Abstract
As a sequel of brain ischemia, selective neuronal loss (SNL)—as opposed to pannecrosis (i.e. infarction)—is attracting growing interest, particularly because it is now detectable in vivo. In acute stroke, SNL may affect the salvaged penumbra and hamper functional recovery following reperfusion. Rodent occlusion models can generate SNL predominantly in the striatum or cortex, showing that it can affect behavior for weeks despite normal magnetic resonance imaging. In humans, SNL in the salvaged penumbra has been documented in vivo mainly using positron emission tomography and 11C-flumazenil, a neuronal tracer validated against immunohistochemistry in rodent stroke models. Cortical SNL has also been documented using this approach in chronic carotid disease in association with misery perfusion and behavioral deficits, suggesting that it can result from chronic or unstable hemodynamic compromise. Given these consequences, SNL may constitute a novel therapeutic target. Selective neuronal loss may also develop at sites remote from infarcts, representing secondary ‘exofocal' phenomena akin to degeneration, potentially related to poststroke behavioral or mood impairments again amenable to therapy. Further work should aim to better characterize the time course, behavioral consequences—including the impact on neurological recovery and contribution to vascular cognitive impairment—association with possible causal processes such as microglial activation, and preventability of SNL.
Keywords: carotid disease, cerebral ischemia, 11C-flumazenil, neuronal death, PET, vascular cognitive impairment
Introduction
The aim of this review is to address selective neuronal loss (SNL) in ischemic cerebrovascular disease, with a focus on anterior circulation stroke and large artery disease. As opposed to ‘pannecrosis'—i.e. necrosis involving all cells (microglia, astroglia, endothelial cells, and neurons), the neuropil, and the white matter and causing eventual tissue dissolution—SNL refers to the death of single neurons with preserved extracellular matrix and tissue bulk.1, 2, 3, 4
Given the complex background, a brief historical note is in order. Although cerebral infarction i.e. pannecrosis, has long been known to result from occlusion of a cerebral artery, the observation that some brain regions including the cornu ammonis of the hippocampus, basal ganglia, and Purkinje cells of the cerebellum display particular neuronal vulnerability to global ischemia was reported almost a century ago and termed elective parenchymal necrosis (in German: ‘elektive Parenchymnekrose').5 As will be seen below, delayed selective neuronal death in the CA1 sector of the hippocampus was subsequently shown to follow global ischemia in rodent models. With regard to focal ischemia, although in 1939 Spatz noted that following short-lasting or moderate degrees of ischemia tissue necrosis could affect only some cells (‘unvollständige Nekrose'),6 SNL after experimental focal ischemia was not addressed until the early 80s.3, 7 In an editorial published in 1982, Lassen proposed that ‘partial/incomplete infarction' could follow acute stroke in the clinical setting,8 and in 1983 he presented two cases of cardioembolic middle cerebral artery (MCA) occlusion (MCAo) in whom there was ‘CT-negative massive periinfarct neuronal loss of the peripheral parts of the MCA territory' at autopsy.9 Although these two cases were never formally published, this conference presentation ignited a strong controversy that led to a series of articles from established neuropathologists that failed to reproduce Lassen's observations (see below). Nevertheless, Garcia, Lassen and others2 revived this topic almost 10 years later in a seminal review article that followed two rodent studies showing for the first time definite striatal SNL after brief proximal MCAo10, 11 and a study on aphasic patients with striatocapsular infarcts in whom slowly progressing atrophy of the insular cortex on CT raised the possibility of incomplete infarction in the penumbra.12 Around the same time, Sette et al13 reported that 11C-flumazenil (FMZ) positron emission tomography (PET) was a sensitive marker of neuronal death following temporary MCAo (tMCAo) in baboons , raising the possibility that even subtle ischemic damage could be studied in vivo. Interest in SNL gained further momentum in the late 90s following the introduction of reperfusion therapies for acute stroke, given that the ischemic penumbra rescued from impending infarction could be affected by SNL, with potential impact on functional recovery. The development of 123I-iomazenil (IMZ), a SPECT analog of FMZ, led to several clinical investigations, more recently complemented by clinical and experimental FMZ PET studies that have established the occurrence of cortical SNL both in the salvaged penumbra and in chronic major arterial obstruction. In parallel, novel experimental investigations of SNL in rodents considerably clarified the overall concept.
Selective neuronal loss is sometimes confused for, but must be differentiated from, cortical (cystic) laminar necrosis,1, 14 which is a specific subtype of infarction seen after cardiac arrest or focal ischemia, and from microinfarcts, which are very small infarcts affecting the cortex,15 although the association of SNL and microinfarcts is plausible. Similarly, the terms ‘partial' or ‘incomplete' infarction should not be used to describe SNL, as they refer to subtypes of/phases toward pannecrosis.16, 17
Another potential source of confusion is that different names have often been given to the same condition over the years, including incomplete ischemic injury, selective neuronal necrosis, selective neuronal death, and of course SNL. However, incomplete ischemic injury is vague and does not explicitly mention neurons, whereas selective neuronal necrosis assumes that ischemic necrosis is the underlying process, whereas as will be seen below both necrosis and apoptosis seem to contribute to the condition. Selective neuronal death would be an acceptable alternative to SNL but is less adequate in our opinion because (i) it is historically connected to, and could therefore be confused with, slow neuronal death after global ischemia, whose underlying mechanism differs from stroke (see below), and (ii), as will be explained below, neuronal death can be identified pathologically only for a relatively short period of time, after which no remaining trace of the dead neuron can be seen, whereas SNL can be identified in principle indefinitely as a genuine ‘loss' by simply counting the remaining neurons.
Three subtypes of SNL in ischemic cerebrovascular diseases will be considered below: (i) selective neuronal loss in the penumbra salvaged from infarction; (ii) selective neuronal loss affecting brain areas remote from the ischemic territory; and (iii) selective neuronal loss in chronic internal carotid artery (ICA)/MCA disease. Findings in both animal models and human patients will be presented. Although outside the scope of the present review, selective neuronal death after global ischemia will also be briefly addressed because of its potential relevance.
Selective neuronal death in global ischemia
Transient global brain ischemia of short duration in rodents causes neuronal death particularly targeting the neocortex (layers 3, 5, and 6), the dorsolateral striatum (small to medium-sized neurons), and the hippocampal CA1 region.18, 19 Importantly, the changes in CA1 pyramidal cells develop very slowly and become recognized on light microscopy only several days after the index event. Bilateral CA1 neuronal death also occurs in humans following cardiac arrest,20 and it alone can cause a massive amnesic syndrome.21 Preferential atrophy of the hippocampal formation can be detected on magnetic resonance imaging (MRI) within days after cardiac arrest in humans22; however, systematic longitudinal studies are still missing. Interestingly, T1-weighted hyperintensities affecting the bilateral basal ganglia, thalami, or substantia nigra (SN) have also been reported23; these do not represent microhemorrhages on T2*-MR (Fujioka et al unpublished data), and their mechanism still remains unclear. Similar T1 hyperintensities can also be seen after hypoglycemic coma,24, 25 which may result in cortical SNL adopting a laminar fashion4, 26; these MR changes have been linked to intense SNL and gliosis at postmortem,25, 27 probably inducing paramagnetic substance deposition (see below for further discussion on this).
Because the apparent delayed onset of CA1 neuronal death could provide a wide therapeutic window, the underlying pathophysiological mechanisms have been extensively studied. Although early studies suggested various potential causative factors including excitotoxicity, ionic imbalance, mitochondrial dysfunction, oxidative and nitrosative stresses, inflammatory reactions, and apoptotic-like mechanisms, its pathogenesis still remains uncertain.28, 29 Various interventions have been tested experimentally, such as promoting neurogenesis by growth factors.30 However, in the clinical setting, hypothermia is so far the only proven effective treatment to improve neurological outcomes after cardiac arrest.31, 32
Selective neuronal loss in the non-infarcted penumbra
Reperfusion therapy in acute stroke is underpinned by undisputable experimental and clinical evidence that preventing infarction of even part of the penumbra strongly fosters functional recovery. Nevertheless, the rescued penumbra, which has been through a phase of severe ischemia, may be affected by SNL, which may both hinder the early clinical benefit and dampen long-term periinfarct plasticity, and is therefore important to study.33 We will first address SNL as directly documented postmortem, and then SNL as inferred from in vivo imaging.
Assessing Selective Neuronal Loss Postmortem
Two main approaches have been used. The classic method uses H&E or Nissl staining, which detects ‘dead neurons' as somas with abnormal morphological and staining features (dead or dying neurons acutely, dark neurons in the chronic stage). However, a limitation of this method is that once the somas have disappeared (phagocytosed), it is very difficult, if not impossible, to detect a loss of neurons on the background of the neuropil and glial cells, although sometimes ‘ghost neurons' can be identified. In addition, ‘dark neurons' are difficult to distinguish from staining or fixation artifacts, and their interpretation has long been debated.29 Cresyl violet is preferable, as it allows focal loss of neurons to be identified on the background of other stained cells, and it can show (micro- and astro-)gliosis that is associated with SNL for several weeks.3 However, the best approach by far is to use specific neuronal antibodies such as NeuN, which stain only neurons against a blank background, so that neuronal loss is clearly visible as lost stain (Figure 1A), in principle indefinitely (but see caveat below). This method also affords straightforward quantification of SNL as reduced cell counts, as well as direct comparison with other antibodies specific for e.g. microglial activation (MA) (Figure 1B) and astrocytosis (Figure 1C). The limitations are first that acutely a neuron may still stain with NeuN yet be already dead, and second that perfusion fixation is, in principle, required for proper tissue processing, which is not applicable to humans. An additional issue with all methods is that when using gyrencephalic brains, SNL can be very difficult to identify given the risk of tangential or oblique cutting of the gyrus, making delineation of a contralateral reference region sometimes impossible.34 Accordingly, poststroke SNL is best assessed in the lissencephalic brain, and after at least 48 hours have elapsed.
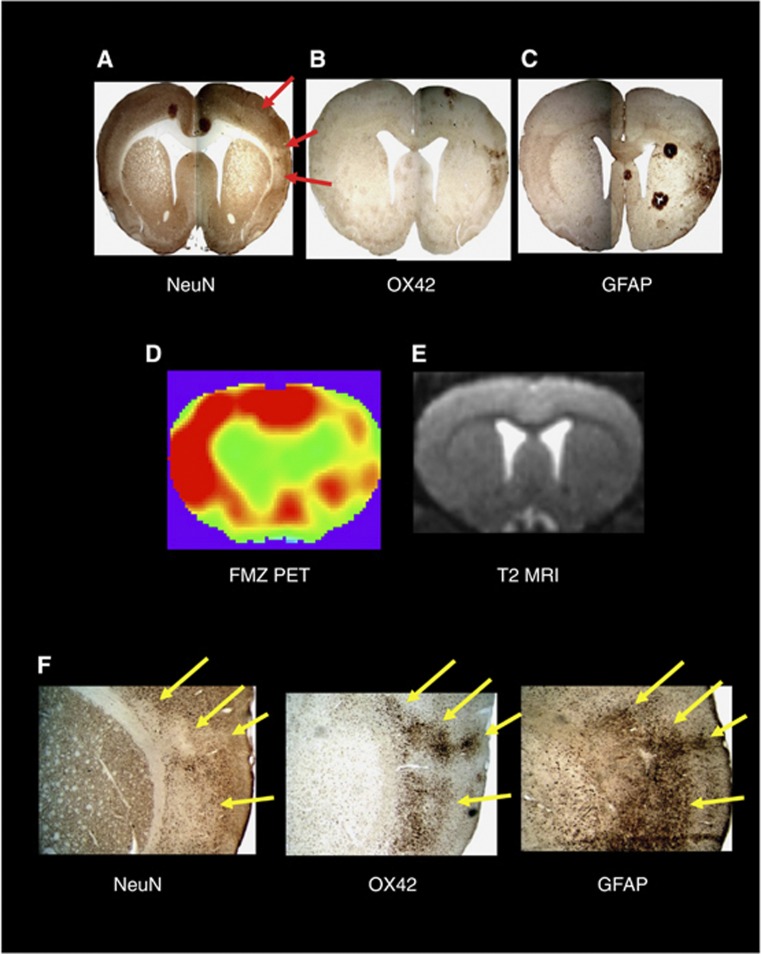
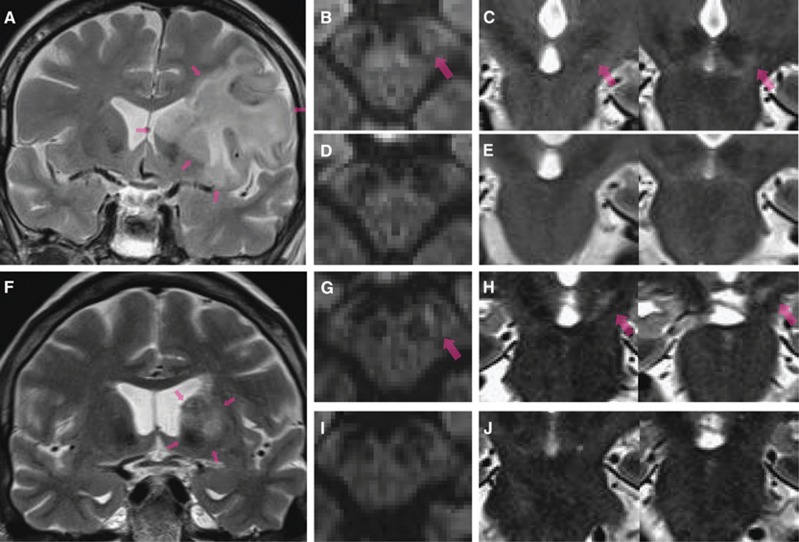
Figure 1.
