Abstract
Background
The Metabolic syndrome (MetS) and/or its individual components have been linked to the development of cancer. Recent studies have suggested a similar link to hepatocellular carcinoma (HCC). The aim of this study was to evaluate the direction and magnitude of the association between the MetS and HCC.
Methods
Two reviewers independently conducted a systemic search to identify available evidence from databases from January 1980 to June 2012. Search terms included ‘Metabolic syndrome’, ‘insulin resistance syndrome’, ‘metabolic abnormalities’ combined with ‘hepatocellular carcinoma’ and ‘liver cancer’. No language restriction was applied to the search. Only studies reporting an effect measure for the association between MetS and HCC were eligible for inclusion. Publication bias was assessed using the Begg and Egger’s tests, with a visual inspection of funnel plot. All analyses were performed using Comprehensive Meta-Analysis version 2 software.
Results
Four studies (3 cohort and 1 case-control) with a total of 829,651 participants were included in the analysis. The age range of participants was between 30 and 84 years. The combined analysis showed an overall 81% increase risk of HCC in cases with MetS (RR: 1.81, 95% CI: 1.37–2.41). After excluding the single case-control study from analysis, the overall risk ratio remained statistically significant (RR: 1.49, 95% CI: 1.27–1.74). Funnel plot inspection, Begg and Egger’s tests showed no evidence of publication bias for combined analysis.
Conclusions
Though studies are scarce, currently available epidemiologic data is suggestive of significantly higher risk of hepatocellular carcinoma among patients with metabolic syndrome.
Keywords: metabolic abnormalities, liver cancer, hepatoma
BACKGROUND
Hepatocellular carcinoma (HCC) accounts for more than half a million new cases worldwide, including around 20,000 new cases in the United States. Incidence of HCC in the United States has tripled over the past decades, notably due to high prevalence of hepatitis C infection. Although viral hepatitis and excessive alcohol use have been identified as major risk factors for HCC, roughly 5% to 30% of HCC cases still lack identifiable causes 1, 2.
Recent studies have shown that nonalcoholic fatty liver disease, the hepatic manifestation of metabolic syndrome, is one of the risk factors for HCC. Metabolic syndrome (MetS) is an assemblage of metabolic abnormalities including high body mass index or truncal obesity, diabetes, insulin resistance, dyslipidemia, and elevated blood pressure 3. It is well known that subjects with MetS are at significant risk of cardiovascular diseases 4. However, recent studies have shown that MetS might have other adverse ramification, notably the development of various types of cancers. The hallmark of this syndrome, hyperinsulinemia, can induce cell proliferation through the interference with several signaling cascades and disruption of the apoptotic pathway. Moreover, derangement in the adipokines such as adiponectin; which is commonly observed in subjects with MetS, might also increase the propensity of carcinogenesis 5. To support this assumption, several reports have demonstrated the increasing risk of several cancers in subjects with MetS 6–8.
There are some studies linking obesity and diabetes with HCC suggesting that MetS might play a role in the carcinogenesis 9, 10. The outcome from a few epidemiological studies estimates that the risk of HCC is in fact increased by 1.5–2.0 folds in those with MetS 11, 12. The accurate estimate on the magnitude of MetS and HCC is of importance given the rise in the epidemic of obesity and MetS in the US population. Even a small increase in risk of HCC from MetS can, therefore, account for significant increase in number of HCC cases which may lead to profound economic impact 13.
In this study, we have conducted the systematic review and meta-analysis to evaluate all the available evidence to determine the association between MetS and HCC.
METHODS
Search Strategy
We performed a literature search using PubMed, EMBASE and Cochrane database from database inception to June 30, 2011 without language restriction using the following search terms: metabolic syndrome, insulin resistance, metabolic abnormalities, primary liver cancer, hepatoma and hepatocellular carcinoma. In addition, we carefully reviewed the reference lists from all the articles to further identify relevant studies. The process of systematic review was conducted in adherence to standards of quality for reporting meta-analyses.
Eligibility criteria
Studies were included in the meta-analysis if they fulfilled the following criteria: (i) the studies are either case-control or cohort design; (ii) the study subjects were ≥ 18 years old; and (iii) they reported relative risk (RR) estimate for HCC in those with MetS. We included all the publications with the use of various definitions of MetS (such as Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 14, World Health Organization 15, International Diabetes Federation 16, and the American Heart Association 17. In the circumstance when the reports did not clearly delineate the definition of MetS, the RR estimate of HCC in individuals with more than or equal to three metabolic abnormalities, based on Adult Treatment Panel III, was considered in the analysis. If the same studied population were used by multiple studies or if data were duplicated, only the estimates from the most recently published reports were considered in the final analysis.
