Abstract
Monocyte-derived antigen presenting cells (APC) are central mediators of the innate and adaptive immune response in inflammatory diseases. As such, APC are appropriate targets for therapeutic intervention to ameliorate certain diseases. APC differentiation, activation and functions are regulated by the NF-κB family of transcription factors. Herein, we examined the effect of NF-κB inhibition, via suppression of the IκB Kinase (IKK) complex, on APC function. Murine bone marrow-derived macrophages and dendritic cells (DC), as well as macrophage and DC lines, underwent rapid programmed cell death (PCD) after treatment with several IKK/NF-κB inhibitors through a TNFα-dependent mechanism. PCD was induced proximally by reactive oxygen species (ROS) formation, which causes a loss of mitochondrial membrane potential and activation of a caspase signaling cascade. NF-κB-inhibition-induced PCD of APC may be a key mechanism through which therapeutic targeting of NF-κB reduces inflammatory pathologies.
Antigen presenting cells (APC), including DC and macrophages, are crucial regulators of the immune system in response to danger signals such as foreign pathogens, aberrant self-proteins, or tissue damage, but when activated can contribute to chronic diseases1,2,3. The transcription factor NF-κB is a central regulator of differentiation, activation, and function of APC, regulating expression of numerous cytokines, chemokines and adhesion molecules4.
In addition to the regulation of APC function, NF-κB also plays an important regulatory role in cellular survival and apoptosis, specifically in cases of infection and inflammation5. NF-κB suppresses programmed cell death (PCD) mediated by TNFα-induced JNK and caspase-8 activation6. Hence, the embryonic lethality of p65(RelA)−/−7, IKKγ−/−8, and IKKβ−/−9 mice is rescued by additionally knocking-out tumor necrosis factor receptor (TNFR)10. It is thought that NF-κB suppresses TNFα-induced apoptosis via transcriptional regulation of several anti-apoptotic genes, including XIAP, Bcl-xL, A1-bfl2, c-FLIP, A20, and GADD45β5,11,12.
In light of numerous mechanisms by which NF-κB suppression alters immune function, it has been the target of therapeutic trials. Several methods of pharmacologic inhibition of NF-κB activation and signaling are currently being examined in models of human disease, including muscular dystrophy13, diabetes mellitus14, Parkinson's disease15, inflammatory bowel disease16, rheumatoid arthritis17, aging18, and cancer19. It is speculated that the beneficial effects of NF-κB suppression in mammalian diseases are related to reduced cytokine signaling in innate immune cells, as well as a reduction in subsequent T-cell activation and signaling, thus leading to decreased tissue damage and improved pathology.
Here we demonstrate that multiple NF-κB inhibitors, acting through varying mechanisms, including inhibition of the IKK complex, suppression of IKKβ activity, or inhibition of proteosomal degradation of IκBα, induce apoptosis specifically in APC. Furthermore, NF-κB-inhibition-induced APC apoptosis is dependent upon TNFα and leads to ROS formation. The accumulation of ROS results in the subsequent loss of mitochondrial membrane potential (ΔΨm) and activation of the caspase-9/3 pathway. These data suggest a novel mechanism of NF-κB-inhibition-induced PCD in APC that is distinct from the canonical TNFα/JNK/Caspase-8 apoptotic pathway. Moreover, our results indicate that APC death, in both macrophages and monocyte-derived DC, may contribute to the anti-inflammatory effects of NF-κB inhibitors observed in mammalian models of disease.
Results
NF-κB suppression results in APC death
Previously, studies by our group demonstrated that chronic treatment of a murine model of inflammatory bowel disease with the Nemo Binding Domain (NBD) peptide, a highly specific NF-κB/IKK inhibitor, fused to a protein transduction domain ameliorated disease20. Moreover, levels of inflammatory cytokines derived from innate cells, including IL-12p40 and TNFα, were reduced in the NBD-treated animals compared with controls20.
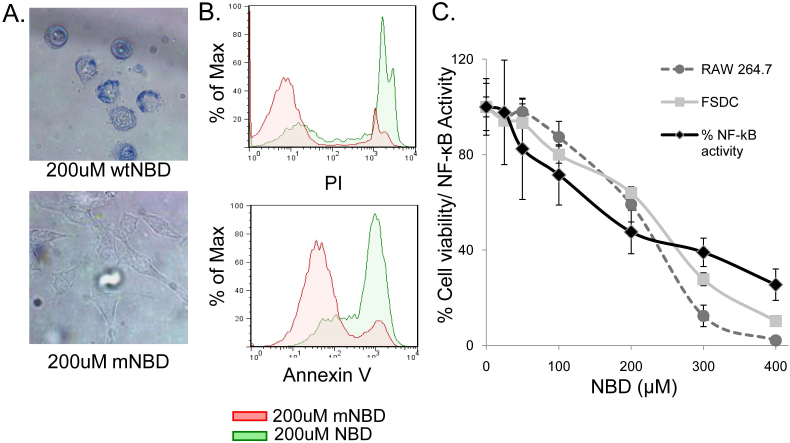
During the course of these experiments, it was observed that treatment with NBD resulted in macrophage cell death. For example, treatment of the RAW264.7 macrophage cell line with NBD conjugated to a protein transduction domain (TAT) resulted in rapid cell death (Figure 1). The majority of the macrophages exhibited characteristics of apoptosis, including membrane blebbing, nuclear condensation, cell shrinkage, and loss of symmetry (Figure 1A, Supplemental Figure 1). Further analysis showed that this cell death occurred rapidly with a majority of RAW cells treated with TAT-NBD, but not an inactivated form of the peptide TAT-mNBD, quantified by PI and Annexin V staining 4 hours after treatment (Figure 1B).
Figure 1. NBD peptide induces NF-κB inhibition-dependent cell death in APC.
(A) RAW264.7 cells were treated with TAT-NBD (NBD) or TAT-mNBD (mNBD) peptide for 12 hours, cells were then stained with trypan blue and images were obtained. The top image indicates a high number of trypan blue positive, dead cells following NBD treatment. For the bottom image, phase-contrast microscopy was utilized in order to visualize mNBD-treated cells, which remain alive and capable of excluding trypan blue. (representative of 5 independent experiments) (B) RAW264.7 cells were treated for 4 hours with NBD or mNBD peptide and analyzed for expression of Annexin V (early apoptotic marker) and PI (late apoptotic and necrotic marker) by flow cytometric analysis (representative of 3 independent experiments). (C) 293NF-κB reporter cell line was utilized to measure relative levels of NF-κB activation 2 hours after TNFα stimulation (10 ng/ml) at varying concentrations of NBD peptide (black lines). RAW264.7 macrophages (dashed line with circles) and FSDC (grey line and squares) were treated with the same doses of NBD. Percent survival was determined by MTT assay after 24 hr incubation. FSDC unlike the 293NF-κB, were not stimulated with exogenous TNFα. Data are representative of 3 independent experiments in triplicate.
This cell death response in macrophages secondary to NF-κB inhibition was unexpected, as we hypothesized that the major effect would be to suppress cytokine signaling and inhibit phagocytosis. To determine whether our observation that rapid induction of cell death in a macrophage cell line was observed in other APC lines, we examined the induction of cell death by NBD using the MTT assay for both RAW264.7 macrophages and fetal skin dendritic cells (FSDC). To demonstrate that the death inducing doses of NBD were equivalent to the relative suppression of NF-κB by TAT-NBD, HEK293 cells stably transfected with a multimerized NF-κB DNA binding element-luciferase reporter (293NF-κB cells; Figure 1C) were used to quantify NF-κB suppression. The survival curves for both FSDC and RAW cells and the NF-κB suppression profiles as determined in the 293NF-κB cells were similar, suggesting that the observed APC death correlates with the level of NF-κB suppression.
