Abstract
Previous studies have highlighted the enormous potential of cell-based therapies for stroke not only to prevent ischemic brain damage, but also to amplify endogenous repair processes. Considering its widespread availability and low immunogenicity human umbilical cord blood (HUCB) is a particularly attractive stem cell source. Our goal was to investigate the neurorestorative potential of cryopreserved HUCB mononuclear cells (MNC) after permanent middle cerebral artery occlusion (MCAO) in spontaneously hypertensive rats (SHR). Human umbilical cord blood MNC or vehicle solution was administered intravenously 24 hours after MCAO. Experimental groups were as follows: (1) quantitative polymerase chain reaction (PCR) of host-derived growth factors up to 48 hours after stroke; (2) immunohistochemical analysis of astroglial scarring; (3) magnetic resonance imaging (MRI) and weekly behavioral tests for 2 months after stroke. Long-term functional outcome and lesion development on MRI were not beneficially influenced by HUCB MNC therapy. Furthermore, HUCB MNC treatment did not change local growth factor levels and glial scarring extent. In summary, we could not demonstrate neurorestorative properties of HUCB MNC after stroke in SHR. Our results advise caution regarding a prompt translation of cord blood therapy into clinical stroke trials as long as deepened knowledge about its precise modes of action is missing.
Keywords: cell therapy, experimental stroke, functional recovery, human umbilical cord blood, neurorestoration, spontaneously hypertensive rat
Introduction
Cerebral ischemia is one of the leading causes for death and disability in industrialized countries. The most crucial limitation of stroke treatment is the narrow time window for effective clot lysis that has often elapsed when stroke patients arrive at hospital. Much effort has thus been made to explore novel therapeutic concepts that permit successful treatment even days after stroke onset. Amongst others, cell-based therapies are considered a promising approach, especially for those patients being ineligible for thrombolysis or missing the thrombolysis time window. Human umbilical cord blood (HUCB) mononuclear cells (MNC) constitute a particularly attractive stem cell source because of little ethical concerns on the use in humans, long-term cryostorage eligibility, and widespread availability.1 Another advantage relates to the immaturity of HUCB MNC and their immune naivete limiting the incidence and severity of graft–versus-host diseases compared with bone marrow transplants.2There is ample experimental evidence for functional improvement after intravenous HUCB MNC transplantation in animal models of stroke from our group3, 4 (using spontaneously hypertensive rats, SHR) and others.5, 6, 7 However, underlying mechanisms are not yet fully understood. Human umbilical cord blood MNC are able to adopt a neuronal phenotype in cell culture, but integration and neuronal differentiation of grafted HUCB MNC in the recipient's central nervous system, if occurring at all, is a rare event.8 Thus, the common understanding is that HUCB MNC exert their beneficial properties via bystander effects rather than cell replacement. A widely discussed mode of action is that HUCB MNC might increase local growth factor levels thereby contributing to a regenerative microenvironment.9 In fact, a recent study revealed that transplanted HUCB MNC enhance local messenger RNA (mRNA) levels of vascular endothelial growth factor and brain-derived neurotrophic factor (BDNF) after perinatal hypoxic–ischemic brain injury in vivo thereby promoting early neuronal survival.10 The cellular origin of these factors is unclear; however, increasing evidence suggests that reactive astrocytes primarily contribute to local growth factor expression,11 an effect that can be further enhanced by bone marrow cells.12
Some well-designed studies were not able to fully reproduce the aforementioned beneficial effects of HUCB MNC after experimental stroke.13, 14 It is conceivable that differences in cell storage and processing may contribute to these controversial results. In fact, there is an ongoing debate whether either the composition or the functionality of cord blood cells or both is altered by the process of cryopreservation.15 This applies all the more, as HUCB consists of many cell types and it is currently unclear which cell subset (or combination of subsets) exactly determines the efficacy of HUCB MNC in ischemic stroke. Although many preclinical studies that reported beneficial effects of HUCB MNC after central nervous system injury used freshly donated cord blood, this approach will not be feasible in the clinical routine. Moreover, long-term assessment of functional endpoints as especially relevant for the human target group was only performed in a minority of studies reporting positive impact of HUCB MNC treatment on stroke recovery.3
Hence, the primary aim of the present study was to assess whether intravenous transplantation of cryopreserved HUCB MNC induces sustained functional benefits after ischemic stroke in SHR, a strain exhibiting comorbidities such as hypertension and hypercholesterolemia representing common stroke risk factors. The potential therapeutic impact was also evaluated by sequential magnetic resonance imaging (MRI) assessing lesion development and secondary hemispheric atrophy. As additional endpoints, transcriptional modulation of endogenous growth expression by HUCB MNC treatment and its influence on postischemic astrogliosis as possible mediators of functional recovery were assessed.
Materials and Methods
Sample Size Calculation
Functional recovery was defined as the primary endpoint of this study. Group sizes were intended to detect an effect size of 25% in the area under the curve analysis of the respective behavioral tests and were calculated via a priori power analysis with α=0.05 and β=0.8. Sigma values (standard deviation, s.d.) were derived from former experiments and set to 20% of the area under the curve for the ladder rung test and 15% for the modified neurologic severity score (mNSS).16 Thus, the required sample size (two-tailed) was 12 animals per group for the ladder rung test and seven animals per group for the mNSS. Secondary endpoints comprised infarct volume and secondary hemispheric atrophy on MRI. Given the computed sample size regarding the primary endpoint, detectable effect sizes were estimated at 35% for both outcome parameters. Changes in local growth factor mRNA expression were considered tertiary endpoints. Analysis was only undertaken exploratory thereby not being sufficiently powered to detect small intergroup differences.
Preparation of Human Umbilical Cord Blood Mononuclear Cells
Human cord blood was obtained from healthy full-term pregnancies after informed consent according to the principles outlined in the Declaration of Helsinki. The mononuclear fraction was separated from the cord blood by density gradient centrifugation. Subsequently, the obtained MNC population was frozen to −80°C in 10% dimethyl sulfoxide and fetal calf serum using a Nalgene cooling box. Before use, vials were thawed in a water bath (37°C). Then, vials were rapidly transferred to 15 mL Falcon tubes with 1 mL of DNAse-containing buffer (75 U/mL DNAse/0.5 mol/L Mg2Cl2). 10 mL of RPMI medium (PAA, Linz, Austria) were added to dilute dimethyl sulfoxide. After centrifugation, the cell pellet was washed three times in RPMI and 10% fetal calf serum. Finally, the number of live and dead cells was determined by trypan blue staining. Viability data and information regarding the content of CD34+ cells among HUCB MNC are provided by Supplementary Table 1 in the Supplementary Information.
