Abstract
The coupling of cerebral blood flow (CBF) to neuronal activity is well preserved during evolution. Upon changes in the neuronal activity, an incompletely understood coupling mechanism regulates diameter changes of supplying blood vessels, which adjust CBF within seconds. The physiologic brain tissue oxygen content would sustain unimpeded brain function for only 1 second if continuous oxygen supply would suddenly stop. This suggests that the CBF response has evolved to balance oxygen supply and demand. Surprisingly, CBF increases surpass the accompanying increases of cerebral metabolic rate of oxygen (CMRO2). However, a disproportionate CBF increase may be required to increase the concentration gradient from capillary to tissue that drives oxygen delivery. However, the brain tissue oxygen content is not zero, and tissue pO2 decreases could serve to increase oxygen delivery without a CBF increase. Experimental evidence suggests that CMRO2 can increase with constant CBF within limits and decreases of baseline CBF were observed with constant CMRO2. This conflicting evidence may be viewed as an oxygen paradox of neurovascular coupling. As a possible solution for this paradox, we hypothesize that the CBF response has evolved to safeguard brain function in situations of moderate pathophysiological interference with oxygen supply.
Keywords: brain, CMRO2, functional hyperemia, neurovascular coupling, oxygen
A quick glance at basic numbers of oxygen and glucose delivery to the brain and cerebral energy metabolism suggests that the most delicate function of cerebral blood flow (CBF) is the delivery of sufficient amounts of oxygen to the tissue where the oxygen reserve is so low that ATP production declines almost immediately once blood flow ceases. Therefore, the intuitive explanation for the rapid and large CBF response to neuronal activation is the supply of the additional oxygen needed. Experimental observations of large CBF responses accompanying small increases of cerebral metabolic rate of oxygen (CMRO2), however, have cast doubt on this intuitive assumption. A number of alternative hypotheses may reconcile the seemingly conflicting findings: (1) A small increase of CMRO2 may only be possible with a large increase in CBF due to physical limitations of oxygen delivery. (2) The large CBF response may be necessary to ensure oxygen delivery to the areas most distant from blood supply. (3) The large CBF response may not be necessary in its full extent but may have evolved as a safety mechanism ensuring sufficient oxygen supply in situations where oxygen delivery to the brain is impaired. (4) The large CBF response may be necessary for reasons other than oxygen delivery.
In this opinion paper, we discuss studies on brain energy metabolism and neurovascular coupling. Based on the available evidence, we hypothesize that the surprisingly large CBF response to neuronal activation has evolved as a safety mechanism for oxygen delivery. To support this hypothesis, we first review basic facts on oxygen and glucose delivery to the brain, which strongly suggest that the CBF response primarily serves oxygen delivery. We then take a look on the discrepancy between CBF and CMRO2 responses to neuronal activation and models of oxygen delivery, which may explain this discrepancy. Subsequently, we discuss pathophysiological limits of oxygen delivery and studies that observed an uncoupling of CBF and CMRO2, both suggesting in contradiction to some models of oxygen supply that CBF operates with a considerable safety factor in many conditions, likely including the physiologic CBF baseline and physiologic CBF increases during neuronal activation. As this ‘safety factor hypothesis' is not compatible with regulation of CMRO2 by oxygen availability as has been suggested by previous research, we then review studies on oxidative phosphorylation to find out whether regulation by other mechanisms is plausible. Finally, we describe the significance of the brain tissue pO2 heterogeneity, determined in recent studies, in relation to our main hypothesis.
Most of the evidence discussed here was derived from experiments in cerebral cortex of mice, rats, or humans. Although other parts of the brain that have developed earlier in the evolutionary process also exhibit functional hyperemia, the structure of neuronal circuitry and details of neurovascular coupling mechanism differ considerably from the cortex. For some brain regions, only few studies have investigated blood flow and oxygen metabolism. A detailed discussion of differences between brain regions is beyond the scope of our article; therefore, we note that some of the principles discussed here may apply only to cerebral cortex but not to other brain regions.
Relevant basic numbers of oxygen and glucose delivery to the brain tissue
In this first section, we will discuss in detail the basic numbers and mechanisms of oxygen and glucose metabolism in the brain and delivery to the brain, as quantitative knowledge of solubilities, concentrations, metabolic rates, diffusion constants, diffusion distances, etc. form the basis for generating hypotheses on the function of neurovascular coupling. Our considerations will lead us to the main conclusion that the brain's supply with oxygen is much more delicate than its supply with glucose pointing to the fact that the rapid adjustment of CBF has evolved to adjust oxygen rather than glucose delivery.
Brain activity, especially synaptic transmission, is highly energy demanding and the brain generates the majority of its ATP via oxidative metabolism of glucose.1, 2 The oxygen–glucose index of the brain is close to six in the resting state,3, 4 thus the brain consumes, on average, six molecules of oxygen per molecule glucose. The high-energy demand generates the need for a large amount of oxygen delivered via the blood stream. As the oxygen solubility in water is very low, a transport molecule, hemoglobin, increases the oxygen content of arterial blood from 150 nmoL/mL dissolved in plasma to around 9,000 nmoL/mL at physiologic arterial oxygen saturation. Even with almost half of the blood volume devoted to oxygen transporting red blood cells (10% of the cardiac workload at rest required to pump oxygen transport molecules through the brain), the number of oxygen molecules in the arterial blood exceeds the number of glucose molecules by only a factor of 1.5 (physiologic glucose concentration around 6,000 nmoL/mL). As the brain extracts six molecules of oxygen per molecule glucose the proportion of oxygen extracted from the blood during capillary passage through the brain tissue (oxygen extraction fraction) largely exceeds the proportion of glucose extracted. Under physiologic conditions, oxygen extraction fraction is around 30% to 50%5, 6 whereas glucose extraction fraction is only around 10%.6
Oxygen Diffusion to the Brain Tissue
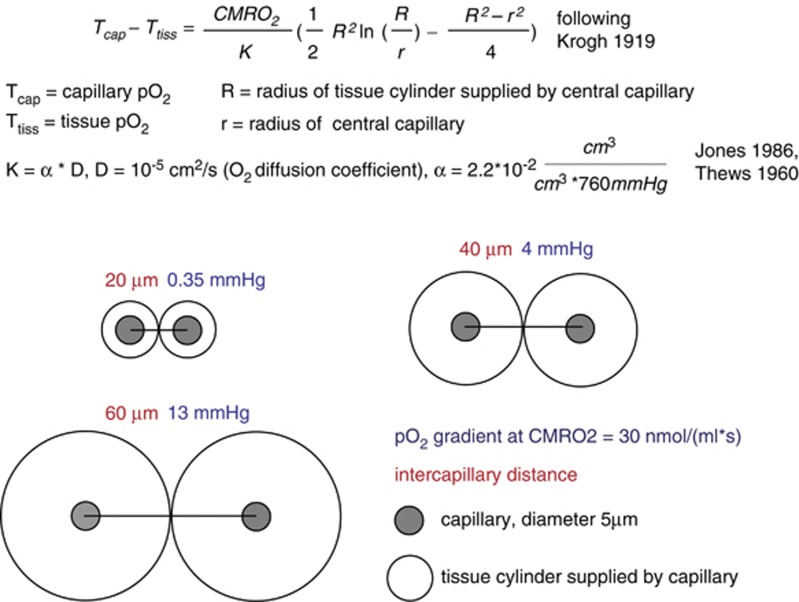
Oxygen is transported from microvessels to the brain tissue by diffusion. The speed of this transport is determined by the oxygen conductivity of the brain tissue, the specific value of which is difficult to interpret intuitively. A simplified description of the diffusion process is provided by the Krogh–Erlang equation.7 The Krogh–Erlang equation relates vascular geometry (radius of a central capillary and radius of the tissue cylinder supplied by this capillary), oxygen diffusion properties of the tissue, and oxygen metabolism to the oxygen gradient from capillary to tissue. Applied to the brain, it enables a simplified calculation of the oxygen gradients necessary to support a given CMRO2 (Figure 1). Krogh developed the model for tissue such as muscle, where the capillaries are organized as parallel tubes. In the brain, capillaries have a more disorderly orientation and the longitudinal pO2 gradient varies among capillaries due in part to capillary transit time heterogeneity (CTTH).8 Hence, the Krogh model does not adequately describe oxygen delivery to the brain tissue.9 The model assumes no diffusion of oxygen in and out of the tissue slice for which the equation describes oxygen supply and no diffusion across cylinder borders from adjacent cylinders within the slice. This likely occurs to a relevant degree in the brain tissue. However, no diffusion of oxygen out of slices with lowest oxygen occurs and sections through the brain suggest that intercapillary distances are not entirely random. Therefore, while underestimating the gradients present in high pO2 microregions with significant O2 flux out of the slice, the model still gives valuable rough information on the lower limit of oxygen gradients from capillary to tissue necessary to support a given CMRO2.