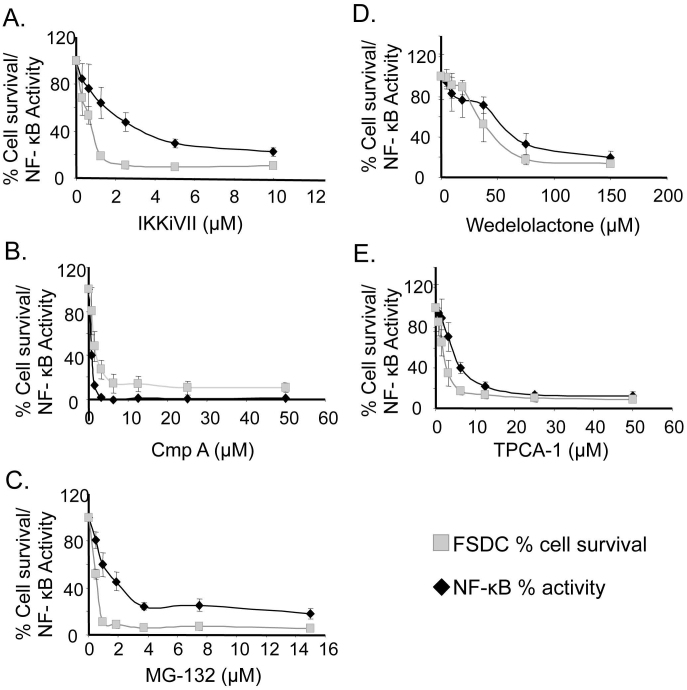
To determine if the rapid induction of apoptosis in macrophages and DC was NF-κB/IKK inhibition-dependent and not an off-target effect of TAT-NBD, five additional NF-κB inhibitory compounds were tested: Compound A (CmpA) and TPCA-1, both of which inhibit IKKβ; MG-132, which inhibits IκBα proteosomal degradation; and IKKiVII and Wedelolactone, which inhibit both IKKα and IKKβ (see Supplemental Table 1). Each of these five inhibitors was evaluated for induction of APC death using FSDC. As with TAT-NBD experiments, the level of NF-κB suppression was quantified using the 293NF-κB reporter cells (Figure 2). For each inhibitor used, the FSDC survival profile was similar to that of the NF-κB inhibition profile, demonstrating a direct correlation between the extent of NF-κB suppression and APC death.
Figure 2. Decreased cell survival is directly correlated with decreased NF-κB activity following treatment with a panel of NF-κB/IKK inhibitors.
293NF-κB reporter cell line was utilized to measure relative levels of NF-κB activation 3 hours after TNFα stimulation (10 ng/ml) at varying concentrations of the respective inhibitors (black lines). FSDC (grey lines) were treated with the same dose of the five NF-κB inhibitors and percent survival was determined by MTT assay after 24 hr incubation. FSDC, unlike the 293NF-κB, were not stimulated with exogenous TNFα. Data are representative of 5 independent experiments in triplicate.
NF-κB inhibition induces apoptotic PCD in APC
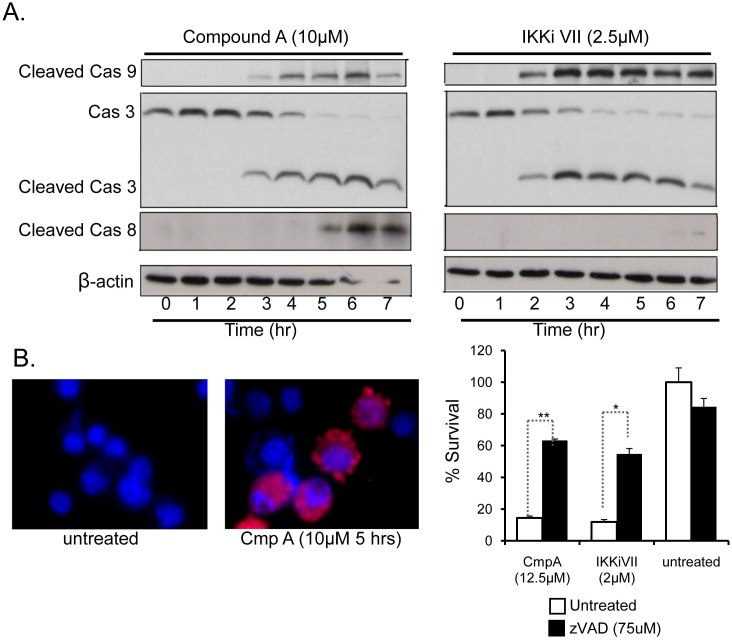
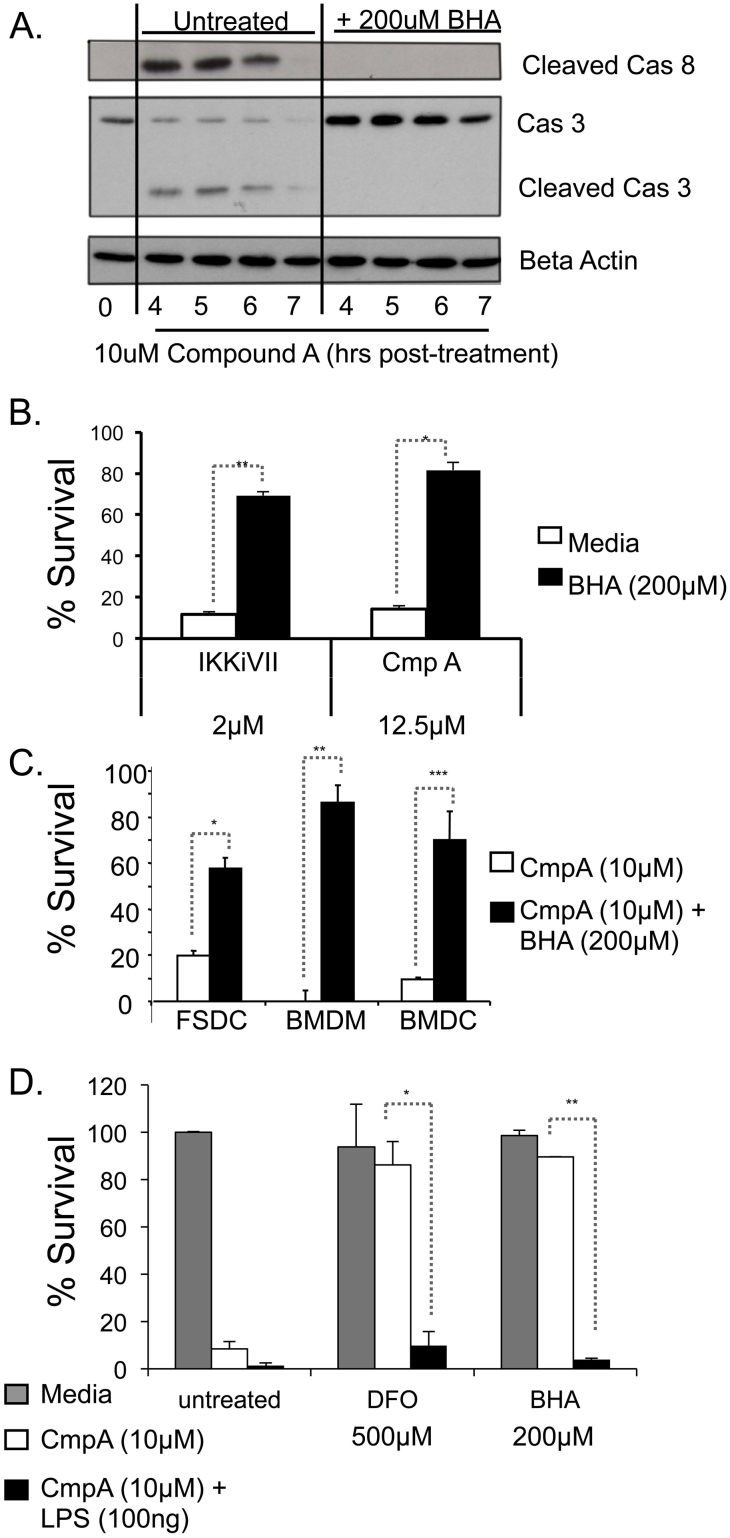
To determine whether NF-κB-inhibition induced PCD or necrosis, initially the morphology of the treated FSDC was examined. The FSDC treated with each of the four NF-κB inhibitors exhibit several features of apoptosis including membrane blebbing, nuclear condensation, cell shrinkage, and loss of symmetry (Figure 1A, Supplemental Figure 1). To further elucidate whether these cells were undergoing necrotic or apoptotic cell death, caspase activation was measured in FSDC treated with two NF-κB inhibitors, Compound A and IKKiVII. Treatment with these inhibitors resulted in a significant increase in caspase-8, caspase-9 and caspase-3 cleavage over a 7-hour time course (Figure 3A). Caspase-3 activation was confirmed by immunofluorescence staining of CmpA treated FSDC (Figure 3B). Primary BMDM also underwent caspase-3 and −9 cleavage following NF-κB inhibition as determined by immunoblot (Supplemental Figure 2A). Pretreatment of FSDC and BMDM with the broad spectrum caspase inhibitor zVAD significantly increased APC survival nearly 4.5 fold with p<0.0002 (Figure 3C and Supporting Figure 2B). These results suggest that the observed death mediated by NF-κB suppression is through a caspase-dependent apoptotic cell death pathway.
