Abstract
Medium chain triglycerides (MCTs) are used to treat neurologic disorders with metabolic impairments, including childhood epilepsy and early Alzheimer's disease. However, the metabolic effects of MCTs in the brain are still unclear. Here, we studied the effects of feeding even and uneven MCTs on brain glucose metabolism in the mouse. Adult mice were fed 35% (calories) of trioctanoin or triheptanoin (the triglycerides of octanoate or heptanoate, respectively) or a matching control diet for 3 weeks. Enzymatic assays and targeted metabolomics by liquid chromatography tandem mass spectrometry were used to quantify metabolites in extracts from the hippocampal formations (HFs). Both oils increased the levels of β-hydroxybutyrate, but no other significant metabolic alterations were observed after triheptanoin feeding. The levels of glucose 6-phosphate and fructose 6-phosphate were increased in the HF of mice fed trioctanoin, whereas levels of metabolites further downstream in the glycolytic pathway and the pentose phosphate pathway were reduced. This indicates that trioctanoin reduces glucose utilization because of a decrease in phosphofructokinase activity. Trioctanoin and triheptanoin showed similar anticonvulsant effects in the 6 Hz seizure model, but it remains unknown to what extent the anticonvulsant mechanism(s) are shared. In conclusion, triheptanoin unlike trioctanoin appears to not alter glucose metabolism in the healthy brain.
Keywords: epilepsy, metabolomics, mitochondria, pentose phosphate pathway, seizure, tricarboxylic acid cycle
Introduction
Impaired energy metabolism in the brain has a role in many neurologic conditions. Ketogenic diets and even medium chain triglycerides (MCTs) are effective in the treatment of several forms of childhood drug-resistant epilepsy1 and are currently used in the treatment of early Alzheimer's disease.2 Uneven MCTs are rare in natural foods, but can be chemically produced from natural oils. They are found to be beneficial in several metabolic disorders,3 anticonvulsant in four mouse seizure models,4, 5, 6 and improve cognitive function in a mouse Alzheimer's disease model.7 However, little is known about the effects of even and uneven MCTs on brain energy metabolism.
The majority of energy required by the brain is provided by the oxidation of glucose via glycolysis and the tricarboxylic acid (TCA) cycle. Glucose-derived pyruvate is either metabolized to acetyl-CoA via pyruvate dehydrogenase or is carboxylated by pyruvate carboxylase to provide oxaloacetate, thereby refilling the TCA cycle pool of C4 intermediates (anaplerosis). Thus, via the TCA cycle, glucose provides the substrates oxaloacetate and α-ketoglutarate for the synthesis of the neurotransmitters, aspartate, glutamate, glutamine, and GABA. Ketone bodies are an important energy source for the developing brain, and under conditions such as prolonged starvation where there are glucose deficiencies, the adult brain can also utilize medium chain fatty acids and ketones as an alternate metabolic substrate.
A considerable body of research suggests that glucose is the primary fuel for the brain, and is sufficient to provide all essential brain energy requirements in an individual consuming a regular diet. There is also evidence suggesting that under given conditions, alternate fuels such as medium-chain fatty acids can be used as a fuel for the TCA cycle. Measurements of radioactivity levels after infusion with both [1-11C]- and [1-14C] octanoate have shown that octanoate can readily cross the blood–brain barrier.8 Further studies have shown that with infusion of [1-14C]- or [1-13C] octanoate at 15% to 25% of caloric requirements, the labeled carbon appears predominantly in glutamine as compared with glutamate. As glutamine is predominantly found in astrocytes, this suggests that medium-chain fatty acids are primarily metabolized by astrocytes.9, 10 In cultures of developing rat brain cells, astrocytes are the only cells capable of metabolizing octanoate through respiration to produce carbon dioxide.11 Although these experimental settings were artificial, they provide evidence that when feeding >15% to 50% of calories as medium chain fats, the brain has the capacity to use them as energy substrates in the presence of normal blood glucose. Also, feeding oral octanoate at doses of 10 to 30 mmol/kg was anticonvulsant in several models, which is most easily explained by diffusion into the brain.12 However, even-chain fatty acids such as octanoate can only supply two carbon atoms to the TCA cycle. In contrast, odd-chain fatty acids such as heptanoate can provide the two and three carbon fragments, acetyl-CoA and propionyl-CoA, to the cycle.
Triheptanoin, the triglyceride of the seven carbon-chain fatty acid heptanoate, provides the TCA cycle with both acetyl-CoA and anaplerotic propionyl-CoA. Propionyl-CoA is carboxylated to methyl-malonyl-CoA, which is then metabolized into succinyl-CoA, thereby refilling the C4 TCA cycle intermediate pool. Several studies have shown that triheptanoin can improve cardiac and skeletal muscle function in some long-chain fatty acid oxidation disorders.7, 13, 14 In these reports, a greater improvement was observed with triheptanoin than with even-chain fatty acids. The efficacy and potency of even versus uneven MCTs in the treatment of neurologic disorders is still unexplored; however, both are effective in animal models of seizures. Triheptanoin has been found to be anticonvulsant in four rodent seizure models.4, 5, 6 The exact mechanisms of the anticonvulsant effects are still unknown for even and uneven MCTs. In the present study, we compared the effects chronic trioctanoin and triheptanoin feeding on glucose and TCA cycle metabolism in the mouse hippocampal formation (HF) and the 6 Hz seizure test.
Materials and Methods
Animals and Diets
Male CD1 mice (Australian Research Council, Western Australia, Australia) were individually caged under a 12-hour light–dark cycle where food and water were given ad libitum. All efforts were made to minimize the suffering and the number of animals used. All experiments were approved by the University of Queensland's Animal Ethics Committee and followed the guidelines of the Queensland Animal Care and Protection Act 2001. This work is written according to the ARRIVE guidelines (http://www.nc3rs.org/ARRIVE).
All diets were matched in protein, mineral, antioxidant, and vitamin content relative to their caloric densities. Standard diet (SF11-027) or matched diets (SF11-079) in which 35% of the calories are from either trioctanoin or triheptanoin oil6 were obtained from Specialty Feeds (Western Australia, Australia). Triheptanoin was a gift from Sasol GmbH (Germany) and trioctanoin from Stepan Lipid Nutrition (Maywood, NJ, USA). The oils replaced the sucrose, some of the complex carbohydrates and long-chain fats in the standard diet.
Animals for Metabolite Analysis
The animals used for metabolite analysis were from a larger cohort used to investigate the mouse pilocarpine status epilepticus model of temporal lobe epilepsy. The mice used were injected with methylscopolamine (2 mg/kg intraperitoneally in 0.9% NaCl; Sigma-Aldrich, St Louis, MO, USA) and 15 minutes later pilocarpine (330 to 345 mg/kg subcutaneously in 0.9% saline; Sigma-Aldrich). After a 90-minute observation period, mice were injected with pentobarbital (22.5 mg/kg intraperitoneally in 0.9% NaCl; Provet, Northgate, Queensland, Australia) to stop status epilepticus. Only mice that did not develop status epilepticus were used for metabolic analysis. Those mice are known to behave normally and show no brain abnormalities.15, 16 At 7 to 8 weeks old, and immediately after pilocarpine treatment, mice were initiated on either standard diet or matched diets in which 35% of the calories are from either trioctanoin or triheptanoin oil. Mice were maintained on these diets for three weeks before being killed.