Figure 1.
Calculation of pO2 gradients according to the Krogh model. Under the simplifying assumption of the Krogh–Erlang equation,7 the pO2 gradients from the capillary surface to the border of the tissue cylinder are calculated, which would accompany a tissue oxygen consumption of 30 nmoL/(mL/second) at different intercapillary distances. Note that these gradients scale linearly with CMRO2. The cylindrical shape does not allow for perfect tissue coverage; therefore, the pO2 gradients into the ‘lethal corners' of the tissue will be a little larger. Diffusion of oxygen in and out of the tissue slice or across the border of the cylinder is not taken into account.
The average capillary oxygen tension in the brain is around 40 to 50 mm Hg.10 The average brain tissue pO2 is around 20 to 30 mm Hg11, 12, 13, 14, 15 with little differences between species.16 Tissue pO2 exhibits significant microregional variability, with considerably lower values in regions distant from microvessels with high pO2.12, 15 The extent of variability of tissue pO2 depends on the geometry of the vascular network, the density of capillaries, the metabolic rate of oxygen, and the capillary oxygen content. In the olfactory bulb, for example, where capillary density is high, intercapillary differences and ‘Krogh cylinders' are small. Parpaleix et al17 have found no major pO2 differences within tissue and between tissue and inter-erythrocyte spaces in nearby capillaries. Figure 1 illustrates the oxygen gradients required for a physiologic CMRO2 of 30 nmoL/(mL/second), following the simplifying assumption of the Krogh–Erlang equation. We have applied the model to enable a more intuitive interpretation of the oxygen conductivity of the brain tissue. The calculations indicate that very small oxygen gradients may suffice if intercapillary distances are short, as observed in the olfactory bulb.17 Considerably larger intercapillary distances bring about a significant heterogeneity of tissue pO2. Capillary density measurements suggest an average intercapillary distance of around 50 to 60 μm in the cerebral cortex15, 18 (with intercapillary distances not so random as may be suggested by the seemingly chaotic three-dimensional topology at first glance). Thus, the average capillary Krogh cylinder may have a radius of around 25 to 30 μm, which would require a pO2 gradient of around 10 to 15 mm Hg in low pO2 regions to support the CMRO2.7, 19, 20 This gradient scales linearly with CMRO2, hence a rather small reduction of tissue pO2 of 1 to 1.5 mm Hg may enable an increase of CMRO2 by 10% when capillary pO2 stays constant. Assuming that oxidative metabolism becomes limited by oxygen availability at a mitochondrial pO2 of a few mm Hg, a capillary pO2 (at the venous end of the capillary) of around 15 to 20 mm Hg would be the critical limit for maintaining CMRO2, if no relevant changes in vascular geometry occur. This theoretical limit fits well with observations on blood oxygenation limits discussed below.
Intracellular Oxygen Gradients, Critical Mitochondrial pO2
Tissue pO2 measurements are usually carried out with oxygen electrodes and sample average pO2 extracellularly within a considerably large sample volume. Thus, the question arises, whether there are significant oxygen gradients within the extracellular space and from there to the mitochondria, where oxygen is ultimately consumed. Mathematical modeling suggests that intracellular oxygen gradients depend on a number of factors such as the extent of mitochondrial clustering within cells but are probably small in the range of at most a few mm Hg.21 Because of the lower fraction of free water within cells as compared with extracellular space, higher viscosity within the cells and diffusional barriers imposed by proteins, diffusion coefficients for small molecules such as oxygen are considerably smaller intracellularly21 than extracellularly. In cardiomyocytes, an intracellular oxygen gradient of only 2 mm Hg has been determined over a wide range of extracellular pO2.22 Vanderkooi et al23 found no significant oxygen gradient at the mitochondrial surface. Experiments comparing oxygen metabolism in suspensions of isolated mitochondria versus cells suggest an oxygen gradient of below 1 mm Hg from extracellular medium to mitochondria.24 There are also no relevant diffusion barriers for oxygen through the walls of microvessels.17 Collectively, these data argue against major diffusion barriers for oxygen along the way from the blood space to the mitochondria.
High-esolution in vivo images of nicotinamide adenine dinucleotide fluorescence in rat brain confirm heterogeneity of oxygen tension measured with extracellular oxygen probes12 for the cellular compartment.20 Patches of tissue distant from supplying arterial vessels with large increases in nicotinamide adenine dinucleotide fluorescence that appear during hypoxia have been described.20 As nicotinamide adenine dinucleotide fluorescence increases sharply below a pO2 of 5 to 10 mm Hg, the unmasking of well-delineated tissue patches during hypoxia indicates tissue pO2 above this limit even in distant tissue regions during normoxia. Assuming no relevant diffusion barriers for oxygen, these data indicate heterogeneity of oxygen tension not only for tissue but also for mitochondria within the brain. However, based on modeling of oxygen transport to the brain tissue and PET measurements of CBF and CMRO2, very low average mitochondrial pO2 around 5 mm Hg have been suggested.25, 26 A similar approach, however, has also yielded substantially higher values around 15 mm Hg.27 In vitro and in vivo experiments on the critical oxygen tension, at which oxidative metabolism becomes limited by oxygen availability, have generated conflicting results. In isolated mitochondria, very low P50 of oxidative metabolism have been determined suggesting a critical pO2 of below 1 mm Hg. However, substantially higher P50 have also been reported indicating the possibility that oxygen availability could limit oxidative metabolism for pO2 up to more than 5 mm Hg, potentially within the range of mitochondrial pO2 in the ‘lethal corners' of the brain tissue under normoxic conditions.28
In summary, the oxygen gradient from the erythrocyte within the brain capillary to the mitochondrion in a nearby neuron or astrocyte enables oxygen delivery by diffusion with no relevant diffusion barriers for oxygen. The gradient depends on metabolic rate of oxygen and capillary density, which varies considerably between tissues. In the cerebral cortex, the minimum gradient required for the physiologic CMRO2 may be around 10 to 20 mm Hg.
Differences of Oxygen and Glucose Supply
In contrast to the muscle or heart, there are no significant amounts of oxygen storage molecules in the brain. Neuroglobin, a protein with oxygen-binding capacity with low P50 has been found in concentrations of less than 1 nmoL/mL in the cortex.29 Significantly higher amounts (50 to 100 nmoL/mL), co-localized with mitochondria, have been detected in the retina,30 which may indicate a role in oxygen supply in the retina.
At the average brain tissue pO2 of around 25 mm Hg11, 13, 15 and with no significant amounts of oxygen-binding proteins, the oxygen content of the brain is very low at around 30 nmoL/mL (oxygen solubility in brain tissue derived from reference31and references therein31). In contrast, due to the high solubility in water, the glucose content of brain tissue under physiologic conditions is by far higher at around 1,000 to 4,000 nmoL/mL.32, 33, 34 The average CMRO2 is around 30 nmoL/(mL/s).4, 35, 36 Hence, the oxygen present in the brain tissue under physiologic conditions would sustain CMRO2 for only around 1 second, if supply from the blood stream would suddenly stop.37 In contrast, the average brain metabolic rate of glucose (CMRGlu) is around one sixth of the CMRO2, 4 to 7 nmoL/mL per second.4, 36 Physiologic baseline CMRGlu could thus be sustained for several minutes, if the glucose supply would suddenly stop (but oxygen supply continue).37
The physiologic blood flow response to increases in neuronal activity is very fast, initiated by an incompletely understood coupling mechanism involving several molecules, neurons, interneurons,38 astrocytes,39 endothelial cells, and possibly pericytes40, 41, 42 (the ‘neurovascular unit'), which signal changes in brain activity to nearby blood vessels in a feed-forward manner.43, 44, 45, 46 Cerebral blood flow increases within seconds after the onset of activation and a rapid decline follows the end of the stimulus. The CBF changes are paralleled by changes in oxygenation and blood volume.47, 48, 49, 50, 51, 52 The temporal dynamics of the CBF response thus suggest a close relation to oxygen rather than glucose availability.