Figure 3. NF-κB suppression in APC leads to caspase-dependent apoptotic cell death.
(A) Levels of caspase-8, -9, and -3 cleavage were determined in FSDC via immunoblot analysis at the indicated time-points following treatment with two different NF-κB inhibitors (Compound A and IKKiVII). β-actin was used as a positive control. The full-length gels are provided in the supplemental information. (B) Immunofluorescence shows an increase in the levels of cleaved caspase-3 (red) in FSDC after 5-hour treatment with 10 μM Compound A. Nuclei were counterstained with DAPI (blue). (C) MTT assays were used to measure survival of FSDC that were untreated or treated with the listed doses of CmpA, or IKKiVII in the presence of 75 μM zVAD. *, p<0.004; **, p<0.00002. Data are representative of 3 independent experiments in triplicate.
Apoptosis induced by NF-κB inhibition is specific to APC populations
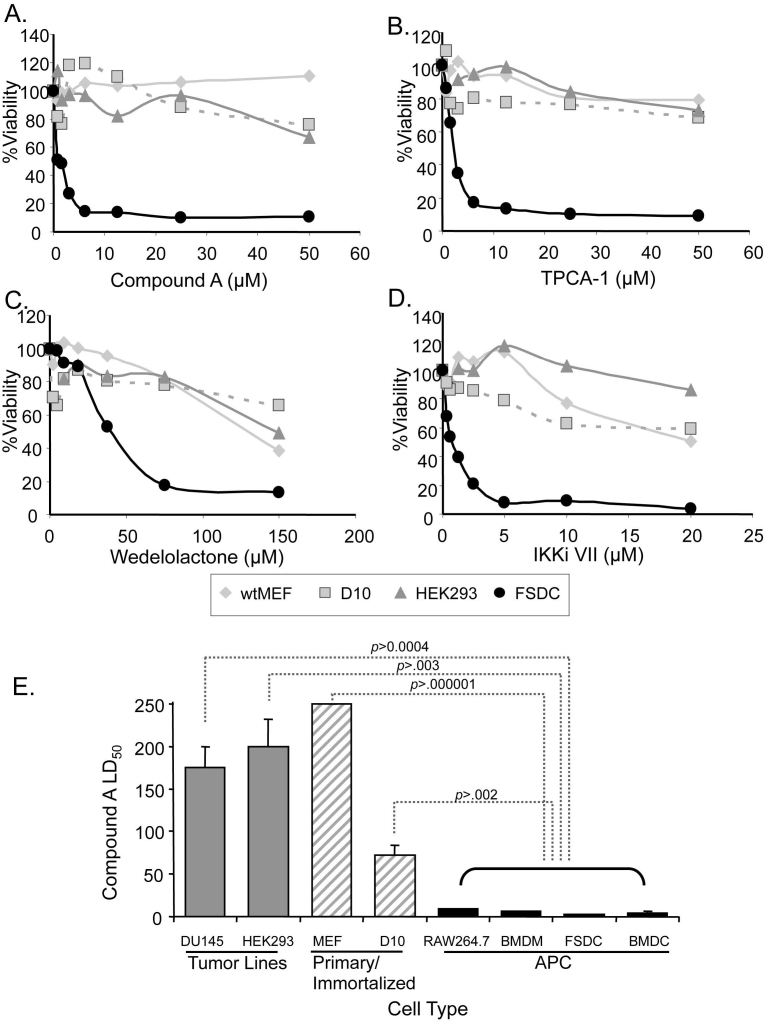
To determine if apoptosis in response to NF-κB inhibition was APC-specific, we measured the ability of four different NF-κB inhibitors to induce cell death of HEK293 cells, immortalized D10 T cells, and primary mouse embryonic fibroblasts (MEFs) and compared their cell survival profiles with FSDC, using the MTT assay. Cell death occurred in FSDC at significantly lower concentrations of inhibitors compared to the three non-APC lines. CmpA and TPCA-1, both IKKβ selective inhibitors, showed very little toxicity in non-APC cell lines (Figure 4A and B). The two IKK complex inhibitors, IKKiVII and Wedelolactone, induced more cell death in FSDC compared to other cell types, but caused some toxicity in non-APC at high concentrations (Figure 4C and 4D). These high concentrations of inhibitors induced morphologic changes in non-APCs that were more consistent with necrosis than apoptosis (data not shown).
Figure 4. IKK inhibitor-induced cell death is APC-specific.
(A-D) Four cell lines, MEF, D10, HEK293, and FSDC, were evaluated for percent survival using a MTT assay in the presence of increasing doses of several IKK inhibitors. Each point was determined by averaging at least 3 measurements at the indicated concentrations of IKKβ-specific inhibitors, CmpA and TPCA-1 (A, B), or IKK complex inhibitors, Wedelolactone and IKKiVII (C,D), respectively. (E) LD50 values were determined for 4 APC and 4 non-APC cell lines in the presence of CmpA. Values were completed by regression analysis and LD50 was determined on a minimum of 3 survival curves for each cell line. p-values were determined by comparing each non-APC cell line with the least susceptible APC cell line (RAW264.7).
To determine if cell death secondary to NF-κB inhibition was unique to APC, other APC lines were tested, including primary bone marrow derived DC (BMDC) and macrophages (BMDM), as well as RAW264.7 and FSDC. The LD50 for CmpA was significantly higher with a minimum p<0.003 in non-APC lines compared to all four APC types (Figure 4E). All non-APC lines tested had LD50 values 10-25-fold higher (Figure 4E), even when treated with CmpA for extended periods of time (supplemental Figure 3). In contrast, the APC lines had significantly lower LD50 values, ranging from 2.7±0.2 μM for FSDC to 8.9±0.4 μM for RAW264.7. These data demonstrate that the NF-κB-inhibition-induced cell death is APC-specific.