Tissue Extraction
Three weeks after the initiation of feeding of the different diets, the mice were killed by focal microwave fixation to the head at 5 kW for 0.80 to 0.87 seconds, denaturing enzymes and other proteins instantaneously (Model MMW-05, Muromachi, Tokyo, Japan). Afterwards, the mice were decapitated and the cerebral cortices and HF were dissected and stored at −80°C until extracted. All HF samples were sonicated in 0.2 mL of methanol using a Vibra Cell sonicator (Model VCX 750, Sonics and Materials, Newton, CT, USA). Polar metabolites were extracted from samples using a modified Bligh-Dyer water/methanol/chloroform extraction procedure at a 1/2/3 ratio, as previously described.17 Samples were lyophilized and liquid chromatography tandem mass spectrometry analysis was performed by investigators masked to the treatment. Metabolite concentrations are within reported ranges18, 19 and the average coefficient of variation of metabolite levels was 21%, which is expected for biologic samples, despite no internal standard being used to monitor extraction efficiency.
Liquid Chromatography Tandem Mass Spectrometry Analysis
Reference standards and tributylamine (puriss plus grade) were purchased from Sigma-Aldrich (Sigma Aldrich, NSW, Australia). HPLC Grade acetonitrile and acetic acid (AR Grade) were purchased from RCI Labscan (Bangkok, Thailand) and Labscan (Gliwice, Poland), respectively. De-ionized water was generated via an Elga Purelab Classic water purification unit (Veolia Water Solutions and Technologies, Saint Maurice Cedex, France).
Liquid chromatography tandem mass spectrometry data were acquired on a Dionex UltiMate 3000 liquid chromatography system (Dionex, Sunnyvale, CA, USA) coupled to an ABSciex 4000 QTRAP mass spectrometer (ABSciex, Concord, ON, Canada). The liquid chromatography system was controlled by Chromeleon software (Dionex) and chromatographic separation was achieved by injecting 10 μL onto a Gemini-NX C18 150 mm × 2 mm internal diameter, 3 μm 110 Å particle column (Phenomenex, Aschaffenburg, Germany) equipped with a precolumn Security Guard Gemini-NX C18 4 mm x 2 mm intradermal cartridge. The column oven temperature was controlled and maintained at 55°C throughout the acquisition and the mobile phases (adapted from Luo et al.,20) were as follows: 7.5 mmol/L aqueous tributylamine adjusted to pH 4.95 (±0.05) with glacial acetic acid (eluent A) and acetonitrile (eluent B). The mobile phase flow rate was maintained at 300 μL/minute throughout the gradient profile (Table 1) and was introduced directly into the mass spectrometer with no split.
Table 1. Mobile phase gradient profile.
| Time (min) | Eluent A (%) |
|---|---|
| 0 | 100 |
| 8 | 100 |
| 20 | 80 |
| 30 | 73 |
| 31 | 0 |
| 33 | 0 |
| 34 | 100 |
| 50 | 100 |
The mass spectrometer was controlled by Analyst 1.5.2 software (ABSciex) and was equipped with a TurboV electrospray source operated in negative ion mode. The following optimized parameters were used to acquire scheduled multiple reaction monitoring data: ionspray voltage−4,500 V, nebulizer (GS1), auxiliary (GS2), curtain (CUR), and collision (CAD) gases were 60, 60, 20, and medium (arbitrary units), respectively, generated via a N300DR nitrogen generator (Peak Scientific, Billerica, MA, USA). The auxiliary gas temperature was maintained at 350°C. The analyte-dependent parameters for the detection of central carbon metabolites are given in Table 2. For all analytes, the entrance potential (EP) was −10 volts.
Table 2. Analyte-dependent parameters for the transitions used in scheduled multiple reaction monitoring data acquisition.
| Analyte | Q1 Mass (daltons) | Q3 Mass (daltons) | RT (minutes) | DP (volts) | CE (volts) | CXP (volts) |
|---|---|---|---|---|---|---|
| Pyruvate | 87.02 | 43 | 12.5 | −45 | −12 | −1 |
| Lactate | 88.95 | 42.9 | 9.0 | −45 | −18 | −5 |
| Fumarate | 115.01 | 70.9 | 21.4 | −45 | −12 | −1 |
| Succinate | 117 | 73 | 19.0 | −45 | −16 | −3 |
| Oxaloacetate | 130.93 | 86.9 | 22.0 | −25 | −10 | −5 |
| Malate | 133 | 70.8 | 20.1 | −40 | −22 | −3 |
| Alpha-ketoglutarate | 144.95 | 100.8 | 20.9 | −40 | −12 | −5 |
| Phosphoenolpyruvate | 166.83 | 79 | 22.3 | −40 | −18 | −5 |
| Glyceraldehyde 3-phosphate | 168.84 | 97.1 | 10.2 | −40 | −10 | −5 |
| Dihydroxyacetone phosphate | 168.84 | 97 | 12.7 | −50 | −14 | −5 |
| Aconitate | 172.94 | 84.9 | 22.7 | −30 | −18 | −5 |
| 2 & 3-phosphoglycerate | 184.91 | 97 | 21.7 | −50 | −20 | −5 |
| Isocitrate | 190.93 | 111.1 | 22.5 | −45 | −20 | −7 |
| Citrate | 190.96 | 110.9 | 22.5 | −50 | −18 | −7 |
| Ribose 5-phosphate | 228.94 | 96.9 | 9.9 | −65 | −18 | −5 |
| Ribulose 5-phosphate | 228.92 | 96.9 | 11.6 | −55 | −16 | −5 |
| Xylulose 5-phosphate | 228.93 | 97 | 11.4 | −50 | −18 | −5 |
| Glucose 1-phosphate | 259.02 | 78.8 | 10.9 | −65 | −48 | −3 |
| Glucose 6-phosphate | 258.89 | 96.7 | 8.9 | −75 | −22 | −5 |
| Fructose 1-phosphate | 259.02 | 96.8 | 10.5 | −55 | −22 | −5 |
| 6-Phosphogluconate | 274.93 | 97.1 | 21.5 | −60 | −24 | −5 |
| Cytidine monophosphate | 322.07 | 78.8 | 12.4 | −65 | −66 | −3 |
| Uridine monophosphate | 323.01 | 78.8 | 14.1 | −60 | −64 | −3 |
| Fructose 1,6-bisphosphate | 339.08 | 96.9 | 22.1 | −35 | −30 | −5 |
| Adenosine monophosphate | 346.02 | 78.6 | 16.5 | −70 | −62 | −3 |