Furthermore, the glucose extraction fraction of the brain is low, around 10%.6, 53 During neuronal activation as evoked by typical experimental paradigms, CMRGlu increases substantially, e.g. 30% to 50% in PET studies during visual stimulation.36, 54 With no concomitant CBF response, the extraction fraction would increase from 10% at baseline to 13% to 15% during stimulation and the average capillary glucose concentration would decrease by only 2% to 3%. When CBF increases match CMRGlu increases during neuronal activation, the extraction fraction of glucose and the average capillary glucose concentration remain constant. Because the transport of glucose depends on the concentration gradient between blood and tissue, even large increases in CBF do not relevantly change the delivery of glucose to the brain. Rather, in the physiologic CBF range with very low glucose extraction fraction, glucose delivery to the brain is largely determined by changes in the brain tissue glucose concentration and not by CBF changes. Based on a reversible Michaelis–Menten model of glucose transport, Choi et al34 have estimated that at physiologic blood glucose levels, the maximally possible CMRglu is around two-thirds above the actually present. This was experimentally supported by Cholet et al55 who found unchanged CMRGlu increases to whisker stimulation in rats, although CBF responses were blocked by 7-NI. Powers et al56 found a significant correlation of resting CBF with CMRO2, but no correlation of CBF with CMRGlu in healthy humans. Cerebral blood flow remains constant during moderate hypoglycemia.53
In contrast to the glucose concentration, CBF increases may relevantly increase the average capillary pO2 and thus oxygen availability for the brain, because the resting oxygen extraction fraction is around 30% to 50%. This fundamental difference for oxygen and glucose delivery is further enhanced by hemoglobin: A 30% oxygen extraction fraction corresponds to a 60% drop in the pO2 (from 100 mm Hg in arteries to around 40 mm Hg in veins, depending on the exact shape of the oxygen-binding curve). As discussed in more detail below, the situation may change during hypoxia, e.g. at high altitude: Within the steep part of the oxygen-binding curve and at high hematocrit, substantial amounts of oxygen may be delivered to the brain with only small accompanying changes of blood pO2 during the capillary passage.
Table 1 gives an overview of key numbers of oxygen and glucose delivery to the brain. We have converted some of the commonly used units (e.g. from mL/(100 g/minute) to nmoL/(mL/second)) to facilitate comparison of concentrations and metabolic rates. In a recent review article, Buxton37 put forward similar numbers on oxygen and glucose delivery and metabolism to conclude that the blood flow response serves the supply of the brain with oxygen and not with glucose. Waste products of brain energy metabolism, such as lactate,57, 58 CO2, or heat59, 60 have a role in neurovascular coupling, but experimental data61, 62 and calculations of the quantitative effects37 (as carried out for oxygen and glucose in detail above) argue against the assumption that the CBF response primarily serves to remove these waste products from brain tissue.
Table 1. Key numbers of oxygen and glucose delivery to the brain.
| Parameter | Value | Reference |
|---|---|---|
| Oxygen–glucose index (resting state) | 6 | 3, 4 |
| CMRO2 | 30 nmoL/(mL/second) | 4, 5, 35, 93, 135 |
| CMRGlu | 4–7 nmoL/(mL/second) | 36, 93 |
| CBF | 6–15 μL/(mLsecond) | 4, 5, 93, 135, 136 |
| Oxygen conc. of brain tissue | 30 nmoL/mL | tpO2=25 mm Hg and oxygen solubility31 |
| Glucose conc. of brain tissue | 1,000–4,000 nmoL/mL | 6, 32, 33, 34, 124 |
| Oxygen conc. of arterial blood | 9,000 nmoL/mL | |
| Glucose conc. of arterial blood | 5,000–6,000 nmoL/mL | 53 |
| Oxygen extraction fraction | 30%–55% | 3, 5, 6, 27, 75, 93 |
| Glucose extraction fraction | 8%–15% | 3, 6 |
| Average brain tissue pO2 | 25 mm Hg | 11, 12, 14, 15 High microregional variability |
| Average capillary pO2 | 45 mm Hg | 10 |
| Average mitochondrial pO2 (mm Hg) | 5–15(−25?) mm Hg | 27, 96 (Only small intracellular gradients?) |
| Coupling ratio (dCBF/dCMRO2) PET | 2–10 | 36, 66, 96, 97 (Few studies found const. CMRO2) |
| Coupling ratio MRI | 1.3–5 | 67, 68, 72, 135, 136 |
| ATP concentration of brain tissue | 1,000–3,000 nmoL/mL | 124, 137, 138 |
| Total ADP concentration of brain tissue | 300 nmoL/mL | 124, 138 |
| Free ADP concentration of brain tissue | 30–35 nmoL/mL | 121, 122, 123 |
| PCr concentration of brain tissue | 4,000–5,000 nmoL/mL | 123, 124, 138 |
| Diffusion constant for O2 in brain | 1–2 × 10−5 cm2/second | 21, 139 difference intra- versus extracellularly |
| Diffusion constant for ATP in brain | 10−6 cm2/second | 21 |
| Diffusion constant for PCr in muscle | 2.8 × 10−6 cm2/second | 134 |
| P50 of cytochrome oxidase for O2 | 0.4 nmoL/mL | 115, 140 Significant variability in studies |
| C50 of cytochrome oxidase for ADP | 56 nmoL/mL | 28 |
| Average intercapillary distance | 50–60 μm | 15, 18 |
| Oxygen gradient needed for CMRO2 according to Krogh model | 10–15 mm Hg | See Figure 1, intercapillary distance 60 μm, CMRO2 30 nmoL/(mL*s) |
CBF, cerebral blood flow; CMRGlu, brain metabolic rate of glucose; CMRO2, cerebral metabolic rate of oxygen; MRI, magnetic resonance imaging; PCr, phosphocreatine; PET, positron-emission tomography.
This table shows key numbers of brain energy metabolism. We have converted commonly used units to facilitate comparison of concentrations with metabolic rates.
Cerebral Blood Flow Regulation by Oxygen?
An intuitive consequence of the facts mentioned above is the hypothesis that a feedback mechanism exists, which senses the tissue pO2 and consequently transmits a signal to the vasculature such that CBF is adjusted to maintain a constant pO2. Evidence for such a mechanism has been found in brain slice experiments demonstrating a switch from vasoconstrictory to vasodilatory signaling of astrocytes when lowering oxygen concentration in the slices.63 However, in an environment of 100% oxygen at 3 to 4 atmospheres of pressure, when tissue pO2 is largely elevated and the brain oxygen supply provided entirely by oxygen dissolved in plasma, we found no change in CBF responses to neuronal activation,64 arguing against a role of an oxygen sensing mechanism for physiologic neurovascular coupling in vivo. Our data further show that the CBF response does not depend on deoxygenation of hemoglobin, which has been proposed as part of a universal mechanism for coupling of oxygen demand and supply.65
Cerebral blood flow and cerebral metabolic rate of oxygen responses to neuronal activation
As some of the findings discussed above were known at that time, the first concurrent CBF and CMRO2 measurements during neuronal activation in the mid 1980s came as a big surprise. Although CBF increased largely upon visual and somatosensory stimulation, a CMRO2 increase was barely detectable.36, 66 The ratio of relative CBF increases divided by relative CMRO2 increases has been termed coupling ratio.37 Later, magnetic resonance imaging (MRI) experiments indicated a lower coupling ratio of 2 to 467, 68, 69, 70 (as opposed to 6 to 10 as measured in the early PET experiments and other PET studies, that not even found a CMRO2 increase at all71). However, CMRO2 determination is challenging both with PET and MRI37 and some recent MRI studies also found comparably high coupling ratios with a refined MRI methodology,72 confirming the counterintuitive observation that CBF increases much more than CMRO2 during a wide variety of increases in neuronal activity. The discrepancy between large CBF and small CMRO2 responses leads to a hyperoxygenation of the activated brain area with a decrease in deoxyhemoglobin and provides the basis for blood–oxygen-level-dependent (BOLD) functional magnetic resonance imaging.73, 74
Models of oxygen delivery: oxygen diffusion limitation and cerebral blood flow responses
An explanation why large CBF responses could be necessary to support small increases in CMRO2 was provided by Buxton and Frank in 1997.75 The Buxton and Frank model assumed constant diffusivity of oxygen in the absence of capillary recruitment76 and complete metabolism of the oxygen entering brain tissue. The model predicted that CBF had to increase much more than CMRO2, because the CBF increases are accompanied by decreases in mean transit time and thus decreases of the oxygen extraction fraction. This elegantly resolved the apparent discrepancy between large CBF responses and small CMRO2 responses. Hyder et al77 later incorporated dynamic changes in oxygen diffusivity, thereby allowing for smaller changes of CBF supporting CMRO2 increases as compared with the Buxton and Frank model. A nonlinear relationship between changes in CBF and CMRO2 was predicted by a subsequent model that stresses low mitochondrial oxygen tension as the basis for a disproportionately large CBF response.78 Assuming mitochondrial oxygen tension close to zero (meaning so low that any further reduction would limit oxidative metabolism), the driving force for oxygen delivery, the gradient between the capillary and mitochondria, can only be increased by an increase in capillary oxygen tension, which in turn requires a decrease of the oxygen extraction fraction. Later, the observation of constant CMRO2 during pharmacological reductions of CBF led to a modification of the model by incorporating potential changes of cytochrome c oxidase affinity to oxygen during increases in neuronal activity.26 Valabrègue et al79 provided a model, which relaxes the assumption of very low mitochondrial pO2 and thus allows for independent variation of CBF and CMRO2 within limits.