NF-κB-inhibition-induced cell death is TNFα dependent, but independent of the TNFα/JNK/Caspase-8 pathway
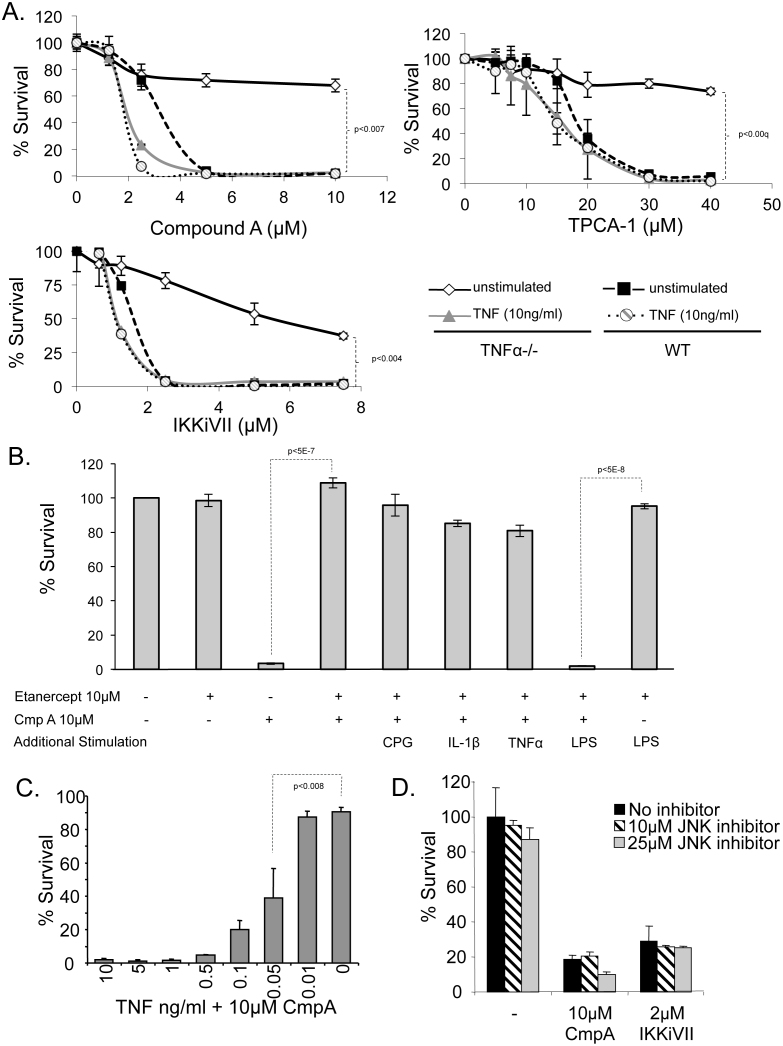
Activation of caspase-8 (Figure 3A) suggests involvement of the TNFα-dependent cell death pathway, which is the most commonly recognized NF-κB inhibition-induced PCD pathway5. However, caspase-8 was activated hours after caspases-9 and -3 in both the CmpA and IKKiVII-treated FSDC (Figure 3A). This suggests that, in the APC death cascade initiated by NF-κB inhibition, caspase-8 may lie downstream of caspase-9, and is therefore not the initiating event. Furthermore, this brings into question whether NF-κB induced APC apoptosis is a TNFα-dependent event. To examine this, WT and TNFα−/− primary macrophages were treated with CmpA, TCPA-1, and IKKiVII to evaluate the involvement of TNF/JNK/caspase-8 pathway activation in APC PCD. TNFα−/− BMDM were highly resistant to NF-κB/IKK inhibition-induced cell death compared to WT cells using three inhibitors (Figure 5A). The resistance of the TNFα−/− BMDM suggests that TNFα is necessary to induce APC death.
Figure 5. NF-κB-inhibition induced APC death is dependent on TNFα.
Cellular survival was measured after 24 hours using MTT assays in the following experiments: (A) TNFα−/− (dark symbols) and WT (light symbols) primary macrophages were evaluated for survival in the presence of NF-κB inhibitors in unstimulated cells (blue) or following stimulation with 10 ng/ml of TNFα (red). (statistical variation was evaluated at max concentration using ANOVA analysis) (B) RAW264.7 cells were treated with etanercept (25 ng/ml) and compound A (10 μM) in the presence of several endogenous and exogenous known NF-κB activators, CpG (7 ng/ml), IL-1β (10 ng/ml), TNFα (10 ng/ml) and LPS (100 ng/ml). MTT assay was used to evaluate survival after overnight incubation. Controls included untreated RAW264.7 cells, etanercept treatment alone, and etanercept + LPS treatment. Representative results are from at least 3 independent experiments in triplicate (p-values determined by student T-test). (C) TNFα−/− macrophage survival was measured by MTT assay in the presence of 10 μM Compound A and varying concentrations of TNFα. Addition of 0.05 ng/ml TNFα led to significant (p<0.008) cell death compared with untreated cells using a student t-test. (D) Cell survival was determined by MTT assay for FSDC pretreated with either 10 or 25 μM of specific JNK inhibitor (SP600125) and in the presence of three NF-κB inhibitors. Data are representative of at least 3 independent experiments in triplicate.
In further support of TNFα-dependence of this PCD pathway, TNFα−/− macrophages were treated with varying concentrations of TNFα, or left untreated in the presence of CmpA. Addition of exogenous TNFα at concentrations as low as 0.05 ng/ml caused TNFα−/− BMDM to become more sensitive to CmpA-induced cell death than WT BMDM treated with CmpA alone (Figure 5A, top panel). This same phenomenon was observed with additional NF-κB inhibitors, TCPA-1 and IKKiVII (Figure 5A). Thus, we hypothesized that endogenous TNFα produced by WT APC contributes to the minimal activation state required for APC apoptosis, and only minor activation of these cells is necessary for induction of NF-κB inhibition-mediated PCD. To test this, TNFα was depleted from WT macrophages 1 hour prior to CmpA treatment using 50 μg of etanercept (anti-TNF antibody), which resulted in inhibition of cell death (p<5E-07) (Figure 5B).
To determine whether other NF-κB activators would also cause apoptosis in APC, RAW264.7 cells were pretreated with etanercept, followed by a number of varying NF-κB activators, including TNFα, IL-1β, CpG, and LPS, prior to the addition of CmpA (Figure 5B). Interestingly, in the presence of etanercept, only LPS allowed for CmpA induced APC death to occur, while none of the other TLR and stimulatory ligands had any potentiating effect on cell death. LPS has previously been shown to induce apoptosis in IKKβ deficient macrophages21, possibly in a PAI-2-dependent manner21. These results illustrate that LPS is capable of inducing cell death in the absence of TNFα. In addition, these results further demonstrate that TNFα is necessary to induce apoptosis in NF-κB-suppressed APC.
We next measured the minimal amount of exogenous TNFα necessary to induce PCD. APC are typically stimulated in culture with 10 ng/ml of TNFα; however, amounts as low as 0.05 ng/ml of TNFα were sufficient to induce significant cell death in CmpA treated TNFα−/− macrophages, suggesting that only minor activation of these cells is necessary for induction of the NF-κB inhibition-induced PCD (Figure 5C).
To determine if the TNFα-dependent PCD is APC specific, the non-APC cell lines HEK293 cells and immortalized D10 T cells were pretreated with 10 ng/ml of TNFα, with RAW264.7 cells used as a positive control. As shown in supplemental Figure 4, NF-κB inhibition of RAW264.7 cells resulted in significant apoptosis. In contrast, there was no effect of TNFα and/or compound A on HEK293 cells. Interestingly, there was a slight increase in cell number following treatment of D10 cells with compound A. Taken together, these results suggest that the effect of the NF-κB inhibitory compounds is specific for APC cell lines, both in the absence and presence of TNFα.
The canonical mechanism of TNFα-mediated apoptosis involves downstream JNK signaling. Therefore, the effect of SP600125, a potent inhibitor of JNKI and JNKII signaling, was evaluated in two APC lines. JNK inhibition had no effect on NF-κB-induced cell death in FSDC (Figure 5D) or in BMDC (data not shown), although it did reduce nitric oxide signaling, demonstrating that the inhibitor was indeed active (data not shown). Thus, APC apoptosis in response to NF-κB inhibition is independent of JNK signaling. Collectively, these data suggest that NF-κB inhibition-induced PCD diverges from the well-documented TNFα/JNK/Caspase-8 cell death pathway, and suggests a novel TNFα-dependent PCD pathway.