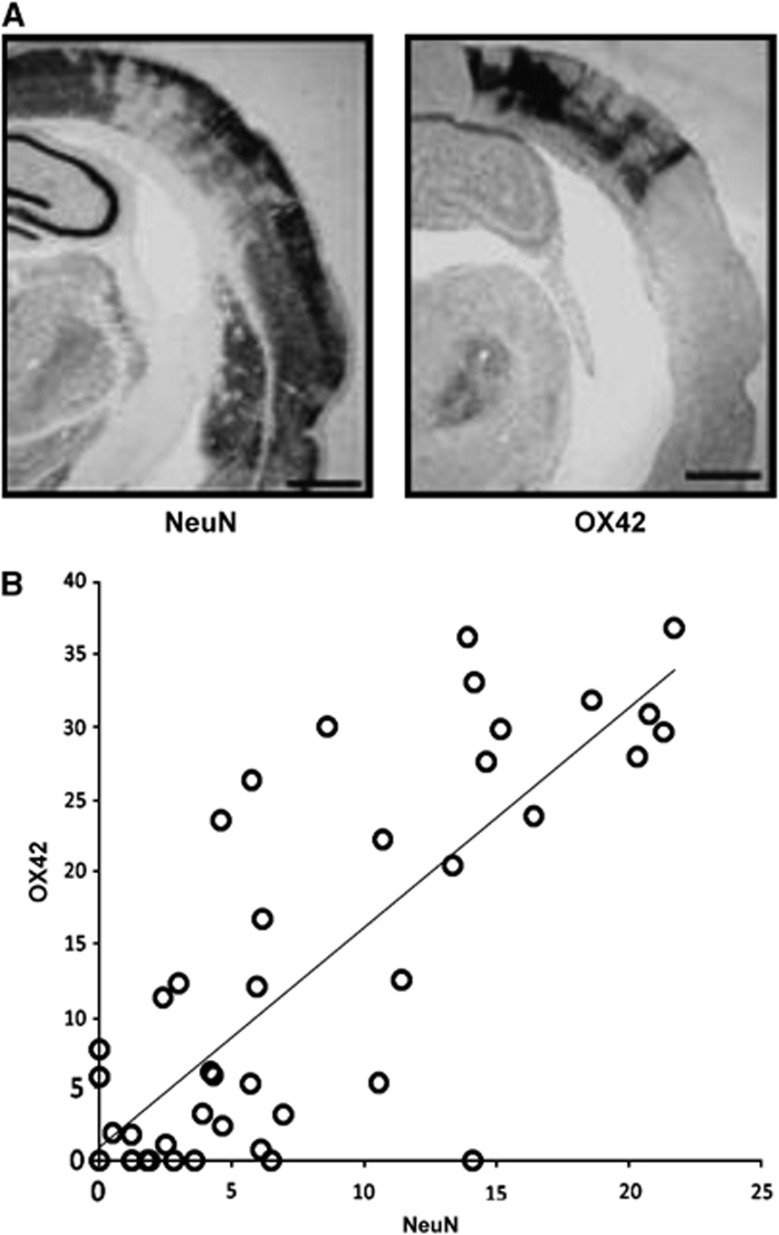
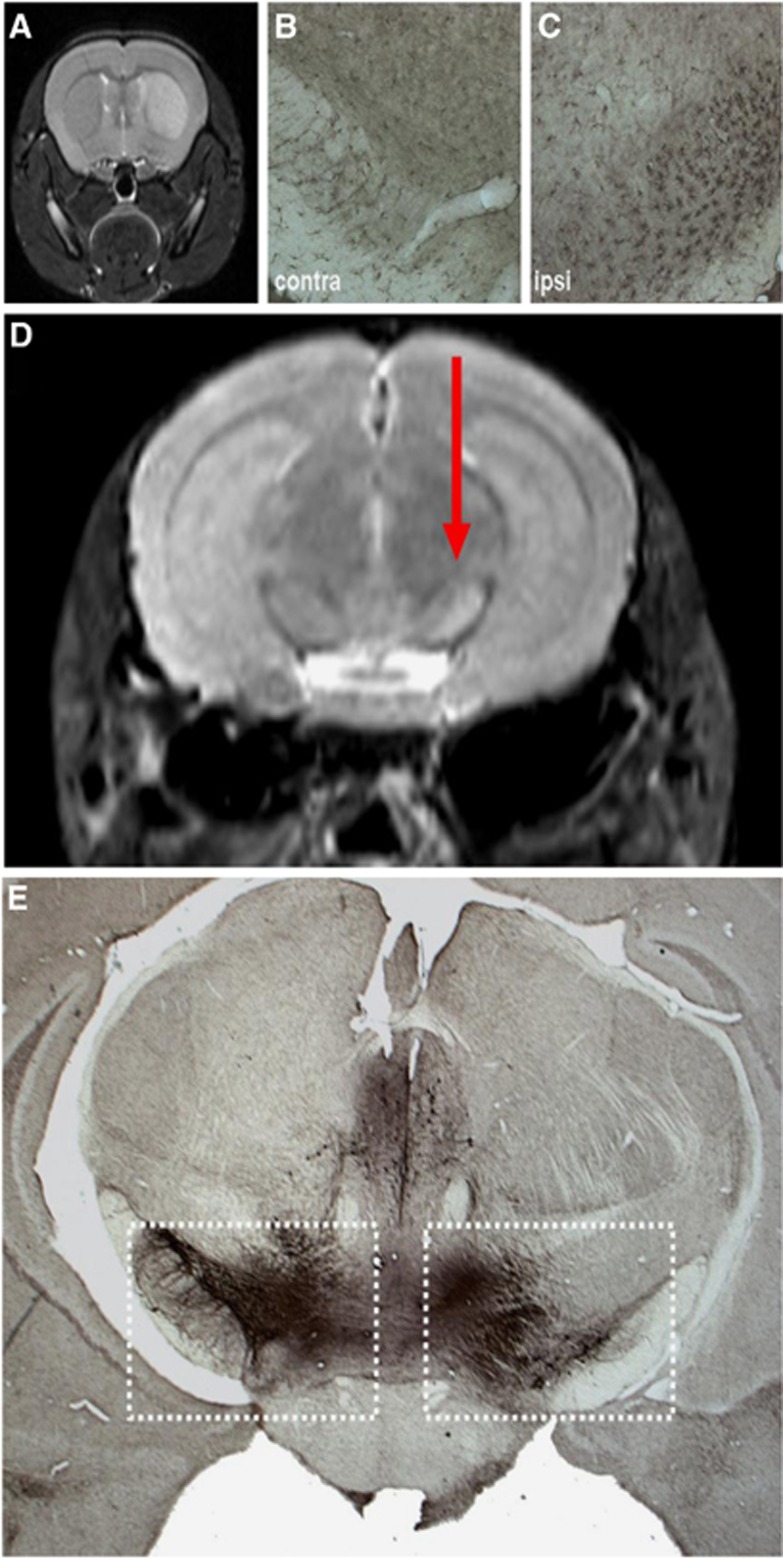
(A) Example of patchy cortical selective neuronal loss (SNL; red arrows) obtained 28 days after 45-minute distal middle cerebral artery (MCA) occlusion in a spontaneously hypertensive rat using immunohistochemistry (IHC) with NeuN (coronal section at bregma +1.00 mm). (B) and (C) OX42 and glial fibrillary acidic protein (GFAP)-stained sections at the same anatomical level, respectively, obtained in the same rat as (A), illustrating the close topographical relationship between the patches of NeuN loss and the areas of increased OX42 and GFAP staining, indicating a close association between SNL, microglial activation, and astrocytosis. (D) and (E) Co-registered 11C-flumazenil (FMZ) positron emission tomography (PET) and T2-weighted magnetic resonance imaging (MRI) coronal sections from the same rat and at the corresponding level as the IHC sections as A, B, and C, obtained 28 days after MCAo, illustrating the excellent topographical concordance between SNL and reduced FMZ binding (acknowledging the difference in spatial resolution), and the normality of T2-weighted MRI in areas of SNL; (F) high-resolution ( × 40) NeuN, OX42, and GFAP stains from the same rat, illustrating the striking colocalization of SNL, microglial activation, and astrocytosis at the microscopic level. The arrows point to four separate patches of SNL and their matching areas of microglial activation and astrocytosis. Modified from Ejaz et al,17 with permission.
Animal models
Rodents. Striatal SNL can be regularly induced by brief proximal tMCAo using the filament model. Here we will discuss only those studies that have allowed sufficient recovery time to afford reliable histopathologic assessment, in practice ⩾48 hours. In the rat, apart from Lehrmann et al (see below), striatal infarction regularly follows occlusion times ⩾45 minutes,10, 35, 36, 37, 38, 39, 40 whereas 30-minute occlusion resulted in either SNL or infarction.10, 37, 40, 41 Twenty-minute occlusion resulted in isolated SNL in two studies,11, 42 whereas a third reported infarction but histological assessment used silver impregnation only.43 Finally, all studies assessing ⩽15-minute occlusions reported isolated SNL,10, 11, 35, 37, 40 except in Zhan et al38 where microinfarcts with as short as 5-minute tMCAo were reported; however, their definition for ‘microinfarct' in fact probably refers to patchy SNL.15 Importantly, the severity of striatal SNL clearly increases with longer occlusion times, from ∼5% up to ∼20% between 10- and 20-minute MCAo.11 Overall, therefore, striatal SNL is the regular outcome following 5- to 20-minute proximal tMCAo in the rat.
Studies of brief tMCAo in mice are few and have reported discrepant results, with one study reporting striatal infarction with MCAo as short as 12.5 minutes and SNL with shorter durations,44 but two studies reporting isolated SNL, albeit severe and associated with striatal atrophy, following 30-minute occlusion.45, 46
Cortical SNL is of particular clinical relevance given that cortical lesions result in more enduring behavioral deficits,47 and that the cortex is usually more prone to rescue by recanalization than the striatum owing to less profound ischemia. Temporary cortical ischemia can be achieved with either proximal or distal MCAo. The latter is preferred because with the former cortical ischemia is not only less severe but also less consistent.39, 48 Using proximal MCAo in rats, scattered patches of cortical SNL but virtually no infarction is observed following ⩽30 minutes of proximal MCAo,10, 11, 35, 36, 37, 40, 42, 49 the only exception being Zhan et al,38 but see caveats above. However, cortical SNL was found not to affect every subject, being for instance observed in 40% of rats subjected to 10- to 25-minute tMCAo.11 In addition, with proximal MCAo, the fraction of dead neurons in the cortex is typically smaller than in the striatum, for instance ∼10% as compared with 15%, respectively.42 Of note, scattered cortical SNL has been observed in association with striatal infarction after up to a 1-hour MCAo in one study.10 Overall, therefore, up to 30-minute proximal MCAo in rats regularly causes cortical SNL but not infarction. Similar findings have been reported in mice.44, 45, 46
Few studies using the distal tMCAo model have investigated short occlusion times. Distal tMCAo of ⩾1-hour duration in spontaneously hypertensive rats (SHRs) consistently results in cortical infarcts, which can be patchy and are surrounded by a thin (0.1 to 1.2 mm) rim of SNL.50, 51 Using NeuN 14 days after 45-minute MCAo in SHRs, Hughes et al52 reported extensive pannecrosis in one rat but isolated, and sometimes striking, cortical SNL affecting the center of the MCA territory in five. There was marked inter-subject variability in the intensity of SNL, probably reflecting differences in the degree of ischemia during MCAo (see below). In a subsequent study using the same experimental design, strain, and occlusion duration, Ejaz et al17 found pure SNL in 1/8 rats only, the remaining being affected by variably extensive infarcts together with SNL both as a periinfarct band and as scattered patches. This difference in histopathological outcome between these two studies suggests that the divide between cortical SNL and infarction can be a fine line39 and that 45-minute distal MCAo in the SHR is not a reliable model for consistently isolated cortical SNL. On investigating shorter occlusions, Ejaz et al17 found that both 30- and 22-minute occlusion times invariably resulted in infarction, whereas 15 minutes consistently resulted in pure SNL.53 Overall, therefore, cortical SNL is consistently observed with shorter occlusion durations in distal as compared with proximal MCAo (∼15 vs ∼25 to 30 minutes, respectively), probably because the former induces more severe ischemia (see below). Importantly, cortical SNL is optimally assessed in the rat within 30 days of tMCAo, as it may become more difficult to detect at later time points.54
Whether SNL may represent a transitional state en route to actual infarction, so-called ‘delayed infarct maturation', is an important issue to address. Although infarct maturation is widely acknowledged to be complete well within 7 days,11, 55, 56, 57 two reports suggest possible exceptions to this rule. Significant infarct growth beyond day 14 after 60-minute distal tMCAo in SHRs (but not after 30 or 45-minute occlusion times) was reported in a conference proceeding58; however, as specific neuronal markers were not used, it is unclear whether infarct growth was preceded by SNL. In another study using Nissl stain,36 although the striatum and piriform cortex were already infarcted 72 hours after 60-minute proximal tMCAo in SHRs, infarction of the parietal cortex was present at 3 months but not at 3 weeks, but as this was assessed in different subjects the finding may reflect well-known intrinsic variability in the ischemic sequelae of tMCAo. In their 15-minute distal tMCAo model in SHRs, Ejaz et al54 found no evidence of SNL progressing to infarct maturation up to 60 days after injury.
Distinct from infarction, slow maturation of striatal SNL has been reported by Fujioka et al59 following 15-minute proximal MCAo in Wistar rats , with NeuN-defined loss of neurons gradually reaching 97% at 16 weeks together with tissue atrophy on MRI, yet not fulfilling the histopathologic criteria for pannecrosis (see below, MR section). These striking findings are reminiscent of Endres group's 30-minute proximal MCAo studies in mice reporting severe SNL (85% to 90% cell loss but sparing the interneurons) in the striatum at 3 weeks45 and causing a 25% tissue atrophy at 10 weeks,46 and of two other similar reports in rats.40, 39 These findings raise the intriguing possibility of an intrinsic difference regarding SNL between the cortex and striatum, such that neuron dropout in the latter may, under some as yet undetermined circumstances, slowly progress to very severe SNL over weeks.
Whether SNL in the rescued penumbra, perhaps in conjunction with neuroinflammation, triggers neurogenesis and/or facilitates survival of migrated neuroblasts, in turn ensuring partial or total neuronal repopulation, is still unknown. This possibility also deserves specific investigations.
Other animal models Partly for technical reasons discussed above, SNL has been reported only rarely in non-rodent species. Following permanent MCAo in cats, extensive periinfarct damage in the form of dark neurons was reported in one study.60 Using both H&E and cresyl violet, genuine SNL was reported following tMCAo durations between 15 minutes and 4 hours in awake monkeys, according to a ‘flea-bitten' (i.e. patchy) pattern and/or ‘adjacent to or tapering away from an area of total necrosis'.3 As already noted, identifying SNL in the gyrencephalic brain, especially using standard stains, can be difficult.34
SNL in man
Few postmortem studies of SNL in humans are available, and all date back from the 80s when autopsies were still commonplace.61, 62, 63 In these studies, reduced neuron counts were observed only as an inconsistent and narrow (<5 to 10 mm) periinfarct rim, except in one case (not further described) where dead neurons on H&E were seen >20 mm from infarct borders. These classic pre-thrombolysis studies are, however, limited by their intrinsic selection bias toward severe rather than recovering strokes, the lack of assessment of the penumbra acutely, and the already mentioned problems with postmortem assessment of SNL in man.
Assessing SNL In Vivo
Assessing SNL in vivo has considerable advantages over postmortem not only because of its direct clinical applicability in patients who survive from stroke, but also because it allows for longitudinal assessment in the same subject (human or not), including multitracer and multimodality imaging (e.g. PET and MRI), which avoids the above-mentioned major limitation of postmortem that it provides only a snapshot of the process at a selected point in time.