Although these models provide important insight into the relationship between CBF and CMRO2, key components such as diffusivity of oxygen, exact mitochondrial oxygen tension, and the relationship of mitochondrial oxygen tension with CMRO2 are difficult to measure in vivo and may change dynamically during the changes in neuronal activity and blood flow. As these factors may have relevant influence on the predicted relationship between CBF and CMRO2, calculations derived from these models should be interpreted with care. The picture gets even more complicated when trying to account for the relevant heterogeneity of capillary blood flow, capillary oxygen tension, and tissue oxygen tension.12 Jespersen and Ostergaard8 have incorporated CTTH as an extension to the simplified Krogh cylinder model and show that changes in CTTH may have substantial influence on the relationship between CBF and CMRO2,, which is of relevance for the understanding of pathophysiological changes of these parameters.80 Specifically, their model predicts that CMRO2 may increase with constant CBF when CTTH decreases, with a CBF increase when CCTH remains constant or any favorable combination of changes in both. However, even a CBF increase may be accompanied by a decrease in oxygen delivery when concomitant CTTH increases extinguish the beneficial effects of blood flow.80, 81 It is therefore important to consider that average CBF measurements deliver incomplete information on tissue perfusion and deviations from the physiologic CTTH in diseases affecting small brain vessels become important when interpreting disease-associated changes in CBF and CMRO2.80, 81
Is the physiologic capillary pO2 a prerequisite for brain function?
Although it is important to keep in mind potential changes of capillary surface area, CTTH and other factors that may influence the CBF–CMRO2 relationship, it is also worthwhile taking a look on the limits of the main driving force of oxygen delivery to the brain: The gradient between capillary and mitochondrial oxygen tension. Grocott et al82 have measured arterial pO2 at 8,400 m altitude during a Mount Everest expedition. They found an arterial pO2 of 25 mm Hg (19 to 30 mm Hg) in four healthy volunteers with grossly normal brain function (able to perform complex tasks). Increases in hemoglobin up to 20 g/dL and a left shift of the oxygen-binding curve due to hyperventilation with massive hypocapnia (pCO2 around 13 mm Hg) guarantee a relatively high arterial oxygen content under these conditions. Nonetheless, the average brain capillary pO2 cannot have exceeded 25 mm Hg and was probably below 20 mm Hg. Considering a further extreme condition, a similar limit has been observed in competitive divers holding their breath for several minutes. While maintaining consciousness, their end-tidal pO2 fell to around 25 mm Hg—with arterial pO2 and brain capillary pO2 probably less.83 Pagani et al84 report mean arterial pO2 of 28 mm Hg during hypobaric hypoxia in healthy volunteers with no mental distress under these conditions. Interestingly, they found only small CBF increases in some parts of the brain. At severe hypoxia in anesthetized piglets (paO2=24 mm Hg), Tichauer et al85 observed largely stable CBF and only a small decrease in CMRO2. Artru and Michenfelder86 report unchanged CMRO2 down to arterial pO2 of 27 mm Hg, but a decrease by 13% at paO2 of 21 mm Hg in anesthetized rabbits, with a 50% increase in CBF. Bailey et al27 suggest largely constant CBF and CMRO2 during hypoxia, whereas the calculated average capillary pO2 dropped from 43 to 33 mm Hg and the calculated average mitochondrial pO2 from 15 mm Hg to 1.4 mm Hg.
Collectively, these data indicate that the physiologic average capillary pO2 of 40 to 50 mm Hg is not a prerequisite for brain function and physiologic CMRO2, at least within short term. It should be noted, however, that because of the steep slope of the oxygen-binding curve at low pO2, a large amount of oxygen can be offloaded within a narrow pO2 range during the capillary passage (this is further enhanced by increases in hemoglobin content and a left shift of the binding curve at high altitude). Hence, under these conditions, the average capillary pO2 drops dramatically, but the pO2 at the venous end of the capillary and the tissue pO2 in the ‘lethal corners' drop probably much less. Still, these data indicate that the lower limit of arterial pO2 for grossly intact brain function lies in the range of 20 to 30 mm Hg, probably indicating a limit of pO2 at the venous end of the capillary somewhere around 15 to 20 mm Hg. This fits well with the theoretically calculated oxygen gradients using the Krogh–Erlang equation and published values for CMRO2, oxygen diffusivity, and intercapillary distances (Figure 1). We stress that these observations indicate oxygen delivery limits for overall CMRO2 and gross brain function on short term, but do not consider more subtle short-term neuropsychological effects or more deleterious long-term effects of these extreme levels of hypoxia, which may arise as a consequence of limitation of important cellular reactions that become limited at higher pO2 levels but do not contribute relevantly to the overall CMRO2. This may have a role, for example, in diseases that affect small brain vessels. Evidence accumulates that dysfunction of the neurovascular unit has a role in Alzheimer's disease and stroke/small vessel disease and impairment of neurovascular coupling may be part of this dysfunction.80, 81, 87, 88 It can be speculated that impaired neurovascular coupling could lead to repeated drops in tissue pO2 upon neuronal activation with initial short-term preservation of normal neuronal function but induction of inflammatory pathways that ultimately lead to long-term tissue damage.80 However, no disease affects neurovascular coupling specifically while leaving baseline CBF, capillary morphology, endothelial function, etc. intact. Therefore, it will be challenging to dissect and quantify the contribution of selective neurovascular coupling impairment in disease progression.
Safety margin of oxygen delivery suggested by uncoupling of cerebral blood flow and cerebral metabolic rate of oxygen
As oxygen supply of the brain is so delicate, it seems likely that evolution has favored an oxygen delivery system that does not fail once a minor dysfunction of one of its many components occurs. In general, evolution balances costs and advantages of safety factors.89 As regional large increases in CBF during functional activation are not accompanied by significant increases of whole brain CBF,57 the costs in terms of increased workload of the heart to provide the additional CBF of a generous CBF response to neuronal activation are low. Thus, a large CBF response could have developed during evolution, even if the potential advantage for the brain may be small. Furthermore, the load applied to the system (changes in CMRO2 during neuronal activation) as well as the capacity of the system (additional O2 delivery via the blood stream during activation) may vary between different types of neuronal activation and between individuals. A relevant genetic variability may exist of receptors, channels, and enzymes involved in the complex interplay between changes in neuronal activity, metabolism, and CBF. As CBF increases are not directly coupled to changes in pO2,64 the feed-forward mechanisms coupling neuronal activity and CBF may have evolved with a safety factor that ensures CBF levels sufficient even in individuals with an unfavorable combination of genes that lead to rather large CMRO2 responses but small CBF responses.
If a safety factor for oxygen delivery to the brain exists, to some degree independent changes of CBF and CMRO2 with either CBF decreases at constant CMRO2 or CMRO2 increases with constant CBF should be possible.
Uncoupling of cerebral blood flow and cerebral metabolic rate of oxygen
In their seminal 1986 PNAS paper, Fox and Raichle66 used the term ‘focal physiological uncoupling' to describe the phenomenon that CBF increased much more than CMRO2 during neuronal activation. Later, it has been pointed out by Buxton and Frank75 that the large CBF increase may in fact be necessary for a small CMRO2 increase because of the physical properties of the brain tissue (oxygen limitation model, see above). Following this argument, physiologic neurovascular coupling including a capillary pO2 increase during activation could be termed ‘tight' and in this sense, the positive BOLD signal that forms the basis of functional imaging as well as the negative BOLD signal observed during stimulus evoked reductions of neuronal activity,90, 91 e.g. ipsilaterally to somatosensory stimulation or in ‘surround' regions of focal activation, could also represent ‘tight coupling' of CBF and CMRO2.