Experimental Stroke and Group Allocation
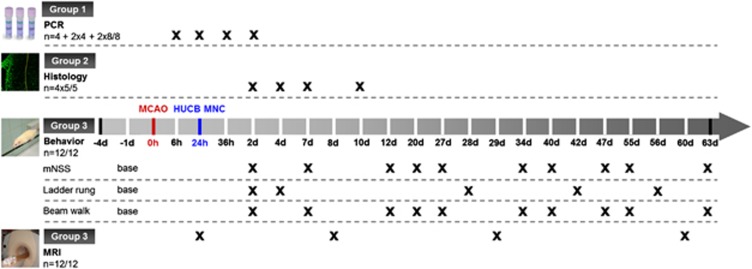
Investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were performed according to the ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). All animal procedures were approved by the appropriate federal agency (protocol number TVV18/07). In total, 108 male SHR at the age of 14 weeks were subjected to permanent distal middle cerebral artery occlusion (MCAO) according to the Tamura model.17 After surgery, animals were randomly assigned to cell therapy or a control group (n=48/60) and subsequently divided into experimental subgroups as follows (Figure 1). Group 1: quantitative real-time PCR of growth factors and astroglial markers (n=16/24; n=4 at 6 hours and 24 hours, and n=8/8 at 36 hours and 48 hours post MCAO, respectively); group 2: immunofluorescence analysis of reactive astrogliosis (n=20/20; n=5/5 at 2, 4, 7, and 10 days after MCAO); group 3: long-term investigation of functional recovery and infarct volume (n=12/12, surveillance period: 63 days after MCAO). Balanced randomization was performed by drawing lots. One day after MCAO, animals allocated to the therapy group received an intravenous injection of 8 × 106 HUCB MNC per kilogram bodyweight dissolved in 0.8 mL phosphate-buffered solution (PBS), while the control group received the same amount of vehicle solution. The injection rate was 0.8 mL/minute.
Figure 1.
Study design. MCAO: middle cerebral artery occlusion; human umbilical cord blood (HUCB) mononuclear cells (MNC): systemic cord blood therapy; polymerase chain reaction (PCR): quantitative real-time PCR; histology: immunofluorescence analysis of astroglial scarring; MRI: magnetic resonance imaging.
Exclusion Criteria
The exclusion criteria were as follows: (i) death within 24 hours after MCAO (ii) lack of an ischemic lesion in the distal supply territory of the MCA in T2-weighted (T2-w) MRI 1 day after stroke induction. To compensate for dropouts, five additional animals were enrolled to the study population, resulting in an overall study population of 113 rats.
Brain Tissue Sampling
Animals were killed according to survival periods. For analysis of mRNA expression (group 1), rats were transcardially perfused with ice-cold PBS. Removed brains were cut into 2 mm thick coronal slices and samples of approximately 8 mm3 were taken from the upper and lower infarct border zone. The infarct border zone could be differentiated from intact brain tissue by slight changes of opacity and color. All specimens were transferred into RNAlater RNA stabilization reagent (Qiagen, Hilden, Germany) and stored at −20°C until further use. Animals randomized to immunofluorescence analysis of astroglial scarring (group 2) were perfused with body-warm PBS and ice-cold formalin solution (4%). Thereafter, brains were removed, vitrified in 30% sucrose, and cryopreserved at −80°C. Further staining procedures were performed on coronal cryosections (20 μm) mounted on coated slides.
Quantitative Real-Time Polymerase Chain Reaction
Gene expression profiles of rodent nerve growth factor, BDNF, basic fibroblast growth factor, insulin-like growth factor 1 (IGF-1), glial fibrillary acidic protein (GFAP), Nestin, and the housekeeping gene YWHAZ were evaluated 6 (n=4), 24 (n=4), 36 (n=8/8), and 48 hours (n=8/8) after onset of ischemia and in age-matched naïve SHR (without MCAO; n=4) using quantitative RT-PCR. RNA was extracted using the RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. DNA was digested by applying one unit of RQ1 DNAse per μg total RNA (Promega, Mannheim, Germany) at 37°C for 30 minutes. RNA concentration and purity were measured using a NanoDrop (Peqlab, Erlangen, Germany) at 260 and 280 nm. Single-strand cDNA copies were generated from 1 μg purified total RNA by using random primers and superscript III reverse transcriptase (Life Technologies, Darmstadt, Germany). Polymerase chain reaction was performed and monitored by ABI 7900 Real-Time PCR System (Life Technologies) applying the following conditions: initial denaturation at 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The primers (Supplementary Table 2, Supplementary Information) were synthesized by Eurofins MWG Operon (Ebersberg, Germany). All PCRs were performed in 20 μL total volume with Power SYBR Green I PCR Master Mix (Life Technologies). Relative quantification was performed after normalization to the housekeeping gene YWHAZ and with samples of healthy controls as reference. All procedures were performed without the knowledge of group allocation.
Immunofluorescence Analysis of Reactive Astrogliosis
The density of GFAP and Nestin-positive structures within the ischemic lesion border was determined 2, 4, 7 and 10 days after stroke onset (n=5/5 each day). Four sections per subject (Bregma anteroposterior +1.6 mm, +0.7 mm, −0.2 mm, and −1.1 mm) were processed for immunofluorescence. The brain sections were mounted on Superfrost slides (Resolab, Wetter, Germany) and pretreated with blocking buffer (PBS, 0.2% Triton X-100 and 5% goat serum) for 1 hour, followed by incubation with mouse monoclonal antibodies for GFAP (1:200, Abcam, Cambridge, UK) and Nestin (1:300, Abcam) for 24 hours at 4°C. Primary antibodies were visualized by incubation with tetramethylrhodamine and fluorescein conjugates of goat anti-mouse immunoglobulin G (1:200; Sigma-Aldrich, Munich, Germany) for 2 hours at room temperature. As negative control, one brain section on each slide was processed analogously to the staining procedure except for the use of primary antibodies. The slides were analyzed using a Zeiss LSM710 (Carl Zeiss Microscopy GmbH, Jena, Germany) (laser: Argon 488, Helium-Neon 543; objective: Plan-Apochromat 63x/1.40 oil). For appraisal of glial reactivity, the region of interest (infarct border zone) was adjusted by identification of a dense band of GFAP and Nestin-positive cells. Per section, two adjacent confocal images (each 135 × 135 μm) were taken in a 1024 × 1024 resolution using fourfold averaging. Image channels were binarized my means of the Otsu algorithm18 embedded in ImageJ (NIH Image, Bethesda, MD, USA). Positive pixels were expressed as percentage of total pixels within a region of interest. All sections from one brain were treated as technical replicates and averaged to one final value.