| Guanosine monophosphate | 362.05 | 78.9 | 14.7 | −60 | −62 | −3 |
| Uridine diphosphate | 403.03 | 78.8 | 22.0 | −60 | −74 | −3 |
| Adenosine diphosphate | 426.07 | 78.8 | 22.5 | −85 | −74 | −3 |
| Guanosine diphosphate | 442.06 | 78.9 | 21.9 | −70 | −76 | −3 |
| Cytidine triphosphate | 481.97 | 158.6 | 29.3 | −75 | −36 | −11 |
| Uridine triphosphate | 483.06 | 158.8 | 30.4 | −65 | −42 | −7 |
| Adenosine triphosphate | 506.1 | 158.7 | 30.5 | −85 | −40 | −11 |
| Guanosine triphosphate | 522 | 158.7 | 30.0 | −80 | −42 | −11 |
| UDP glucose | 565.18 | 323 | 21.3 | −90 | −34 | −7 |
| UDP glucuronate | 579.14 | 79.1 | 29.7 | −90 | −108 | −1 |
| NAD | 662.25 | 540 | 13.6 | −50 | −20 | −9 |
| NADH | 664.2 | 78.8 | 22.7 | −110 | −98 | −1 |
| NADP | 742.2 | 620 | 21.7 | −45 | −24 | −11 |
| NADPH | 744.1 | 79.1 | 30.3 | −120 | −116 | −1 |
| Acetyl CoA | 808.17 | 79.1 | 32.3 | −125 | −54 | −5 |
| Cyclic AMP | 328.088 | 134 | 17.4 | −80 | −36 | −9 |
| Glyoxylate | 72.815 | 45.2 | 6.0 | −45 | −12 | −1 |
| Glycolate | 74.799 | 46.9 | 6.3 | −35 | −14 | −3 |
| Creatine phosphate | 209.738 | 78.8 | 19.7 | −35 | −16 | −3 |
| UDP N-acetylglucosamine | 605.857 | 78.7 | 21.4 | −95 | −106 | −1 |
| Tiglylglycine | 156.053 | 112 | 19.4 | −80 | −16 | −7 |
CE, collision energy; CXP, collision cell exit potential; DP, declustering potential; NAD and NADH, nicotinamide adenine dinucleotide; NADP and NADPH, nicotinamide adenine dinucleotide phosphate; Q, quadrupole; RT, retention time.
The samples were run with sample- and analyte-relevant calibration standards and pooled quality control samples21, 22 to control for reproducibility of data acquisition and to ensure data integrity. Analyte stock solutions were prepared in purified water (Veolia) and aliquots of each solution were mixed to achieve a final calibrant solution at 400 μmol/L for organic acids and at 50 μmol/L for all other metabolites. This calibrant solution was serially diluted and the dilutions used as calibration standards from 0.006 to 400 μmol/L, constituting ⩽17 calibration points for organic acids and ⩽14 calibration points for all other analytes to account for differential responses in the mass spectrometer. Data were processed in MultiQuant 2.1 software (ABSciex).
Enzymatic Metabolite Assays
Glucose and β-hydroxybutyrate (BHB) levels in the HF extracts were measured by quantitative enzyme assays. Briefly both metabolite levels were measured through endpoint enzymatic assays, measuring the NADH produced from glucose metabolized by hexokinase and then glucose-6-phosphate dehydrogenase (Sigma-Aldrich, Ann Arbor, MI, USA), and BHB metabolized by BHB dehydrogenase (Pointe Scientific, Canton, MI, USA). Please note that this assay cannot distinguish between BHB and β-hydroxypentanoate (BHP), a metabolite of heptanoate. However, BHP levels are comparatively low at >20-fold less than BHB.
6 Hz Seizure Test
Fifteen 7-week-old CD1 mice were placed on either standard diet, or diets containing 35% of calories as triheptanoin or trioctanoin. The 6 Hz seizure model was performed as previously described20 on the same mice on days 2, 7, and 21 after diet initiation. The critical current required to induce seizures in 50% of mice (CC50) was determined using the ‘up and down method' varying electrical current intensities at 2-mA intervals. A topical anesthetic (0.5% tetracaine hydrochloride ophthalmic solution) was applied to corneas 15 minutes before a 3-seconds corneal stimulation with 0.2 millisecond-duration pulses at 6 Hz (ECT Unit 57800, Ugo Basile). Mice were held manually during stimulation and released to observe behavioral responses. Seizures were classified as a stunned or fixed posture, rearing, forelimb clonus, or twitching.
Data Analysis
The levels of each metabolite were normalized to the weight of extracted tissue in grams. All statistics were performed by GraphPad Prism software (La Jolla, CA, USA) using a one-way analysis of variance, with a Newman–Keuls post test. P<0.05 was considered statistically significant in post test if the analysis of variance was P<0.05. All data are represented as mean±s.e.m. We chose to use 9 to 11 animals in each group for metabolite analyses and 15 mice per group in the 6 Hz model, based on previous experience of variations in metabolite levels4, 19 and the 6 Hz model.6, 20
Results
Body Weights and Brain Levels of Glucose and Ketones
The changes in body weights were similar to control diet and found to not be significantly different when feeding 35% trioctanoin or triheptanoin diets for up to 21 days (Figure 1). To assess the effects of the ingestion of the even and odd carbon length MCTs trioctanoin and triheptanoin on metabolic pathways in the mouse brain, concentrations of metabolites were analyzed in HF extracts through liquid chromatography tandem mass spectrometry and enzymatic assays.
Figure 1.
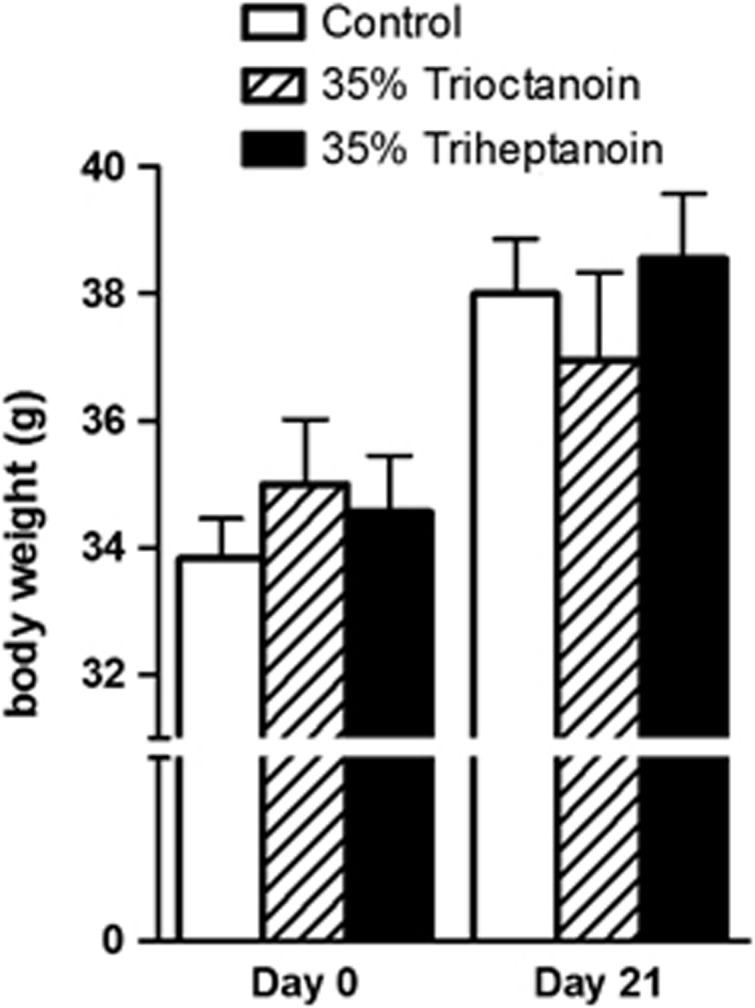
Dietary medium chain triglycerides do not alter body weight in mice. Body weights of CD1 mice, before diet induction and after 3 weeks of feeding a control (white bars, n=11), 35% trioctanoin (striped bars, n=10), or triheptanoin (black bars, n=9) diet ad libitum. Each bar is representative of the mean weight±s.e.m.