Data extraction
The following data were extracted from each study: publication data [first author’s last name and first name initials, year of publication and country where the study was performed, sample size, and participants’ demographic data], type of study design, number of cases and controls (for case-control studies), number of exposed and unexposed (for cohort studies), definitions of metabolic syndrome, risk estimates with their corresponding confidence intervals (CIs), and all the covariates (if any) being used in the multivariate analyses and modeling. We carefully reviewed the potential confounders, notably the use of alcohol; that might be associated with the risk of HCC in the studied population. Odds ratios (ORs) from case-control studies were considered as estimate of relative risk 18. Two independent reviewers (RJ and SP) reviewed the studies and any discrepancies regarding inclusion/exclusion or risk estimates were resolved through the discussion by the review team. The agreement between reviewers for inclusion/exclusion of specific studies was assessed using Cohen’s kappa coefficient 19.
Assessment of methodological quality
The Newcastle-Ottawa Scale was used for assessment of methodological quality of all the publications that were included in the final analysis. The scales allocate stars, maximum of nine, for quality of selection, comparability, exposure and outcome of study participants 20.
Statistical analysis
Summaries of relative risk (RR) estimates were evaluated using both fixed- and random-effects methods. Initial analysis was performed to look for association between MetS and HCC. The heterogeneity of all the publications was evaluated with Cochran’s Q-test and I2-statistic 21. Publication bias was assessed by (i) construction and visual inspection of funnel plot and (ii) employing the Egger’s22 and Begg and Mazumdar tests 23. Duval and Tweedie’s trim and fill method was utilized to obtain RR after adjustments in the presence of publication bias. The p value of < 0.05 indicated statistical significance. All analyses were performed using Comprehensive Meta-analysis Version 2 (Biostat, Englewood, New Jersey).
RESULTS
Literature Search and Study Characteristics
The schematic diagram of the detailed steps of our literature search is shown in Figure 1. Briefly, we identified 1,500 studies from different databases, either in full publications or abstract forms, using the methodology and the search terms described above [760 studies from Pubmed, 120 studies from Cochrane database, 2 from EMBASE, and 600 abstracts from the proceedings of the national meetings such as Digestive Disease Week and American College of Gastroenterology]. After title appraisal, 141 publications were considered to be potentially relevant. Of these, we excluded 55 review articles, 20 animal studies, 19 letters, 15 case reports, 5 pathological reports and 24 studies which did not provide RR estimate. Three studies were considered for full article assessment and during this process one additional relevant report was identified during the reference list review. A total of 4 studies (3 cohort and one case-control studies) from the United States, Europe, and Japan were included in the final analyses 11, 12, 24, 25. The observed Cohen’s kappa for the agreement between reviewers was 0.75 26.
Figure 1.
Study selection
Association of metabolic syndrome and Hepatocellular carcinoma
Due to evidence of heterogeneity of the 4 studies (Q= 23.95, p value for heterogeneity = 0.001, I2=79.1%), we employed random-effect model to calculate the pooled RR. The definitions of MetS that were used in these studies are outlined in Table 1. Among the 3 cohort studies, there were 630,049 participants, of which 4,055 had HCC. One case-control study was published in 2011 using the SEER Medicare database comprising a total of 3,649 cases and 195,953 controls. The age of participants ranged from 30 to 84 years old. The pooled RR (among 829,651 participants from 4 studies) for HCC among subjects with MetS was 1.81 (95% CI 1.37–2.41) (Figure 2). The association remained significant when we only considered 3 cohort studies in the analysis (RR: 1.49, 95% CI 1.27–1.74).
Table 1.