APC NF-κB-inhibition-induced death is dependent on ROS production
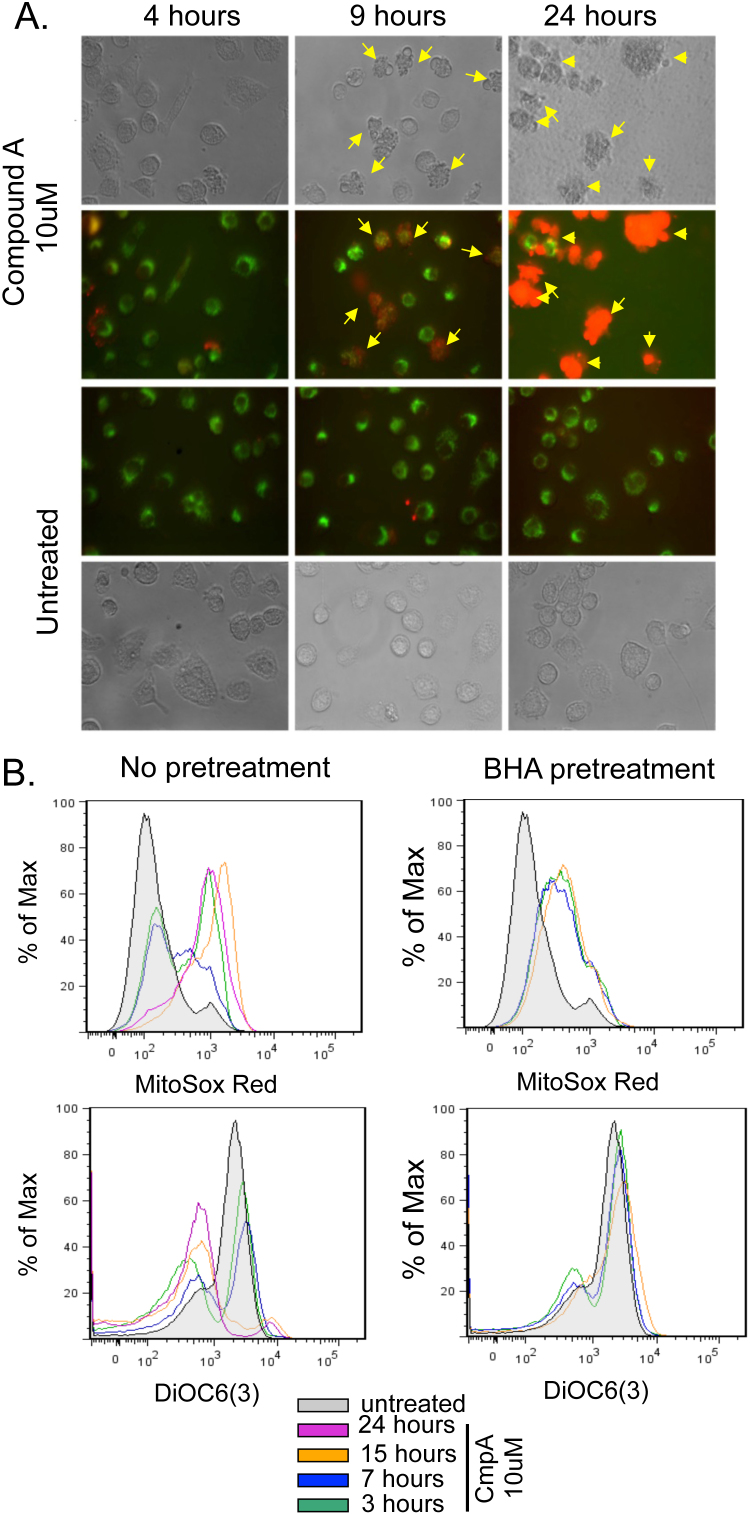
The activation of caspase-9 during NF-κB inhibition-induced PCD (Figure 3A, Supplemental Figure 2A) suggested a mitochondrial dependent apoptotic response12. Thus, mitochondrial ROS and membrane potential (ΔΨm) were measured using MitoSOX Red and DiOC6(3), respectively. FSDC were treated with 10 μM CmpA and fluorescence levels of MitoSOX Red and DiOC6(3) were visualized by microscopy, demonstrating a significant increase in ROS (red fluorescence) after incubation with CmpA, in particular in cells with apoptotic morphology (Figure 6A). Additionally, levels of MitoSOX Red and DiOC6(3) were measured by FACS at multiple time points (0, 3, 7, 15, and 24 hours) after treating cells with 10 μM CmpA. Following NF-κB inhibition, there was a progressive increase in ROS and a concomitant loss in ΔΨm, noted by decreased fluorescent levels of DiOC6 (Figure 6B).
Figure 6. IKK suppression results in increased ROS production and secondary loss of ΔΨm.
(A) FSDC were treated with 10 μM Compound A and at the indicated time points, MitoSOX (red) and DiOC6(3) (green) were analyzed by fluorescent microscopy. Cells with apoptotic morphology are marked with yellow arrows. (B) FSDCs were treated with 10 μM Compound A. These FSDCs were either grown in serum alone or pretreated with 200 μM BHA. At 3, 7, 15, and 24 hours, flow cytometry was performed to analyze staining with MitoSOX Red [ROS production] and DiOC6(3) [mitochondrial membrane potential, ΔΨm]. The results are representative of 3 independent experiments.
Increased mitochondrial ROS is often considered a consequence of loss of ΔΨm. However, a few recent reports suggest that increased mitochondrial ROS can precede loss of ΔΨm22,23. To elucidate the order of events, 200 μM BHA, an ROS scavenger that reduces O2−24, was added to the cells for 1 hour prior to the treatment with CmpA (Figure 6C). ROS levels increased in FSDC at 3 hours post-treatment with BHA + CmpA, then stabilized and remained constant through the 15-hour time point, while ROS increased continually in samples treated with CmpA alone. Furthermore, pretreatment of FSDC with BHA protected against loss of ΔΨm in response to CmpA (Figure 6B/C), suggesting that loss of ΔΨm is ROS-dependent.
ROS production occurs upstream of caspase activation
To determine if ROS production was upstream or downstream of caspase activation, FSDC were pretreated with BHA or left untreated and analyzed for caspase cleavage at 4-7 hours after inhibition of NF-κB with CmpA. Caspase-8 and -3 cleavage was observed in cells treated with CmpA alone, but not in those treated with BHA plus CmpA (Figure 7A). Therefore, these results demonstrate that, like ΔΨm, caspase activation is ROS-dependent. These data also support the observation that NF-κB inhibition-induced PCD is independent of the TNFα/JNK/Casapse-8 pathway. Furthermore, BHA treatment of FSDC results in suppression of cell death in response to both CmpA and IKKiVII (Figure 7B). This phenomenon was also observed in additional APC cell types, BMDM, BMDC and FSDC, treated with CmpA + BHA (Figure 7C). Another anti-oxidant deferoxamine (DFO)25, also suppressed NF-κB-inhibitor-induced PCD in primary WT macrophages (Figure 7D). However, neither BHA or DFO inhibited CmpA-induced cell death in APCs treated with LPS (Figure 7D), providing further evidence that LPS indeed acts by a separate mechanism to induce APC PCD in the presence of NF-κB inhibition. We also have demonstrated that treatment with the anti-oxidant nordihydroguaiaretic acid (NDGA), but not inhibitors of NADPH oxidase, diphenylene iodine (DPI) or apocynin, was able to prevent CmpA-induced APC death (Supplemental Figure 5). Taken together, these data suggest that caspase activation occurs secondary to ROS formation and loss of ΔΨm, and that ROS is the initiating event in this process.
Figure 7. ROS production is upstream of caspase activation in NF-κB-inhibition-induced APC death.