The levels of glucose were similar in HF extracts of mice in each treatment group (Table 3). In contrast, the levels of BHB were significantly higher in animals that were fed MCTs, specifically 1.7-fold higher for triheptanoin (P<0.05, n=9) and 1.6-fold for trioctanoin (P<0.05, n=10) compared with those fed a control diet with 92.2±10.1 nmol/g tissue (n=11).
Table 3. Glucose metabolism via glycolysis in extracts of the hippocampal formation from CD1 mice fed a control, 35% trioctanoin, or 35% triheptanoin diets.
| nmol/g tissue | Control n=11 | 35% Trioctanoin n=10 | 35% Triheptanoin n=9 |
|---|---|---|---|
| Glucose | 1860±218 | 2090±255 | 2170±286 |
| Glucose 6-phosphate | 36.3±10.0 | 59.3±10.1a | 14.7±1.6b |
| Fructose 6-phosphate | 32.7±10.1 | 49.1±9.4a | 10.1±1.2b |
| Fructose 1,6-biphosphate | 105±11.6 | 62±4.5c | 84±8.9 |
| Dihydroxyacetone phosphate | 0.50±0.1 | 0.16±0.02a | 0.39±0.09 |
| 2-and 3-phosphoglycerate | 21.8±3.8 | 11.5±1.1a | 26.9±12.1 |
| Phosphoenolpyruvate | 13.5±1.5 | 11.3±2.5 | 8.3±1.9 |
| Lactate | 3400±371 | 3150±346 | 2860±330 |
| Acetyl-CoA | 1.4±0.08 | 1.3±0.1 | 1.3±0.1 |
| β-hydroxybutyrate | 92±10.1 | 153±7.6a | 160±21.4d |
Metabolite levels were quantified in polar metabolite extracts from hippocampal tissue from CD1 mice fed a standard, or 35% trioctanoin or triheptanoin diet for three weeks using liquid chromatography tandem mass spectrometry and enzymatic assays. Values represent mean±s.e.m.
indicates P<0.05 and between trioctanoin and control groups.
indicates that P<0.05 between triheptanoin and trioctanoin treatments.
indicates P<0.01 between trioctanoin and control.
indicates P<0.05 between triheptanoin and control-fed mice.
Metabolism of Glucose Via Glycolysis
The levels of several metabolites produced through the metabolism of glucose via the glycolytic pathway were measured (Table 3). In mice fed a diet where 35% calories are supplied from trioctanoin, a 1.6- (P<0.05) and fourfold (P<0.05) increase was observed in the levels of glucose 6-phosphate, and a 1.5- (P<0.05) and 4.8-fold (P<0.05) increase in fructose 6-phosphate levels in comparison to those fed a control diet and the odd-chain triglyceride, triheptanoin, respectively. Furthermore the levels of downstream metabolites in this pathway, including those of fructose 1,6-bisphosphate were reduced by 40% (P<0.01), dihydroxyacetone phosphate by 67% (P<0.05) and 2- and 3-phosphoglycerate (measured together) by 48% (P<0.05), in mice fed trioctanoin as compared with those fed a control diet. No statistically significant differences were observed in the values for these metabolites between the 35% triheptanoin and the control treatment group.
Metabolites of the Pentose Phosphate Pathway
Xylulose 5-phosphate, ribulose 6-phosphate, and 6-phosphogluconate, three intermediates of the pentose phosphate pathway (PPP) were quantified in the HF (Figure 2). In the HF extracts of mice fed trioctanoin, the concentration of ribulose 5-phosphate was reduced by 60% (P<0.05) and 57% (P<0.05) compared with control and triheptanoin-fed mice, respectively. Similarly, xylulose 5-phosphate levels were reduced in trioctanoin-fed mice by 60% (P<0.05) and 55% (P<0.05). This trend was also observed in the metabolite 6-phosphogluconate, where compared with the control and 35% (P>0.05) triheptanoin diets, the levels were decreased by 25% (P>0.05) in trioctanoin-fed mice; however, the difference was not statistically significant. The concentrations of another important metabolite in this pathway, ribose 5-phosphate, were below the detectable limits of this experiment. Again, no statistically significant differences were observed in the values for these metabolites between the 35% triheptanoin and the control treatment group.
Figure 2.
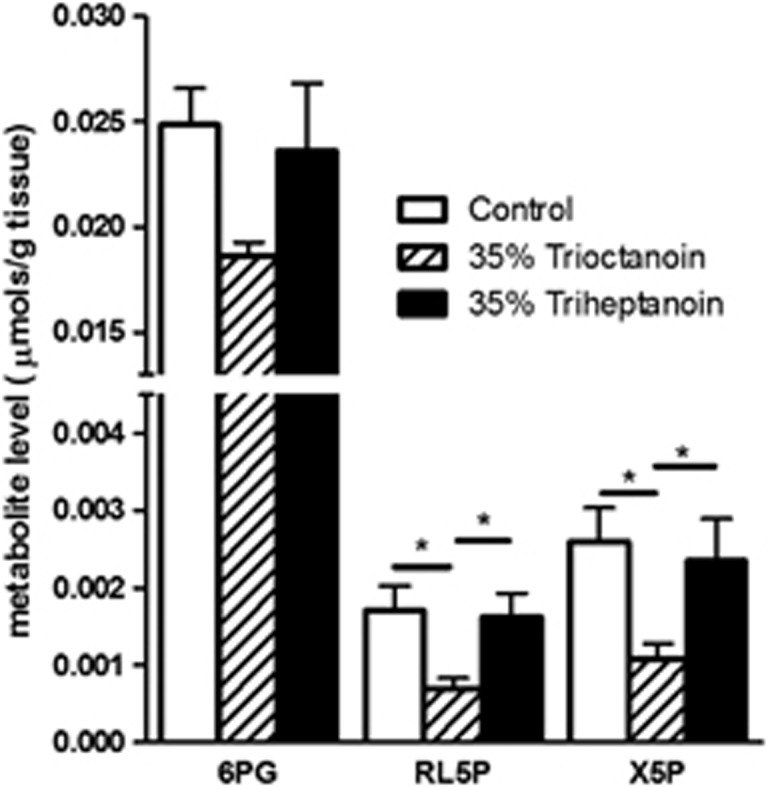
Alterations of levels of the pentose phosphate pathway metabolites after feeding medium-chain triglycerides. Metabolite levels of 6-phosphogluconate (6PG), ribulose 5-phosphate (RL5P), and xylulose 5-phosphate in hippocampal formation brain extracts from mice fed control (white bars, n=11), 35% trioctanoin (striped bars, n=10), and 35% triheptanoin (black bars, n=9) diets for 3 weeks. Data represent mean±s.e.m, with *indicating P<0.05.