Baseline characteristics of included studies
| Author, year, country, reference | Type of study | Study population | Definition of MetS | RR, 95% CI | Controlled variables | Study characteristics+ | Newcastle-Ottawa quality assessment scale |
|---|---|---|---|---|---|---|---|
| Welzel et al., 2011, USA12 | Case-control | 3649 HCC cases and 195,953 controls from SEER Medicare databases | NCEP-ATP III | Total, 2.58, 2.40–2.76 | Age, sex, race, geographic location, Medicare/Medicaid dual enrollment | Cases: Age (mean): 76.1 years Male – 67% Non-Hispanic white 73% Controls: Age (mean) 77.9 years Male – 63.7% Non-Hispanic white – 86.3% |
6 |
| Borena et al., 2011, Europe 11 | Cohort | 578700 people from Norway, Austria and Sweden (266 HCC cases) | WHO | Total, 1.35, 1.12–1.63 | Age, smoking | Age (Mean): Men-43.9 years, Women- 44.1 years Current smokers: 31% men and 25% women |
8 |
| Osaki et al., 2011, Japan25 | Cohort | 23625 Japanese men and women (1,931 HCC cases) | NCEP-ATP III | m- 1.89, 1.11–3.22 f-3.67, 1.78–7.57 |
Age, smoking status, heavy drinking | Age (mean): 58.6 years Current smokers-13.3% Heavy drinkers-1.9% |
7 |
| Inoue et al., 2008, Japan24 | Cohort | 27724 total Japanese men and women (1,858 HCC cases) | AHA | m-1.73, 1.03–2.91 f- 1.18, 0.55–2.51 |
Age, study area, smoking status, weekly ethanol intake, total serum cholesterol | Age (mean): men – 56.5 years, women – 55.5 years Smoking (past or current) – Men 27.6%, Women 1.3% Alcohol use ≥ 300g/week: men 22.8%, women 0.5% |
8 |
MetS – Metabolic syndrome, HCC – hepatocellular carcinoma, RR – relative risk, SEER – Surveillance Epidemiology and End Results, NCEP-ATP III – National Cholesterol Education Program-Adult Treatment Panel III, AHA – American Heart Association, WHO – World Health Organization, m - male, f - female
Figure 2.
Forest plot: Association between metabolic syndrome and Hepatocellular carcinoma
Publication quality and bias
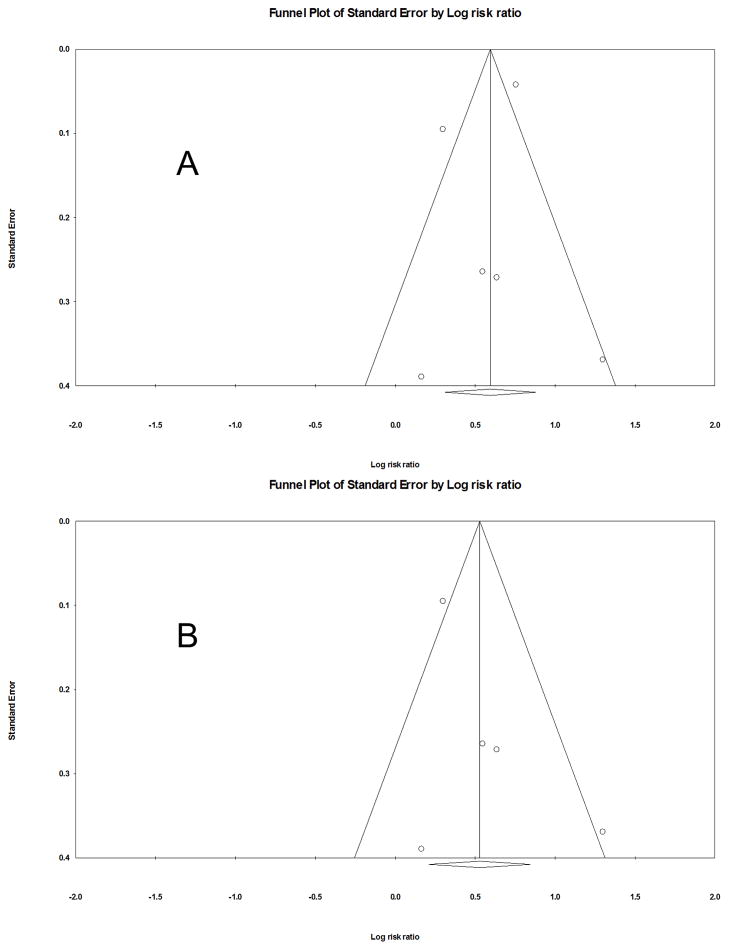
The Newcastle-Ottawa Scale (range, 1–9 stars) to assess the publication quality revealed that the average 7.7 stars for the three cohort studies and 6 for the one case-control study (Table 1). Visual inspection of funnel plot (Figures 2) and further evaluation with Egger’s and Begg and Mazumdar tests for combined analysis of all 4 studies did not suggest evidence of publication bias. (Begg and Mazumdar test: p = 0.85; Egger’s test: p = 0.57).
