Abstract
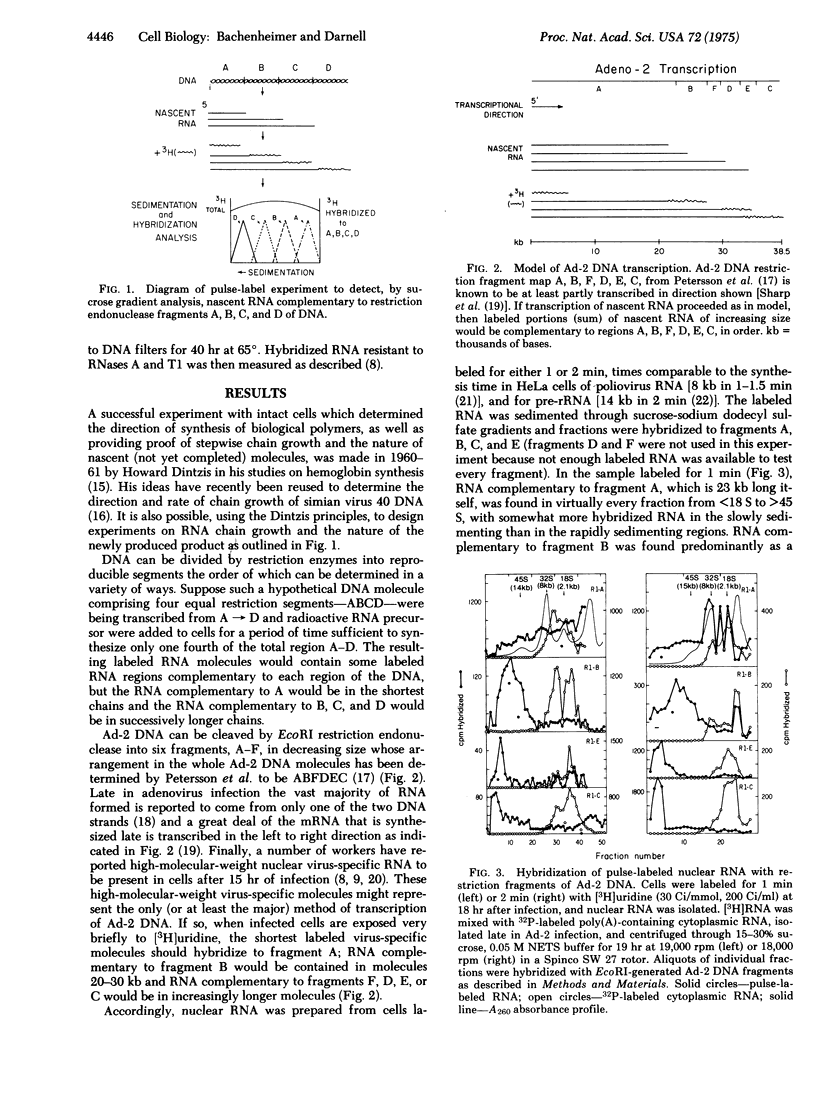
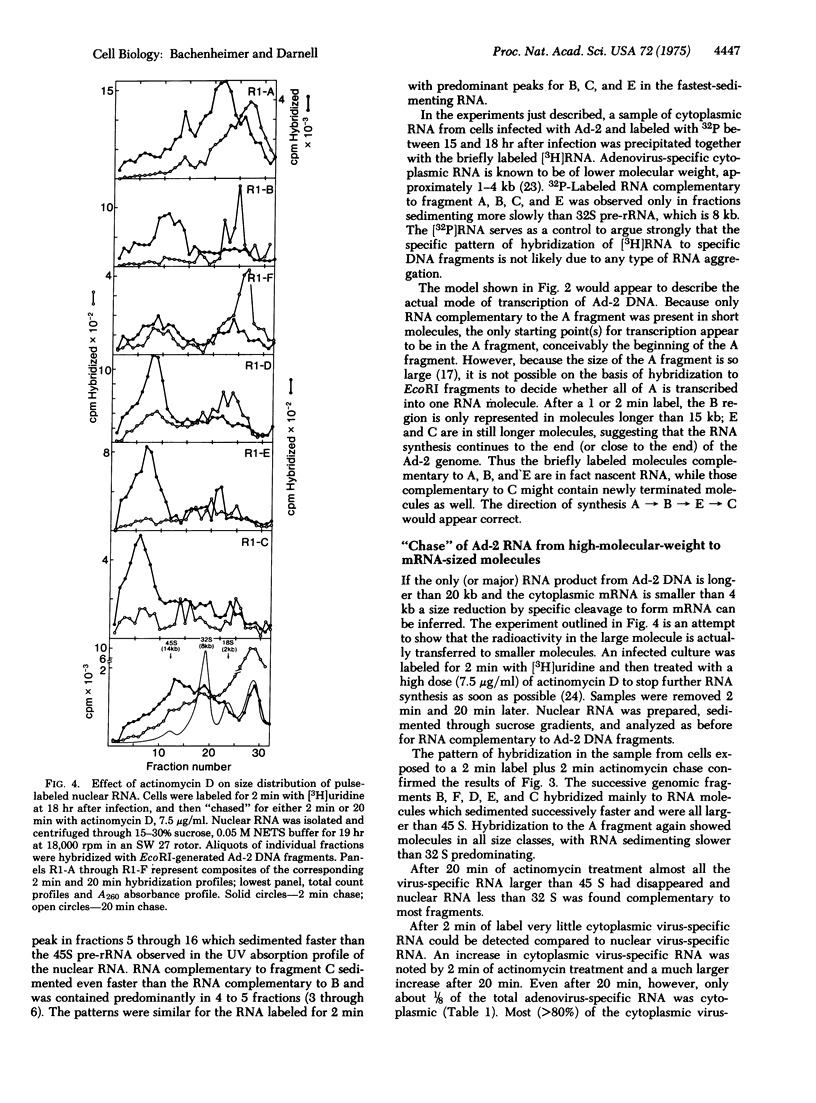
The order of transcription and the length of nascent RNA transcripts from adenovirus-2 (Ad-2) DNA in the nucleus of infected cells has been deduced by labeling the growing RNA chains in vivo for a very brief period, separating the RNA on the basis of size, and hybridizine to the ordered EcoRI restriction endonuclease fragments derived from Ad-2 DNA. The majority of the virus-specific RNA molecules are synthesized as very high-molecular-weight units beginning at a common point at least 25-30,000 base pairs from one end of the Ad-2 DNA. These molecules can be reduced in size without further RNA synthesis. The experiments indicate the obligatory origin of Ad-2 mRNA from a high-molecular-weight precursor molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhardt D., Darnell J. E., Jr tRNA synthesis in HeLa cells: a precursor to tRNA and the effects of methionine starvation on tRNA synthesis. J Mol Biol. 1969 May 28;42(1):43–56. doi: 10.1016/0022-2836(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Clason A. E. Intracellular location and molecular characteristics of tumour cell transfer RNA precursors. J Mol Biol. 1969 Jan 14;39(1):113–124. doi: 10.1016/0022-2836(69)90337-4. [DOI] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr The origin of mRNA and the structure of the mammalian chromosome. Harvey Lect. 1973;(69):1–47. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Darnell J. E. SV40-specific RNA in the nucleus and polyribosomes of transformed cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1089–1096. doi: 10.1073/pnas.65.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. M., Swart C., Hodge L. D. Adenovirus messenger RNA in mammalian cells: failure of polyribosome association in the absence of nuclear cleavage. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1578–1582. doi: 10.1073/pnas.69.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Pemberton R. E. New method of studying the precursor-product relationship between high molecular weight RNA and messenger RNA. Nat New Biol. 1972 Apr 12;236(67):172–174. doi: 10.1038/newbio236172a0. [DOI] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUM E. L. Effect of actinomycin D on cellular nucleic acid synthesis and virus production. Science. 1961 Aug 25;134(3478):556–557. doi: 10.1126/science.134.3478.556. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E. RNA processing and the control of gene expression. Brookhaven Symp Biol. 1975 Jul;(26):240–266. [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]