Tricarboxylic Acid Cycle Intermediates and High-Energy Metabolites
Few changes were observed in the concentrations of high-energy metabolites after even and uneven MCT feeding (Table 4). Levels of ADP were lowered by 17% in mice fed triheptanoin with respect to those fed a control diet (P<0.05). However, the ratio of ATP to all adenosine nucleotides was not found to be statistically significant among any of the treatment pairs. The concentrations of the TCA cycle intermediates were only reduced in mice fed trioctanoin, namely there were decreases in succinate (50%, P<0.01) and malate (41%, P<0.05) levels compared with control diet. No changes in the levels of TCA cycle intermediates were observed in the HF extracts of mice fed triheptanoin.
Table 4. High-energy metabolite and tricarboxylic acid cycle metabolite levels in CD1 HF extracts from mice fed a control, 35% trioctanoin, or 35% triheptanoin diet.
| μmol/g tissue | Control n=11 | 35% Trioctanoin n=10 | 35% Triheptanoin n=9 |
|---|---|---|---|
| ATP | 1.15±0.04 | 1.34±0.08 | 0.97±0.16 |
| ADP | 0.77±0.02 | 0.69±0.02 | 0.65±0.05a |
| AMP | 0.20±0.008 | 0.22±0.04 | 0.37±0.09 |
| ∑(ATP, ADP, AMP) | 2.15±0.05 | 2.19±0.09 | 1.80±0.15 |
| ATP/∑(ATP, ADP, AMP) | 0.54±0.01 | 0.58±0.02 | 0.49±0.06 |
| NAD+ | 0.24±0.03 | 0.19±0.05 | 0.24±0.02 |
| NADH | 0.010±0.004 | 0.006±0.002 | 0.009±0.001 |
| NADPH | 0.097±0.007 | 0.083±0.008 | 0.086±0.009 |
| Citrate | 0.20±0.03 | 0.20±0.02 | 0.16±0.03 |
| Succinate | 0.12±0.01 | 0.06±0.007b | 0.13±0.01c |
| Fumarate | 0.048±0.004 | 0.045±0.005 | 0.055±0.01 |
| Malate | 0.23±0.02 | 0.13±0.07d | 0.22±0.03e |
NAD and NADH, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate.
Metabolite levels (μmol/g tissue) were quantified using liquid chromatography tandem mass spectrometry of polar metabolite extracts from the hippocampal formation of CD1 mice fed one of a standard diet, a 35% trioctanoin diet, or a 35% triheptanoin diet for 3 weeks. Values represent mean±s.e.m. for each metabolite
P<0.05 between control and triheptanoin treatments.
P<0.01 between these trioctanoin and control.
P<0.01 between trioctanoin and triheptanoin groups.
statistically significant difference between trioctanoin and control groups (P<0.05).
indicates a statistically significant difference between trioctanoin and triheptanoin-fed groups (P<0.05).
Seizure Susceptibility
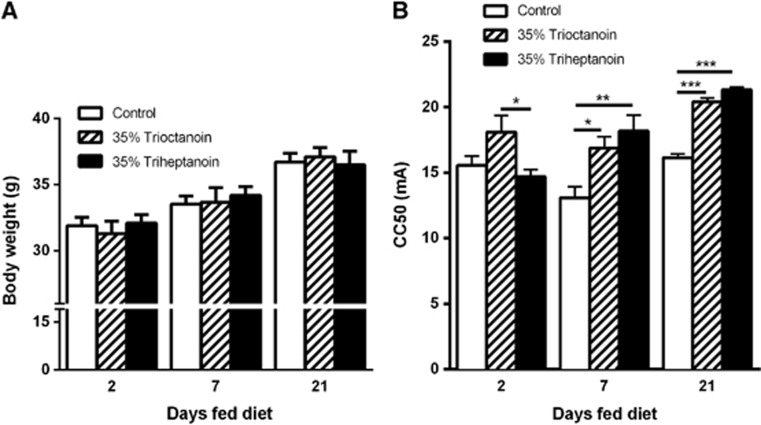
The body weights of mice between each treatment group were similar at each given time point (Figure 3A). The 6 Hz test was used to compare the effects of triheptanoin versus trioctanoin on seizure susceptibility in mice after feeding these oils for 2, 7, and 21 days (Figure 3B). After two days of feeding, trioctanoin-fed mice showed a higher seizure threshold by 3.41 mA relative to triheptanoin (P<0.05). At the later time points, both MCT oils increased the seizure thresholds in a similar fashion compared with control diets. After 7 and 21 days of feeding, the CC50 value was increased by 3.79 mA (P<0.05) and 4.3 mA (P<0.001) with trioctanoin and by 5.09 mA (P<0.01) and 5.2 mA (P<0.001) with triheptanoin respectively, as compared with the control diet.
Figure 3.
Similar anticonvulsant effects with even and uneven medium-chain triglycerides in the 6 Hz model. (A) Body weights of mice in each treatment group 2, 7, and 21 days after feeding of control (white bars, n=15), 35% trioctanoin (striped bars, n=15), or 35% triheptanoin (black bars, n=15) diets. No significant difference was observed in the weights of mice between treatment groups at any of the time points. (B) Effects of 2, 7 days and 21 days feeding of 35% trioctanoin (striped bars, n=7), 35% triheptanoin (black bars, n=6) and control (white bars, n=7) diets in the 6 Hz test in CD1 mice. The critical currents required for 50% of animals to seize were calculated using the up-and-down method, varying the current intensity by 2 mA. *represents P<0.05; **represents P<0.01; ***represents P<0.001. Means±s.e.m are shown.