DISCUSSION
In this comprehensive review and meta-analysis, we found that metabolic syndrome significantly increased the risk of HCC.
Besides from cardiovascular risks, MetS is being recognized as the risk factors for several malignancies such as endometrial, colon, and prostate cancers 6–8. Our meta-analysis including a large number of participants attests the results of previous studies on the link between MetS and cancers suggesting the association between the presence of metabolic abnormalities and the risk of HCC.
Despite our findings, it is still debatable on whether the increase in risk is in fact from the specific components of metabolic derangement as opposed to the abnormalities in aggregate. Many reports found that the high glucose level is associated with HCC 27, 28. This finding is in fact supported by the recent meta-analysis showing the increasing in the relative risk of HCC by 2.3-fold in those with diabetes 10. On the other hand, the association between the level of cholesterol and HCC is conflicting. The study from Japan finds the positive association between low levels of cholesterol and HCC 24; while the study from Italy reports the contrary 11.
The pathogenesis of HCC in patients with metabolic syndrome is complex and multi factorial. One plausible explanation is that it might be related to the presence of non-alcoholic steatohepatitis and cirrhosis, a known risk factor for HCC 2. Whether patients with MetS may develop HCC in the absence of cirrhosis is not well-defined. However, there are some anecdotal reports supporting this circumstance and identifying MetS as an independent predictor of HCC development. Study by Paradis et al 29 reported distinct pathological characteristics of HCC in patients with MetS as the only risk factor, as compared to patients with chronic liver disease who follow fibrosis-cirrhosis-HCC pathway. Even among the MetS subgroup, HCC occurred mainly in the setting of non-fibrotic liver which supported the hypothesis of specific molecular pathway of liver tumorigenesis in MetS individuals 29.
Several molecular mechanisms, such as oxidative stress and reactive oxygen species production, have been postulated for the risk of tumorigenesis among these individuals. Reactive oxygen species can induce cancer promoting mutations such as mutation of p53 tumor suppressor gene and cause DNA damage 5. Another potential mechanism involves the high level of insulin growth factor-1 in these subjects. This growth factor has been shown to increase cell turnover and inhibits apoptosis; which may place individual at higher risk for cancer development 30. Lastly such association might be associated with dysregulation of inflammatory cytokines, i.e., tumor necrotic factor-alpha and interleukin-6, among subjects with MetS 31. These cytokines have direct effect on hepatocytes and have been linked to HCC development in animal model32.
Our systematic review has limitations. First, there are multiple factors that were not taken into consideration for the pooled RR analysis such as dietary habits, family history of HCC, and other genetic risk factors due to the unavailability of these variables in the original studies. Second, the information on the underlying viral hepatitis is not obtainable, except for the study by Welzel et al 12. Hepatitis C infection is a well-defined risk factor for HCC and is an independent predictor of metabolic syndrome. One report showed no or minimal changes in risk after further adjustment for hepatitis C infection on the association between obesity or diabetes and liver cancer 33. It is still plausible that, the hepatitis C infection, if present, might confound the reported magnitude of association between MetS and HCC in our analysis. Third, it is also possible that subjects with alcoholic liver disease might be included in the meta-analysis. Among the 4 studies11–2, 24–5, only the studies by Osaki and Inoue24–5 described the definition of alcohol use in their studies. Inoue et al24, stratified subjects (n =27,724) into 4 groups based in the weekly alcohol intake (non-drinkers, < 150 grams per week, 150–<300 grams per week, and ≥ 300 grams per week). On the other hand, Osaki et al25, classified subjects as heavy drinking when the amount of alcohol consumed ≥ 60 grams per day. Despite the difference in definitions, alcohol drinking status had been adjusted in the Cox proportional hazard models in both studies. In our analysis, the potential association between the use of alcohol in the study cohort and the risk of HCC has been accounted for as we used the adjusted hazard ratios (adjusted for status of alcohol use) from those two studies 24–5; when we calculated the relative risk estimates.
In summary, this meta-analysis shows that higher rates of HCC in subjects with MetS. Because the increasing incidence of MetS in the Unites States; such association cannot be disregarded. Timely preventive interventions targeted at reducing metabolic risk factors can perhaps reduce the risk of HCC.
Figure 3.