Neurofunctional Tests
Animals allocated to group 3 (n=12/12) were subjected to three behavioral tests. The mNSS system allowed the evaluation of various sensory, motor and reflex parameters. Neurologic deficits were further ascertained by the beam walk test as described recently by our group19 and the ladder rung test.20 In the latter, animals crossed a horizontal ladder with randomly mounted rungs spacing between 1 and 5 cm. Each run (three per animal and day) was video recorded and subsequently analyzed for each step and limb separately. The total number of steps and paw placing errors were ascertained and averaged for the particular animal at one time point.
After a training period of 3 days, the performance 1 day before MCAO was taken as baseline value. The ladder rung test was performed 2, 4, 28, 42, and 56 days after experimental stroke while mNSS and beam walk test were carried out 2, 7, 12, 20, 27, 34, 40, 47, 55, and 63 days post MCAO. All behavioral tests were conducted at the same time (6 pm) and location by an investigator masked to group allocation.
Magnetic Resonance Imaging
Magnetic resonance imaging measurements of the ischemic lesion were performed at a clinical 1.5 T scanner (Gyroscan Intera human whole-body spectrometer equipped with a 47 mm loop RF-Coil, Philips, Hamburg, Germany) 1, 8, 29, and 60 days post MCAO in all animals assigned to group 3 (n=12/12). Infarct volumes were analyzed by T2-w sequences (T2-TSE) consisting of 20 transverse slices (matrix: 224 × 224; field of view: 50 mm; slice thickness: 1 mm). The edema-corrected lesion volume (%HLVe) and the space-occupying effect due to brain edema (%HSE) were calculated as described previously21 and expressed as percentage of the ipsilateral hemisphere. Only rats with a hyperintense ischemic lesion in the distal supply territory of the right MCA in T2-w MRI 24 hours after MCAO were included into the study.
Statistical Analysis
All data are shown as mean±s.d. SigmaPlot 11.0 (Systat Software, San Jose, CA, USA) was used for statistical analysis. To address the response to HUCB MNC treatment over the entire observation period data from behavioral tests was summarized as area under the curve analysis integrating all times points from day 2. Area under the curve data were analyzed for normality by Shapiro Wilk test. Afterwards, an unpaired t-test was used to reveal statistical differences between the HUCB MNC-treated and control group. For MRI investigations, a two-way repeated measures analysis of variance (ANOVA) with group allocation and time as variable factors with post hoc Tukey's multiple comparison test was performed. The temporal profile of growth factor expression in the control group was analyzed using a one-way ANOVA with Tukey's post hoc test. Polymerase chain reaction and immunohistochemistry data were compared using two-way ANOVA with group allocation and measuring time as comparison factors and post hoc Tukey's multiple comparison test. P values <0.05 were considered statistically significant and indicated by single symbols. Asterisks (*) indicate statistical differences as compared with the control group, whereas the pound sign (#) indicates intragroup differences.
Results
Mortality and Group Heterogeneity
Three animals died during the induction of the MCAO and two rats were later excluded because of the absence of an ischemic lesion on MRI. These animals were immediately replaced by new subjects that were randomly allocated to the experimental groups, but have not been part of the initial study population.
Treatment with Human Umbilical Cord Blood Mononuclear Cells Does Not Induce a Growth-Promoting Milieu in Spontaneously Hypertensive Rats up to 48 hours after Stroke
In order to investigate whether HUCB MNC transplantation influences the expression of endogenous growth factors in the ischemic border zone, mRNA levels of four trophic factors were compared with vehicle-treated rats at 36 hours and 48 hours after stroke injury. Earlier time points were additionally examined to determine the intrinsic neurotrophic activity after cerebral ischemia.
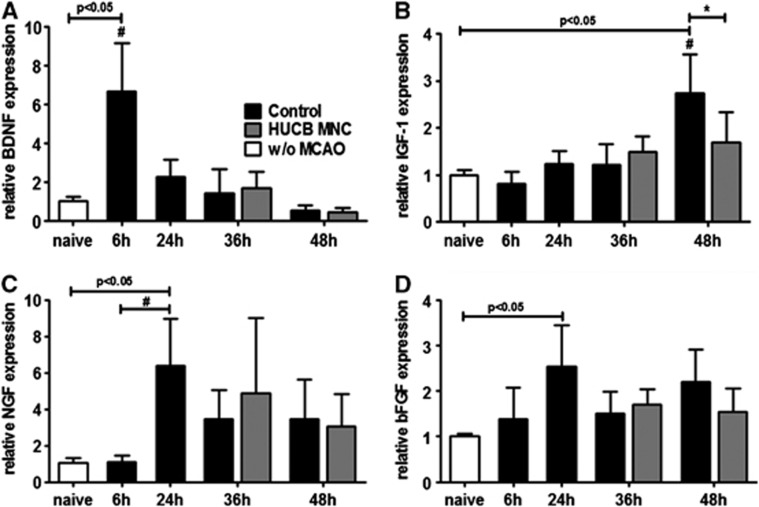
The ischemic stimulus led to an early increase of BDNF mRNA expression in the ischemic border zone with the highest level 6 hours after stroke, followed by a rapid decline (Figure 2A, one-way ANOVA, P<0.0001). By contrast, IGF-1 mRNA levels were not significantly elevated in the penumbra until 48 hours after stroke (Figure 2B, one-way ANOVA, P<0.0001). Relative mRNA expression of nerve growth factor increased by 24 hours post MCAO and slightly diminished thereafter (Figure 2C, one-way ANOVA, P=0.004). In addition, elevated levels of basic fibroblast growth factor mRNA expression were detected 24 hours after induction of MCAO compared with the premorbid situation (Figure 2D, one-way ANOVA, P=0.019). Most importantly, treatment with HUCB MNC did not induce a significant change in the transcriptional levels of BDNF (P=0.553) and nerve growth factor (P=0.370) at 36 hours and 48 hours after stroke compared with the vehicle-treated controls (Figures 2A, 2C, and 2D two-way ANOVA, time × treatment). Surprisingly, relative expression of rodent IGF-1 mRNA was significantly decreased in the HUBC MNC-treated group (Figure 2B, two-way ANOVA, time × treatment P=0.008), whereas reduction of basic fibroblast growth factor levels in the therapy group slightly failed to reach significance (Figure 2D, two-way ANOVA, time × treatment P=0.051).