SNL has been widely assessed in vivo using IMZ or FMZ, two selective ligands of the α2,3/β/γ2 subtype of the central benzodiazepine receptor (cBZR, a component of the exclusively neuronal GABAa complex), which is dense in the cerebral cortex, sparse in basal ganglia, and absent in white matter.64 Although FMZ PET allows fully quantitative studies,64, 65 IMZ SPECT is affected by less favorable binding properties and tissue kinetics and worse spatial resolution and inaccurate attenuation correction, and hence provides less reliable data.66 Although one in vitro 3H-FMZ study and another ex vivo 123I-IMZ rodent study have reported decreased cBZR binding in areas affected by SNL,45, 67 validation of in vivo binding as a surrogate for histopathologically defined SNL has been provided only recently in a rat study using FMZ PET 28 days after tMCAo.17 Consistent with an in vitro rat study,45 in one clinical study around 10 to 15 days elapsed until IMZ binding was maximally reduced in SNL areas,68 suggesting that at least a fraction of the cBZRs remain for this time interval on doomed or already dead but not yet dismantled neurons. Interestingly, recent studies even suggest a period lasting around 2 weeks of high or normal FMZ binding in infarcted areas.69, 70, 71 Thus, it is recommended when studying poststroke SNL in vivo that at least 3 weeks have elapsed.
Beyond actual loss of neurons, reductions in IMZ/FMZ binding may reflect ultrastructural neuronal damage such as dendritic spine loss, which has been reported following brief focal ischemia.72, 73 It is understood that although in what follows the term SNL will be used generically when referring to reduced in vivo cBZR mapping, this marker may in fact reveal neuronal damage in addition to actual death.
Rodent studies
In a rat with pure cortical SNL on NeuN staining, Ejaz et al17 found reduced in vivo FMZ binding 28 days following 45-minute distal tMCAo, topographically matching the patches of SNL (Figure 1D).
Non-human primates
Sette et al13 reported reduced FMZ binding in both periinfarct tissue and CT-normal cortical areas overlying striatocapsular infarcts, suggesting SNL; histopathological correlates, however, were not reported. Importantly, non-infarcted cortical areas affected by diaschisis, as shown by coupled reduced perfusion and oxygen metabolism, showed no reduction in cBZR binding, suggesting that FMZ PET is able to differentiate damaged from simply disconnected cortex. In another baboon study following 20-hour proximal tMCAo, Giffard et al34 reported significant chronic-stage reductions in FMZ binding in ipsilateral cortical areas remote for the infarct; interestingly, these areas exhibited hypoperfusion and high oxygen extraction fraction acutely, suggesting that they indeed represented surviving penumbra . However, using H&E, no definite histopathological correlates were found, probably owing to the above-discussed issues with gyrencephalic brains.
In man
The presence in some patients of mild reduction in chronic-stage IMZ binding in periinfarct areas (such as the insular cortex in striatocapsular infarction), as opposed to markedly reduced within the infarcts, was reported in several early SPECT studies and inferred to reflect SNL in the non-infarcted penumbra.74, 75, 76, 77 However, none of these studies directly mapped perfusion acutely. These studies also confirmed unchanged cBZR binding in areas of diaschisis, such as the contralateral cerebellum. In a seminal study of 14 acute MCA stroke patients—some thrombolysed—Nakagawara et al68 reported mild (mean 11%) but significant reductions in IMZ binding 5 days to 23 months post stroke, involving cortical areas that displayed hypoperfusion (or hyperperfusion as a marker of prior ischemia) on acute-stage 133Xe SPECT but no evident infarct on follow-up CT. Although this study strongly suggested SNL in the surviving penumbra, it was limited by the use of IMZ SPECT and the lack of co-registration among imaging modalities or correction for poststroke atrophy; also, the relationship between IMZ binding and acute cerebral blood flow (CBF) was not presented. More recently, Saur et al78 reported significantly reduced IMZ binding (mean: 5%, maximum 11%) in the whole non-infarcted MR diffusion–perfusion mismatch (with TTP>4 seconds) 5 to 15 days after stroke in 12 patients; a weak and nonsignificant correlation with whole mismatch perfusion was mentioned. Guadagno et al79 carried out a PET FMZ study of seven hyperacute MCA stroke patients with extensive penumbra on acute CT perfusion who went on to have rapid spontaneous or post-thrombolysis recovery and small infarcts on follow-up MR (6/7 subcortical), indicating early recanalization. In six patients, there was significantly reduced FMZ binding of generally mild degree, affecting the non-infarcted but initially hypoperfused cortical MCA territory (Figure 2); FMZ values significantly correlated with acute-stage perfusion (see below). Of note, these findings were unchanged following correction for cortical atrophy. Importantly, in both Saur et al78 and Guadagno et al,79 periinfarct tissue was excluded from the analysis to avoid partial volume effects. These findings have been recently replicated in a PET FMZ study of seven additional patients.80 Overall, therefore, the above clinical studies have consistently documented reduced cBZR binding, likely representing SNL, in the non-infarcted penumbra, according to robust methodology. Importantly, reductions in cBZR binding were consistently mild to moderate, suggesting that SNL in the rescued penumbra not only can be absent, but that when present it is of mild-to-moderate degree—although it may be more severe in small spots beyond PET resolution and/or near the infarct but missed by the analysis. New-generation PET scanners with higher spatial resolution may help resolve this issue.
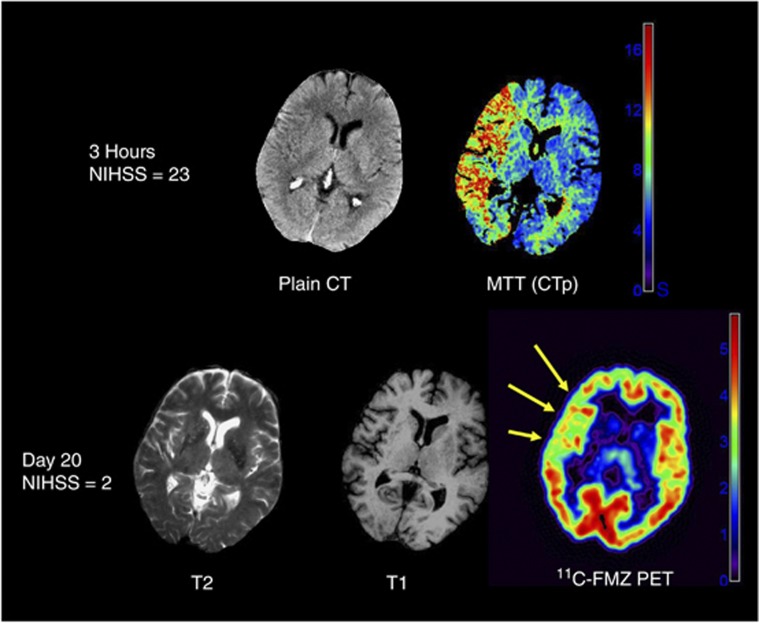
Figure 2.
Illustrative images from one patient with reduced 11C-flumazenil (FMZ) binding potential (BPND) in the initially severely ischemic but eventually non-infarcted middle cerebral artery (MCA) cortex. Shown is one representative slice from the co-registered acute plain CT, CT perfusion (mean-transit time, MTT) map (both obtained 120 minutes after stroke onset), and outcome T2- and T1-weighted magnetic resonance imaging (MRI) and FMZ BPND map (obtained 20 days later). This patient had a severe MCA stroke (NIH stroke scale score 23 at admission) but made a rapid and excellent spontaneous recovery in a few days (NIH stroke scale at day 20: 2), with only a small basal ganglia infarct on follow-up MRI, but showed extensive and statistically significant reductions in BPND in the MCA cortical ribbon. The pseudo-color scales show the range of MTT (seconds) and BPND (standard units). NIHSS, National Institutes of Health Stroke Scale (clinical scale that ranges from 0, no deficit, to 42, maximal deficit). From Guadagno et al,79 with permission.
Magnetic resonance correlates
T1- and T2-weighted magnetic resonance
Increased T2 signal from around 3 to 6 hours and persisting into the chronic stage is the hallmark of infarction in both the experimental and the clinical setting.29 In contrast, numerous rodent studies have demonstrated that SNL, be it striatal or cortical, does not display chronically increased T2 signal17, 37, 39, 42, 49 (Figure 1E), although transiently present slight signal change may be present at day 1 post MCAo.39 By definition, changes on follow-up standard MRI disqualify SNL in clinical studies.68, 78, 79, 80
At variance, in four patients with ‘spectacular shrinking deficit', i.e. a sudden-onset major hemispheric syndrome followed by rapid and near-complete clinical recovery within hours indicating early recanalization, Fujioka et al81 reported hyperintensities on T1-weighted associated with hypointensity on T2-weighted MRI, without corresponding changes on X-ray CT. These changes affected the caudoputamen and cortex, appeared around 1 week after the stroke and disappeared in the following weeks to months together with progressive tissue atrophy (Figure 3A). These authors subsequently reported59 six additional clinical cases who, despite full neurological recovery, showed a decline in global cognitive functions at 12 months of follow-up. They replicated these MR findings in two experimental studies in Wistar rats subjected to 15-minute proximal MCAo,35, 59 where a striatal T2W hypointense signal appeared at day 5 to 7 but was preceded by transient hyperintensity at day 1, indicating vasogenic edema, whereas the T1W hyperintensity peaked at ∼4 weeks and then subsided slowly over months (Figure 3B). Histology showed a severe and progressive SNL affecting the striatum, evolving from a mean of ∼20% of surviving neurons at 4 hours and day 3 down to only 3% at 16 weeks, together with marked and sustained astrocytosis and MA, yet not fulfilling criteria for pannecrosis. These slowly evolving striatal changes were associated with increasingly impaired behavioral tests, and biochemical analyses showed high tissue amounts of manganese. This pattern of MR changes, which strikingly differs from known poststroke changes but is reminiscent of findings in anoxic or hypoglycemic coma (see above), has been replicated once regarding T1,82 while transient day 1 striatal T2-hyperintense lesion, but no T1 changes, was observed in some rats in Wegener et al39 60-minute proximal MCAo study , associated with very severe SNL and inflammation as in Fujioka's study. This MR pattern may reflect accumulation in tissues of agents that shorten T1 and T2 relaxation times, such as iron and manganese59, 82 or reactive oxygen species. Why these changes have not been more regularly replicated so far is intriguing but may reflect differences in experimental protocol and possibly genetic factors in humans, as well as possibly magnetic field strength, which strongly influences signal. Importantly, however, they appear to correspond to very severe and slowly evolving SNL, functionally not really different from infarction, and may be specific to the striatum. Further studies are needed to elucidate these issues.
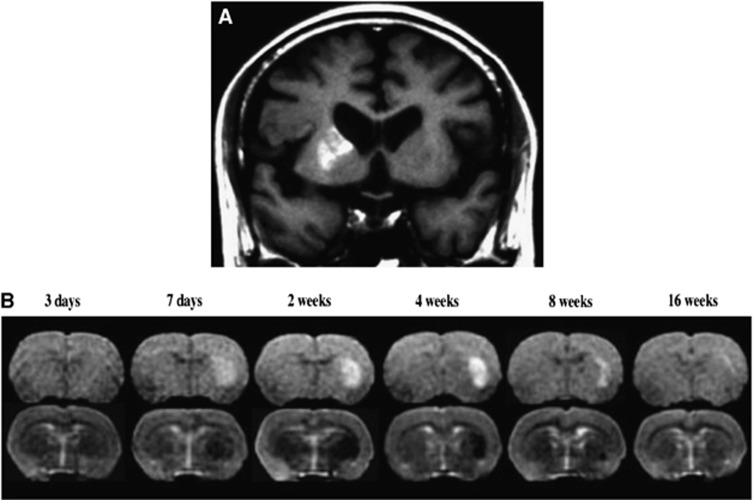
Figure 3.
Delayed ischemic T1 hyperintensity representing selective neuronal loss and glial proliferation after brief and/or mild ischemia. (A) Delayed ischemic hyperintensity on T1-weighted magnetic resonance imaging in the striatum in a patient 10 days after brief focal ischemia. (B) Chronological changes in T1- and T2-weighted (top and bottom rows, respectively) magnetic resonance imaging (MRI) in one rat after 15-minute proximal middle cerebral artery occlusion. Modified from original Figures 1 and 2A in Fujioka et al.59
Diffusion-weighted imaging
Experimentally, areas that develop histopathological SNL ⩾48 hours after tMCAo regularly display apparent diffusion coefficient/diffusion-weighted imaging (DWI) lesions acutely, indicating severe ischemia.37, 40, 42 Diffusion-weighted imaging lesions affected by SNL completely vanish after reperfusion37, 42 and do not reappear secondarily42 (Figure 4). Conversely, DWI lesions that reappear secondarily are generally T2-hyperintense and exhibit pannecrosis histopathologically,40, 41 although very severe SNL in the caudoputamen has been reported in some rats with this imaging pattern40 (Figure 4). Thus, permanently normalized DWI lesions after TIAs or (spontaneously or therapy-induced) recanalized stroke in man83, 84 probably harbor SNL, whereas late reappearances85 probably represent infarction or very severe SNL with similar functional consequences. Clinical studies specifically designed to address these issues would be of considerable interest.
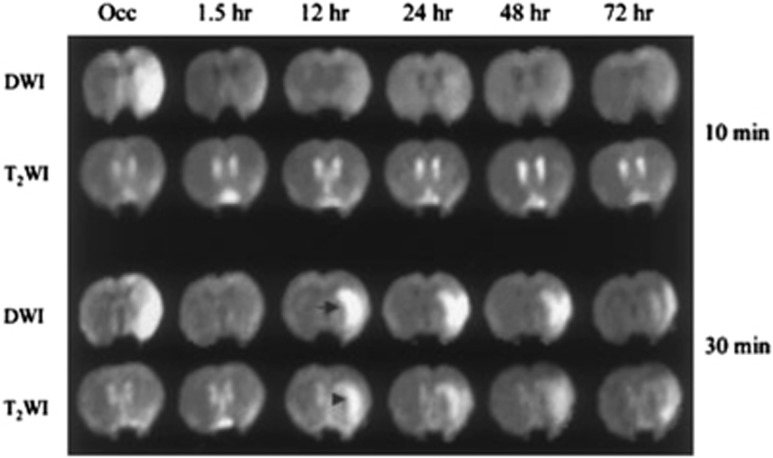
Figure 4.
Representative diffusion-weighted images (DWI) and T2-weighted images (T2WI) at different time points after 10-minute (top) and 30-minute (bottom) middle cerebral artery (MCA) occlusion in Sprague–Dawley rats. In both groups, DWI hyperintensity was seen during occlusion in the lateral caudoputamen and overlying cortex and completely disappeared 1.5 hours after reperfusion. The DWI and T2WI were normal thereafter in the 10-minute group, but secondary DWI lesions (arrow), accompanied by hyperintensity on T2WI (arrowhead), occurred in the 30-minute group at 12 hours after reperfusion and were still present at 72 hours. Note that secondary DWI lesions first developed in the caudoputamen and then gradually spread to the cortex. Occ indicates during occlusion. Histopathology showed moderate selective neuronal death (4% to 28% necrotic neurons) in the caudoputamen with no instance of infarction in the 10-minute group, and massive neuronal death or pannecrosis in all 30-minute group subjects (88% to 100% necrotic neurons). From Li et al,40 with permission.