(A) FSDC were untreated or pretreated for 1 hr with BHA and samples were collected at 0 hours (no CmpA) or 4, 5, 6, and 7 hours post-treatment with CmpA (10 μM), and caspase-8 and caspase-3 activation were evaluated via immunoblot. β-actin was used as a loading control. All samples were run on the same gel. The full-length gels are provided in the supplemental information. (B) MTT assays were used to measure survival of FSDC that were untreated or treated with the listed doses of CmpA, or IKKiVII in the presence of 200 μM BHA. *, p<0.004; **, p<0.00002 (3 independent experiments in triplicate). (C) FSDC, BMDM, and BMDC were treated with CmpA ± 200 μM BHA and analyzed for survival via MTT assay. *, p<0.00005; **, p<0.0002; ***, p<0.001 (3 independent experiments in triplicate). (D) Survival of WT macrophages was measured by MTT in cells treated with the Media or CmpA ± LPS 100 ng, in the presence of 500 μM DFO or 200 μM BHA. *, p<0.02; **, p<0.001 (3 independent experiments in triplicate).
Discussion
The NBD peptide, when fused to a PTD, is therapeutic in animal models of inflammatory bowel disease (IBD), arthritis, type I diabetes, multiple sclerosis, Parkinson's disease and muscular dystrophy. While examining the mechanism of action of NBD in a mouse model of IBD, we observed that treatment of APC with the NBD peptide results in rapid and extensive apoptosis. These results suggest a novel NF-κB-inhibition-induced cell death response in APC. The previously observed NF-κB-inhibition-induced PCD response was reported to occur via the TNFα/JNK/caspase-8 signaling pathway. However, in APC, while it appears that the process is TNFα dependent, secondarily there is increased ROS formation, which leads to a subsequent loss of ΔΨm (Figure 6) and activation of caspase-9/3/8 (Figures 3 & 7). Using TNFα−/− primary macrophages, we demonstrated that a minimal level of NF-κB activation is necessary for NF-κB inhibition-induced cell death, which is equivalent to basal levels of TNFα (0.05 ng/ml) produced by WT macrophages in cell culture26. Further, non-APC cell lines treated with exogenous TNFα in the presence of NF-κB inhibition did not undergo cell death, thus this process is likely specific for APCs. While LPS induces cell death to a similar extent as that induced by the addition of TNFα in TNFα−/− BMDM (data not shown), other NF-κB activators including IL-1β and CpG did not induce similar effects. Additionally, LPS-induced death in APC with suppressed NF-κB activity could not be reversed by the anti-oxidants BHA and DFO. Thus, the LPS and TNFα dependent death pathways in APCs are likely different.
JNK inhibition had no effect on APC PCD and caspase-8 activation occurred subsequent to ROS production (Figure 5) and likely downstream of activation of both caspases-9 and -3. Taken together, these data provide evidence that this PCD pathway is independent of TNFα/JNK/Caspase-8 and death receptors, and suggests a novel TNFα/NF-κB inhibition-induced, APC-specific apoptotic pathway.
Our results suggest that ROS formation is a proximal component of this NF-κB inhibition-induced PCD pathway as demonstrated by the early increase in superoxide formation in cells (Figure 6) and the fact that addition of the antioxidant BHA to cell culture caused a stabilization of mitochondrial membrane potential, and prevented caspase activation (Figures 6 & 7). NF-κB is known to transcriptionally regulate several anti-oxidant genes including SOD1, SOD2 and FHC27,28,29. Further, it is known that NADPH oxidase is up-regulated in activated APC30, and that macrophages up-regulate iNOS and COX-2 during activation as a part of their immune function31. Thus, as APC apoptosis was dependent on NF-κB inhibition as well as TNFα activation, there are numerous possible mechanisms by which NF-κB suppression in the presence of stimulation may lead to elevation of intracellular ROS. However, our results suggest that NADPH oxidase regulation is not involved in the observed effect of NF-κB inhibition, since treatment with the NADPH oxidase inhibitors DPI and apocynin had no effect on NF-κB inhibition-induced cell death in APCs (Supplemental Figure 5).
Our data support and extend the findings of two previous studies, which evaluated the mechanisms of NF-κB inhibition-induced cell death in macrophages. Mannick et al, observed that a non-specific NF-κB inhibitor, PTDC, caused RAW264.7 macrophages to undergo cell death in culture32. This finding was expanded upon by Pagliari et al., who also used PTDC, but additionally examined NF-κB suppression using an adenovirus expressing IκBα-DN, which induced a clear collapse of ΔΨm. However, when using PTDC, it was observed that the cells were not rescued from apoptosis using caspase inhibitors, possibly due to zVAD toxicity in RAW264.7 cells. In addition, they did not observe caspase-3 cleavage, leading to the suggestion of a caspase-3-independent pathway, and did not address the role of ROS production in this apoptotic response12. Furthermore, since PTDC also has anti-oxidant properties33, the role of ROS in NF-κB-inhibition-induced cell death pathway was not examined. Pagliari et al suggested a major role for A1/bfl-1, a bcl-2-like anti-apoptotic protein, in preventing NF-κB-inhibition-induced macrophage cell death. We also observed a slight downregulation of A1/bfl-1 at the mRNA level following treatment with CmpA with no effect on Bcl-2 and Bcl-XL mRNA levels. However, an increase in A1/bfl-1 protein levels was observed by immunofluorescence and immunoblot after NF-κB suppression (Supplemental Figure 6).
The findings presented here may help to explain physiologic and therapeutic responses to NF-κB inhibition. PCD secondary to NF-κB inhibition in APC may explain responses observed in specific pathogen infections. A few well-known pathogens, including Yersinia bacteria and Vaccinia virus, cause macrophage and DC apoptosis after infection, and are known to produce NF-κB inhibitory compounds. Studies indicate that APC death is induced secondary to one of two NF-κB inhibitors produced by Yersinia, YopP and YopJ, as a loss of these molecules leads to improved APC survival34,35. Additionally NF-κB inhibition-induced PCD was enhanced by LPS signaling34. Vaccinia virus inhibits NF-κB signaling via two proteins, N1L36 and B1437. Furthermore, the N1L protein is deleted in attenuated Vaccinia virus, which does not induce APC death38. Therefore, APC death induced by NF-κB inhibition may further elucidate the cause of macrophage cell death responses associated with other pathogen infections.
The apoptotic response following NF-κB inhibition is biologically important not only in regard to APC responses or protection against infection, but also in evaluating therapeutics for cancer and inflammatory/autoimmune diseases. Recently, several groups have begun to examine the efficacy of NF-κB inhibition in treating myeloid leukemia, such as acute and chronic myeloid leukemia (AML and CML). AML and CML derive from myeloid progenitors, which give rise to non-lymphocyte white blood cells, including macrophages and DC39. In Imatinib-resistant CML cell lines, NF-κB/IKK was activated19,40,41, and as expected these cells underwent caspase-dependent cell death in response to NF-κB/IKK suppression. Furthermore, mice injected with CML cell lines showed reduced tumor burdens when treated with an IKKβ inhibitor41.
The NF-κB initiated inflammatory response is an obvious pharmacologic target for the treatment of autoimmune and inflammatory diseases. Not surprisingly, numerous NF-κB inhibitory compounds have been evaluated in the treatment of inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis, muscular dystrophy, Type-1 diabetes, as well as Parkinson's and osteoporosis. In each case, APC play a potent role in disease initiation or progression. Therefore, inhibition-induced APC death is a possible mechanism that could explain the ameliorating effects of NF-κB inhibitor treatment. In two studies evaluating the NBD peptide, NF-κB inhibition resulted in decreased APC number. Microglial cells, which are APCs present in the central nervous system, were reduced in number after treatment with NBD in a model of Parkinson's disease15. Similarly, there was a reduced number of infiltrating macrophages following NBD treatment in a mouse model of muscular dystrophy13, which may be the result of increased APC death. Additionally, experiments utilizing mice with floxed IKKβ promoters in macrophages showed that cells with partial deletion of IKKβ were selected for and that cells with complete knockdown of IKKβ were counter-selected42, suggesting that macrophages with complete loss of NF-κB/IKK after differentiation are not viable.