Figure 2.
Growth factor expression after middle cerebral artery occlusion (MCAO) is not beneficially altered by human umbilical cord blood (HUCB) mononuclear cells (MNC) treatment. Growth factor expression was investigated in the infarct border zone of control and HUCB MNC-treated rats. While brain-derived neurotrophic factor (BDNF) expression increased 6 hours after MCAO in the control group (A, #P<0.05 6 hours versus any other time point) and declined thereafter, insulin-like growth factor 1 (IGF-1) (B, #P<0.05 48 hours versus 6 hours and 36 hours) and nerve growth factor (NGF) (C, #P<0.05 24 hours versus 6 hours) messenger RNA (mRNA) levels peaked later. Relative basic fibroblast growth factor (bFGF) mRNA expression was only increased 24 hours after MCAO compared with naive spontaneously hypertensive rats without MCAO (D, P<0.05). Treatment with HUCB MNC did not significantly change transcriptional levels of BNDF, NGF, and bFGF at 36 hours and 48 hours after stroke onset. Insulin-like growth factor 1 expression was even decreased in the HUCB MNC-treated group (D, *P<0.05 versus control). n=4 per group at 0 to 24 hours, n=8 per group at 36 hours and 48 hours.
Treatment with Human Umbilical Cord Blood Mononuclear Cells Does Not Influence Reactive astrogliosis in Spontaneously Hypertensive Rats
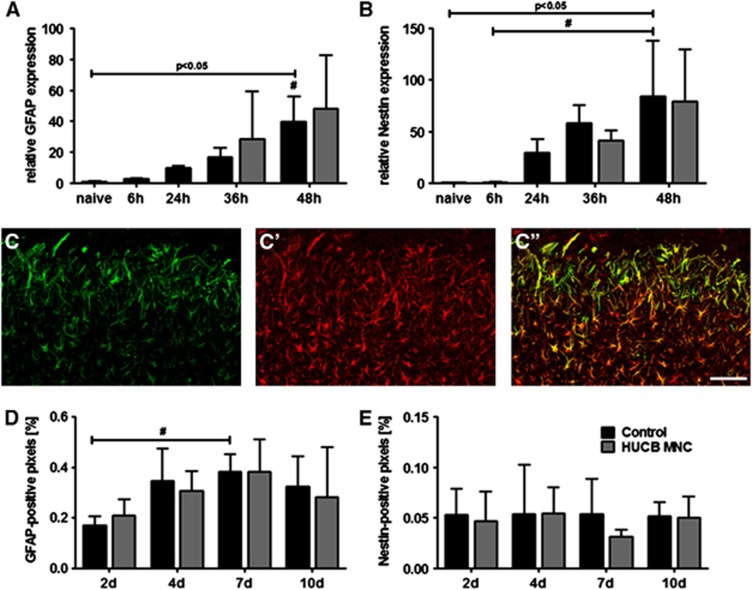
To evaluate the effects of HUCB MNC transplantation on reactive astrogliosis, brain samples were analyzed for mRNA expression of GFAP and Nestin. Levels of GFAP mRNA in the infarct border zone successively increased within the first 48 hours after stroke induction (Figure 3A, one-way ANOVA, P<0.0001). A similar picture arose for local mRNA expression of Nestin (Figure 3B, one-way ANOVA, P=0.002). Post hoc Tukey's multiple comparison tests revealed a significant rise of GFAP and Nestin mRNA only at 48 hours post MCAO. Human umbilical cord blood MNC transplantation did neither change relative GFAP (P=0.857) nor Nestin (P=0.720) mRNA expression in the ischemic penumbra at 36 hours and 48 hours after MCAO (Figure 3A, B, two-way ANOVA, time × treatment).
Figure 3.
Human umbilical cord blood (HUCB) mononuclear cells (MNC) treatment does not influence glial scar development after stroke in spontaneously hypertensive rats. Relative messenger RNA expression of glial fibrillary acidic protein (GFAP) (A, #P<0.05 48 hours versus any other time point) and Nestin (B, #P<0.05 hours 48 hours versus 6 hours) increased significantly 48 hours after middle cerebral artery occlusion (MCAO) in the control group. At neither time points, relative GFAP and Nestin expression differed as a consequence of treatment (A–B). Illustration of GFAP (C′) and Nestin (C) immunoreactive cells and their colocalization (C′′) within the ischemic border zone 4 days after MCAO (scale bar: 100 μm). Quantitative analysis revealed an increase of the GFAP (D, #P<0.05 2 days versus 7 days), but not Nestin (E) positive volume over time in the control group. However, HUCB MNC treatment did not alter the development of the astroglial scar at any investigated time point (D–E). n=5 per group and day.
Likewise, no differences were found at GFAP and Nestin protein levels among both experimental groups. Morphologically, a dense meshwork of GFAP and Nestin-positive cells was present in the ischemic hemisphere already 2 days after MCAO (Figure 3C′′). The distribution of the GFAP signal differed from that of Nestin-positive cells. Whereas Nestin immunoreactivity was predominantly located in the ischemic border zone (Figure 3C), GFAP expression was more evenly distributed among the ischemic lesion (Figure 3C′). Quantitative analysis of marker expression revealed that the GFAP immunoreactive volume significantly increased over time in the infarct border zone. However, the GFAP volume did not change time-dependently as a consequence of treatment (Figure 3D, two-way ANOVA, factor ‘time' P=0.008, time × treatment P=0.853). In addition, analysis of Nestin immunoreactivity within the infarct border zone disclosed a stable picture from 2 up to 10 days after induction of MCAO in the control and in the HUCB MNC-treated group. No significant differences in Nestin immunoreactivity were observed between both groups at any investigated time point (Figure 3D, one-way ANOVA, factor ‘time', P=0.843, time × treatment P=0.808).