Functional MRI
Whether SNL hampers neuronal activation in the rescued penumbra is a clinically important question given the likely behavioral relevance. In their 20-minute tMCAo rat study, Sicard et al42 found that passive forepaw stimulation-induced S1 activation was initially impaired but had returned to normal at 24 hours. As the somatosensory area showed mild but significant SNL at postmortem, this finding would imply that SNL did not have long-lasting effects on neuronal activation. In contrast, in a small-scale clinical study, Carrera et al80 found that, within the rescued penumbra, the areas affected by SNL as detected by FMZ PET had significantly reduced fMRI activation 1–3 months post stroke. These apparent discrepancies might reflect species differences but also the small degree of neuronal death in S1, the lack of co-registration between fMRI and histopathology, and the fact that fMRI was performed under general anesthesia, in the Sicard study.42
Behavioral Correlates
Although avoiding infarction of the penumbra markedly benefits behavioral outcome,86, 87 whether SNL may limit or delay functional recovery and plastic processes in the immediate periinfarct tissue, known to partly underlie recovery after cortical stroke,86, 88, 89 is an important issue. In rats subjected to 20-minute proximal tMCAo inducing striatal SNL together with mild and inconsistent cortical SNL, Sicard et al42 reported initial impairment but eventual full recovery in 3 weeks of subtle motor functions assessed by the adhesive test, which has been suggested to be specific to cortical lesions.47 As already mentioned, Fujioka et al59 reported slowly increasing behavioral deficits (circling behavior, delayed reaction time) over 16 weeks following 15-minute proximal MCAo in Wistar rats, in parallel with slow neuronal attrition in the striatum reaching 93% yet not fulfilling the criteria for pannecrosis. Regarding distal MCAo, which induces more severe and consistent cortical ischemia, Hughes et al52 reported no evident neurological deficit following 45-minute occlusion in SHRs. However, given that even extensive cortical infarcts after 60-minute MCAo in this rat strain induce only mild neurological deficits,90 subtle behavioral tests are required to assess the effects of cortical SNL. Similar to Sicard et al's42 findings, Ejaz et al17 recently reported significant deficits on the adhesive test that lasted 3 to 4 weeks, in association with mild cortical SNL, following 15-minute distal MCAo in SHRs.54 Taken together, these two rodent studies indicate that SNL affecting the striatum and, probably more importantly, the cortex can induce subtle but significant and long-lasting, but eventually reversible, motor deficits. However, a detailed correlation of these behavioral effects with individual degree, extent, and topography of SNL is still lacking. Whether these animals remain more liable to reinstate their deficit with subsequent ischemic injuries also remains to be studied.
Mild focal ischemia in the mouse causing severe striatal SNL sometimes associated with small infarcts also leads to subtle, although long-lasting, sensorimotor deficits,91, 92 as well as cognitive changes including deficits in strategy switching,93 and long-term neuropsychiatric sequelae such as anxious and hyperactive phenotype.46
The clinical correlates of SNL are difficult to address because in the vast majority of patients there is an associated infarct, albeit small, in most. For instance, Nakagawara et al68 mentioned ‘clinical signs of cortical dysfunction', i.e. global aphasia, in one patient with reduced 123I-IMZ uptake in non-infarcted cortex, but this was associated with infarction ‘in small areas of frontal cortex'. As already mentioned, Fujioka et al59 reported late cognitive decline despite initial complete recovery in patients with spectacular shrinking deficit and delayed striatal MRI changes, but these may represent very severe SNL (see above). Both Guadagno et al79 and Carrera et al80 underlined their patients' excellent-to-full neurological recovery despite sometimes extensive cortical SNL, impeding neuronal activation. Two main, mutually nonexclusive explanations for this apparent discrepancy were proposed, namely that plastic processes may develop over time so that the neural functions impaired by SNL are taken over by neighboring neurons/areas, and second that standard clinical stroke scales may not capture subtle cognitive or sensorimotor impairments, as suggested in recent studies after minor stroke or transient ischemic attacks.94, 95 Thus, longitudinal studies using subtle behavioral tests will be necessary to document whether, akin to the above rodent studies, SNL delays recovery without affecting eventual outcome in man. Nonetheless, even if SNL did not affect the final outcome and ultimately appears clinically ‘silent', it might reduce the subject's overall ‘cognitive reserve' and enhance the detrimental behavioral effects from any subsequent brain injury or neuronal dysfunction such as further strokes, white matter ischemic damage, and Alzheimer's pathology, potentially contributing to poststroke cognitive decline and, more generally, vascular cognitive impairment. Whether SNL also has a role in the recently described but still vague concept of delayed poststroke cognitive impairment96 is another intriguing possibility.
Mechanisms of Neuronal Death in the Non-Infarcted Penumbra
The mechanism(s) underlying SNL in the salvaged penumbra, including why neurons die but other cells do not, are incompletely understood. Even the time course of SNL, i.e. when exactly the neurons die after reperfusion, is not well known, and systematic serial studies are missing, although in a mice study loss of NeuN labeling was already present 1 day after tMCAo.45 At the biochemical level, the very high energy consumption of neurons makes them more sensitive to oxygen/glucose deprivation than other CNS cells, and hence more susceptible to ischemic injury during the occlusion phase; conversely, their very high post-reperfusion mitochondrial activity would make them prone to oxygen radical production and damage. Also, glutamate excitotoxicity or glutamate/GABA imbalance are also more likely to affect neurons than glial cells. One major hypothesis therefore is that SNL reflects irreversible cell damage ignited during the period of severe ischemia, which, if true, would imply that the degree of SNL should be proportional to both the severity and duration of hypoperfusion. The major alternative hypothesis is that SNL is secondary to detrimental processes triggered by reperfusion—so-called ‘reperfusion injury'—such as oxygen radicals or inflammatory processes, particularly microglia activation. A dual mechanism, requiring first the ischemic insult and then secondary reperfusion injury, may also be at play, and only intervention studies will eventually identify the culprit mechanism(s), with potential applicability to prevention of SNL in man. The main putative mechanisms are briefly reviewed below.
Relationship with perfusion
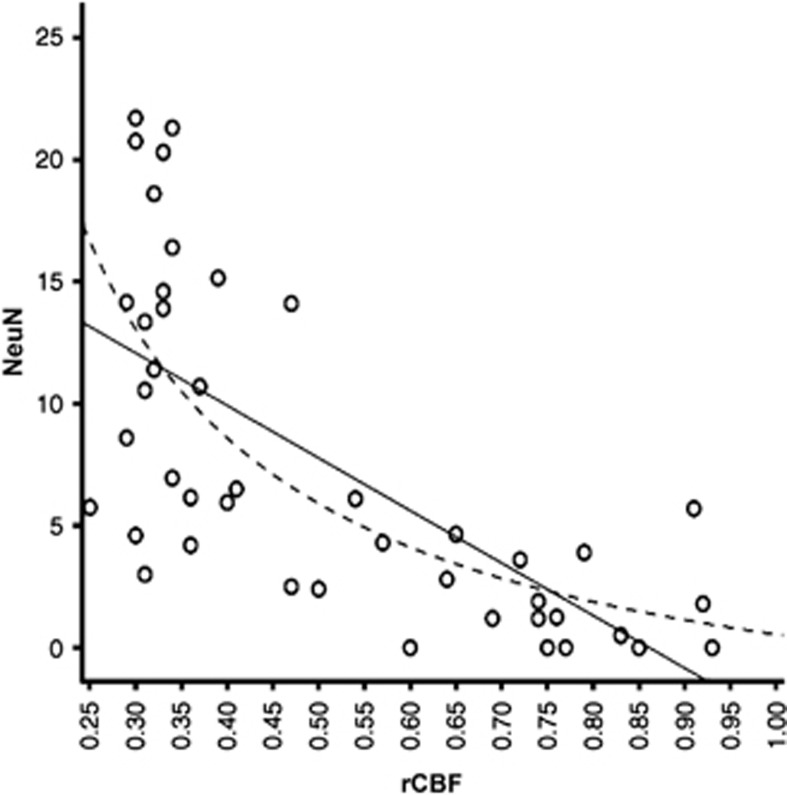
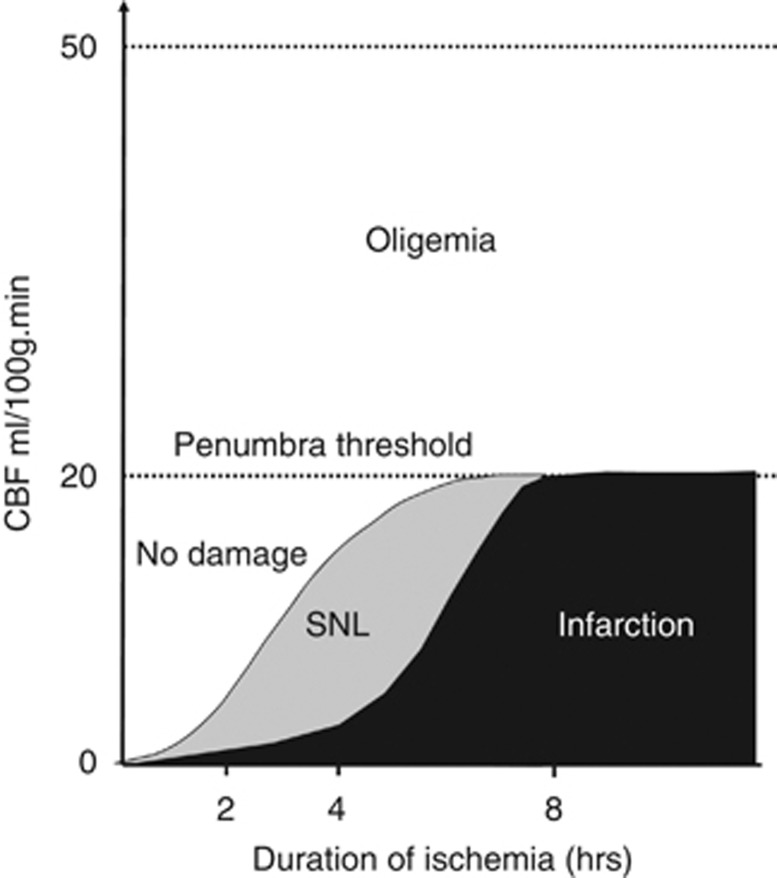
In a seminal cat study, Heiss and Rosner7 found that death of single neurons during MCAo was a function of both severity and duration of ischemia, with, for instance, neurons subjected to CBF <30% of normal for 45 minutes having poor chance of recovery. In awake monkeys subjected to temporary proximal MCAo, apart from infarction and integrity, tissue outcome could also include SNL, which occurred only for short durations of occlusion (30 minutes to 4 hours).3 In proximal tMCAo studies described above, Sicard et al42 found greater SNL in the striatum than in the neocortex, which had higher CBF during occlusion. Following 45 minutes of distal tMCAo in SHRs, Hughes et al52 reported a strong correlation between occlusion CBF and intensity of SNL, with the latter sharply rising below CBF ∼40% of normal, close to the penumbra threshold in this strain97 (Figure 5). In humans, reductions in FMZ binding in non-infarcted areas have been found to be proportional to the severity of acute-stage hypoperfusion, again with an inflexion around the expected penumbra threshold.79 In this study, the precise duration of occlusion was unknown, which future investigations should try and determine. Altogether, these experimental and clinical findings strongly support the idea that SNL in the rescued penumbra is related to the severity (and probably duration) of the initial ischemic insult, which could trigger an irreversible ‘ischemic cascade' ending up in neuronal death some time after reperfusion. In turn, the classic (CBF × time) core/penumbra model98 may be extended to include SNL, i.e. penumbral areas that do not progress to frank infarction may develop SNL, depending on the severity and duration of hypoperfusion (Figure 6). Said otherwise, for a given decrease in CBF, tissue outcome may range from integrity to infarction, with SNL in between these two extremes, depending on the duration of occlusion. In agreement with this model, Ejaz et al17 recently reported in rats a nonlinear relationship between occlusion CBF and severity of ischemic damage, ranging from SNL to partial and complete infarction.
Figure 5.
Linear correlation (P<0.0001) between NeuN scores and relative cerebral blood flow (rCBF) during occlusion (affected/unaffected side ratios), obtained in two different sets of rats, across a fixed template of 44 regions of interest (ROIs). Nonlinear 1/x regression, shown as dotted line, provided better fit and depicts a sharp rise in SNL for rCBF <40%, i.e. the expected penumbral threshold. Modified from Hughes et al,52 with permission.
Figure 6.
Idealized representation of the classical core/penumbra model, but including selective neuronal loss. From Guadagno et al,79 with permission.
Inflammation
Inflammation is an invariable consequence of neuronal injury or death of any cause, and accordingly it is consistently observed in association with SNL.3 For instance, in studies by Endres and co-workers, high in vitro binding of 3H-PK11195 (a selective ligand for activated microglia) was found in the striatal areas showing reduced NeuN staining.45 Strong reactive gliosis with morphologic changes and proliferation of GFAP- and nestin-positive cells was also reported.99 Interestingly, these cells also changed their electrophysiologic properties (‘astron-like'), although did not differentiate into a neuronal phenotype.99, 100 In addition, there was profound activation, proliferation, and invasion of monocytic inflammatory cells (both resident microglia and bone marrow-derived monocytes), which remained in the ischemic area into late time points. In their in vivo PET studies following tMCAo in the baboon, Sette et al13 reported increased 11C-PK11195 colocalized with decreased FMZ binding in periinfarct regions, although the time course of these two ligands was different, with 11C-PK11195 peaking around day 25 and then slowly declining over weeks, whereas FMZ plateaued from day 3 onward. In their distal tMCAo study in SHRs, Hughes et al52 reported high cortical OX42 staining (a specific marker of MA) adopting the same regional distribution as NeuN loss (Figure 7A), with a strong quantitative correlation between the two antibodies (Figure 7B). Interestingly, increased 11C-PK11195 in vivo binding was found in the same sample 14 days after distal tMCAo, which strongly correlated with postmortem OX42.101 In a recent high-resolution IHC study, Ejaz et al further documented this striking colocalization of NeuN loss and OX42 increases within SNL patches17 (Figure 1F).
Figure 7.
(A) Example of pseudo-columnar selective neuronal loss (SNL) assessed using NeuN 14 days after 45-minute distal MCA occlusion in a spontaneously hypertensive rat, and OX42 immunostain obtained at the same coronal level illustrating the matching microglial activation (MA); (B) linear correlation (P<0.0001) between NeuN SNL and OX42 MA scores obtained in a template of 44 regions of interest (data averaged across five rats from the same study).