Following this line of thinking, ‘uncoupling' of CBF and CMRO2 could be demonstrated more convincingly by studies, which find, to some extent, independent variations of CBF and CMRO2. In the following section, we will review these studies. We think that the most conclusive evidence can be derived from studies, which combine measurements of CBF and CMRO2 in at least two different experimental states of one brain region. Experimental manipulations used include substances interfering with neurovascular coupling (e.g. indomethacin, caffeine), hyperventilation, and variations of stimulus paradigms.
For example, indomethacin decreased CBF in healthy volunteers by around 25% to 40% with constant or minimally reduced CMRO2.26, 92 Hyperventilation reduced CBF in healthy controls and meningitis patients significantly (by around 30%), but CMRO2 remained constant.93 Caffeine increased CMRO2 by 20% while decreasing CBF by 25% in moderate caffeine consumers who abstained from coffee for at least 12 hours.67 The CMRO2 levels found during neuronal activation before caffeine administration were equal to baseline CMRO2 values after caffeine, which were supported by a 20% lower CBF. In another study, caffeine decreased CBF by 35% with a non-significant +5% change in CMRO2.94 Reductions of CBF by 15% accompanied by increases in CMRO2 by 8% were observed after cortical spreading depression in rats.95
Several functional activation studies indicate that CBF may be considerably above the level needed to support CMRO2 during a variety of specific stimulus conditions. After 10 minutes of continuous finger tapping, CBF remained elevated by 20% to 25% as compared with the resting state, but CMRO2 decreased below the baseline in a PET experiment on healthy volunteers.96 During continuous visual stimulation, Mintun et al97 found an initial 41% CBF increase accompanied by a 5% increase in CMRO2. After 25 minutes, the CBF level declined to 26%, but CMRO2 increased to 15% above baseline. Visual stimulation at 4 Hz increased CBF by 38% and CMRO2 by 15% compared with baseline. However, at 8 Hz stimulation frequency, CMRO2 increase was only 5% whereas the CBF increase reached 42%.78 This PET observation has been confirmed with MRI by Lin et al.98 Other studies have found tight coupling of CBF and CMRO2 with varied stimulus paradigms. For example, in a recent study, Leontiev et al99 did not find a significant difference in CBF/CMRO2 coupling between two visual stimuli that activate different neuron populations characterized histologically by differences in cytochrome c distribution. Hoge et al100 found close coupling of CBF with CMRO2 over a large number of different visual stimuli.
Several groups have found transient decreases of tissue oxygen concentration,11, 101, 102, 103 deoxyhemoglobin increases or BOLD signal decreases,48, 52, 104 or decreases of vascular pO217, 105 during the first second(s) of neuronal activation, when CBF has not yet significantly increased, indicating the possibility of increased CMRO2 with constant CBF on very short term. However, this ‘initial dip' has not been detected in all studies106, 107, 108 and methodological issues remained owing to the complexity of the methods employed.
During partial pharmacological inhibition of CBF responses (attenuated by around two-thirds) and with constant baseline CBF, we found constant CMRO2 responses to neuronal activation in the somatosensory cortex of rats.109 In the cerebellum, Offenhauser et al11 found larger initial decreases of tissue pO2 during inhibition of CBF responses. Mathiesen et al102 demonstrated initially unaffected CMRO2 responses during pharmacologically reduced CBF responses after climbing fiber stimulation in the cerebellum. During severe hypotension and with increased baseline CBF, increases in CMRO2 with absent CBF responses were observed.14, 110 Accordingly, a negative BOLD signal indicating increased CMRO2 with no concomitant CBV and thus presumably absent CBF responses were detected using MRI in hypotensive rats.111 Baker et al112 observed uncoupling of CBF and CMRO2 responses during progressive cerebral ischemia.
Collectively, these studies demonstrate that under a variety of conditions, most likely including the physiologic baseline, CBF is higher than needed to support CMRO2. This provides evidence against the hypothesis that a disproportionately large CBF response is a prerequisite for a small increase in CMRO2 during neuronal activation. In contrast, these data suggest that oxidative metabolism can increase within limits without a concomitant CBF increase.
The oxygen paradox of neurovascular coupling
The low oxygen reserves in brain tissue compared with high glucose reserves and the almost exclusive ATP production via oxidative phosphorylation suggest that the rapid CBF response serves oxygen delivery. The uncoupling data reviewed above could thus be interpreted as an ‘oxygen paradox of neurovascular coupling': basic physiologic numbers and facts of energy supply to the brain reviewed in detail above indicate that the role of the CBF response is oxygen delivery, but accumulating evidence from recent studies suggests that it is not needed (at least not in its full extent and on short term) for oxygen delivery. As argued above, one possible explanation in terms of ‘ultimate causation'89 is a safety margin function. That is, because oxygen delivery is so delicate, it is too dangerous for the brain to operate at a CBF level that does not allow increases in CMRO2 without perfect neurovascular coupling.
Has Neurovascular Coupling Evolved to Keep Tissue pO2 Constant?
An alternative perspective using a similar explanation has been suggested in a recent review. In this review, Buxton37 notes that although CBF increases much more than CMRO2, there is little change in tissue pO2. Thus, the CBF response may have evolved to keep average tissue pO2 constant during variations of neuronal activity. Keeping average tissue pO2 constant could serve to maintain oxygen-dependent reactions other than ATP production that operate at a considerably higher P50 and are thus more susceptible to reductions from baseline levels. Another slightly different theory developed by Devor et al12 based on high-resolution tissue pO2 measurements holds that the CBF response may have evolved to prevent pO2 from falling in the lethal corners of the tissue, where energy production may operate at oxygen levels so low that they are rate limiting for ATP production. Our hypothesis of a safety margin function shares the view that functional hyperemia has evolved to balance oxygen demand and supply. However, we think that the accumulating data reviewed above indicates that constant average brain tissue pO2 or constant pO2 in the lethal corners is not a short-term prerequisite for maintaining brain function and CMRO2.
Regulation of oxidative phosphorylation and nonzero mitochondrial pO2
In order to gain a more detailed understanding of whether O2 availability (in the ‘lethal corners' of brain tissue) limits ATP production, we will discuss studies on the regulation of brain oxidative phosphorylation in the following paragraph. Although many details remain unknown to date, the available evidence suggests that at the pO2 levels observed in the brain, regulatory mechanisms other than O2 availability are involved to a substantial degree.
Nerve cells need to rapidly adjust ATP production to demand, which fluctuates with the cells signaling activity. The vast majority of ATP is used for signaling and it is likely that almost the entire short-term fluctuation of CMRO2 is due to changes in signaling activity113 so that CMRO2 is closely linked to neuronal activity. It seems unlikely that the way developed during evolution to accomplish the adjustment of ATP production to neuronal activity is the detour of first increasing CBF, which then ‘passively' drives an increased ATP production by increased oxygen delivery. Such a regulation would introduce a high vulnerability of brain function depending totally on intact neurovascular coupling. A combination of regulatory mechanisms may be more safe and effective, separately adjusting CMRO2 and ATP production first and then CBF generously to safeguard oxygen delivery when the amplitude of the CMRO2 increases cannot be exactly predicted or measured by the coupling mechanisms.
Cerebral metabolic rate of oxygen depends, among other factors, on mitochondrial pO2 as well as the dynamics of the respiratory cascade for oxygen (Jmax and P50).114 If pO2 is much higher than the P50, further increases of mitochondrial pO2 do no longer lead to relevant increases of the CMRO2. Adding to the uncertainties of mitochondrial pO2, the exact P50 of the respiratory cascade for oxygen in neurons in vivo is unclear. Experiments with isolated mitochondria and various cell types in vitro found variable P50 of the respiratory cascade for oxygen,115 with oxygen dependence of oxidative metabolism potentially extending into the range of mitochondrial pO2 levels in the brain. Therefore, a relevant influence of the mitochondrial pO2 on CMRO2 is possible within the physiologic range. However, an increase of CMRO2 could also be achieved with constant or even decreasing mitochondrial pO2 via adjustment of the P50 and/or Jmax of the respiratory cascade for oxygen by other factors. A simple example is limitation of other substrates: during normoxic hypoglycemia, reduced glucose availability may reduce the Jmax of the respiratory cascade for oxygen with constant or even increasing mitochondrial pO2. As argued above, increases in CMRO2 with concomitant decreases in mitochondrial pO2 are suggested by experiments demonstrating decreases in tissue pO2 while CBF remains constant during neuronal activation.