Treatment with Human Umbilical Cord Blood Mononuclear Cells Does Not influence Motor Recovery and Infarct Volume in Spontaneously Hypertensive Rats
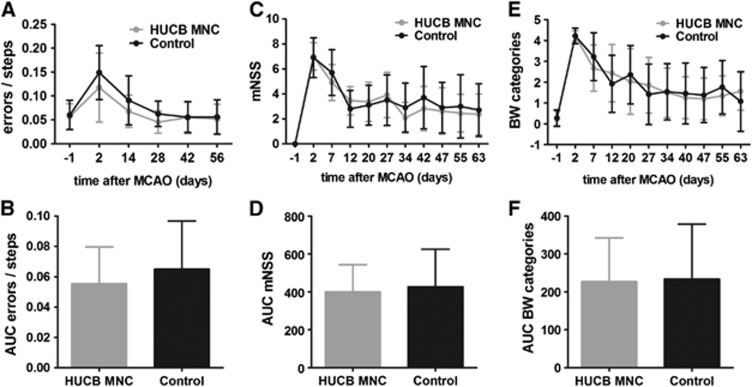
To investigate the therapeutic effects of HUCB MNC on stroke recovery in SH rats, a long-term experiment combining serial behavioral phenotyping by three tests and repeated measurement of the infarct volume as well as the space-occupying effect by MRI was performed (group 3). Compared with baseline values the ladder rung test showed a 2.1-fold increase of gait errors 2 days after induction of MCAO in the control group versus a 2.5-fold rise in the HUCB MNC-treated group (Figure 4A). The frequency of gait errors returned to basic values after 4 weeks in both experimental groups and then remained stable until the end of the experiment (day 56: HUCB MNC 0.052±0.031 versus control 0.056±0.036). The cumulated error rate over time did not significantly differ between the HUCB MNC-treated rats and the control group (Figure 4B, t-test, P=0.445). Analogously, permanent MCAO caused a clear increase of the mNSS score (Figure 4C) and beam walk categories (Figure 4E) 2 days after ischemia onset in both experimental groups. Scores of the HUCB MNC and the vehicle-treated animals dropped over time in each of the two tests, but did not reach baseline levels until the end of the experiment (Figures 4C and 4E). Considering the area under the curve analysis as measure of the cumulative treatment response neither functional test revealed a significant impact of intravenous HUCB MNC administration on sensorimotor performance (Figures 4D and 4F, t-test P=0.713 for mNSS, t-test P=0.908 for beam walk test).
Figure 4.
Human umbilical cord blood (HUCB) mononuclear cells (MNC) transplantation does not exert amelioration of functional deficits after ischemic stroke. Development of functional deficits measured by ladder rung test (A–B), modified neurologic severity score (mNSS; C, D), and beam walk (BW) test (E, F). Permanent middle cerebral artery occlusion (MCAO) caused an increase of gait errors (A), mNSS scores (C) and BW categories (E) 2 days after stroke onset. Performances in each of the three tests improved during the remaining experiment, but did not reach baseline levels indicating an incomplete functional recovery (A, C, E). The area under the curve (AUC) analysis did not reveal any significant differences between the HUCB MNC-treated and control group for ladder rung test (B), mNSS (D), and BW test (F). n=12 per group.
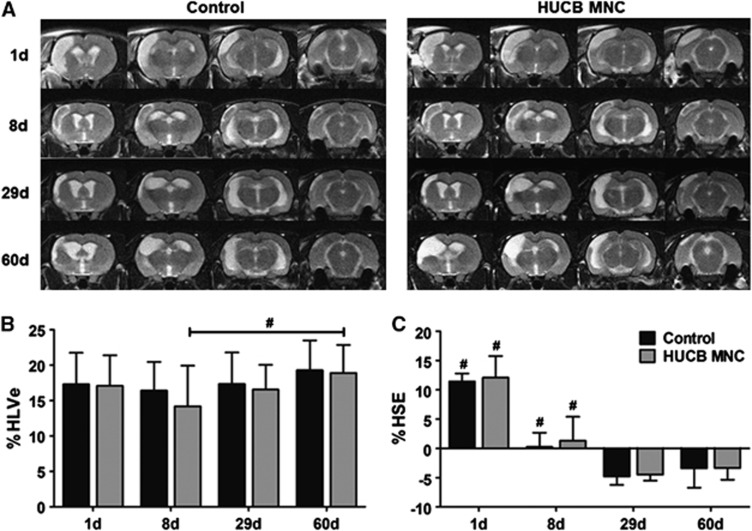
A typical depiction of the ischemic lesion and its development were seen in all animals subjected to MCAO. Exemplary examples are given in Figure 5A. The mean infarct volume as percentage of the ipsilateral hemisphere corrected for edema (%HLVe) did not significantly differ among both groups up to 60 days after stroke corroborating the absent therapeutic effect of HUCB MNC transplantation (two-way ANOVA-RM, time × treatment P=0.829). Lesion volumes before treatment induction were comparable in both experimental groups (Figure 5B; HUCB MNC-treated group 16.9%±4.3% versus control group 17.3%±4.1%, two-way repeated measures ANOVA with Tukey's post hoc multiple comparison, P=0.923). Interestingly, the corrected infarct volume remained virtually unchanged until the end of the experiment in the control group while there was a delayed increase of the lesion volume from day 8 to day 60 in rats treated with HUCB MNC (Figure 5B, two-way repeated measures ANOVA, factor ‘time' P=0.029).
Figure 5.
Development of ischemic lesion volume and space-occupying effect on T2-w magnetic resonance imaging (MRI) in the control and human umbilical cord blood (HUCB) mononuclear cells (MNC)-treated group. (A) Representative series of T2-w images showing the hyperintense ischemic lesion 1, 8, 29, and 60 days after middle cerebral artery occlusion (MCAO) in untreated (control) and HUCB MNC-treated rats. The mean edema-corrected infarct volume as percentage of the hemispheric lesion volume (%HLVe) did not change during the observation period in the control group whereas there was a delayed infarct growth in HUCB MNC-treated rats (B, #P<0.05 8 days versus 60 days). Expectably, the space-occupying effect expressed as volume change of the affected hemisphere (%HSE) was increased 1 day after MCAO, but declined successively attaining negative values at days 29 and 60 in both groups (C, #P<0.05 1 day and 8 days versus any other time point). At neither investigated time point, cord blood therapy had a significant impact on lesion volume (B), edema development (C), and hemispheric atrophy (C) assessed by MRI. n=12 per group.