Discussion
Reduction of Glucose Utilization with Trioctanoin Feeding
The various pathways of glucose metabolism were analyzed through the determination of the concentration of intermediates in extracts of the HF in CD1 male mice that were fed ad libitum either a standard diet or matched diets containing 35% of calories as MCTs trioctanoin or triheptanoin. No statistically significant differences were observed in body weights of mice over the 3-week feeding period, which correlates with the earlier findings that the diets are equally palatable and metabolically adequate.4 Surprisingly, we found that trioctanoin, but not triheptanoin, feeding changed several HF levels of metabolites involved in glycolysis, the PPP and TCA cycle, suggesting that HF glucose utilization is reduced in mice fed trioctanoin. As the levels of brain glucose were similar across all treatment groups, we believe that it is unlikely that the observed changes are due solely to a loss of glucose uptake in the brain. Furthermore, MCTs replaced 32% of calories from the provided carbohydrates and the even-chain fatty acids are not thought to be gluconeogenic. Therefore, an increase in fatty acid oxidation and reduction of glucose utilization is expected to some extent. The notion that the brain has the capacity to use octanoate as a fuel is corroborated by the previous findings that in the developing brain, astrocytes can use octanoate to produce carbon dioxide via respiration.11 Furthermore via isotopic analysis octanoate has been shown to be metabolized to both glutamate and glutamine in the brain.9 In addition, the effects of trioctanoin on the metabolism of the HF appear to be similar to those found in muscle metabolism. In muscle, it is generally accepted that an increased supply of even MCTs (mainly consisting of triglycerides of octanoic and decanoic acids) leads to the subsequent inhibition of carbohydrate utilization, independent of blood glucose levels.23, 24 This effect may not happen with triheptanoin because uneven fatty acids are gluconeogenic and anaplerotic.25 Therefore, relative to trioctanoin, triheptanoin is expected to result in more glucose utilization.
Triheptanoin does not Appear to Alter Glucose Metabolism
Levels of intermediates of glucose and TCA cycle metabolism were largely unaltered by triheptanoin in the hippocampus of mice without neurologic problems in this and previous studies.4 Heptanoate is β-oxidized to acetyl-CoA and propionyl-CoA, both of which can supply carbons to the TCA cycle. Acetyl-CoA is a direct substrate for the TCA cycle, whereas propionyl-CoA can be metabolized through the anaplerotic propionyl-CoA carboxylation pathway to provide the cycle with succinyl-CoA. In addition, the liver uses propionyl-CoA for the formation of glucose via gluconeogenesis. Long-term feeding elevated the levels of propionyl- and methylmalonyl-CoA in brains of mice in the chronic stage of the pilocarpine-induced status epilepticus models.4 Heptanoate metabolism appears to maintain glycolysis and not inhibit phosphofructokinase activity in healthy tissue, most likely because it is gluconeogenic and anaplerotic.25 When [3,4,5-13C] heptanoate was provided though intracarotid infusion, which provided 50% of caloric requirements, most of the labeling derived from heptanoate was found in glutamine, again indicating that MCTs are metabolized primarily in astrocytes. As astrocytic end feet surround the capillaries, it can be proposed that heptanoate would be mostly taken up into the astrocytes, where through the propionyl-CoA carboxylation pathway or through metabolism to acetyl-CoA, it can enter the TCA cycle. The carbon skeleton of α-ketoglutarate produced in the TCA cycle is then incorporated into glutamate by transamination reactions or by glutamate dehydrogenase, and further converted to glutamine by glutamine synthetase. Glutamine can then provide a source of energy to the neuronal TCA cycle through the glutamate–glutamine shuttle.26 Conversely [1,2-13C] glucose that was derived from the labeled heptanoate was primarily metabolized in neurons, indicated by the higher levels of label found in glutamate.19 The lack of triheptanoin's effect on these major metabolic pathways would appear to be beneficial, as fewer metabolic side effects would be expected to result from the therapeutic administration of triheptanoin.
Reduction of Phosphofructokinase Activity by Trioctanoin
A key finding of this study was the loss of fructose 1,6-bisphosphate and downstream metabolites of the glycolytic pathway in mice fed trioctanoin. This coupled with the findings that fructose 6-phosphate and glucose 6-phosphate levels were increased, indicates that phosphofructokinase activity is inhibited in the HF in mice that were fed trioctanoin but not triheptanoin. This is consistent with previous studies showing that both octanoate and ketone bodies inhibit the activity of phosphofructokinase both in vitro and in vivo.27, 28 In the isolated rat heart, perfusion of fatty acids or ketone bodies results in the depression of phosphofructokinase activity and glucose phosphorylation.27 Both fructose 6-phosphate and glucose 6-phosphate accumulate with a loss of phosphofructokinase activity, leading to inhibition of hexokinase and subsequent reduction of glucose metabolism and anaplerosis via pyruvate carboxylation.
One proposed mechanism for this inhibition of phosphofructokinase is through the accumulation of two negative regulators, acetyl-CoA and citrate, that are produced through the β-oxidation of MCTs or even chained ketone bodies.29 β-oxidation as well as production of acetyl-CoA and citrate are proposed to occur mostly in glia,11 whereas glucose metabolism occurs in both glia and neurons. However, this does not correlate with our findings that both acetyl-CoA and citrate levels were similar in our three diet groups, suggesting this mechanism may be more complex. Nishimura and Uyeda30 provided evidence for a more complex activation mechanism of glycolysis in the rat liver, where xylulose 5-phosphate, an intermediate of the PPP, increases glycolytic activity. Xylulose 5-phosphate, a metabolite shown to be reduced by trioctanoin feeding, activates a specific protein phosphatase 2A, which in turn increases production of fructose 2,6-bisphosphate, a potent activator of phosphofructokinase.30, 31 In contrast, incubation with propionate, a possible metabolite of heptanoate, does not influence the levels of xylulose 5-phosphate or fructose 2,6-bisphosphate, and thus does not inhibit phosphofructokinase activity.32
Even-Chain Fatty Acids Alter Metabolism Through the Pentose Phosphate Pathway
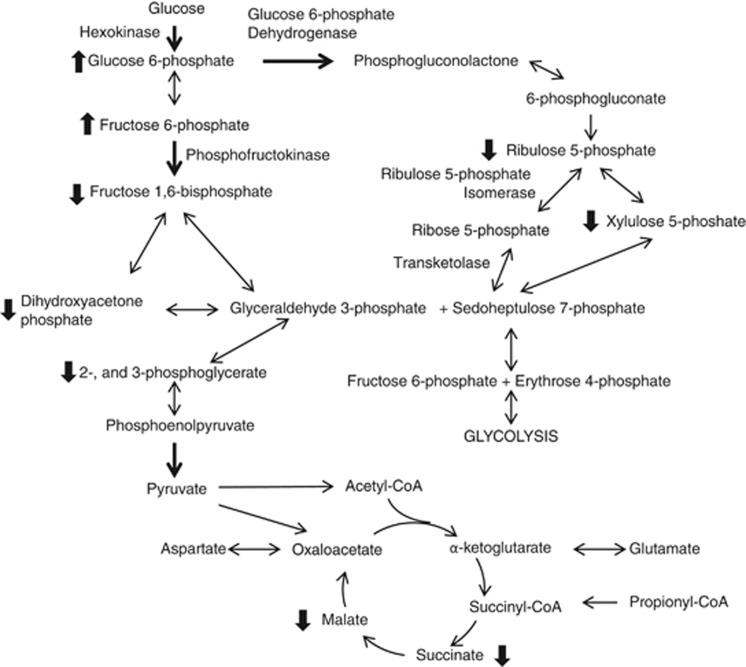
In the HF, the reduction in xylulose 5-phosphate levels coincided with a decrease in ribulose 5-phosphate levels in mice fed trioctanoin. Similar to the glycolytic intermediates, these reductions were not observed with triheptanoin. These findings can be interpreted either as a (1) decrease or (2) increase of the PPP activity with even MCT feeding. (1) Glucose 6-phosphate dehydrogenase, which catalyzes the reaction of glucose 6-phosphate to 6-phosphogluconolactone, is the limiting reaction in the PPP pathway. There is evidence that diets high in polyunsaturated fatty acids limit the synthesis of this enzyme,33, 34 which would lead to a decrease in PPP activity. Little is known of the effect of MCT on brain PPP metabolism. In light of the data from this study, it will be of interest to investigate the effect of medium-chain fatty acids on glucose 6-phosphate dehydrogenase. (2) Alternatively, the loss of PPP intermediates could reflect an increase in PPP activity to compensate for the reduction of glycolytic activity to facilitate the metabolism of glucose. Citrate levels increase acutely after the application of octanoate in guinea pig hearts.35 This initial increase was associated with the inhibition of phosphofructokinase activity, reducing the production of downstream glycolytic intermediates, such as glyceraldehyde-3-phosphate, which is also a product of the PPP. We did not observe increases in citrate levels in our steady-state measurements. However, it is possible that it was missed because of fast turnover rate of TCA cycling. The loss of glyceraldehyde 3-phosphate may drive the reactions by ribulose 5-phosphate isomerase and transketolase to completion, which would explain the observed loss in xylulose 5-phosphate and ribulose 5-phosphate by an increase in PPP activity (Figure 4).