In the following, we will discuss mechanisms of CMRO2 regulation at the mitochondrial level other than O2 availability. A number of potential mechanisms have been proposed. Several are probably involved at the same time.116, 117 An obvious candidate mechanism is regulation by ADP availability,118, 119 which has been termed the ‘first mechanism of respiratory control'.120 Magnetic resonance spectroscopy measurements of free ADP concentrations in vivo suggest values around 30 to 35 nmoL/mL in the human hippocampus and occipital lobe.121 Similar low values have been found in dog and rat brain.122, 123 Keeping in mind that one molecule of oxygen generates, on average, six ATP in oxidative phosphorylation, these ADP values are very low even when compared with the low oxygen content of the brain. At these low concentrations, the total amount of free ADP available would be converted to ATP within 160 milliseconds. Total ATP levels are higher by a factor of around 100.121, 124 By analytical biochemistry methods, substantially higher ADP concentrations around 300 nmoL/mL124 are found, because these also capture the large amount of ADP bound to proteins.122 The mitochondria, however, sense the free ADP.125 Measurements obtained on isolated mitochondria indicate a c50 of the respiratory cascade for ADP of 56 nmoL/mL.28 Thus, it seems likely that free ADP concentrations influence the rate of oxidative phosphorylation at physiologic levels.
ATP has been found to act as an allosteric inhibitor of cytochrome c oxidase.126 This allosteric inhibition could increase the p50 of the respiratory cascade for oxygen and thus reduce ATP production at a given mitochondrial pO2 when energy requirements of the cell are low and ATP accumulates (in addition to the effect of reduced ADP availability under these conditions). Acin-Perez et al127 suggested that inhibition of cytochrome c by ATP is modulated by phosphorylation of an ATP binding site. Moderate changes in CMRO2 may not change the high physiologic ATP level relevantly. However, the phosphorylation may reduce inhibitory ATP binding to cytochrome c oxidase and thus increase cytochrome c oxidase activity at a constant ATP level.
Nitric oxide has been demonstrated to inhibit cytochrome c oxidase.128 A reduction of NO levels accompanied by reduced cytochrome c oxidase inhibition during decreases in CBF could thus explain constant CMRO2 whereas pO2 gradients could be maintained despite increased oxygen extraction fraction by a decrease in mitochondrial pO2.26 However, during neuronal activation, NO levels increase (participating in neurovascular coupling129) and thus this mechanism does not explain CMRO2 increases with constant CBF, e.g. during the first second of neuronal activation.
Several mechanisms have been identified by which changes in cellular Ca2+ concentration influence oxidative phosphorylation.130, 131 As neuronal activity is accompanied by transient increases in intracellular calcium, this would represent a rapid link between neuronal activity and oxidative phosphorylation. However, in the cerebellum, CMRO2 changes during climbing fiber stimulation remained unchanged when stimulus-induced increases in intracellular calcium were pharmacologically reduced.102
The complex interplay of processes involved in the regulation of oxidative phosphorylation remains incompletely understood. Nevertheless, it seems likely that factors other than oxygen availability are involved in the control of CMRO2 and may adjust ATP supply rapidly and independently of CBF within the limits set by oxygen delivery.
The significance of heterogeneous tissue pO2 in the brain
In this section, we briefly discuss the relevant heterogeneity of brain tissue pO2 detected in recent studies as a potential limitation when interpreting studies that measure average CBF and CMRO2. Because of limited spatial resolution of measurements of molecules relevant for brain energy metabolism and because of the complexity of modeling heterogeneities within small tissue regions, research on brain energy supply has focused on average values. It has recently been demonstrated experimentally in fine detail,12, 20 however, that oxygen concentration is highly heterogeneous in the brain. Keeping in mind the low intracellular oxygen gradients,21, 22 the oxygen measurements implicate high variability also of mitochondrial pO2. Mitochondria located close to diving arterioles may operate at a pO2 as high as 60 mm Hg, whereas those supplied by venous ends of capillaries in ‘lethal corners' of the tissue may have pO2 around 5 to 10 mm Hg. It is very likely that ATP production is not limited by oxygen availability for mitochondria located in the areas of higher oxygen concentration. It could still be, given the potentially very low P50 of the respiratory cascade for oxygen, that from the physiologic baseline even the mitochondria with lowest pO2 may increase their ATP production despite a further decrease in pO2. At the venous end of capillaries, pO2 of around 40 mm Hg has been determined on the brain surface with oxygen electrodes.10 However, a considerable variability was also detected with pO2 as low as 20 mm Hg on the surface of some vessels. High-resolution three-dimensional oxygen measurements using phosphorescence quenching indicate substantial variability with pO2 as low as 20 to 30 mm Hg at the surface of microvessels with lowest oxygen content.12, 132 Hence, the concentration gradient between blood vessels and tissue areas with lowest pO2 may be around 15 to 20 mm Hg, still enabling physiologic CMRO2 levels, as discussed above. Keeping in mind the potentially very low P50 of the respiratory cascade for oxygen, a further decrease in the tissue pO2 by 2 to 3 mm Hg may thus allow for a relevant increase of this concentration gradient by 10% to 20% and thus an increase in CMRO2 even without changes in CBF. Given the substantial heterogeneity of pO2 in microvessels, a decrease in CTTH may also increase oxygen delivery to areas with lowest pO2 while maintaining constant average CBF.8
In principle, diffusion of ATP and/or phosphocreatine (PCr) could deliver energy from microregions with high pO2 to those with low pO2. Both high-energy phosphates are found in the brain in relevant concentrations (see Table 1). ATP and PCr may diffuse over significant distances and may have a role in energy transport in addition to energy buffering.133 In muscle, average diffusion distances of ATP and PCr have been estimated at around 20 (ATP) to 60 μm (PCr),134 which is in the range of intercapillary distances in the brain. However, a significant proportion of energy is consumed in synapses and diffusion to synapses from the next extrasynaptical mitochondria would have to occur along dendrites or axons over much larger distances. Hence, although confirmation by experimental data would be interesting, a relevant contribution of diffusion of ATP or PCr to ‘lethal corners' of brain tissue may be unlikely.
The heterogeneity of tissue pO2 introduces a potential limitation to studies exploring effects of changes in average CBF and CMRO2. When reducing CBF or arterial pO2, microregions close to the arterial side of capillaries will maintain constant CMRO2 longer than the ‘lethal corners' of the tissue. Oxygen limitation of energy metabolism may be subtle and restricted to small microregions at the beginning and may have subtle effects on brain function not detected in most of the studies discussed above. To explore this further, experiments using high-resolution imaging of oxidative metabolism and neuronal activity (capable of distinguishing ‘lethal corners' from the rest of the tissue) during manipulations of oxygen supply to the brain are needed.
Conclusions
Relevant details of oxygen supply to the brain have not yet been fully elucidated. Specifically, it remains unclear to what extent CMRO2 is limited by oxygen availability under physiologic conditions. Studies on the limits of oxygen supply during hypoxia, experimental studies of baseline changes of CBF and CMRO2, studies of transient changes of oxygen concentration during physiologic blood flow responses to neuronal activation and studies of inhibited blood flow responses suggest that a moderate safety margin of oxygen delivery to the brain exists and that CBF at rest is not the sole determinant of CMRO2. Regulation of oxidative phosphorylation to some extent independent from blood flow seems possible. Potential regulatory mechanisms include changes of ADP availability, inhibitory effects of ATP and NO on cytochrome c oxidase and changes in intracellular Ca2+ concentrations. However, detailed in vivo studies on the complex regulation of oxidative phosphorylation in the brain are difficult and many details remain unknown. Further complicating the picture, relevant heterogeneity of oxygen availability exists within the brain tissue, indicating that regulation of CMRO2 may vary significantly between areas close to microvessels with high pO2 and those more distant. Especially in these areas, the brain's oxygen reserves are low and while the quick and large CBF response to neuronal activation may not be necessary in its full extent, it may have evolved to ensure that brain function is not immediately lost when oxygen delivery to tissue is impaired.
The authors declare no conflict of interest.