The novel mechanism of NF-κB-inhibition-induced PCD in APC described here offers a potential explanation for many of the observed therapeutic effects following treatment with NF-κB/IKK inhibitors. Many of the inhibitors which suppress NF-κB signaling have extremely short half-lives in vivo, but can be injected only a few times per week and still maintain their immunosuppressive effects. Thus, we hypothesize that this APC death may result in a fundamental change in the immune system that prolongs the efficacy of NF-κB/IKK inhibition. In addition, our observations that minimal stimulation is required for cell death to occur would potentially mean that only activated APC would undergo apoptosis with this NF-κB inhibitor therapy, further reducing side effects, and improving the therapeutic potential of NF-κB inhibitors.
Methods
NF-κB inhibitors used include: Compound A (gift from Bayer), IKK inhibitor VII (Calbiochem), IKK2 inhibitor IV (Calbiochem), Wedelolactone (Calbiochem), and MG-132 (Calbiochem). Caspase activation was inhibited by zVAD-fmk (Calbiochem). ROS production and apoptosis was inhibited by butylated hydroxyanisole (BHA) (Sigma), deferoxamine (DFO) (Calbiochem) and nordihydroguaiaretic acid (NDGA) (Aldrich). The NADPH oxidase inhibitors diphenylene iodine (DPI) and apocynin were obtained from Sigma. All compounds were diluted as suggested by manufacturers in DMSO and then diluted in desired media. MTT (Sigma) was diluted to 5 mg/ml in Opti-mem media (Invitrogen). Etanercept was obtained from Wyeth Pharmaceuticals. The JNKI/II inhibitor, SP600125, was obtained from Sigma. NF-κB activators were obtained as follows TNFα (Ebioscience), LPS (Sigma), CpG (Invitrogen), IL-1β (Peprotech).
Peptides
Peptide NF-κB inhibitors were synthesized in association with the HIV TAT-protein transduction domain (PTD) or the 8-lysine (8 K) PTD. The peptides TAT-NEMO Binding Domain (NBD; (YGRKKRRQRRRGGTALDWS WLQTE-amide) and inactive (mutant) TAT-NBD (mNBD; YGRKKRRQRRRGGTALDAS ALQTE-amide), were synthesized by the peptide synthesis facility at the University of Pittsburgh. Underlined amino acids represent tryptophan to alanine mutations. Peptides were purified and characterized by reversed-phase high performance liquid chromatography and mass spectrometry. For in vitro experiments, TAT-NBD and TAT-mNBD peptides were used, while in vivo 8K-NBD and 8K-mNBD peptides were used due to differing transduction rates.
Murine macrophages, DC and other cell lines
Wild-type bone marrow (BM)-derived macrophages (BMDM) were isolated from the femurs of C57BL/6J mice, both WT and TNFα−/−. BM was flushed with washing medium (RPMI 1640 supplemented with 1% penicillin/streptomycin), passed through a 70 μm nylon cell strainer into a 50-ml conical tube, and spun down at 1500 rpm for 5 min. RBC were lysed using ACK lysis buffer for 15 min, and resuspended in complete medium (washing medium with 10% FBS). BM cells were seeded in conditioned L-cell media (consisting of 20% precondition L-cell media, 60% DMEM, 20% FBS), supplemented with 1% L-glut, 1% sodium pyruvate, and 1% penicillin/streptomycin added to the media. Cells were seeded in 10 cm dishes, media was replaced after 3 days and cells were collected on day 7. Cells were passaged every 3–4 days and were discarded after one month.
Bone marrow-derived dendritic cells (BMDC) were isolated from mice as described for BMDM. BM cells were seeded at 5e6 cells per well in 6 well plates in complete RPMI supplemented with 10 ng/ml of GM-CSF (Cell Sciences) and 20 g/ml IL-4 (Cell Sciences). Media was replaced on Day 3 (supplemented with GM-CSF and IL-4) and cells were collected on Day 7. Cells were then isolated using MACs columns (Miltenyi Biotech) with positive selection using CD11c beads. Isolated cells were then seeded in complete RPMI media and used for experiments.
Other cell lines used included Fetal Skin Dendritic Cells (FSDC), an immortalized murine DC cell line (maintained in RPMI with 10% FBS and 1% P/S), RAW264.7, a murine macrophage cell line (maintained in RPMI with 10% FBS and 1% P/S), an immortalized T-cell line (D10; maintained in RPMI with 10% FBS, 1% NEAA, 1% Pen/strep, 1% HEPES, 1% Sodium Pyruvate, and 0.1% β-ME, and supplemented with 1:2000 50,000U IL-2), and primary mouse embryonic fibroblasts (MEFs) derived as described43 (maintained in DMEM with 44% Ham's F10, 10% FBS, 1% P/S and 1% NEAA). HEK293 cells were maintained in DMEM with 10% FBS and 1% P/S.
Animals were maintained in a pathogen-free animal facility at University of Pittsburgh Biotechnology Center. All animal-related experiments were conducted in strict accordance with the guidelines for the care and use of Laboratory Animals of the National Institutes of Health and animal protocol 0804421B-1 was approved by the University of Pittsburgh Institutional Animal Care and Use Committee, assurance number A3187-01. Mice were euthanized using CO2 for harvesting of organs.
NF-κB luciferase assay
HEK293 cells stably transfected with a multimerized NF-κB DNA binding element-luciferase reporter (293NF-κB cells), were pretreated for 1 hour with varying NF-κB inhibitory compounds (in Materials), and were activated for 3 h with 10 ng/ml TNFα (R&D Systems). The cells were lysed in reporter lysis buffer and luciferase activity was measured with a luciferase assay system (Promega) using an AutoLumat Luminometer (Berthold Technologies). Due to the high binding affinity of the PTD fragments of the peptides, peptides were administered in Opti-MEM prior to addition of TNFα. All other NF-κB inhibitors were administered in the maintenance media of the specific cell type being treated.
MTT Assay
Cells were seeded in 96 well plates at a concentration of 40,000 cells per well for FSDC, 10,000 cells/well for MEFs, 30,000 cells/well for D10, 30,000 cells/well for BMDM and BMDC and 30,000 cells/well for 293NF-κB cells. Cells were treated with listed doses of the NF-κB inhibitors and allowed to grow for 24 hours or as described. 10 μl of MTT working solution (5 mg/ml) was added to each well and incubated for 2 hours. Excess media was removed and crystals were dissolved in 20 μl of DMSO and then diluted in dH20. Absorbance was measured at 530 nm on MRX Revelation microplate reader (Dynex Technologies). Values were then normalized to untreated controls and blank wells.
Western blotting
Cells were treated over a time course with varying doses of NF-κB inhibitors as described in the results. Cells were collected by scraping, then pelleted and lysed using Reporter Lysis Solution (Promega). Protein concentration was determined via Bradford assay (Pierce). Western blots were performed with the following antibodies: Caspase 3 (Cell Signaling), Cleaved Caspase-9 (Cell Signaling), Cleaved Caspase-8 (Cell Signaling), β-actin (Abcam), A1/bfl-1 (Cell Signaling).
Mitochondrial Membrane Potential and Cellular ROS production
FSDC were treated with desired compounds as listed in individual experiments. At indicated time points, cells were treated with 40 nM DiOC6(3) (Invitrogen), which was made from a 40 mM stock in DMSO, and 5 mM MitoSOX Red (Invitrogen), used according to manufacturers' instructions. Cells were incubated in these solutions diluted in complete IMDM media for 30 min at 37°C protected from light. Cells were then washed two times in complete IMDM media and were subsequently analyzed by flow cytometry and fluorescence microscopy. Flow cytometry was performed using a LSR2 flow cytometer (BD Bioscience) and analyzed using FlowJo software (TreeStar, Inc.). Fluorescence imaging was performed using an Axiovert 200 Microscope and Axiovision software (Zeiss). To remove background fluorescence, all images had equally altered red and green channel levels for imaging but not quantification.