The space-occupying effect of the ischemic lesion expressed as volume change of the affected hemisphere (%HSE) expectedly was most pronounced at the first day after MCAO and almost vanished until day 8 in both experimental groups (Figure 5C, HUCB MNC 1.3%±4.1% versus control 0.3%±2.4%). At later time points, %HSE attained negative values (Figure 5C; day 60 HUCB MNC −3.4%±2.0% versus control −3.4%±3.3%), indicative of secondary atrophy of the ischemic hemisphere. Volume changes of the ischemic hemisphere either provoked by brain edema in the early phase or by shrinkage in the late stage were not significantly influenced by HUCB MNC treatment (two-way repeated measures ANOVA, time × treatment P=0.959).
Discussion
In this study, we aimed to investigate whether cryopreserved HUCB MNC transplantation 24 hours after focal cerebral ischemia has sustained impact on recovery in SHR. Long-term functional outcome as assessed by three behavioral tests as well as lesion development and hemispheric atrophy on MRI were not beneficially influenced by cord blood cell therapy. Furthermore, HUCB MNC treatment neither did relevantly change host-derived mRNA levels of various growth factors in the infarct border zone early after stroke nor did it affect the extent of glial scarring.
As delayed administration was shown to be efficient in several preclinical trials,22 HUCB MNC are assumed not only to prevent damage, but also to promote neurorestorative effects after stroke injury. From a mechanistic perspective, cell-based therapies might amplify endogenous processes of brain plasticity as well as vascular regeneration and recruitment of progenitor cells.23 While the regulation of ischemia-induced neurogenesis is poorly understood, various growth factors are known to be implicated in precursor cell proliferation in the subventricular zone as well as in chemotaxis toward the sites of injury and survival of neuroblasts in the ischemic border zone.24 In this context, the ability of HUCB MNC to increase local growth factor levels after stroke, either by direct secretion or by inducing host expression,9 is supposed to be a major mediator of the reported beneficial effects. In this study, we found a rapid increase of endogenous BDNF, nerve growth factor, and basic fibroblast growth factor mRNA after the ischemic injury; however, HUCB MNC treatment did not further augment these levels within 48 hours after stroke. To our surprise, local IGF-1 mRNA expression was yet decreased in HUCB MNC-treated rats early after stroke. Considering the previously described neuroprotective properties of IGF-1,25 this finding implies a rather detrimental, although functionally irrelevant effect of HUCB MNC transplantation. Insulin-like growth factor 1 expression after stroke is largely ascribed to subpopulation of infiltrating macrophages.26 As experimental evidence suggests that HUCB MNC treatment diminishes leukocyte migration to the ischemic brain,7 one might speculate that lower IGF-1 mRNA levels in HUCB MNC-treated rats result from changes of the poststroke immune response. However, inflammatory mechanisms were clearly beyond the scope of the present study and thus not further investigated.
In the time frame analyzed here (up to 10 days after the onset of stroke), astrogliosis is considered a primarily protective process. First, the dense meshwork of GFAP and Nestin-positive reactive astrocytes in the infarct border zone prevents dispersion of deleterious processes to the surrounding tissue. Additionally, reactive astrocytes were shown to contribute to a regenerative microenvironment adjacent to the lesion, thereby promoting synapto- and neurogenesis as well as the repair of the blood–brain barrier.11 However, in line with our primary and secondary endpoints, we did not observe a significant impact of HUCB MNC transplantation on astroglial scarring.
As a potential limitation of our study, primer pairs used for PCR analysis only recognized rodent mRNA. Thus, direct release of human neurotrophins by cord blood cells as shown in in vitro experiments27 cannot be ruled out by our study design and might account for discrepancies with the literature.10, 28 But even if present, it would not have had an effect on the primary and secondary endpoints assessed. Moreover, we performed analysis of growth factor mRNA expression only up to 2 days after stroke, as previous studies suggested that gene expression functionally related to processes of brain plasticity is initiated very quickly after stroke.29 We cannot exclude later changes in the expression levels of these factors, but again, any delayed increase would not have been transferred into functional benefits in our study.
Functional deficits after ischemic stroke are predominantly assessed by simple reflex tests during the early phase of infarction. In the present study, we applied a combination of three behavioral tests to provide a more comprehensive picture of the motor capacity of SHR after MCAO. Besides the modified neurologic severity score (mNSS) the beam walk test measuring hindlimb function as well as the ladder rung test for fore- and hindlimb stepping and interlimb coordination were used. Furthermore, we performed a long-term followup of functional recovery up to 2 months after stroke to discern sustained benefits from a mere delay in the appearance of deficits. Chronic behavioral testing, though rarely performed, is considered especially critical when brain plasticity is therapeutically addressed.30 However, while there was a long-term recovery in both experimental groups neither its course nor the cumulative degree of impairment was substantially affected by HUCB MNC treatment.
In the present study, untreated SHR rapidly developed a stabilized ischemic lesion without relevant infarct expansion over 60 days. This was previously ascribed to the poor collateral flow in this model.31 Interestingly, we found a delayed increase of the lesion volume from day 8 to day 60 in rats treated with HUCB MNC, possibly providing evidence for transient beneficial effects on the lesion size. However, cord blood therapy had no significant impact on lesion volume as well as on hemispheric atrophy 60 days after MCAO. This corroborates the functional data that failed to prove any sustained influence of HUCB MNC treatment on poststroke recovery. Only few studies addressed late-stage volume changes on MRI as outcome parameter despite the fact that cerebral atrophy is considered a common feature in the chronic phase of stroke.32 Instead, most experimental stroke studies focus on changes of the infarct volume as a measure of treatment efficacy even though attempts to correlate behavioral tests and infarct volume often failed in the past.33 Apart from the primary lesion, diffuse neuronal death in areas remote from the lesion, diaschisis, and metabolic changes might also contribute to poorer outcome in the chronic stage stroke,34 underlining the need for more sophisticated structural MRI analysis.