Figure 4.
Simplified schematic representation of the alterations within glucose metabolism pathways in the brain after feeding of medium-chain triglycerides. The metabolism of glucose by glycolysis and the pentose phosphate pathway is shown, along with the TCA cycle. Dark arrows represent regulatory steps in the pathway. Only the enzymes involved in reactions where changes in metabolite levels were observed are labeled. Thick arrows next to metabolites indicate significant differences between trioctanoin- and control diet-fed mice in the direction of arrow.
Levels of Tricarboxylic Acid Cycle Intermediates and Adenosine Nucleotides after Medium-Chain Triglyceride Feeding
Feeding of trioctanoin reduced the levels of two of the intermediates in the TCA cycle, succinate and malate, which suggests that the TCA cycling is altered. However, as only static levels of metabolites were measured, we were unable to determine whether this is caused by an increase in flux because of increased acetyl-CoA entry via citrate synthase or a reduction in the cycling resulting from a loss of glucose utilization. Triheptanoin did not result in a reduction of these intermediates levels, which correlates with previous studies in mice fed a 35% triheptanoin diet for 3 weeks.4 Triheptanoin may restore the levels of the TCA cycle intermediates through the supply of succinyl-CoA through the anaplerotic propionyl-CoA pathway. In addition, as previously stated, triheptanoin appears not to inhibit glycolysis, and thus the flux through the TCA cycle may not be altered in these mice.
Levels of ADP were lowered by 17% in mice fed triheptanoin. This was surprising, because it was not observed in a previous study investigating the metabolism of 13C-glucose after triheptanoin feeding in the same mouse strain (data unpublished). In both studies, the total amount of all adenosine nucleotides and the ratio of ATP against all adenosine nucleotides remained unchanged as compared with control-fed mice.
Anticonvulsant Effects of Even versus Uneven Medium-Chain Triglycerides and Mechanism of Anticonvulsant Effects
Despite the differences in the metabolite concentrations in the HF after trioctanoin versus triheptanoin feeding in this study, both MCTs showed similar anticonvulsant effects in the acute 6 Hz seizure model. These anticonvulsant effects are similar in magnitude to those found after ketogenic diet feeding in this model.20 In a previous study of CD1 mice, no consistent anticonvulsant effects of triheptanoin were observed in the 6 Hz model.6 A possible explanation for this discrepancy is that CD1 mice used in previous studies were from a local mouse colony; however, the mice used in this study obtained from a different colony at the Australian Research Council, which is genetically slightly different. As we do not have a comparison of efficacy of trioctanoin versus triheptanoin versus ketogenic diet in several seizure models, it is difficult to speculate on the anticonvulsant mechanism(s) of these dietary treatments. We concluded earlier that the anticonvulsant mechanism(s) of the ketogenic diet in mice may involve a reduction of glucose utilization as fuel.20 The anticonvulsant actions of 2-deoxyglucose and fructose 1,6-bisphosphate found in animal models36, 37, 38 also suggest that reduced glycolysis and/or increased PPP can be anticonvulsant. As trioctanoin appears to alter glucose utilization through glycolysis and the PPP, it is possible that these metabolic alterations are involved in increasing the seizure threshold in naïve mice in this model with trioctanoin but not triheptanoin. Also, it is possible that the anticonvulsant mechanisms observed by both MCTs in this model are similar and could be explained by anticonvulsant effects of BHB. Similar BHB levels were reached in the HF by both oils to those achieved by the ketogenic diet in a similar mouse strain.39 Furthermore, this would suggest that the anticonvulsant effects in the 6 Hz model are independent of the possible anaplerotic effects of triheptanoin. However, the anaplerotic potential of triheptanoin is expected to add advantages in chronic disease models with metabolic disturbances, and in clinical settings.3, 4, 13, 14
Previously, it has been hypothesized that anaplerosis is an advantage, specifically to the ‘epileptic' brain in which TCA cycle intermediates levels are reduced.4, 19 So far, to our knowledge trioctanoin has not been tested in chronic epilepsy models. From this study, one could expect that trioctanoin may be less effective than triheptanoin in chronic epilepsy models because trioctanoin reduces the levels of TCA cycle intermediates and is not anaplerotic. As discussed above, the reduced PPP metabolite levels found after trioctanoin feeding are difficult to interpret. However, if they are an indication of increased flux through the PPP, this would permit glucose catabolism, resulting in increased production of NADPH, which increases the potential to reduce glutathione disulfide by glutathione reductase, and allows production of anaplerotic pyruvate. Although we did not find an increase in the NADPH levels with trioctanoin, the conclusions from these data are limited, as NADP+ levels were below detectable levels. Increased flux through the PPP is a proposed mechanism for the anticonvulsant effect observed by fructose-1,6-bisphosphate.40
Conclusion
In comparison with trioctanoin, triheptanoin feeding had only few significant effects on metabolite levels in the HF, predicting few metabolic side effects for triheptanoin. Both MCTs showed similar anticonvulsant effects in healthy brains, thus the anticonvulsant effects appear to be independent of changes in glucose metabolism. The HF metabolite level changes found with trioctanoin raise several questions, mostly in terms of alteration in PPP activity and its effects and mechanism of action in models of chronic epilepsy and Alzheimer's disease.