Funnel plots for publication bias: A) all studies, B) only cohort studies
Acknowledgments
Grant Support: This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, and Central Society for Clinical Research Career development award, and Research Support Fund Grant (S.L).
Abbreviations
- MetS
Metabolic syndrome
- HCC
Hepatocellular carcinoma
- RR
Relative risk
- OR
Odds ratio
Footnotes
Authors contribution:
RJ and SL – study design
RJ and SP – literature search, review and collection of data
RJ and SL – analysis and interpretation of data
RJ and SP – drafted manuscript
SL – made critical revisions to manuscript
Conflict of interest: none
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. doi: 10.1056/NEJMra1001683. Epub 2011/10/14. [DOI] [PubMed] [Google Scholar]
- 2.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651–61. doi: 10.1002/cncr.24687. Epub 2009/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. Epub 2002/12/18. [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. Epub 2002/12/04. [DOI] [PubMed] [Google Scholar]
- 5.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169(5):1505–22. doi: 10.2353/ajpath.2006.051090. Epub 2006/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila) 2011;4(11):1873–83. doi: 10.1158/1940-6207.CAPR-11-0218. Epub 2011/06/24. [DOI] [PubMed] [Google Scholar]
- 7.Laukkanen JA, Laaksonen DE, Niskanen L, et al. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1646–50. Epub 2004/10/07. [PubMed] [Google Scholar]
- 8.Rosato V, Zucchetto A, Bosetti C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22(4):884–9. doi: 10.1093/annonc/mdq464. Epub 2010/10/13. [DOI] [PubMed] [Google Scholar]
- 9.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97(7):1005–8. doi: 10.1038/sj.bjc.6603932. Epub 2007/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Kang D, Cao W, et al. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–22. doi: 10.1002/dmrr.1291. Epub 2011/09/08. [DOI] [PubMed] [Google Scholar]
- 11.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200. doi: 10.1002/ijc.26338. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 12.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54(2):463–71. doi: 10.1002/hep.24397. Epub 2011/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang K, Danchenko N, Gondek K, et al. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50(1):89–99. doi: 10.1016/j.jhep.2008.07.029. Epub 2008/11/04. [DOI] [PubMed] [Google Scholar]
- 14.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. Epub 2001/05/23. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. Epub 1998/08/01. [DOI] [PubMed] [Google Scholar]
- 16.Federation ID. The IDF consensus worldwide definition of the metabolic syndrome. [Google Scholar]
- 17.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. Epub 2004/01/28. [DOI] [PubMed] [Google Scholar]
- 18.Hogue CJ, Gaylor DW, Schulz KF. Estimators of relative risk for case-control studies. Am J Epidemiol. 1983;118(3):396–407. doi: 10.1093/oxfordjournals.aje.a113646. Epub 1983/09/01. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. Epub 2002/07/12. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. Epub 1997/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. Epub 1994/12/01. [PubMed] [Google Scholar]
- 24.Inoue M, Noda M, Kurahashi N, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18(3):240–7. doi: 10.1097/CEJ.0b013e3283240460. Epub 2009/06/06. [DOI] [PubMed] [Google Scholar]
- 25.Osaki Y, Taniguchi S, Tahara A, et al. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012;36(2):141–7. doi: 10.1016/j.canep.2011.03.007. Epub 2011/09/06. [DOI] [PubMed] [Google Scholar]
- 26.Cohen’s Kappa calculator. [cited 2012]; Available from: http://www.vassarstats.net/kappa.html.
- 27.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–80. doi: 10.1016/j.cgh.2005.12.007. Epub 2006/03/11. [DOI] [PubMed] [Google Scholar]
- 28.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116(8):1938–46. doi: 10.1002/cncr.24982. Epub 2010/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851–9. doi: 10.1002/hep.22734. Epub 2008/12/31. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836–42. doi: 10.1093/ajcn/86.3.836S. Epub 2008/02/13. [DOI] [PubMed] [Google Scholar]
- 31.Leu CM, Wong FH, Chang C, et al. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22(49):7809–18. doi: 10.1038/sj.onc.1207084. Epub 2003/10/31. [DOI] [PubMed] [Google Scholar]
- 32.Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124(12):2766–70. doi: 10.1002/ijc.24281. Epub 2009/03/10. [DOI] [PubMed] [Google Scholar]
- 33.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12(1):13–21. doi: 10.1023/a:1008995217664. Epub 2001/03/03. [DOI] [PubMed] [Google Scholar]