The results of this study are in clear contrast with previously reported findings from our3 and other groups6, 28 that indicated a noticeable benefit of experimental HUCB MNC transplantation after stroke. However, next to a considerable body of indications corroborating the therapeutic efficacy of HUCB MNC after stroke, some investigators were unable to reproduce therapeutic findings, especially when applying more demanding functional tests as in the present study.13 Furthermore, differences in cell storage and processing may potentially impair the efficacy of the treatment. In fact, while we recently failed to detect neuroprotective effects of cryopreserved HUCB MNC early after MCAO,17 previous studies reporting beneficial results were generally conducted with freshly isolated HUCB MNC.4, 6, 10
Human umbilical cord blood preservation techniques have originally been introduced to create a valuable source of hematopoietic stem and progenitor cells being capable of repopulating the hematopoietic system after treatment of devastating malignancies, but also to replenish bone marrow stocks. Consequently, studies assessing the effects of HUCB cryopreservation exclusively focused on the hematopoietic potential of the cells. Albeit this was found unaffected in most studies, the use of dimethyl sulfoxide as a cryoprotectant or non-automated, slow-cooling techniques, both being common in experimental studies, can cause a significant decrease in viable cell yields after thawing.35 This effect is particularly prominent for CD34+ hematopoietic progenitor cells.15 Loss of CD34+ cells was even reported when applying automated cooling techniques according to industrial good manufacturing practice standards.36 Though not being the only effector cell population within HUCB MNC, CD34+ are supposed to exert neuroprotective as well as regenerative capabilities and are further discussed to have an important role during the early phase of HUCB MNC stroke therapy.3, 37 A higher number of circulating CD34+ cells was also associated with improved functional outcome in human stroke patients.38 Given the supposed relevance of this population among the transplant, it is important to consider that most studies reporting a distinct therapeutic effect after application of cryopreserved HUCB MNC samples relied on preparations with increased CD34+ content of up to 70%.6 Hence, a moderate loss of CD34+ cells during cryopreservation and subsequent thawing may have been compensated by the cell enrichment strategies applied. Given positive results from previous studies using freshly prepared or CD34+-enhanced HUCB MNC, the use of more informative control groups must be recommended for future studies. A direct comparison of cryopreserved HUCB MNC against these populations (positive controls) as well as freeze–thawed killed HUCB MNC rather than PBS (negative controls) is particularly rational.
Another possible and obvious explanation for the absent therapeutic impact may be the study design, which employed a xenotransplantation paradigm without an accompanying immunosuppression regime to prevent graft rejection. Immunosuppression in experimental transplantation studies is mainly achieved by application of cyclosporin A or tacrolimus, which are highly effective and widely used in common rodent models. However, both agents were shown to exert neuroprotective effects after ischemic brain injury. Moreover, cyclosporin A may also detrimentally affect poststroke recovery by enhancing the risk of convulsions in human patients and rodents.39 We therefore decided to omit immunosuppression to prevent confounding effects on the chosen primary endpoints. However, long-term engraftment of HUCB MNC is not a prerequisite for functional improvement after MCAO5 and sustained lesion size reduction as well as functional improvement have previously been observed after treatment with human HUCB cells in the absence of immunosuppression.28 Hence, omission of immunosuppression alone may be considered unlikely to explain the observed lacking therapeutic impact.
The use of animals with risk factors that match the clinical stroke population such as in the present study is highly recommended for preclinical trials.40 Although cell-based therapies using freshly prepared HUCB MNC have already been performed in SHR, pathologic hypertension and other genetically determined abnormalities in the strain may have confounded results obtained in primary and secondary endpoints, at least to some extent. For example, chronic cerebrovascular damage,41 reduced expression of neurotrophic factors and impaired neuroneogenesis42 as well as divergent neuroendocrine responses to distress stimuli have been reported in SHR as compared with normotensive controls.43 The impact of these differences on poststroke functional recovery in SHR remains for further investigation.
Recent failure in translation has often been attributed to inadequate preclinical study design and severe methodological shortcomings. By contrast, this study strictly adhered to current recommendations for basic stroke trials,40 which among others included randomization, rater-masked evaluation and full disclosure of dropout rates. Furthermore, to minimize the risk for false-negative interpretation of the results a priori, sample size calculations were performed based on the data derived from previous studies of our group.16 In fact, hardly any previous study applying cord blood therapy for ischemic injury reported on sample size calculations and if so, these studies were powered to detect improvements in primary outcome parameters of ⩾40%.44 Nevertheless, it has to be mentioned that s.d. values in the present study were higher than expected from previous data, thereby potentially limiting the predictive value of our results. However, we desisted from increasing the sample size because of missing differences among the means of both groups rendering significant impact of HUCB treatment even with higher sample sizes unlikely. In this context, the fact that preclinical studies need to be large enough to provide useful information must always be thoroughly weighed up against ethical concerns to minimize the size of individual animal experiments.45
Taken together, the results reported herein advise caution regarding a swift translation of HUCB MNC therapies for ischemic stroke into a clinical setting before achieving clarity about the precise modes of action of these cells and the possible impact of long-term cryopreservation on the neuroprotective or neurorestorative abilities of these cells. Future experimental studies should reveal the exact circumstances under which a HUCB MNC-based therapeutic approach can exert clinical benefit for human stroke patients before commencing clinical trials. Taking into account aspects of cell preparation and preservation, which have been mostly unconsidered so far, may be not only be expedient but mandatory when designing such experiments.
Acknowledgments
The authors would like to thank Doreen Reich for providing the cells and Annekatrin Kränkel for excellent technical support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by structural founds of the European Union granted by the Development Bank of Saxony.