Acknowledgments
The authors are grateful to Mussie Hadera for comments on the manuscript and funding by the Australian National Health and Research Council (Grants 63145 and 104407 to KB). We thank Sasol and Stepan Lipid Nutrition for providing triheptanoin and trioctanoin in kind.
KB has filed for a complete US patent regarding triheptanoin as a treatment for seizures. No other authors have any interests/conflicts to disclose.
References
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31–55. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CR, Mochel F. Anaplerotic diet therapy in inherited metabolic disease. J Inherit Metab Dis. 2006;29:332–340. doi: 10.1007/s10545-006-0290-3. [DOI] [PubMed] [Google Scholar]
- Willis S, Stoll J, Sweetman L, Borges K. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis. 2010;40:565–572. doi: 10.1016/j.nbd.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Borges K, Petrou S, Reid CA. Triheptanoin reduces seizure susceptibility in a syndrome-specific mouse model of generalized epilepsy. Epilepsy Res. 2013;103:101–105. doi: 10.1016/j.eplepsyres.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Thomas NK, Willis S, Sweetman L, Borges K. Triheptanoin in acute mouse seizure models. Epilepsy Res. 2012;99:312–317. doi: 10.1016/j.eplepsyres.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Aso E, Semakova J, Joda L, Semak V, Halbaut L, Calpena A, et al. Triheptanoin supplementation to ketogenic diet curbs cognitive impairment in APP/PS1 mice used as a model of familial Alzheimer's disease. Curr Alzheimer Res. 2013;10:290–297. doi: 10.2174/15672050112099990128. [DOI] [PubMed] [Google Scholar]
- Kuge Y, Yajima K, Kawashima H, Yamazaki H, Hashimoto N, Miyake Y. Brain uptake and metabolism of [1-11C] octanoate in rats: pharmacokinetic basis for its application as a radiopharmaceutical for studying brain fatty acid metabolism. Ann Nucl Med. 1995;9:137–142. doi: 10.1007/BF03165040. [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy Contribution of Octanoate to Intact Rat Brain Metabolism Measured by 13C Nuclear Magnetic Resonance Spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer JE, Teal HM, Heath DF, Cavanagh JB. The influence of portocaval anastomosis on the metabolism of labeled octanoate, butyrate and leucine in rat brain. J Neurochem. 1977;28:215–222. doi: 10.1111/j.1471-4159.1977.tb07729.x. [DOI] [PubMed] [Google Scholar]
- Edmond J, Robbins R, Bergstrom J, Cole R, De Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res. 1987;18:551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- Wlaź P, Socała K, Nieoczym D, Łuszczki JJ, Żarnowska I, Żarnowski T, et al. Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology. 2012;62:1882–1889. doi: 10.1016/j.neuropharm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Shan Z, She D, Foong S, Kurniawan N, Reutens D.MRI changes and complement activation correlate with epileptogenicity in a mouse model of temporal lobe epilepsy Brain Struct Funct 2013. doi: 10.1007/s00429-013-0528-4(e-pub ahead of print). [DOI] [PubMed]
- Le Belle JE, Harris NG, Williams SR, Bhakoo KK. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed. 2002;15:37–44. doi: 10.1002/nbm.740. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Smeland OB, Hadera MG, McDonald TS, Sonnewald U, Borges K. Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J Cereb Blood Flow Metab. 2013;33:1090–1097. doi: 10.1038/jcbfm.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J Chromatogr A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Sangster T, Major H, Plumb R, Wilson AJ, Wilson ID. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC–MS-based metabonomic analysis. Analyst. 2006;131:1075–1078. doi: 10.1039/b604498k. [DOI] [PubMed] [Google Scholar]
- Hodson MP, Dear GJ, Griffin JL, Haselden JN. An approach for the development and selection of chromatographic methods for high-throughput metabolomic screening of urine by ultra pressure LC-ESI-ToF-MS. Metabolomics. 2009;5:166–182. [Google Scholar]
- Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décombaz J, Arnaud MJ, Milon H, Moesch H, Philippossian G, Thélin AL, et al. Energy metabolism of medium-chain triglycerides versus carbohydrates during exercise. Eur J Appl Physiol. 1983;52:9–14. doi: 10.1007/BF00429018. [DOI] [PubMed] [Google Scholar]
- Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab. 2013;33:175–182. doi: 10.1038/jcbfm.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Randle PJ, Manchester KL. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature. 1962;193:270–271. doi: 10.1038/193270a0. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A, Bowman RH. Regulation of phosphofructokinase activity by citrate in normal and diabetic muscle. Biochem Biophys Res Commun. 1963;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes and starvation. Nature. 1963;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Uyeda K. Purification and characterization of a novel xylulose 5-phosphate-activated protein phosphatase catalyzing dephosphorylation of fructose-6-phosphate,2-kinase: fructose-2,6-bisphosphatase. J Biol Chem. 1995;270:26341–26346. doi: 10.1074/jbc.270.44.26341. [DOI] [PubMed] [Google Scholar]
- Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci USA. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet S, Morand C, Besson C, Rémésy C, Demigné C. Effects of propionate on rat hepatocyte metabolism. J Nutr Biochem. 1998;9:652–658. [Google Scholar]
- Stabile LP, Klautky SA, Minor SM, Salati LM. Polyunsaturated fatty acids inhibit the expression of the glucose-6-phosphate dehydrogenase gene in primary rat hepatocytes by a nuclear posttranscriptional mechanism. J Lipid Res. 1998;39:1951–1963. [PubMed] [Google Scholar]
- Salati LM, Amir-Ahmady B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr. 2001;21:121–140. doi: 10.1146/annurev.nutr.21.1.121. [DOI] [PubMed] [Google Scholar]
- Nakatsu K, Mansour TE. Effect of perfusion with different substrates and with isoproterenol on phosphofructokinase activity in the isolated guinea pig heart. Mol Pharmacol. 1973;9:405–413. [PubMed] [Google Scholar]
- Gasior M, Yankura J, Hartman AL, French A, Rogawski MA. Anticonvulsant and proconvulsant actions of 2-deoxy-D-glucose. Epilepsia. 2010;51:1385–1394. doi: 10.1111/j.1528-1167.2010.02593.x. [DOI] [PubMed] [Google Scholar]
- Lian X-Y, Khan FA, Stringer JL. Fructose-1, 6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27:12007–12011. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2,deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Klein J, Borges K. The ketogenic diet changes metabolite levels in hippocampal extracellular fluid. Neurochem Int. 2011;58:5–8. doi: 10.1016/j.neuint.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Xu K. Possible mechanisms for the anticonvulsant activity of fructose-1,6-diphosphate. Epilepsia. 2008;49:101–103. doi: 10.1111/j.1528-1167.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]