References
- Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak RS, Herold S, Petty RK, Morgan-Hughes JA. The cerebral metabolism of glucose and oxygen measured with positron tomography in patients with mitochondrial diseases. Brain. 1988;111 (Pt 5:1009–1024. doi: 10.1093/brain/111.5.1009. [DOI] [PubMed] [Google Scholar]
- Poulsen PH, Smith DF, Ostergaard L, Danielsen EH, Gee A, Hansen SB, et al. In vivo estimation of cerebral blood flow, oxygen consumption and glucose metabolism in the pig by [15O]water injection, [15O]oxygen inhalation and dual injections of [18F]fluorodeoxyglucose. J Neurosci Methods. 1997;77:199–209. doi: 10.1016/s0165-0270(97)00127-1. [DOI] [PubMed] [Google Scholar]
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jónsdottir KY, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab. 2012;32:1177–1187. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol (Lond) 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2011;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Bassingthwaighte JB. Capillary supply regions. Math Biosci. 2001;173:103–114. doi: 10.1016/s0025-5564(01)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 1999;437:617–623. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol (Lond) 2005;565:279–294. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Sakadzic S, Saisan PA, Yaseen MA, Roussakis E, Srinivasan VJ, et al. ‘Overshoot' of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J Neurosci. 2011;31:13676–13681. doi: 10.1523/JNEUROSCI.1968-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Wilson DF, Greenberg JH, Detre JA. Dynamic changes in cerebral blood flow, O2 tension, and calculated cerebral metabolic rate of O2 during functional activation using oxygen phosphorescence quenching. J Cereb Blood Flow Metab. 2001;21:511–516. doi: 10.1097/00004647-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Vazquez A, Wang P, Kim S-G. Trial-by-trial relationship between neural activity, oxygen consumption, and blood flow responses. NeuroImage. 2008;40:442–450. doi: 10.1016/j.neuroimage.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–1219. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Parpaleix A, Houssen YG, Charpak S. Imaging local neuronal activity by monitoring PO(2) transients in capillaries. Nat Med. 2013;19:241–246. doi: 10.1038/nm.3059. [DOI] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schröck H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986;251:H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Demchenko IT, Luchakov YI, Moskvin AN, Gutsaeva DR, Allen BW, Thalmann ED, et al. Cerebral blood flow and brain oxygenation in rats breathing oxygen under pressure. J Cereb Blood Flow Metab. 2005;25:1288–1300. doi: 10.1038/sj.jcbfm.9600110. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Intracellular diffusion gradients of O2 and ATP. Am J Physiol. 1986;250:C663–C675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- Wittenberg BA, Wittenberg JB. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem. 1985;260:6548–6554. [PubMed] [Google Scholar]
- Vanderkooi JM, Wright WW, Erecińska M. Oxygen gradients in mitochondria examined with delayed excited-state triplet probes. Biochemistry. 1990;29:5332–5338. doi: 10.1021/bi00474a018. [DOI] [PubMed] [Google Scholar]
- Wilson DF. Contribution of diffusion to the oxygen dependence of energy metabolism in cells. Experientia. 1990;46:1160–1162. doi: 10.1007/BF01936927. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S, Vafaee M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cereb Blood Flow Metab. 2002;22:1–14. doi: 10.1097/00004647-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Johannsen P, Cold GE, Østergaard L. Cerebral metabolic response to low blood flow: possible role of cytochrome oxidase inhibition. J Cereb Blood Flow Metab. 2005;25:1183–1196. doi: 10.1038/sj.jcbfm.9600113. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Lundby C, Pedersen BK, Rasmussen P, et al. Cerebral formation of free radicals during hypoxia does not cause structural damage and is associated with a reduction in mitochondrial PO2; evidence of O2-sensing in humans. J Cereb Blood Flow Metab. 2011;31:1020–1026. doi: 10.1038/jcbfm.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol. 2001;128:277–297. doi: 10.1016/s0034-5687(01)00307-3. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Green BA, Gonzalez-Carvajal M, Mora J, Veraa RP. Local blood flow, oxygen tension, and oxygen consumption in the rat spinal cord. Part 1: oxygen metabolism and neuronal function. J Neurosurg. 1983;58:516–525. doi: 10.3171/jns.1983.58.4.0516. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Diemer NH. Autoradiographic determination of regional brain glucose content. J Cereb Blood Flow Metab. 1983;3:303–310. doi: 10.1038/jcbfm.1983.45. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecińska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Fukuda H. Human cerebral circulation: positron emission tomography studies. Ann Nucl Med. 2005;19:65–74. doi: 10.1007/BF03027383. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2013;305:H609–H619. doi: 10.1152/ajpheart.00359.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:pii:5. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage. 2012;62:1040–1050. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. NeuroImage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Royl G, Leithner C, Sellien H, Müller JP, Megow D, Offenhauser N, et al. Functional imaging with laser speckle contrast analysis: vascular compartment analysis and correlation with laser Doppler flowmetry and somatosensory evoked potentials. Brain Res. 2006;1121:95–103. doi: 10.1016/j.brainres.2006.08.125. [DOI] [PubMed] [Google Scholar]
- Hillman EMC, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. NeuroImage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füchtemeier M, Leithner C, Offenhauser N, Foddis M, Kohl-Bareis M, Dirnagl U, et al. Elevating intracranial pressure reverses the decrease in deoxygenated hemoglobin and abolishes the post-stimulus overshoot upon somatosensory activation in rats. NeuroImage. 2010;52:445–454. doi: 10.1016/j.neuroimage.2010.04.237. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. NeuroImage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Lubow JM, Piñón IG, Avogaro A, Cobelli C, Treeson DM, Mandeville KA, et al. Brain oxygen utilization is unchanged by hypoglycemia in normal humans: lactate, alanine, and leucine uptake are not sufficient to offset energy deficit. Am J Physiol Endocrinol Metab. 2006;290:E149–E153. doi: 10.1152/ajpendo.00049.2005. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Rundle MM, Mintun MA. Human brain glucose metabolism may evolve during activation: findings from a modified FDG PET paradigm. NeuroImage. 2006;33:1036–1041. doi: 10.1016/j.neuroimage.2006.06.065. [DOI] [PubMed] [Google Scholar]
- Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Videen TO, Markham J, Walter V, Perlmutter JS. Metabolic control of resting hemispheric cerebral blood flow is oxidative, not glycolytic. J Cereb Blood Flow Metab. 2011;31:1223–1228. doi: 10.1038/jcbfm.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Rundle MM, Raichle ME. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci USA. 2004;101:659–664. doi: 10.1073/pnas.0307457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido Y, Chang K, Woolsey TA, Williamson JR. NADH: sensor of blood flow need in brain, muscle, and other tissues. FASEB J. 2001;15:1419–1421. doi: 10.1096/fj.00-0652fje. [DOI] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA. 2000;97:7603–7608. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Brull R, Alsop DC, Marquis RP, Lenkinski RE. Limits on activation-induced temperature and metabolic changes in the human primary visual cortex. Magn Reson Med. 2006;56:348–355. doi: 10.1002/mrm.20972. [DOI] [PubMed] [Google Scholar]
- Royl G, Füchtemeier M, Leithner C, Megow D, Offenhauser N, Steinbrink J, et al. Hypothermia effects on neurovascular coupling and cerebral metabolic rate of oxygen. NeuroImage. 2008;40:1523–1532. doi: 10.1016/j.neuroimage.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Yücel MA, Devor A, Akin A, Boas DA. The Possible role of CO(2) in producing A post-stimulus CBF and BOLD undershoot. Front Neuroenergetics. 2009;1:7. doi: 10.3389/neuro.14.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, Macvicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Leithner C, Kaasch H, Rohrer B, Foddis M, Füchtemeier M, et al. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J Cereb Blood Flow Metab. 2010;30:757–768. doi: 10.1038/jcbfm.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffeth VEM, Perthen JE, Buxton RB. Prospects for quantitative fMRI: investigating the effects of caffeine on baseline oxygen metabolism and the response to a visual stimulus in humans. NeuroImage. 2011;57:809–816. doi: 10.1016/j.neuroimage.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. NeuroImage. 2007;36:1110–1122. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Krüger G, Neumann-Haefelin T, Glover GH, Moseley ME. Changes of cerebral blood flow, oxygenation, and oxidative metabolism during graded motor activation. NeuroImage. 2002;15:74–82. doi: 10.1006/nimg.2001.0916. [DOI] [PubMed] [Google Scholar]