One obvious key question is whether MA, albeit initially triggered by neuronal injury, perpetuates and exacerbates the latter through the release of neurotoxic chemokines over days or even perhaps weeks.102 Firm evidence for this scenario is, however, lacking to this day, although Fujioka's findings59 of slowly progressive SNL with unusually long-lasting, slowly progressive and eventually massive inflammation over 16 weeks post MCAo are intriguing. The strong correlation between SNL and MA found by Hugues et al (Figure 7B) would be consistent with either or both MA locally causing neuron death, or dying neurons locally triggering MA. In addition, activated microglia also have definite pro-repair effects,102 which seem to predominate in the early days post stroke, followed by the more neurotoxic effects.103 Given the still unclear time course of these complex processes and the lack of agents that can selectively block MA's neurotoxic effects, drug interventions carry the risk of also interfering with MA's beneficial effects and other protective processes such as astrocytosis. Among these stands delayed angiogenesis, which consists of both an in situ response and invasion by bone marrow-derived endothelial progenitor cells.104 Angiogenesis can be augmented by a number of therapeutic strategies and is associated with increased density of perfused microvessels, increased absolute blood flow levels, and improved sensory-motor scores.105
Additional histopathological clues
Following 30 minutes of proximal tMCAo in 129/SV mice, Endres and co-workers reported that SNL evolved in a delayed manner after 48 to 72 hours in the lateral striatum.45, 106 Dying neurons showed early markers of apoptosis such as activation of caspase-3 at 9 to 12 hours and became TUNEL positive at 48 to 72 hours.106, 107, 108 This correlated exactly to the time window at which caspase inhibitors are able to protect the ischemic brain.107 Also, there is evidence of abortive cell cycle re-entry as a prelude to delayed neuronal death109: dying neurons exhibit an early loss of endogenous cyclin-dependent kinases such as p16, which is followed by upregulation of cycling D1, activation of cyclin-dependent kinases, and subsequent cell death. A small fraction of dying neurons even seems to enter S phase before delayed cell death demonstrated by uptake of bromodeoxyuridine in TUNEL-positive cells.109 In contrast, however, electron microscopic imaging failed to demonstrate bona fide apoptotic morphology (Auer and Endres, unpublished observation).
The group of Endres also looked at whether neurotransmitter subtypes of neurons are more susceptible to selective death. Selective neuronal loss in the striatum was found to affect almost exclusively GABAergic medium spiny neurons (type I and type II both in the patch and matrix component) and to a much lesser degree also parvalbuminergic interneurons, whereas cholinergic, GABAergic, and somatostatin-containing interneurons were resistant to ischemic cell death.45, 46 No similar study has looked at cortical SNL yet.
Hughes et al52 found in some rats a striking pseudo-columnar pattern of cortical NeuN staining loss (Figure 7A), raising the possibility of a susceptibility to SNL from differences in e.g. basal neuronal activity and/or microvasculature. A similar pattern of alternatingly increased and decreased glucose utilization has been reported under global hypoxia and systemic hypotension in rats with unilateral common carotid occlusion, underpinned by the radial anatomy of cortical arterioles.110 Along the same line, cortical HSP-70 responses after 5- to 10-minute MCAo in mice also adopt a striking columnar pattern.38 Of note, this pseudo-columnar pattern was not observed in all rats of Hughes et al's52 study, nor in any rat in a recent replication study, with the other rats exhibiting the classic patchy and/or pseudo-laminar pattern, with marked intra- and inter-subject variability.17
Concluding Remarks
It is clear from above that SNL, in isolation or in association with infarction, is a frequent, if not invariable, consequence of brief focal ischemia across species, affecting both the striatum and the cortex, although perhaps with different characteristics. Studying SNL postmortem was difficult until recently owing to the complex histopathological procedures required, and consequently it has been elusive in primates and humans. However, in vivo PET/SPECT studies using validated ligands have documented its presence in stroke patients enjoying early recanalization, despite good clinical recovery. Although in rodents SNL has definite behavioral effects, lasting a few weeks only, the clinical implications of SNL in humans remain unclear. It is however likely, although it will be difficult to document, that also in humans SNL affects the speed of recovery, periinfarct plasticity, and potentially final outcome as well. It in turn likely reduces the ‘neuronal reserve' that normally mitigates the motor, gait, and cognitive effects of age-related brain injury including small strokes, white matter damage, and neurodegenerative pathology. In this sense, among other established vascular factors, SNL following stroke and perhaps also transient ischemic attacks may contribute to poststroke cognitive decline,111 vascular cognitive impairment, and old-age cognitive and motor decline.94 Specific studies using adequate designs are needed to address these issues. In turn, SNL in the surviving penumbra may develop as an important therapeutic target in the aftermath of ischemic stroke, particularly following reperfusion therapy.
SNL in remote areas after focal ischemia
Neuropathology
In addition to the primary ischemic lesion, remote neuronal cell death (exofocal postischemic neuronal cell death, EPND) may develop in a number of brain regions, in particular the thalamus and the midbrain, and may represent a novel target for neuroprotection and stroke management.112 The phenomenon in which remote, but connected, brain regions may be functionally impaired after a focal ischemic lesion was initially termed ‘diaschisis'.113 Although diaschisis in principle describes a reversible functional phenomenon and does not imply morphologic changes,114 it has become clear that reversible or irreversible histopathologic changes may develop in remote connected areas with alterations such as cellular swelling and changes in neuronal morphology.112
Apart from the primary ischemic lesion affecting the lateral striatum following 30-minute tMCAo in the SV mouse, scattered EPND developed in several remote brain regions, with a shrunken and acidophilic neuronal phenotype detectable in the ipsilateral and contralateral forebrain, hippocampus, limbic system, hypothalamus, and cholinergic forebrain nuclei.46 Most interestingly, the number of ipsilateral SN and ventral tegmental area neurons was reduced.46, 115 Midbrain changes associated with the loss of basal ganglia neurons have long been described in experimental models of brain ischemia in rats,116, 117, 118, 119, 120, 121 mice,115, 122 gerbils,123 and cynomolgus monkeys.124 Interestingly, several reports suggest that secondary cell death in the midbrain can be prevented (see below), may be reversible, but may in fact depend on the extent of the ischemic injury. In addition, a few reports from human autopsy material indicate neuronal loss in the ipsilateral SN in the chronic phase after large basal ganglia infarcts.125, 126
Several reports also indicate secondary degeneration/EPND in the thalamus.127, 128, 129, 130 Similar to midbrain lesions, thalamic lesions also display delayed and selective neuronal death leading to reduced neuronal cell density with neuropil rarefaction and reactive microgliosis and astrogliosis.128
Mechanisms and Behavioral Implications
Although underpinned by a combination of retrograde and anterograde degeneration, the phenomenon of remote degeneration is incompletely understood. With regard to midbrain lesions, while experimental stroke studies in rats mainly point to involvement of the pars reticulata of the SN, histopathological and clinical MRI studies found secondary changes to be especially prominent in the pars compacta (SNpc).115, 131 This apparent discrepancy may be explained by a number of factors including age, species, or the exact location of the primary ischemic lesion.131 The striatonigral pathway predominantly projects to the ipsilateral pars reticulata of the SN.132 Destruction of this inhibitory (GABAergic) projection may result in disinhibition and, in turn, delayed transneuronal (i.e. transsynaptic) degeneration, primarily of neurons in the pars reticulata of the SN117, 133). By contrast, however, disruption of the nigrostriatal pathway may as well result in retrograde degeneration of SNpc dopaminergic neurons.134 It is thus conceivable that either mechanism may be relevant and may also depend on the exact neuroanatomic location and severity of the primary striatal lesion.
There is controversy regarding whether remote changes may be reversible. Planas and co-workers119 reported that striatal infarction in the rat causes a transient reduction of tyrosine hydroxylase immunoreactivity in the ipsilateral SN, whereas others reported permanent cell loss . However, the severity of the primary insult may correlate not only with the extent of the secondary lesion but may also explain reversibility.117 Interestingly, Witte and co-workers135 elegantly described widespread and long-lasting dysregulation of the GABAergig system after focal cortical infarcts in regions with and without delayed exofocal cell death.
At present, the functional significance of EPND is not entirely clear. In a model of 30-minute tMCAo in the mouse, Kronenberg et al115 observed delayed cell death in the ipsilateral midbrain confined to dopaminergic neurons in the SNpc and ventral tegmental area . This dopaminergic degeneration was accompanied by reduced dopamine concentrations and decreased levels of dopamine transporter density in the ischemic striatum. Most notably, animals developed a ‘depressive phenotype' following left, but not right, MCAo after 14 weeks that was characterized by traits of anxiety, despair, and anhedonia, which correlated with increased dynorphin messenger RNA expression in nucleus accumbens.115 Interestingly, in addition to loss of dopamine, an increase of brain-derived neurotrophic factor was detected in the lesioned striatum, and increased brain-derived neurotrophic factor signaling specifically in ventral striatum has been linked to depressive-like behavior.115 At present, however, it is unclear whether these remote changes in the mesolimbic reward system are causative for ‘poststroke depression', and whether they have a role in human pathophysiology and hence represent a novel target for therapy (vide infra).
Magnetic Resonance/Positron Emission Tomography to Detect Exofocal Postischemic Neuronal Cell Death In Vivo?
Both CT and MRI have been used to demonstrate EPND in experimental studies and in humans. In the clinical setting, Tamura et al130 demonstrated secondary damage to the thalamus after MCA stroke on CT . Higher-resolution MRI studies in animals and stroke patients have since replicated and expanded these initial findings,128, 131, 136, 137 for instance, demonstrating transient exofocal signal changes in ipsilateral midbrain in rodents, consisting of delayed decreases in apparent diffusion coefficient and corresponding increases in T2 values in the ipsilateral midbrain115, 127, 138 (Figure 8), probably reflecting cell swelling and cytotoxic edema. In addition, the exofoxal T2 hyperintensity in the midbrain was associated with activation of microglia and clearly preceded overt neuron loss.115 Similar findings have been reported in stroke patients, e.g. striatal strokes induce a subacute T2-weighted hyperintensity in the ipsilateral midbrain128, 131, 136, 137 (Figure 9). These secondary T2 midbrain changes are detectable from ∼2 weeks after stroke onset,131 although at higher field strengths they are detectable in the SN from the first week. It is important for clinicians to be aware of these delayed MRI changes so as not to confuse them with a second stroke. Similarly, DWI137 and diffusion tensor imaging139 may be of value in detecting EPND in the thalamus or midbrain. Interestingly, diffusion tensor imaging demonstrated increases in mean diffusivity but not in fractional anisotropy in secondary lesions.139, 140 Finally, PET with markers of MA such as 11C-PK11195 can also detect poststroke EPND in thalamus and brainstem in vivo in rodents and man.141, 142
Figure 8.
Secondary exofocal neurodegeneration after striatal infarction in mice. (A) At 24 hours after 30-minute middle cerebral artery occlusion (MCAo)/reperfusion, a T2 hyperintensity demarcates the ischemic left striatum. This characteristic magnetic resonance (MR) signal of the primary lesion fades during the first week after MCAo. (B, C, D) At 7 days after MCAo, an activation of Iba1-expressing microglia in the ipsilateral (C) as compared with the contralateral (B) midbrain was observed. This coincided with a secondary T2 hyperintensity that emerged toward the end of the first week after stroke (arrow in panel D). (E) Loss of tyrosine hydroxylase-expressing neurons on the stroke side in the midbrain at 16 weeks after experimental ischemia (adapted from Kronenberg et al115.)
Figure 9.
Abnormal lesion signal normalization in remote areas at follow-up: brain infarcts in the middle cerebral artery (MCA) territory affecting the striatum on coronal T2w magnetic resonance imaging (MRI) on day 10 in two selected patients (A, F). Hyperintense signal in the ipsilateral midbrain is evident on day 10 in axial diffusion-weighted imaging (DWI) (B, G) and 2 adjacent coronary T2w sections (C, H), which disappeared in corresponding sections on day 72 (D, E) and day 90 (I, J).
Therapeutic Implications
As EPND develops in a delayed manner, while patients are recovering from their deficits, and is uncoupled from the maturation of the primary ischemic lesion, it may be a target for (secondary) neuroprotection. At present, however, it is not entirely clear whether EPND has any functional relevance, how it could be prevented, and, most importantly, whether it has any clinical relevance. In experimental models, a number of pathophysiologic mechanisms and corresponding therapeutic targets have been implicated in EPND (recently reviewed in Zhang et al.112 Over 25 years ago, Saji and Reis117 reported that delayed transneuronal death of SN neurons can be prevented by GABA agonists. Other therapeutic approaches include anti-excitotoxic treatment by either NMDA or AMPA receptor antagonists, but also activators of cerebral peroxisome proliferator-activated receptors gamma.120, 143, 144 Another interesting approach involves antidepressant treatment to simultaneously reduce EPND and prevent poststroke depression but perhaps also interfere with maturation of the primary ischemic lesion. For instance, chronic treatment with the selective serotonin-reuptake inhibitor citalopram following 30-minute MCAo in mice initiated as late as 7 days reversed the behavioral ‘depressive' phenotype, prevented degeneration of dopaminergic midbrain neurons, and attenuated lesion maturation and striatal atrophy at 4 months.115 Hence, prevention of exofocal remote dopaminergic cell death in the midbrain may emerge as a novel target for neuroprotection by antidepressants.
In addition, there may be endogenous mechanisms of protection: Interestingly, Bidmon et al145 using a model of photothrombotic stroke demonstrated transient increases of manganese-superoxide dismutase in several remote brain areas connected via afferents and efferents with the primary lesion site, including the perilesional cortex, corpus callosum, hippocampus, thalamus, and homologous contralateral cortex. The authors speculate that manganese-superoxide dismutase may protect these remote cells from retrograde or anterograde spread of neurotoxicity. In addition, Schroeter et al129 found that mice deficient in osteopontin, a cytokine-like glycoprotein that binds to various integrins and CD44 variants, have similar primary ischemic lesion sizes to control mice but develop significantly aggravated secondary thalamic degeneration associated with increased MA and inflammatory gene expression.
Concluding Remarks
Exofocal postischemic neuronal cell death in the ipsilateral SN and thalamus depends on stroke topography and becomes detectable by MRI and also histologically days and weeks after stroke. Diffusion-weighted imaging and T2-weighted imaging at 3T offer reliable detection of these secondary changes. The study of these delayed processes of lesion progression in the clinical setting may aid research in developing new strategies aimed at preventing secondary neuron loss and improving functional and affective outcome after stroke.
Selective neuronal loss in chronic arterial obstructive disease
In patients with chronic atherosclerotic ICA or MCA occlusive disease, low perfusion or microembolism may produce selective neuronal damage/loss.
Neuropathology
Neuropathological studies have demonstrated cortical granular atrophy or watershed territory microemboli in the cerebral cortex distal to large cerebral arterial occlusion, particularly in the watershed areas.146 However, SNL in patients with ICA or MCA disease has not been confirmed pathologically in the human brain so far.