Supplementary Material
References
- Kobylka P, Ivanyi P, Breur-Vriesendorp BS. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation. 1998;65:1275–1278. doi: 10.1097/00007890-199805150-00024. [DOI] [PubMed] [Google Scholar]
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- Boltze J, Reich DM, Hau S, Reymann KG, Strassburger M, Lobsien D, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant. 2012;21:723–737. doi: 10.3727/096368911X586783. [DOI] [PubMed] [Google Scholar]
- Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant. 2012;21:1199–1211. doi: 10.3727/096368911X589609. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, de MD, Collier L, Bickford PC, Sanberg CD, et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Meier C. Umbilical cord blood cell transplantation after brain ischemia—from recovery of function to cellular mechanisms. Ann Anat. 2011;193:371–379. doi: 10.1016/j.aanat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Kumbruch S, Tenbusch M, Marcus K, Marschner K, Dermietzel R, et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue Res. 2012;348:429–438. doi: 10.1007/s00441-012-1401-0. [DOI] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Mäkinen S, Kekarainen T, Nystedt J, Liimatainen T, Huhtala T, Narvanen A, et al. Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 2006;1123:207–215. doi: 10.1016/j.brainres.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Lukasiuk K, Machaj EK, Pojda Z, Kaminska B. Lack of migration and neurological benefits after infusion of umbilical cord blood cells in ischemic brain injury. Acta Neurobiol Exp (Wars ) 2009;69:46–51. doi: 10.55782/ane-2009-1728. [DOI] [PubMed] [Google Scholar]
- Beshlawy AE, Metwally HG, Khalek KA, Hammoud RF, Mousa SM. The effect of freezing on the recovery and expansion of umbilical cord blood hematopoietic stem cells. Exp Clin Transplant. 2009;7:50–55. [PubMed] [Google Scholar]
- Wagner DC, Bojko M, Peters M, Lorenz M, Voigt C, Kaminski A, et al. Impact of age on the efficacy of bone marrow mononuclear cell transplantation in experimental stroke. Exp Transl Stroke Med. 2012;4:17. doi: 10.1186/2040-7378-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelsberger UM, Deten A, Posel C, Zille M, Kranz A, Boltze J, et al. Intravenous human umbilical cord blood transplantation for stroke: impact on infarct volume and caspase-3-dependent cell death in spontaneously hypertensive rats. Exp Neurol. 2011;227:218–223. doi: 10.1016/j.expneurol.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Kozlowski C, Weimer RM. An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One. 2012;7:e31814. doi: 10.1371/journal.pone.0031814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz A, Wagner DC, Kamprad M, Scholz M, Schmidt UR, Nitzsche F, et al. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010;1315:128–136. doi: 10.1016/j.brainres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Bachmann A, et al. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004;35:566–571. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- Yu G, Borlongan CV, Stahl CE, Hess DC, Ou Y, Kaneko Y, et al. Systemic delivery of umbilical cord blood cells for stroke therapy: a review. Restor Neurol Neurosci. 2009;27:41–54. doi: 10.3233/RNN-2009-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci. 2012;6:70. doi: 10.3389/fncel.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini MJ, Herenu CB, Goya RG, Garcia-Segura LM. Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. J Neuroinflammation. 2011;8:21. doi: 10.1186/1742-2094-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirkin A, Matsumoto H, Takahashi H, Inoue A, Tagawa M, Ohue S, et al. Iba1(+)/NG2(+) macrophage-like cells expressing a variety of neuroprotective factors ameliorate ischemic damage of the brain. J Cereb Blood Flow Metab. 2010;30:603–615. doi: 10.1038/jcbfm.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Sanberg CD, Sanberg PR. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199:201–208. doi: 10.1016/j.expneurol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Dai J, Zhang C, Yan T, et al. Combination treatment of stroke with sub-therapeutic doses of Simvastatin and human umbilical cord blood cells enhances vascular remodeling and improves functional outcome. Neuroscience. 2012;227:223–231. doi: 10.1016/j.neuroscience.2012.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Gill R, Certa U. Temporal and spatial gene expression patterns after experimental stroke in a rat model and characterization of PC4, a potential regulator of transcription. Mol Cell Neurosci. 2003;22:353–364. doi: 10.1016/s1044-7431(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legos JJ, Lenhard SC, Haimbach RE, Schaeffer TR, Bentley RG, McVey MJ, et al. SB 234551 selective ET(A) receptor antagonism: perfusion/diffusion MRI used to define treatable stroke model, time to treatment and mechanism of protection. Exp Neurol. 2008;212:53–62. doi: 10.1016/j.expneurol.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kraemer M, Schormann T, Hagemann G, Qi B, Witte OW, Seitz RJ. Delayed shrinkage of the brain after ischemic stroke: preliminary observations with voxel-guided morphometry. J Neuroimaging. 2004;14:265–272. doi: 10.1177/1051228404264950. [DOI] [PubMed] [Google Scholar]
- Roof RL, Schielke GP, Ren X, Hall ED. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke. 2001;32:2648–2657. doi: 10.1161/hs1101.097397. [DOI] [PubMed] [Google Scholar]
- Pantano P, Formisano R, Ricci M, Di Piero V, Sabatini U, Di PB, et al. Motor recovery after stroke. Morphological and functional brain alterations. Brain. 1996;119 (Pt 6:1849–1857. doi: 10.1093/brain/119.6.1849. [DOI] [PubMed] [Google Scholar]
- Djuwantono T, Wirakusumah FF, Achmad TH, Sandra F, Halim D, Faried A. A comparison of cryopreservation methods: slow-cooling versus rapid-cooling based on cell viability, oxidative stress, apoptosis, and CD34+ enumeration of human umbilical cord blood mononucleated cells. BMC Res Notes. 2011;4:371. doi: 10.1186/1756-0500-4-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholbach J, Schulz A, Westphal F, Egger D, Wege AK, Patties I, et al. Comparison of hematopoietic stem cells derived from fresh and cryopreserved whole cord blood in the generation of humanized mice. PLoS One. 2012;7:e46772. doi: 10.1371/journal.pone.0046772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Nakagomi N, Matsuyama T, Kikuchi-Taura A, Yoshikawa H, Kasahara Y, et al. Circulating CD34-positive cells have prognostic value for neurologic function in patients with past cerebral infarction. J Cereb Blood Flow Metab. 2009;29:34–38. doi: 10.1038/jcbfm.2008.92. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Shuto H, Dohgu S, Nakano Y, Egawa T, Kataoka Y. Cyclosporin A aggravates electroshock-induced convulsions in mice with a transient middle cerebral artery occlusion. Cell Mol Neurobiol. 2005;25:923–928. doi: 10.1007/s10571-005-4956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Liao YJ, Onodera M, Huang CL, Miyanaka H, et al. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004;122:131–137. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- Pietranera L, Lima A, Roig P, De Nicola AF. Involvement of brain-derived neurotrophic factor and neurogenesis in oestradiol neuroprotection of the hippocampus of hypertensive rats. J Neuroendocrinol. 2010;22:1082–1092. doi: 10.1111/j.1365-2826.2010.02058.x. [DOI] [PubMed] [Google Scholar]
- Roman O, Seres J, Pometlova M, Jurcovicova J. Neuroendocrine or behavioral effects of acute or chronic emotional stress in Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Endocr Regul. 2004;38:151–155. [PubMed] [Google Scholar]
- de Paula S, Vitola AS, Greggio S, de PD, Mello PB, Lubianca JM, et al. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr Res. 2009;65:631–635. doi: 10.1203/PDR.0b013e31819ed5c8. [DOI] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke. Trends Neurosci. 2007;30:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.