- Fujita H, Kuwabara H, Reutens DC, Gjedde A. Oxygen consumption of cerebral cortex fails to increase during continued vibrotactile stimulation. J Cereb Blood Flow Metab. 1999;19:266–271. doi: 10.1097/00004647-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Lin A-L, Fox PT, Hardies J, Duong TQ, Gao J-H. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Villringer A, Them A, Lindauer U, Einhäupl K, Dirnagl U. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ Res. 1994;75:55–62. doi: 10.1161/01.res.75.1.55. [DOI] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab. 2000;20:747–754. doi: 10.1097/00004647-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Valabrègue R, Aubert A, Burger J, Bittoun J, Costalat R. Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. J Cereb Blood Flow Metab. 2003;23:536–545. doi: 10.1097/01.WCB.0000055178.31872.38. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Aamand R, Gutiérrez-Jiménez E, Ho Y-CL, Blicher JU, Madsen SM, et al. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Jespersen SN, Mouridsen K, Mikkelsen IK, Jonsdottír KÝ, Tietze A, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab. 2013;33:635–648. doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott MPW, Martin DS, Levett DZH, McMorrow R, Windsor J, Montgomery HE, et al. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Lundgren CEG. Alveolar gas composition before and after maximal breath-holds in competitive divers. Undersea Hyperb Med. 2006;33:463–467. [PubMed] [Google Scholar]
- Pagani M, Ansjön R, Lind F, Uusijärvi J, Sumen G, Jonsson C, et al. Effects of acute hypobaric hypoxia on regional cerebral blood flow distribution: a single photon emission computed tomography study in humans. Acta Physiol Scand. 2000;168:377–383. doi: 10.1046/j.1365-201x.2000.00649.x. [DOI] [PubMed] [Google Scholar]
- Tichauer KM, Elliott JT, Hadway JA, Lee DS, Lee T-Y, St, Lawrence K. Using near-infrared spectroscopy to measure cerebral metabolic rate of oxygen under multiple levels of arterial oxygenation in piglets. J Appl Physiol. 2010;109:878–885. doi: 10.1152/japplphysiol.01432.2009. [DOI] [PubMed] [Google Scholar]
- Artru AA, Michenfelder JD. Canine cerebral metabolism and blood flow during hypoxemia and normoxic recovery from hypoxemia. J Cereb Blood Flow Metab. 1981;1:277–283. doi: 10.1038/jcbfm.1981.32. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Diamond J. Quantitative evolutionary design. J Physiol (Lond) 2002;542:337–345. doi: 10.1113/jphysiol.2002.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, et al. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M, Poulsen PH, Treiber A, Delahaye S, Tankisi A, Cold GE, et al. No influence of the endothelin receptor antagonist bosentan on basal and indomethacin-induced reduction of cerebral blood flow in pigs. Acta Anaesthesiol Scand. 2003;47:200–207. doi: 10.1034/j.1399-6576.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- Møller K, Strauss GI, Thomsen G, Larsen FS, Holm S, Sperling BK, et al. Cerebral blood flow, oxidative metabolism and cerebrovascular carbon dioxide reactivity in patients with acute bacterial meningitis. Acta Anaesthesiol Scand. 2002;46:567–578. doi: 10.1034/j.1399-6576.2002.460515.x. [DOI] [PubMed] [Google Scholar]
- Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. NeuroImage. 2008;40:237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Vang K, Bergersen LH, Gjedde A. Oxygen consumption and blood flow coupling in human motor cortex during intense finger tapping: implication for a role of lactate. J Cereb Blood Flow Metab. 2012;32:1859–1868. doi: 10.1038/jcbfm.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Shulman GL, Snyder AZ. Time-related increase of oxygen utilization in continuously activated human visual cortex. NeuroImage. 2002;16:531–537. doi: 10.1006/nimg.2002.1114. [DOI] [PubMed] [Google Scholar]
- Lin A-L, Fox PT, Yang Y, Lu H, Tan L-H, Gao J-H. Evaluation of MRI models in the measurement of CMRO2 and its relationship with CBF. Magn Reson Med. 2008;60:380–389. doi: 10.1002/mrm.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Buracas GT, Liang C, Ances BM, Perthen JE, Shmuel A, et al. Coupling of cerebral blood flow and oxygen metabolism is conserved for chromatic and luminance stimuli in human visual cortex. NeuroImage. 2013;68:221–228. doi: 10.1016/j.neuroimage.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Thomsen K, Hoogland TM, Witgen BM, Brazhe A, et al. Activity-dependent increases in local oxygen consumption correlate with postsynaptic currents in the mouse cerebellum in vivo. J Neurosci. 2011;31:18327–18337. doi: 10.1523/JNEUROSCI.4526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoq J, Tiret P, Najac M, Shepherd GM, Greer CA, Charpak S. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J Neurosci. 2009;29:1424–1433. doi: 10.1523/JNEUROSCI.4817-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science. 1999;286:1555–1558. doi: 10.1126/science.286.5444.1555. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Royl G, Leithner C, Kühl M, Gold L, Gethmann J, et al. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. NeuroImage. 2001;13:988–1001. doi: 10.1006/nimg.2000.0709. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Leithner C, Royl G, Offenhauser N, Füchtemeier M, Kohl-Bareis M, Villringer A, et al. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez AL, Fukuda M, Kim S-G. Evolution of the dynamic changes in functional cerebral oxidative metabolism from tissue mitochondria to blood oxygen. J Cereb Blood Flow Metab. 2012;32:745–758. doi: 10.1038/jcbfm.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, et al. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J Cereb Blood Flow Metab. 2006;26:1043–1051. doi: 10.1038/sj.jcbfm.9600251. [DOI] [PubMed] [Google Scholar]
- Baker WB, Sun Z, Hiraki T, Putt ME, Durduran T, Reivich M, et al. Neurovascular coupling varies with level of global cerebral ischemia in a rat model. J Cereb Blood Flow Metab. 2013;33:97–105. doi: 10.1038/jcbfm.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- Gnaiger E. Oxygen conformance of cellular respiration. Adv Exp Med Biol. 2003;543:39–55. doi: 10.1007/978-1-4419-8997-0_4. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258:C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Kent J, McCully K, Nioka S, Clark BJ, et al. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA. 1986;83:9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardy HA, Wellman H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952;195:215–224. [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217:409–427. [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Vogt S. High efficiency versus maximal performance—The cause of oxidative stress in eukaryotes: a hypothesis. Mitochondrion. 2013;13:1–6. doi: 10.1016/j.mito.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- Veech RL, Lawson JW, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem. 1979;254:6538–6547. [PubMed] [Google Scholar]
- Nioka S, Chance B, Hilberman M, Subramanian HV, Leigh JS, Veech RL, et al. Relationship between intracellular pH and energy metabolism in dog brain as measured by 31P-NMR. J. Appl Physiol. 1987;62:2094–2102. doi: 10.1152/jappl.1987.62.5.2094. [DOI] [PubMed] [Google Scholar]
- Katsura K, Folbergrová J, Gidö G, Siesjö BK. Functional, metabolic, and circulatory changes associated with seizure activity in the postischemic brain. J Neurochem. 1994;62:1511–1515. doi: 10.1046/j.1471-4159.1994.62041511.x. [DOI] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, et al. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci USA. 1985;82:8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur J Biochem. 1997;249:350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]
- Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Gellerich FN, Gizatullina Z, Trumbekaite S, Korzeniewski B, Gaynutdinov T, Seppet E, et al. Cytosolic Ca2+ regulates the energization of isolated brain mitochondria by formation of pyruvate through the malate-aspartate shuttle. Biochem J. 2012;443:747–755. doi: 10.1042/BJ20110765. [DOI] [PubMed] [Google Scholar]
- Tarasov AI, Griffiths EJ, Rutter GA. Regulation of ATP production by mitochondrial Ca(2+) Cell Calcium. 2012;52:28–35. doi: 10.1016/j.ceca.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakadžić S, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7:755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281 (Pt 1:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Watari H, Radda GK. Role of phosphocreatine in energy transport in skeletal muscle of bullfrog studied by 31P-NMR. Biochim Biophys Acta. 1990;1051:144–150. doi: 10.1016/0167-4889(90)90186-h. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Wey H-Y, Wang DJ, Duong TQ. Baseline CBF, and BOLD, CBF, and CMRO2 fMRI of visual and vibrotactile stimulations in baboons. J Cereb Blood Flow Metab. 2011;31:715–724. doi: 10.1038/jcbfm.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Thews G. A method for determination of oxygen diffusion coefficients, oxygen conductivity and oxygen solubility coefficients in brain tissue. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:227–244. [PubMed] [Google Scholar]
- Gnaiger E, Méndez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci USA. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]