [PubMed] [Google Scholar]
- Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia. 1993;34:284–288. doi: 10.1111/j.1528-1157.1993.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Barrett RD, Bennet L, Naylor A, George SA, Dean JM, Gunn AJ. Effect of cerebral hypothermia and asphyxia on the subventricular zone and white matter tracts in preterm fetal sheep. Brain Res. 2012;1469:35–42. doi: 10.1016/j.brainres.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Bennet L, Roelfsema V, George S, Dean JM, Emerald BS, Gunn AJ. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol. 2007;578:491–506. doi: 10.1113/jphysiol.2006.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Fernandez-Lizarbe S, Guerri C. Role of TLR4 in ethanol effects on innate and adaptive immune responses in peritoneal macrophages. Immunol Cell Biol. 2011;89:716–727. doi: 10.1038/icb.2010.163. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Crossley KJ, Walker DW, Beart PM, Hirst JJ. Characterisation of GABA(A) receptors in fetal, neonatal and adult ovine brain: region and age related changes and the effects of allopregnanolone. Neuropharmacology. 2000;39:1514–1522. doi: 10.1016/s0028-3908(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Brien JF, Kapoor A, Matthews SG, Reynolds JN. Chronic prenatal ethanol exposure increases glucocorticoid-induced glutamate release in the hippocampus of the near-term foetal guinea pig. J Neuroendocrinol. 2006;18:826–834. doi: 10.1111/j.1365-2826.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson I, Mesples B, Bac P, Vamecq J, Evrard P, Gressens P. Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann Neurol. 2002;51:82–92. doi: 10.1002/ana.10072. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Bennet L, Hunter CJ, Power GG, Gunn AJ. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol. 2006;572:131–139. doi: 10.1113/jphysiol.2005.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am J Physiol. 1997;273:H1530–H1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- Tapias V, Escames G, Lopez LC, Lopez A, Camacho E, Carrion MD, et al. Melatonin and its brain metabolite N(1)-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res. 2009;87:3002–3010. doi: 10.1002/jnr.22123. [DOI] [PubMed] [Google Scholar]
- Bennet L, Roelfsema V, Pathipati P, Quaedackers J, Gunn AJ. Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. J Physiol. 2006;572:141–154. doi: 10.1113/jphysiol.2006.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–e139. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.