In Vivo Magnetic Resonance
In cerebrovascular disease, brain atrophy may be a reflection of ischemic changes. Computed tomography studies in ICA disease have disclosed only hemiatrophy in some patients.147 In patients with ICA or MCA disease including Moyamoya, MRI studies have shown decreases in cortical thickness in the ipsilateral hemisphere.148
Atrophy of the corpus callosum may be an indirect but particularly sensitive indicator of ischemic cortical neuronal loss. The largest fraction of neurons projecting through the corpus callosum is the large pyramidal cells in layer 3, which is among the cortical neurons most vulnerable to ischemia.146 Therefore, callosal atrophy may result from cortical SNL. Yamauchi et al149, 150, 151, 152 showed that a bilateral decrease in cortical cerebral metabolic rate of oxygen (CMRO2) occurs in association with callosal atrophy in patients with ICA disease, suggesting that SNL may contribute to the reduced CMRO2.
In Vivo Positron Emission Tomography/Single-Photon Emission Computed Tomography
Reduced cortical CMRO2 in the normal-appearing cortex, which surgical reperfusion cannot improve, has been demonstrated in ICA/MCA disease using PET, suggesting underlying SNL.153, 154 A decrease in glucose or oxygen metabolism in the normal-appearing cortex is one result of ischemic damage to the tissue, but it can also be caused by diaschisis,114 indicating inadequate specificity.
Several small studies in patients with chronic atherosclerotic ICA or MCA disease demonstrated a reduction in cBZR binding in the normal-appearing cortical areas on CT or MRI, including the center of the MCA territory, suggesting the presence of SNL.74, 75, 76, 155, 156, 157, 158 123I-iomazenil uptake significantly but weakly correlated with CMRO2 but not with CBF, cerebrovascular reactivity, or CMRglu (cerebral metabolic rate for glucose utilization), suggesting that the reduction of IMZ uptake may be due to SNL that may not be reliably revealed by other imaging tracers.74, 76, 155 Similar findings were also shown using FMZ and PET.157, 158 A decrease in cortical cBZR binding was correlated with corpus callosum atrophy, which supported that the latter may reflect cortical neuronal damage.156
Recent studies by Yamauchi et al159, 160, 161, 162, 163 involving larger samples addressed the incidence, extent, and severity of SNL demonstrated as decreased cBZR binding, and the factors responsible for the decreased cBZR . They documented a high incidence of SNL, the degree of which was associated with chronic hemodynamic compromise. Subcortical infarcts per se did not decrease cBZR in the overlying cerebral cortex, ruling out interference by diaschisis.
Mechanisms
In patients with atherosclerotic ICA or MCA disease, chronic hemodynamic compromise increases the risk of cerebral ischemic damage.164, 165, 166, 167, 168 In this hemodynamic situation, perfusion may occasionally fall below the penumbra threshold and in turn cause SNL. In 105 patients with atherosclerotic ICA or MCA occlusive disease and no cortical infarction on MRI, Yamauchi et al160 showed an association between SNL, as demonstrated by decreased FMZ binding in the normal-appearing cerebral cortex, and increased oxygen extraction fraction as shown by 15O PET (i.e. misery perfusion) (Figure 10). They also demonstrated a correlation between higher oxygen extraction fraction and progression of SNL in a longitudinal study.160 These findings strongly suggest that misery perfusion may contribute to SNL. This hemodynamic mechanism was also supported by the correlation of cortical neuronal damage with low-flow infarcts. In ICA or MCA occlusive disease, decreased cortical FMZ binding was associated with the presence of borderzone infarcts, suggesting that hemodynamic ischemia leading to borderzone infarcts may cause SNL.159, 161
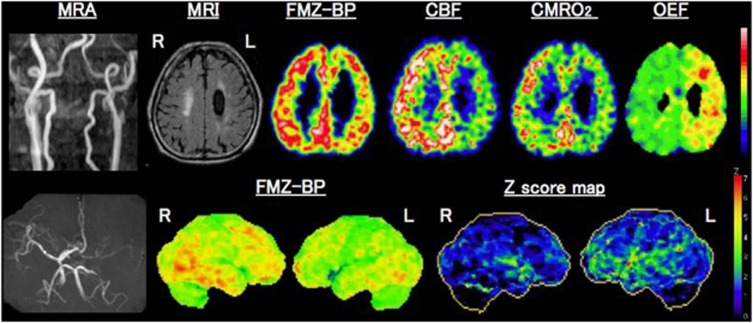
Figure 10.
Benzodiazepine receptor binding and misery perfusion. Upper row: examples of positron emission tomography (PET) images showing decreases in 11C-flumazenil-binding potential (FMZ-BP), cerebral blood flow (CBF), and CMRO2 with increased oxygen extraction fraction (OEF) in a patient with left (L) internal carotid artery occlusion on magnetic resonance angiography(MRA), left) who showed internal borderzone infarction on the corresponding MRI images. Lower row: Three-dimensional-SSP images and Z-score maps showing a decrease of FMZ-BP in the left hemisphere. Modified from Yamauchi et al.160
Microemboli may, however, contribute to SNL in borderzone areas, particularly in cases of arterial stenosis.169, 170 It is less likely, however, that microembolism, which preferentially targets the borderzone, causes SNL beyond the borderzone itself, which is consistently observed in chronic hemodynamic compromise.
Functional/Behavioral Correlates
In atherosclerotic ICA or MCA disease, reduced cortical cBZR binding has been reported in the Broca or Wernicke area in patients with aphasia and subcortical infarcts,75, 155 and global reduction of cortical cBZR binding has been shown to be correlated with callosal atrophy, which is associated with global cognitive impairment.151, 156 In addition, cerebral hyperperfusion after carotid endarterectomy was found to result in a decrease in IMZ uptake that correlated with postoperative cognitive impairment.171 Therefore, in addition to the co-existing subcortical infarcts, SNL may contribute to the development of subtle poststroke cognitive impairment, depending on the degree of neuronal damage.
Using PET and 11C-FMZ in patients with ICA or MCA disease, Yamauchi et al162 demonstrated that decreases in cBZR binding in the non-infarcted cerebral cortex was associated with impaired executive functions.
Treatment/Prevention
Vascular reconstruction surgery can improve chronic hemodynamic compromise, which may in turn prevent the development or worsening of SNL, or even perhaps reverse neuronal damage and improve cognitive impairment. For instance, one study reported improvement of hemodynamic compromise after carotid endarterectomy that was associated with postoperative increases in IMZ uptake in correlation with improved cognitive impairment.172 Another study reported an improvement in cortical thinness after bypass surgery in patients with ICA or MCA occlusive disease including Moyamoya, and severely impaired vasoreactivity.173
Concluding Remarks
Misery perfusion contributes to SNL, demonstrated as decreased cBZR in the normal-appearing cerebral cortex. Selective neuronal loss may have considerable impact on the functional outcomes. Therapeutic strategies for preventing neuronal damage are needed, especially in situations of chronic hemodynamic impairment.
Conclusions
The main point that emerges from the present review is that SNL is a widespread phenomenon in cerebrovascular diseases, yet one that eludes routine imaging procedures such as structural MRI. In acute stroke, it almost invariably occurs in the initially ischemic but eventually non-infarcted striatal and cortical areas, particularly in the setting of reperfusion therapy, but also develops in remote connected areas as a marker of neuronal degeneration, and occurs in the whole hemisphere, perhaps with a predilection for the watershed areas, in patients with chronic obstructive ICA or MCA disease causing hemodynamic impairment. As such, this wide prevalence of SNL throughout the brain, ipsilaterally but also contralaterally, may partly account for the increasingly reported diffuse cortical atrophy present in cerebrovascular disease regardless of underlying etiology, and contribute to poststroke cognitive decline, vascular cognitive impairment, and vascular/mixed dementia. For instance, the strong impact of vascular risk factors and vascular pathology on the incidence of clinically diagnosed Alzheimer's disease174 may be in part explained by SNL, even if it is apparently ‘silent' clinically. It may also reduce the potential for plasticity and recovery in case of further strokes, as well as with age-related neurodegeneration. Studies designed to address these issues are warranted, but also better animal models of SNL. Possible studies to move forward in the field of SNL have been suggested within each section above. In addition, although SNL can be detected in vivo using radionuclide imaging, the search for non-ionizing biomarkers such as MR-based spectroscopy (e.g. NAA82) is an important goal. Similarly, whether SNL can be mitigated by neurogenesis is another important issue. Above all, a better understanding of the underlying mechanisms is required if therapeutic measures to prevent SNL are envisaged.
The authors declare no conflict of interest.
Footnotes
J-CB was supported by the UK Medical Research Council (grants G0001219 and G0500874), the EU (EUSTROKE) and the Cambridge Biomedical Research Centre. J-CB acknowledges his PhD students and post docs, particularly N Watanabe, C Giffard, JL Hughes, JV Guadagno, RS Morris, J Emmrich, and S Ejaz for their work on selective neuronal loss over the last 15 years. ME was supported by the Deutsche Forschungsgemeinschaft (ExcellenceCluster NeuroCure, SFB TR43, KFO 247, KFO 213), the BMBF (Center for Stroke Research Berlin), the EU (European Stroke Network), and the Volkswagen Foundation (Lichtenberg Program). ME acknowledges his PhD students and post docs, particularly Karen Gertz, Juri Katchanov, Christoph Harms, Golo Kronenberg, and Benjamin Winter for their work on SNL.
References
- Graham DI, Peter PL.Greenfield's Neuropathology7th ednArnold publisher: London; 20021251–258. [Google Scholar]
- Garcia JH, Lassen NA, Weiller C, Sperling B, Nakagawara J. Ischemic stroke and incomplete infarction. Stroke. 1996;27:761–765. doi: 10.1161/01.str.27.4.761. [DOI] [PubMed] [Google Scholar]
- DeGirolami U, Crowell RM, Marcoux FW. Selective necrosis and total necrosis in focal cerebral ischemia. Neuropathologic observations on experimental middle cerebral artery occlusion in the macaque monkey. J Neuropathol Exp Neurol. 1984;43:57–71. doi: 10.1097/00005072-198401000-00005. [DOI] [PubMed] [Google Scholar]
- Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24:699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- Spielmeyer W. Zur pathogenese örtlich elektiver gehirnveränderungen. Z Ges Neurol Psych. 1925;99:756–776. [Google Scholar]
- Spatz H. Pathologische anatomie der kreislaufstörungen. Z Neurol. 1939;167:301–324. [Google Scholar]
- Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294–301. doi: 10.1002/ana.410140307. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Incomplete cerebral infarction—focal incomplete ischemic tissue necrosis not leading to emollision. Stroke. 1982;13:522–523. doi: 10.1161/01.str.13.4.522. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Olsen T.S, Hojgaard K, Skriver E. Incomplete infarction: a CT-negative irreversible ischemic brain lesion. J Cereb Blood Flow Metab. 1983;3:S602–S603. [Google Scholar]
- Nakano S, Kogure K, Fujikura H. Ischemia-induced slowly progressive neuronal damage in the rat brain. Neuroscience. 1990;38:115–124. doi: 10.1016/0306-4522(90)90378-h. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ye ZR, Gutierrez JA. Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2303–2309. doi: 10.1161/01.str.28.11.2303. [DOI] [PubMed] [Google Scholar]
- Weiller C, Willmes K, Reiche W, Thron A, Isensee C, Buell U, et al. The case of aphasia or neglect after striatocapsular infarction. Brain. 1993;116:1509–1525. doi: 10.1093/brain/116.6.1509. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barre L, et al. In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke. 1993;24:2046–2057. doi: 10.1161/01.str.24.12.2046. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ogawa T, Yoshida Y, Tamura H, Kado H, Okudera T. Curvilinear t1 hyperintense lesions representing cortical necrosis after cerebral infarction. Neuroradiology. 2005;47:647–651. doi: 10.1007/s00234-005-1398-0. [DOI] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer RN.Histopathology Of Cerebral IschemiaIn: Mohr DC J. P, Grotta James, Philip Wolf eds. Stroke: Pathophysiology, diagnosis and management London: Churchill Livingstone; 2004821–828. [Google Scholar]
- Ejaz S, Williamson DJ, Ahmed T, Sitnikov S, Hong YT, Sawiak SJ, et al. Characterizing infarction and selective neuronal loss following temporary focal cerebral ischemia in the rat: a multi-modality imaging study. Neurobiol Dis. 2013;51:120–132. doi: 10.1016/j.nbd.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field ca1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Nishio K, Miyamoto S, Hiramatsu KI, Sakaki T, Okuchi K, et al. Hippocampal damage in the human brain after cardiac arrest. Cerebrovasc Dis. 2000;10:2–7. doi: 10.1159/000016018. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Okuchi K, Sakaki T, Hiramatsu K, Miyamoto S, Iwasaki S. Specific changes in human brain following reperfusion after cardiac arrest. Stroke. 1994;25:2091–2095. doi: 10.1161/01.str.25.10.2091. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28:584–587. doi: 10.1161/01.str.28.3.584. [DOI] [PubMed] [Google Scholar]
- Aoe H, Takeda Y, Kawahara H, Tanaka A, Morita K. Clinical significance of T1-weighted MR images following transient cerebral ischemia. J Neurol Sci. 2006;241:19–24. doi: 10.1016/j.jns.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Auer RN, Wieloch T, Olsson Y, Siesjo BK. The distribution of hypoglycemic brain damage. Acta Neuropathol. 1984;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol. 2012;12:139. doi: 10.1186/1471-2377-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Zille M, Farr TD, Przesdzing I, Muller J, Sommer C, Dirnagl U, et al. Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives. J Cereb Blood Flow Metab. 2012;32:213–231. doi: 10.1038/jcbfm.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Group HaCAS Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Baron JC. How healthy is the acutely reperfused ischemic penumbra. Cerebrovasc Dis. 2005;20 (Suppl 2:25–31. doi: 10.1159/000089354. [DOI] [PubMed] [Google Scholar]
- Giffard C, Landeau B, Kerrouche N, Young AR, Barre L, Baron JC. Decreased chronic-stage cortical 11c-flumazenil binding after focal ischemia-reperfusion in baboons: a marker of selective neuronal loss. Stroke. 2008;39:991–999. doi: 10.1161/STROKEAHA.107.489419. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Taoka T, Matsuo Y, Hiramatsu KI, Sakaki T. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999;30:1043–1046. doi: 10.1161/01.str.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J Comp Neurol. 1997;386:461–476. [PubMed] [Google Scholar]
- Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, et al. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol. 1999;46:333–342. doi: 10.1002/1531-8249(199909)46:3<333::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Sprenger C, Wiedermann D, et al. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2006;26:38–47. doi: 10.1038/sj.jcbfm.9600166. [DOI] [PubMed] [Google Scholar]
- Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke. 2000;31:946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32:2362–2369. doi: 10.1161/hs1001.096058. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Henninger N, Fisher M, Duong TQ, Ferris CF. Long-term changes of functional MRI-based brain function, behavioral status, and histopathology after transient focal cerebral ischemia in rats. Stroke. 2006;37:2593–2600. doi: 10.1161/01.STR.0000239667.15532.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Knollema S, van der Worp HB, Ter Horst GJ, De Wildt DJ, Berkelbach van der Sprenkel JW, et al. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke. 1998;29:695–704. doi: 10.1161/01.str.29.3.695. [DOI] [PubMed] [Google Scholar]
- Pedrono E, Durukan A, Strbian D, Marinkovic I, Shekhar S, Pitkonen M, et al. An optimized mouse model for transient ischemic attack. J Neuropathol Exp Neurol. 2010;69:188–195. doi: 10.1097/NEN.0b013e3181cd331c. [DOI] [PubMed] [Google Scholar]
- Katchanov J, Waeber C, Gertz K, Gietz A, Winter B, Bruck W, et al. Selective neuronal vulnerability following mild focal brain ischemia in the mouse. Brain Pathol. 2003;13:452–464. doi: 10.1111/j.1750-3639.2003.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B, Juckel G, Viktorov I, Katchanov J, Gietz A, Sohr R, et al. Anxious and hyperactive phenotype following brief ischemic episodes in mice. Biol Psychiatry. 2005;57:1166–1175. doi: 10.1016/j.biopsych.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Wiedermann D, Kandal K, et al. Subcortical lesions after transient thread occlusion in the rat: T2-weighted magnetic resonance imaging findings without corresponding sensorimotor deficits. J Magn Reson Imaging. 2005;21:340–346. doi: 10.1002/jmri.20270. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Henninger N, Fisher M, Duong TQ, Ferris CF. Differential recovery of multimodal MRI and behavior after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1451–1462. doi: 10.1038/sj.jcbfm.9600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A. A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992;23:273–279. doi: 10.1161/01.str.23.2.273. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Brint S, Tanabe J, Jacewicz M, Wang XJ, Pulsinelli W. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke. 1991;22:1032–1039. doi: 10.1161/01.str.22.8.1032. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Beech JS, Jones PS, Wang D, Menon DK, Baron JC. Mapping selective neuronal loss and microglial activation in the salvaged neocortical penumbra in the rat. Neuroimage. 2010;49:19–31. doi: 10.1016/j.neuroimage.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Ejaz S, Williamson D.J, Jensen-Kondering U, Ahmed T, Sawiak S.J, Baron JC.Development of a MCA occlusion rodent model of pure cortical selective neuronal loss: neuropathological validation XXVIth International Conference on Cerebral Blood Flow, Metabolism and Brain FunctionShanghai2013
- Ejaz S, Emmrich J, Sawiak S.J, Williamson DJ, Baron JC.A novel rodent model of pure cortical selective neuronal loss following temporary MCA occlusion (tMCAO): behavioral, histopathological and MRI characterization XXVIth International Symposium on Cerebral Blood Flow, Metabolism and FunctionShanghai2013
- Aspey BS, Cohen S, Patel Y, Terruli M, Harrison MJ. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathol Appl Neurobiol. 1998;24:487–497. doi: 10.1046/j.1365-2990.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, Mc Cullough LD. TTC, fluoro-jade b and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M. Neuronal injury in the infarct border: a neuropathological study in the rat. Acta Neuropathol. 1987;73:267–274. doi: 10.1007/BF00686621. [DOI] [PubMed] [Google Scholar]
- Buchan AM. Delayed Maturation Of Cortical Infarction: Role Of Caspases And Nf-Kb Mediated Transcription. Springer-Verlag: Berlin, Heidelberg; 2001. [Google Scholar]
- Fujioka M, Taoka T, Matsuo Y, Mishima K, Ogoshi K, Kondo Y, et al. Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol. 2003;54:732–747. doi: 10.1002/ana.10751. [DOI] [PubMed] [Google Scholar]
- Mies G, Auer LM, Ebhardt G, Traupe H, Heiss WD. Flow and neuronal density in tissue surrounding chronic infarction. Stroke. 1983;14:22–27. doi: 10.1161/01.str.14.1.22. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Vorstrup S, Astrup J. Cell density in the border zone around old small human brain infarcts. Stroke. 1986;17:1129–1137. doi: 10.1161/01.str.17.6.1129. [DOI] [PubMed] [Google Scholar]
- Torvik A, Svindland A. Is there a transitional zone between brain infarcts and the surrounding brain? A histological study. Acta Neurol Scand. 1986;74:365–370. doi: 10.1111/j.1600-0404.1986.tb03527.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J, Klinken L. Cell density and cortex thickness in the border zone surrounding old infarcts in the human brain. Stroke. 1984;15:1033–1039. doi: 10.1161/01.str.15.6.1033. [DOI] [PubMed] [Google Scholar]
- Abadie P, Baron JC, Bisserbe JC, Boulenger JP, Rioux P, Travere JM, et al. Central benzodiazepine receptors in human brain: estimation of regional BMAX and KD values with positron emission tomography. Eur J Pharmacol. 1992;213:107–115. doi: 10.1016/0014-2999(92)90239-z. [DOI] [PubMed] [Google Scholar]
- Pappata S, Samson Y, Chavoix C, Prenant C, Maziere M, Baron JC. Regional specific binding of [11c]ro 15 1788 to central type benzodiazepine receptors in human brain: quantitative evaluation by pet. J Cereb Blood Flow Metab. 1988;8:304–313. doi: 10.1038/jcbfm.1988.65. [DOI] [PubMed] [Google Scholar]
- Videbaek C, Friberg L, Holm S, Wammen S, Foged C, Andersen JV, et al. Benzodiazepine receptor equilibrium constants for flumazenil and midazolam determined in humans with the single photon emission computer tomography tracer [123i]iomazenil. Eur J Pharmacol. 1993;249:43–51. doi: 10.1016/0014-2999(93)90660-a. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakano T, Yutani K, Nishimura H, Kusuoka H, Nakamura H, et al. Detection of viable cortical neurons using benzodiazepine receptor imaging after reversible focal ischaemia in rats: comparison with regional cerebral blood flow. Eur J Nucl Med. 2000;27:308–313. doi: 10.1007/s002590050038. [DOI] [PubMed] [Google Scholar]
- Nakagawara J, Sperling B, Lassen NA. Incomplete brain infarction of reperfused cortex may be quantitated with iomazenil. Stroke. 1997;28:124–132. doi: 10.1161/01.str.28.1.124. [DOI] [PubMed] [Google Scholar]
- Abe K, Kashiwagi Y, Tokumura M, Hosoi R, Hatazawa J, Inoue O. Discrepancy between cell injury and benzodiazepine receptor binding after transient middle cerebral artery occlusion in rats. Synapse. 2004;53:234–239. doi: 10.1002/syn.20057. [DOI] [PubMed] [Google Scholar]
- Fukumoto D, Hosoya T, Nishiyama S, Harada N, Iwata H, Yamamoto S, et al. Multiparametric assessment of acute and subacute ischemic neuronal damage: a small animal positron emission tomography study with rat photochemically induced thrombosis model. Synapse. 2011;65:207–214. doi: 10.1002/syn.20836. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Pareto D, Herance JR, Abad S, Ruiz A, et al. Positron emission tomography with 11c-flumazenil in the rat shows preservation of binding sites during the acute phase after 2 h-transient focal ischemia. Neuroscience. 2011;182:208–216. doi: 10.1016/j.neuroscience.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Yanagihara T. Electron microscopic investigation of the cerebral cortex after cerebral ischemia and reperfusion in the gerbil. Brain Res. 1992;598:87–97. doi: 10.1016/0006-8993(92)90171-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Fukuyama H, Nabatame H, Yamauchi H, Shibasaki H, Yonekura Y. Assessment of benzodiazepine receptors using iodine-123-labeled iomazenil single-photon emission computed tomography in patients with ischemic cerebrovascular disease. A comparison with PET study. Stroke. 1997;28:1776–1782. doi: 10.1161/01.str.28.9.1776. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Satoh T, Shimosegawa E, Okudera T, Inugami A, Ogawa T, et al. Evaluation of cerebral infarction with iodine 123-iomazenil SPECT. J Nucl Med. 1995;36:2154–2161. [PubMed] [Google Scholar]
- Sasaki M, Ichiya Y, Kuwabara Y, Yoshida T, Fukumura T, Masuda K. Benzodiazepine receptors in chronic cerebrovascular disease: comparison with blood flow and metabolism. J Nucl Med. 1997;38:1693–1698. [PubMed] [Google Scholar]
- Takahashi W, Ohnuki Y, Ohta T, Hamano H, Yamamoto M, Shinohara Y. Mechanism of reduction of cortical blood flow in striatocapsular infarction: studies using [123i]iomazenil spect. Neuroimage. 1997;6:75–80. doi: 10.1006/nimg.1997.0284. [DOI] [PubMed] [Google Scholar]
- Saur D, Buchert R, Knab R, Weiller C, Rother J. Iomazenil-single-photon emission computed tomography reveals selective neuronal loss in magnetic resonance-defined mismatch areas. Stroke. 2006;37:2713–2719. doi: 10.1161/01.STR.0000244827.36393.8f. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Jones PS, Aigbirhio FI, Wang D, Fryer TD, Day DJ, et al. Selective neuronal loss in rescued penumbra relates to initial hypoperfusion. Brain. 2008;131:2666–2678. doi: 10.1093/brain/awn175. [DOI] [PubMed] [Google Scholar]
- Carrera E, Jones PS, Morris RS, Alawneh J, Hong YT, Aigbirhio FI, et al. Is neural activation within the rescued penumbra impeded by selective neuronal loss. Brain. 2013;136:1816–1829. doi: 10.1093/brain/awt112. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Taoka T, Hiramatsu KI, Sakaguchi S, Sakaki T. Delayed ischemic hyperintensity on t1-weighted MRI in the caudoputamen and cerebral cortex of humans after spectacular shrinking deficit. Stroke. 1999;30:1038–1042. doi: 10.1161/01.str.30.5.1038. [DOI] [PubMed] [Google Scholar]
- Wang X, Qian J, He R, Wei L, Liu N, Zhang Z, et al. Delayed changes in T1-weighted signal intensity in a rat model of 15-minute transient focal ischemia studied by magnetic resonance imaging/spectroscopy and synchrotron radiation x-ray fluorescence. Magn Reson Med. 2006;56:474–480. doi: 10.1002/mrm.20985. [DOI] [PubMed] [Google Scholar]
- Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, et al. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Muir KW, Buchan A, von Kummer R, Rother J, Baron JC. Imaging of acute stroke. Lancet Neurol. 2006;5:755–768. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci USA. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fmri stroke study. Brain. 2005;128:1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- Ashioti M, Beech JS, Lowe AS, Hesselink MB, Modo M, Williams SC. Multi-modal characterization of the neocortical clip model of focal cerebral ischaemia by MRI, behaviour and immunohistochemistry. Brain Res. 2007;1145:177–189. doi: 10.1016/j.brainres.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Balkaya M, Krober J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods. 2013;213:179–187. doi: 10.1016/j.jneumeth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Balkaya M, Krober JM, Rex A, Endres M. Assessing poststroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33:330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B, Bert B, Fink H, Dirnagl U, Endres M. Dysexecutive syndrome after mild cerebral ischemia? Mice learn normally but have deficits in strategy switching. Stroke. 2004;35:191–195. doi: 10.1161/01.STR.0000107188.29688.2C. [DOI] [PubMed] [Google Scholar]
- Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–2836. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal cognitive assessment versus face-to-face Montreal cognitive assessment and neuropsychological battery. Stroke. 2012;44:227–229. doi: 10.1161/STROKEAHA.112.673384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasi M, Poggesi A, Salvadori E, Pantoni L. Post-stroke dementia and cognitive impairment. Front Neurol Neurosci. 2012;30:65–69. doi: 10.1159/000333412. [DOI] [PubMed] [Google Scholar]
- Jacewicz M, Tanabe J, Pulsinelli WA. The CBF threshold and dynamics for focal cerebral infarction in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12:359–370. doi: 10.1038/jcbfm.1992.53. [DOI] [PubMed] [Google Scholar]
- Jones TH, Morawetz RB, Crowell RM, Marcoux FW, FitzGibbon SJ, DeGirolami U, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Wang LP, Synowitz M, Gertz K, Katchanov J, Glass R, et al. Nestin-expressing cells divide and adopt a complex electrophysiologic phenotype after transient brain ischemia. J Cereb Blood Flow Metab. 2005;25:1613–1624. doi: 10.1038/sj.jcbfm.9600156. [DOI] [PubMed] [Google Scholar]
- Wang LP, Cheung G, Kronenberg G, Gertz K, Ji S, Kempermann G, et al. Mild brain ischemia induces unique physiological properties in striatal astrocytes. Glia. 2008;56:925–934. doi: 10.1002/glia.20660. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Jones PS, Beech JS, Wang D, Menon DK, Aigbirhio FI, et al. A micropet study of the regional distribution of [(11)c]-pk11195 binding following temporary focal cerebral ischemia in the rat. Correlation with postmortem mapping of microglia activation. Neuroimage. 2012;59:2007–2016. doi: 10.1016/j.neuroimage.2011.10.060. [DOI] [PubMed] [Google Scholar]
- Kriz J, Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Gertz K, Kronenberg G, Kalin RE, Baldinger T, Werner C, Balkaya M, et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain. 2012;135:1964–1980. doi: 10.1093/brain/aws075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Fink K, Zhu J, Namura S, Shimizu-Sasamata M, Endres M, Ma J, et al. Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activation. J Cereb Blood Flow Metab. 1998;18:1071–1076. doi: 10.1097/00004647-199810000-00003. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchanov J, Harms C, Gertz K, Hauck L, Waeber C, Hirt L, et al. Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J Neurosci. 2001;21:5045–5053. doi: 10.1523/JNEUROSCI.21-14-05045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA, Duffy TE. Local cerebral glucose metabolism during controlled hypoxemia in rats. Science. 1979;204:626–629. doi: 10.1126/science.432667. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Wadling S, Silver LE, Mehta Z, Rothwell PM. Transient cognitive impairment in TIA and minor stroke. Stroke. 2011;42:3116–3121. doi: 10.1161/STROKEAHA.111.621490. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management. Stroke. 2012;43:1700–1705. doi: 10.1161/STROKEAHA.111.632448. [DOI] [PubMed] [Google Scholar]
- von Monakow C. Aphasie und diaschisis. Neurol Centralbl. 1906;25:1026–1038. [Google Scholar]
- Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Balkaya M, Prinz V, Gertz K, Ji S, Kirste I, et al. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry. 2012;72:273–281. doi: 10.1016/j.biopsych.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Tamura A, Kirino T, Sano K, Takagi K, Oka H. Atrophy of the ipsilateral substantia nigra following middle cerebral artery occlusion in the rat. Brain Res. 1990;510:154–157. doi: 10.1016/0006-8993(90)90744-v. [DOI] [PubMed] [Google Scholar]
- Saji M, Reis DJ. Delayed transneuronal death of substantia nigra neurons prevented by gamma-aminobutyric acid agonist. Science. 1987;235:66–69. doi: 10.1126/science.3798095. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Blau AD, Wessel TC, Saji M. Delayed histopathological neuronal damage in the substantia nigra compacta (nucleus a9) after transient forebrain ischaemia. Neurobiol Dis. 1995;2:119–127. doi: 10.1006/nbdi.1995.0012. [DOI] [PubMed] [Google Scholar]
- Soriano MA, Justicia C, Ferrer I, Rodriguez-Farre E, Planas AM. Striatal infarction in the rat causes a transient reduction of tyrosine hydroxylase immunoreactivity in the ipsilateral substantia nigra. Neurobiol Dis. 1997;4:376–385. doi: 10.1006/nbdi.1997.0166. [DOI] [PubMed] [Google Scholar]
- Zuhayra M, Zhao Y, von Forstner C, Henze E, Gohlke P, Culman J, et al. Activation of cerebral peroxisome proliferator-activated receptors gamma (ppargamma) reduces neuronal damage in the substantia nigra after transient focal cerebral ischaemia in the rat. Neuropathol Appl Neurobiol. 2011;37:738–752. doi: 10.1111/j.1365-2990.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Saji M, Volpe BT. Delayed histologic damage and neuron death in the substantia nigra reticulata following transient forebrain ischemia depends on the extent of initial striatal injury. Neurosci Lett. 1993;155:47–50. doi: 10.1016/0304-3940(93)90670-g. [DOI] [PubMed] [Google Scholar]
- Boutin H, Catherine A, Mackenzie ET, Jauzac P, Dauphin F. Long-term alterations in MU, delta and kappa opioidergic receptors following middle cerebral artery occlusion in mice. Acta Neuropathol. 2007;114:491–500. doi: 10.1007/s00401-007-0269-7. [DOI] [PubMed] [Google Scholar]
- Hall ED, Andrus PK, Oostveen JA, Althaus JS, VonVoigtlander PF. Neuroprotective effects of the dopamine d2/d3 agonist pramipexole against postischemic or methamphetamine-induced degeneration of nigrostriatal neurons. Brain Res. 1996;742:80–88. doi: 10.1016/s0006-8993(96)00968-7. [DOI] [PubMed] [Google Scholar]
- Hirouchi Y, Suzuki E, Mitsuoka C, Jin H, Kitajima S, Kohjimoto Y, et al. Neuroimaging and histopathological evaluation of delayed neurological damage produced by artificial occlusion of the middle cerebral artery in cynomolgus monkeys: establishment of a monkey model for delayed cerebral ischemia. Exp Toxicol Pathol. 2007;59:9–16. doi: 10.1016/j.etp.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Forno LS. Reaction of the substantia nigra to massive basal ganglia infarction. Acta Neuropathol. 1983;62:96–102. doi: 10.1007/BF00684925. [DOI] [PubMed] [Google Scholar]
- Ohara S, Kondo K, Kagoshima M, Yanagisawa N. [secondary degeneration of substantia nigra following massive basal ganglia infarction] Rinsho Shinkeigaku. 1989;29:1352–1356. [PubMed] [Google Scholar]
- Nakane M, Tamura A, Miyasaka N, Nagaoka T, Kuroiwa T. Astrocytic swelling in the ipsilateral substantia nigra after occlusion of the middle cerebral artery in rats. AJNR Am J Neuroradiol. 2001;22:660–663. [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Okudera T, Inugami A, Noguchi K, Kado H, Yoshida Y, et al. Degeneration of the ipsilateral substantia nigra after striatal infarction: evaluation with MR imaging. Radiology. 1997;204:847–851. doi: 10.1148/radiology.204.3.9280270. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Zickler P, Denhardt DT, Hartung HP, Jander S. Increased thalamic neurodegeneration following ischaemic cortical stroke in osteopontin-deficient mice. Brain. 2006;129:1426–1437. doi: 10.1093/brain/awl094. [DOI] [PubMed] [Google Scholar]
- Tamura A, Tahira Y, Nagashima H, Kirino T, Gotoh O, Hojo S, et al. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke. 1991;22:615–618. doi: 10.1161/01.str.22.5.615. [DOI] [PubMed] [Google Scholar]
- Nakane M, Teraoka A, Asato R, Tamura A. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke. 1992;23:328–332. doi: 10.1161/01.str.23.3.328. [DOI] [PubMed] [Google Scholar]
- Tulloch IF, Arbuthnott GW, Wright AK. Topographical organization of the striatonigral pathway revealed by anterograde and retrograde neuroanatomical tracing techniques. J Anat. 1978;127:425–441. [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Tamura A, Kanazawa I, Sano K. Time-sequential change of amino acid neurotransmitters—gaba, aspartate and glutamate—in the rat basal ganglia following middle cerebral artery occlusion. Neurol Res. 1990;12:231–235. doi: 10.1080/01616412.1990.11739949. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Beck KD, Araujo DM, Irwin I, Langston JW, Hefti F. Chronic intranigral administration of brain-derived neurotrophic factor produces striatal dopaminergic hypofunction in unlesioned adult rats and fails to attenuate the decline of striatal dopaminergic function following medial forebrain bundle transection. Neuroscience. 1993;53:639–650. doi: 10.1016/0306-4522(93)90612-j. [DOI] [PubMed] [Google Scholar]
- Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(a)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22:1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Moritani T, Shrier DA, Wang HZ, Hiwatashi A, Numaguchi Y, et al. Secondary degeneration of the substantia nigra and corticospinal tract after hemorrhagic middle cerebral artery infarction: diffusion-weighted MR findings. Magn Reson Med Sci. 2002;1:175–178. doi: 10.2463/mrms.1.175. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hirano T, Terasaki T, Uchino M. Signal change of the substantia nigra on diffusion-weighted imaging following striatal infarction. Intern Med. 2010;49:65–68. doi: 10.2169/internalmedicine.49.2694. [DOI] [PubMed] [Google Scholar]
- Abe O, Nakane M, Aoki S, Hayashi N, Masumoto T, Kunimatsu A, et al. MR imaging of postischemic neuronal death in the substantia nigra and thalamus following middle cerebral artery occlusion in rats. NMR Biomed. 2003;16:152–159. doi: 10.1002/nbm.823. [DOI] [PubMed] [Google Scholar]
- Herve D, Molko N, Pappata S, Buffon F, LeBihan D, Bousser MG, et al. Longitudinal thalamic diffusion changes after middle cerebral artery infarcts. J Neurol Neurosurg Psychiatry. 2005;76:200–205. doi: 10.1136/jnnp.2004.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffon F, Molko N, Herve D, Porcher R, Denghien I, Pappata S, et al. Longitudinal diffusion changes in cerebral hemispheres after MCA infarcts. J Cereb Blood Flow Metab. 2005;25:641–650. doi: 10.1038/sj.jcbfm.9600054. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by pet and [11c]pk1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Jones PS, Beech JS, Wang D, Menon DK, Aigbirhio FI, et al. A micropet study of the regional distribution of [11c]-pk11195 binding following temporary focal cerebral ischemia in the rat. Correlation with postmortem mapping of microglia activation. Neuroimage. 2012;59:2007–2016. doi: 10.1016/j.neuroimage.2011.10.060. [DOI] [PubMed] [Google Scholar]
- Ni JW, Takahashi M, Yatsugi S, Shimizu-Sasamata M, Yamaguchi T. Effects of ym872 on atrophy of substantia nigra reticulata after focal ischemia in rats. Neuroreport. 1998;9:3719–3724. doi: 10.1097/00001756-199811160-00027. [DOI] [PubMed] [Google Scholar]
- Yamada K, Goto S, Yoshikawa M, Okamura A, Ushio Y. Involvement of N-methyl-D-aspartate receptor in the delayed transneuronal regression of substantia nigra neurons in rats. Brain Res. 1996;743:233–239. doi: 10.1016/s0006-8993(96)01052-9. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Kato K, Schleicher A, Witte OW, Zilles K. Transient increase of manganese-superoxide dismutase in remote brain areas after focal photothrombotic cortical lesion. Stroke. 1998;29:203–210. doi: 10.1161/01.str.29.1.203. [DOI] [PubMed] [Google Scholar]
- Adams JHDL. Greenfield's Neuropathology. Edward Arnold: London; 1992. [Google Scholar]
- Radu EW, Moseley IF. Carotid artery occlusion and computed tomography. A clinicoradiological study. Neuroradiology. 1978;17:7–12. doi: 10.1007/BF00345262. [DOI] [PubMed] [Google Scholar]
- Fierstra J, Poublanc J, Han JS, Silver F, Tymianski M, Crawley AP, et al. Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry. 2010;81:290–293. doi: 10.1136/jnnp.2009.188078. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nabatame H, Harada K, Kimura J. Callosal atrophy with reduced cortical oxygen metabolism in carotid artery disease. Stroke. 1993;24:88–93. doi: 10.1161/01.str.24.1.88. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Pagani M, Fukuyama H, Ouchi Y, Nagahama Y, Matsuzaki S, et al. Progression of atrophy of the corpus callosum with deterioration of cerebral cortical oxygen metabolism after carotid artery occlusion: a follow up study with MRI and pet. J Neurol Neurosurg Psychiatry. 1995;59:420–426. doi: 10.1136/jnnp.59.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Dong Y, Konishi J, et al. Atrophy of the corpus callosum associated with cognitive impairment and widespread cortical hypometabolism in carotid artery occlusive disease. Arch Neurol. 1996;53:1103–1109. doi: 10.1001/archneur.1996.00550110039011. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Oyanagi C, Okazawa H, Ueno M, et al. Long-term changes of hemodynamics and metabolism after carotid artery occlusion. Neurology. 2000;54:2095–2102. doi: 10.1212/wnl.54.11.2095. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Martin WRW, Herscovitch P, Raichle ME, Grubb RL. Extracranial-intracranial bypass surgery: hemodynamic and metabolic effects. Neurology. 1984;34:1168–1174. doi: 10.1212/wnl.34.9.1168. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Kimura J, Konishi J, Kameyama M. Hemodynamics in internal carotid artery occlusion examined by positron emission tomography. Stroke. 1990;21:1400–1406. doi: 10.1161/01.str.21.10.1400. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Matsumoto M, Hashikawa K, Oku N, Ishida M, Seike Y, et al. Iodine-123-iomazenil and iodine-123-iodoamphetamine spect in major cerebral artery occlusive disease. J Nucl Med. 1998;39:1348–1353. [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Dong Y, Nabatame H, Nagahama Y, Nishizawa S, et al. Atrophy of the corpus callosum associated with a decrease in cortical benzodiazepine receptor in large cerebral arterial occlusive diseases. J Neurol Neurosurg Psychiatry. 2000;68:317–322. doi: 10.1136/jnnp.68.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Shiga T, Ishikawa T, Houkin K, Narita T, Katoh C, et al. Reduced blood flow and preserved vasoreactivity characterize oxygen hypometabolism due to incomplete infarction in occlusive carotid artery diseases. J Nucl Med. 2004;45:943–949. [PubMed] [Google Scholar]
- Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N, et al. Cerebral oxygen hypometabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery diseases. Stroke. 2006;37:393–398. doi: 10.1161/01.STR.0000198878.66000.4e. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Kudoh T, Kishibe Y, Iwasaki J, Kagawa S. Selective neuronal damage and borderzone infarction in carotid artery occlusive disease: a 11c-flumazenil pet study. J Nucl Med. 2005;46:1973–1979. [PubMed] [Google Scholar]
- Yamauchi H, Kudoh T, Kishibe Y, Iwasaki J, Kagawa S. Selective neuronal damage and chronic hemodynamic cerebral ischemia. Ann Neurol. 2007;61:454–465. doi: 10.1002/ana.21104. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Hemodynamic compromise as a cause of internal border-zone infarction and cortical neuronal damage in atherosclerotic middle cerebral artery disease. Stroke. 2009;40:3730–3735. doi: 10.1161/STROKEAHA.109.560011. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Selective neuronal damage and Wisconsin card sorting test performance in atherosclerotic occlusive disease of the major cerebral artery. J Neurol Neurosurg Psychiatry. 2011;82:150–156. doi: 10.1136/jnnp.2010.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Silent cortical neuronal damage in atherosclerotic disease of the major cerebral arteries. J Cereb Blood Flow Metab. 2011;31:953–961. doi: 10.1038/jcbfm.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal ‘misery-perfusion syndrome' by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15o positron emission tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Fujimoto N, Nabatame H, Kimura J. Significance of low perfusion with increased oxygen extraction fraction in a case of internal carotid artery stenosis. Stroke. 1992;23:431–432. doi: 10.1161/01.str.23.3.431. [DOI] [PubMed] [Google Scholar]
- Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol. 2010;9:1008–1017. doi: 10.1016/S1474-4422(10)70185-X. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Higashi T, Kagawa S, Nishii R, Kudo T, Sugimoto K, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease. Brain. 2012;135:2515–2526. doi: 10.1093/brain/aws131. [DOI] [PubMed] [Google Scholar]
- Moustafa RR, Izquierdo-Garcia D, Jones PS, Graves MJ, Fryer TD, Gillard JH, et al. Watershed infarcts in transient ischemic attack/minor stroke with⩾or=50% carotid stenosis: hemodynamic or embolic. Stroke. 2010;41:1410–1416. doi: 10.1161/STROKEAHA.110.580415. [DOI] [PubMed] [Google Scholar]
- Moustafa RR, Momjian-Mayor I, Jones PS, Morbelli S, Day DJ, Aigbirhio FI, et al. Microembolism versus hemodynamic impairment in rosary-like deep watershed infarcts: a combined positron emission tomography and transcranial Doppler study. Stroke. 2011;42:3138–3143. doi: 10.1161/STROKEAHA.111.616334. [DOI] [PubMed] [Google Scholar]
- Chida K, Ogasawara K, Suga Y, Saito H, Kobayashi M, Yoshida K, et al. Postoperative cortical neural loss associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: 123i-iomazenil spect study. Stroke. 2009;40:448–453. doi: 10.1161/STROKEAHA.108.515775. [DOI] [PubMed] [Google Scholar]
- Chida K, Ogasawara K, Aso K, Suga Y, Kobayashi M, Yoshida K, et al. Postcarotid endarterectomy improvement in cognition is associated with resolution of crossed cerebellar hypoperfusion and increase in 123i-iomazenil uptake in the cerebral cortex: a spect study. Cerebrovasc Dis. 2010;29:343–351. doi: 10.1159/000278930. [DOI] [PubMed] [Google Scholar]
- Fierstra J, Maclean DB, Fisher JA, Han JS, Mandell DM, Conklin J, et al. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke. 2011;42:1631–1637. doi: 10.1161/STROKEAHA.110.608521. [DOI] [PubMed] [Google Scholar]
- Soros P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol. 2013;9:174–178. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]