Immunofluorescence
FSDC were grown on poly-L-lysine-coated coverslips. Cells were fixed for 15 minutes using 2% paraformaldehyde (Sigma) and then washed with PBS. The cells were then permeabilized with 0.1% Triton X-100 (USB) in PBS and then washed and blocked with 2% BSA for 45 minutes. Cells were treated with indicated primary antibodies for 1 hour and then secondary antibodies conjugated to either Cy3 (1:1000) or SA488 (1:500) (Jackson) for 1 hour. DAPI stain was then added to cells for 30 seconds and coverslips were affixed to glass slides using gelvatol solution. Confocal microscopy was completed using an Olympus FluoView 1000.
mRNA analysis
FSDC and BMDM were grown in 6 well plates and collected by trypsinization. mRNA was isolated from cell pellets using the RNAqueous Kit (Ambion). mRNA was then quantified using a NanoDrop (Thermo Scientific). Samples were then analyzed for A1/bfl-1 (Fwd: 5′-AATTCCAACAGCCTCCAGATATG; Rev: 5′-GAACAAAAT ATCTGCAACTCTGG), Bcl2 (Fwd: 5′-TACCGTCGTGACTTCGCAGAG; Rev: 5′-GGCAGGCTGAGCAGGGTCTT), BclXL (Fwd: 5′-AGGCAGGCGATGAGTTT GAAC; Rev: 5′-GAACCACACCAGCCACAGTCA). Samples were run for 30 cycles using a Techgene PCR machine (Techne).
Statistical analysis
Experiments shown are representative of 3–5 independent experiments as described in each experiment with p = values being determined in those experiments with at least three replicates per independent experiment. Error bars in all experiments represent standard deviation. p-values were determined using the student T-test in cases of two variable analysis, and using ANOVA tukey analysis for multi-variable comparisons. Statistics were derived using SPSS (SPSS Inc., Chicago IL).
Author Contributions
J.S.T., L.J.N., S.E.P. and P.D.R. designed the experiments; J.S.T., D.F.G., J.Z. and S.H.D. performed the experiments; J.S.T., L.J.N. and P.D.R. analyzed the data, J.S.T. wrote the manuscript and D.F.G., J.Z., S.E.P., L.J.N. and P.D.R. edited the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK54452, R41 DK074193 and P30 DK34987 to S.E.P., ES016114 to L.J.N., U01 NS058451, P30 AG024827, R01 CA103730, R01 AR051456, R21 AG033907 and DOD grants 17-03-1-0488 and 17-03-0142 to P.D.R., National Research Service Awards F30 ES013617 to S.H.D. and F30 AG032816 to J.S.T., a post-doctoral fellowship from the Juvenile Diabetes Research Foundation (JDRF) to D.F.G. and funding from the Broad Medical Research Program to S.E.P.
References
- Nathan D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353, 2643–2653 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grip O., Janciauskiene S. & Lindgren S. Macrophages in inflammatory bowel disease. Curr Drug Targets Inflamm Allergy 2, 155–160 (2003). [DOI] [PubMed] [Google Scholar]
- Perry V. H. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun 18, 407–413 (2004). [DOI] [PubMed] [Google Scholar]
- Gilmore T. Rel/NF-kB Transcription Factors. http://www.nf-kb.org (accessed December 30 2009).
- Dutta J., Fan Y., Gupta N., Fan G. & Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 25, 6800–6816 (2006). [DOI] [PubMed] [Google Scholar]
- Papa S. et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ 13, 712–729 (2006). [DOI] [PubMed] [Google Scholar]
- Beg A. A., Sha W. C., Bronson R. T., Ghosh S. & Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 376, 167–170 (1995). [DOI] [PubMed]
- Rudolph D. et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev 14, 854–862 (2000). [PMC free article] [PubMed] [Google Scholar]
- Li Q., Van Antwerp D., Mercurio F., Lee K. F. & Verma I. M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284, 321–325 (1999). [DOI] [PubMed] [Google Scholar]
- Doi T. S. et al. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci U S A 96, 2994–2999 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A., Heyninck K. & Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol 72, 1090–1101 (2006). [DOI] [PubMed] [Google Scholar]
- Pagliari L. J., Perlman H., Liu H. & Pope R. M. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol 20, 8855–8865 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya S. et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117, 889–901 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K. K. et al. Protection of islets by in situ peptide-mediated transduction of the Ikappa B kinase inhibitor Nemo-binding domain peptide. J Biol Chem 278, 9862–9868 (2003). [DOI] [PubMed] [Google Scholar]
- Ghosh A. et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 104, 18754–18759 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S. H. et al. Amelioration of Chronic Murine Colitis by Peptide-Mediated Transduction of the I{kappa}B Kinase Inhibitor NEMO Binding Domain Peptide. J Immunol 179, 7852–7859 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Hirayama T., Abbas S. & Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem 279, 37219–37222 (2004). [DOI] [PubMed] [Google Scholar]
- Adler A. et al. Motif module map reveals enforcement of aging by continual NF-kB activity. Genes Dev 21 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E. A. et al. IkappaB kinase beta inhibition induces cell death in Imatinib-resistant and T315I Dasatinib-resistant BCR-ABL+ cells. Mol Cancer Ther 7, 391–397 (2008). [DOI] [PubMed] [Google Scholar]
- Dave S. H. et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol 179, 7852–7859 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten F. R. et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130, 918–931 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y. & Park J. H. ROS-dependent caspase-9 activation in hypoxic cell death. FEBS Lett 549, 94–98 (2003). [DOI] [PubMed] [Google Scholar]
- Bubici C., Papa S., Dean K. & Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25, 6731–6748 (2006). [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Morgan M. J., Choksi S. & Liu Z. G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 26, 675–687 (2007). [DOI] [PubMed] [Google Scholar]
- Shimoni E., Armon R. & Neeman I. Antioxidant properties of deferoxamine. J. Am. Oil Chem. Soc. 71, 641–644 (1994). [Google Scholar]
- Hsu L. C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A 105, 7803–7808 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K. C., Lewis-Molock Y. & White C. W. Activation of NF-kappa B and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am J Physiol 269, L588–602 (1995). [DOI] [PubMed] [Google Scholar]
- Xu Y. et al. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol 18, 709–722 (1999). [DOI] [PubMed] [Google Scholar]
- Kwak E. L., Larochelle D. A., Beaumont C., Torti S. V. & Torti F. M. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem 270, 15285–15293 (1995). [DOI] [PubMed] [Google Scholar]
- Cathcart M. K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24, 23–28 (2004). [DOI] [PubMed] [Google Scholar]
- Suh N. et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res 58, 717–723 (1998). [PubMed] [Google Scholar]
- Mannick E. E. et al. Inhibitors of nuclear factor kappa B cause apoptosis in cultured macrophages. Mediators Inflamm 6, 225–232 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham C. G. et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119, 529–542 (2004). [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K. et al. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J Immunol 166, 1823–1831 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ting A. T., Marcu K. B. & Bliska J. B. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol 174, 7939–7949 (2005). [DOI] [PubMed] [Google Scholar]
- DiPerna G. et al. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. J Biol Chem 279, 36570–36578 (2004). [DOI] [PubMed] [Google Scholar]
- Chen R. A., Ryzhakov G., Cooray S., Randow F. & Smith G. L. Inhibition of IkappaB kinase by vaccinia virus virulence factor B14. PLoS Pathog 4, e22 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J. N. & Quesnel-Hellmann A. Host-pathogen interactions: a biological rendez-vous of the infectious nonself and danger models? PLoS Pathog 2, e44 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. D., Liebermann D. A. & Hoffman B. Egr-1 abrogates the E2F-1 block in terminal myeloid differentiation and suppresses leukemia. Oncogene 27, 98–106 (2008). [DOI] [PubMed] [Google Scholar]
- Reuther J. Y., Reuther G. W., Cortez D., Pendergast A. M. & Baldwin A. S. Jr A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev 12, 968–981 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounnas N. et al. NF-kappaB inhibition triggers death of imatinib-sensitive and imatinib-resistant chronic myeloid leukemia cells including T315I Bcr-Abl mutants. Int J Cancer 125, 308–317 (2009). [DOI] [PubMed] [Google Scholar]
- Kanters E. et al. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest 112, 1176–1185 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information